Metal Nanoparticles Formation from Nickel Hydroxide
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Jeevanandam, J.; Barhoum, A.; Chan, Y.S.; Dufresne, A.; Danquah, M.K. Review on nanoparticles and nanostructured materials: History, sources, toxicity and regulations. Beilstein J. Nanotechnol. 2018, 9, 1050–1074. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shahbazi, R.; Ozcicek, I.; Ozturk, G.; Ulubayram, K. Functionalized gold nanoparticles manifested as potent carriers for nucleolar targeting. Nanotechnology 2017, 28, 025103. [Google Scholar] [CrossRef] [PubMed]
- Dzidziguri, E.L.; Sidorova, E.N.; Inkar, M.; Yudin, A.G.; Kostitsyna, E.V.; Ozherelkov, D.Y.; Slusarsky, K.V.; Nalivaiko, A.Y.; Gromov, A.A. Cobalt nanoparticles synthesis by cobalt nitrate reduction. Materials Research Express. Mater. Res. Express 2019, 6, 105081. [Google Scholar] [CrossRef]
- Luong, N.H.; Long, N.N.; Vu, L.V.; Hai, N.H.; Nghia, P.T.; van Anh, N.T. Metallic nanoparticles: Synthesis, characterisation and application. Int. J. Nanotechnol. 2011, 8, 227–240. [Google Scholar] [CrossRef]
- Bai, Y.; Williams, C.B. The effect of inkjetted nanoparticles on metal part properties in binder jetting additive manufacturing. Nanotechnology 2018, 29, 395706. [Google Scholar] [CrossRef]
- Sebastian, M.; Aravind, A.; Mathew, B. Green silver-nanoparticle-based dual sensor for toxic Hg(II) ions. Nanotechnology 2018, 29, 355502. [Google Scholar] [CrossRef]
- Wu, X.; Xing, W.; Zhang, L.; Zhuo, S.; Zhou, J.; Wang, G.; Qiao, S. Nickel nanoparticles prepared by hydrazine hydrate reduction and their application in supercapacitor. Powder Technol. 2012, 224, 162–167. [Google Scholar] [CrossRef]
- Dzidziguri, E.L.; Sidorova, E.N.; Yahiyaeva, J.E.; Ozherelkov, D.Y.; Gromov, A.A.; Nalivaiko, A.Y. Low-temperature oxidation of metal nanoparticles obtained by chemical dispersion. Micro Nano Lett. 2020, 15, 461–464. [Google Scholar] [CrossRef]
- Chen, D.-H.; Wu, S.-H. Synthesis of Nickel Nanoparticles in Water-in-Oil Microemulsions. Chem. Mater. 2000, 12, 1354–1360. [Google Scholar] [CrossRef] [Green Version]
- Pajor-Swierzy, A.; Socha, R.; Pawłowski, R.; Warszynski, P.; Szczepanowicz, K. Application of metallic inks based on nickel-silver core-shell nanoparticles for fabrication of conductive films. Nanotechnology 2019, 30, 225301. [Google Scholar] [CrossRef]
- Alymov, M.I.; Rubtsov, N.M.; Seplyarskii, B.S.; Zelenskii, V.A.; Ankudinov, A.B. Synthesis of Nickel Nanopowders Under Dynamic Conditions. Nanotechnol. Russ. 2018, 13, 557–560. [Google Scholar] [CrossRef]
- Shih, Y.-L.; Wu, C.-L.; Wu, T.-Y.; Chen, D.-H. Electrochemical fabrication of nickel phosphide/reduced graphene oxide/nickel oxide composite on nickel foam as a high performance electrode for supercapacitors. Nanotechnology 2019, 30, 115601. [Google Scholar] [CrossRef] [PubMed]
- Peng, B.; Zhang, X.; Aarts, D.G.A.L.; Dullens, R.P.A. Superparamagnetic nickel colloidal nanocrystal clusters with antibacterial activity and bacteria binding ability. Nat. Nanotechnol. 2018, 13, 478–482. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, S.K.; Kaur, R.; Bharti, S.; Gawri, I.; Kaur, J. Recent Advances in Synthesis, Properties and Applications of Magnetic Oxide Nanomaterials. Solid State Phenom. 2015, 232, 1–44. [Google Scholar] [CrossRef]
- McHenry, M.E.; Laughlin, D.E. Nano-scale materials development for future magnetic applications. Acta Mater. 2000, 48, 223–238. [Google Scholar] [CrossRef]
- Sportelli, M.C.; Clemente, M.; Izzi, M.; Volpe, A.; Ancona, A.; Picca, R.A.; Palazzo, G.; Cioffi, N. Exceptionally stable silver nanoparticles synthesized by laser ablation in alcoholic organic solvent. Colloids Surf. A 2018, 559, 148–158. [Google Scholar] [CrossRef]
- Shalichah, C.; Khumaeni, A. Synthesis of nickel nanoparticles by pulse laser ablation method using Nd:YAG laser. J. Phys. Conf. Ser. 2018, 1025, 012002. [Google Scholar] [CrossRef]
- Dong, X.; Ji, X.; Wu, H.; Zhao, L.; Li, J.; Yang, W.J. Shape control of silver nanoparticles by stepwise citrate reduction. Phys. Chem. C 2009, 113, 6573–6576. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, Q.; Liu, Z.; Libor, Z. Highly efficient size reduction of nanoparticles by the shock wave method. Funct. Mater. Lett. 2010, 3, 299–302. [Google Scholar] [CrossRef]
- Ji, X.; Song, X.; Li, J.; Bai, Y.; Yang, W.; Peng, X. Size Control of Gold Nanocrystals in Citrate Reduction: The Third Role of Citrate. J. Am. Chem. Soc. 2007, 129, 13939–13948. [Google Scholar] [CrossRef]
- Bhattacharyya, R.; Bhanja, K.; Mohan, S. Mathematical analysis of reduction of copper oxide pellets by hydrogen using the shrinking core model. Fusion Eng. Des. 2015, 100, 560–564. [Google Scholar] [CrossRef]
- Kar, P. A comparison of the pseudo-steady-state and shrinking-core model for the reduction of titanium dioxide to titanium. J. Solid State Electrochem. 2008, 12, 1611–1617. [Google Scholar] [CrossRef]
- Villen-guzman, M.; Paz-garcia, J.M.; Arhoun, B.; Cerrillo-gonzalez, M.M.; Rodriguez-maroto, J.M.; Vereda-alonso, C.; Gomez-lahoz, C. Chemical Reduction of Nitrate by Zero-Valent Iron: Shrinking-Core versus Surface Kinetics Models. Int. J. Environ. Res. Public Health 2020, 17, 1241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vilardi, G. Mathematical modelling of simultaneous nitrate and dissolved oxygen reduction by Cu-nZVI using a bi-component shrinking core model. Powder Technol. 2019, 343, 613–618. [Google Scholar] [CrossRef]
- Vyatskikh, A.; Delalande, S.; Kudo, A.; Zhang, X.; Portela, C.M.; Greer, J.R. Additive manufacturing of 3D nano-architected metals. Nat. Commun. 2018, 9, 593. [Google Scholar] [CrossRef] [Green Version]
- Gu, D.; Wang, H.; Dai, D.; Yuan, P.; Meiners, W.; Poprawe, R. Rapid fabrication of Al-based bulk-form nanocomposites with novel reinforcement and enhanced performance by selective laser melting. Scr. Mater. 2015, 96, 25–28. [Google Scholar] [CrossRef]
- Li, X.P.; Ji, G.; Chen, Z.; Addad, A.; Wu, Y.; Wang, H.W.; Vleugels, J.; van Humbeeck, J.; Kruth, J.P. Selective laser melting of nano-TiB2 decorated AlSi10Mg alloy with high fracture strength and ductility. Acta Mater. 2017, 129, 183–193. [Google Scholar] [CrossRef]
- Im, D.H.; Park, S.Y.; Hyun, S.H.; Lee, B.Y.; Kim, Y.H. Aqueous dispersion stability of nickel powders prepared by a chemical reduction method. J. Mater. Sci. 2004, 39, 3629–3633. [Google Scholar] [CrossRef]
- Kuznetsov, D.V.; Lysov, D.V.; Levina, V.V.; Kondrat'eva, M.N.; Ryzhonkov, D.I.; Kaloshkin, S.D. Structural special features in nanodispersed Ni-SiO2 composite materials produced by method of chemical dispersion. Inorg. Mater. Appl. Res. 2010, 1, 57–63. [Google Scholar] [CrossRef]
- Volynsky, V.V.; Lopashev, A.V.; Kazarinov, I.A.; Tsimbalenko, E.V.; Kolesnikov, I.V. Structural and electrochemical properties of nickel hydroxides. Electrochem. Energy 2004, 4, 179–194. (In Russian) [Google Scholar]
- NIST X-ray Photoelectron Spectroscopy Database; NIST Standard Reference Database 20, Version 4.1./Data Comp. and Eval; Naumkin, A.V.; Kraut-Vass, A.; Gaarenstroom, S.W.; Powell, C.J. (Eds.) The National Institute of Standards and Technology NIST: Gaithersburg, MD, USA, 2012; pp. 1–49. [Google Scholar]

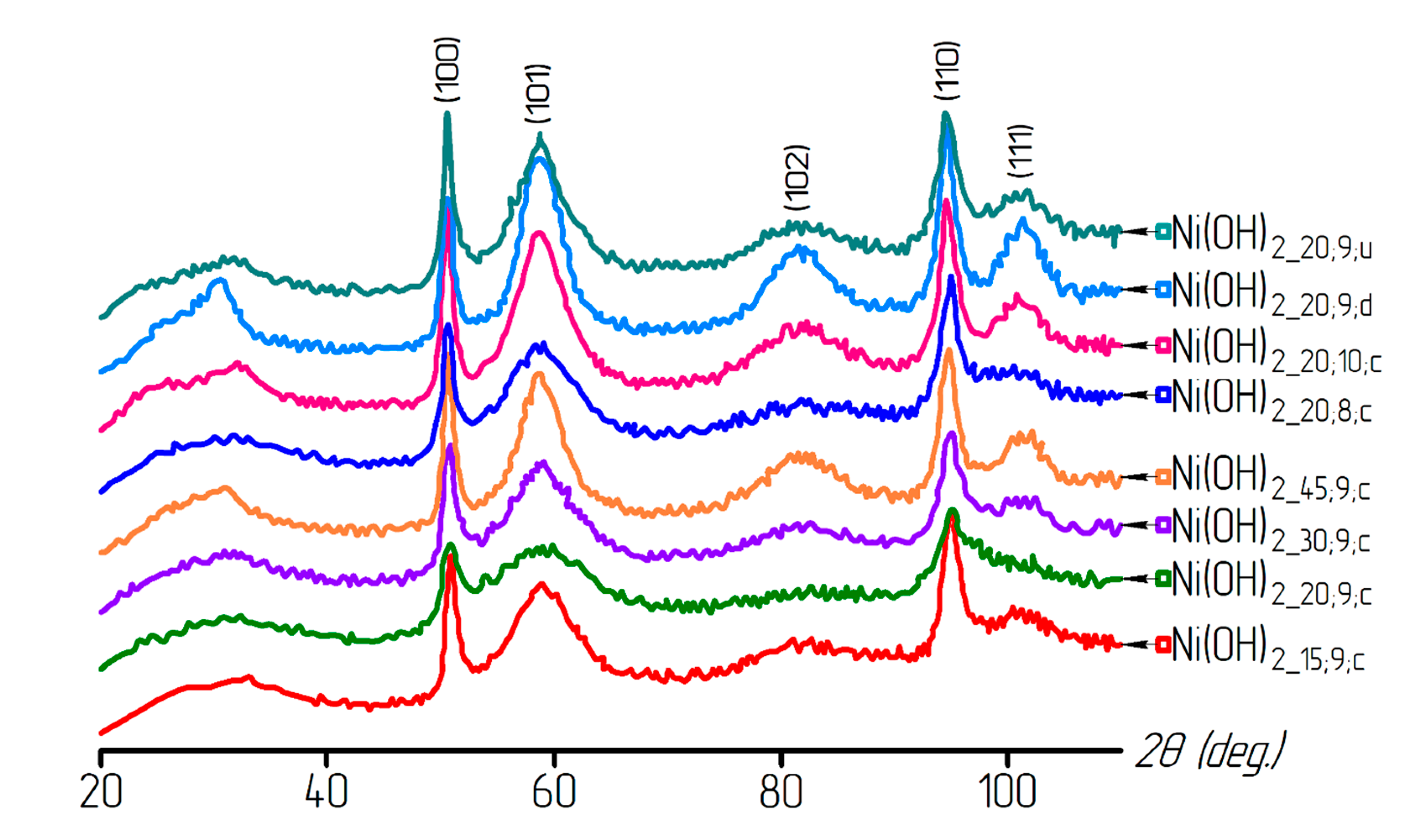

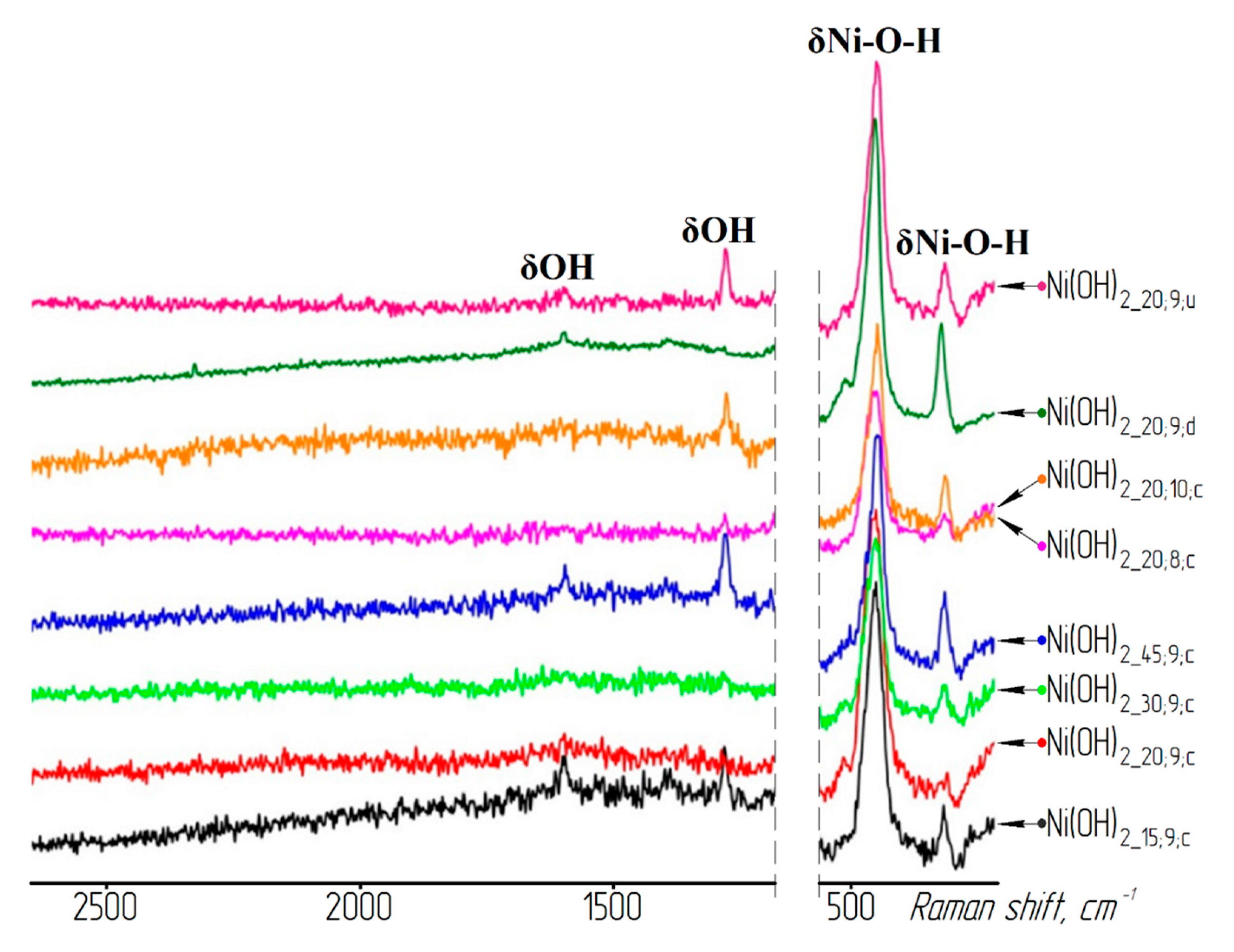


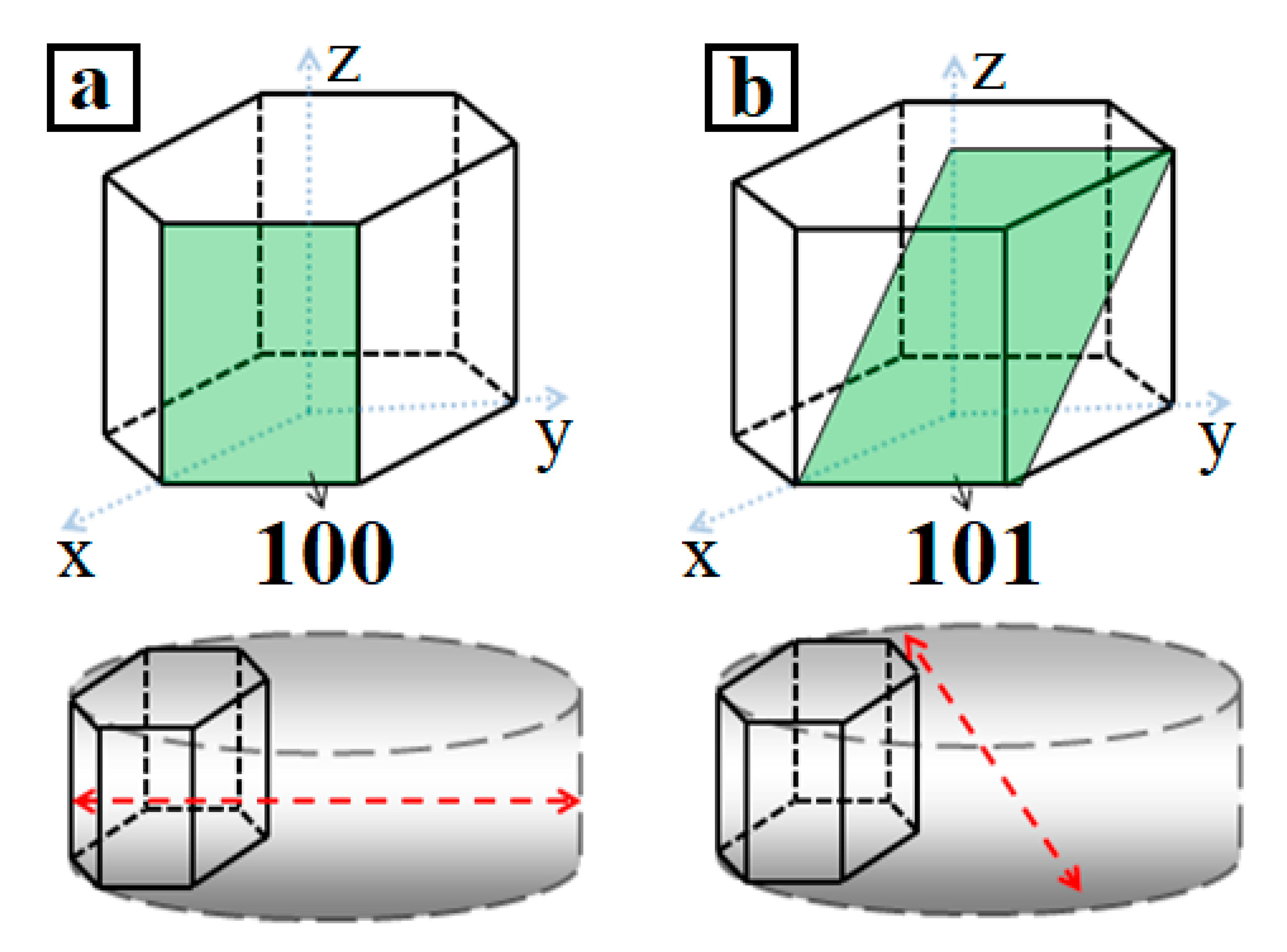
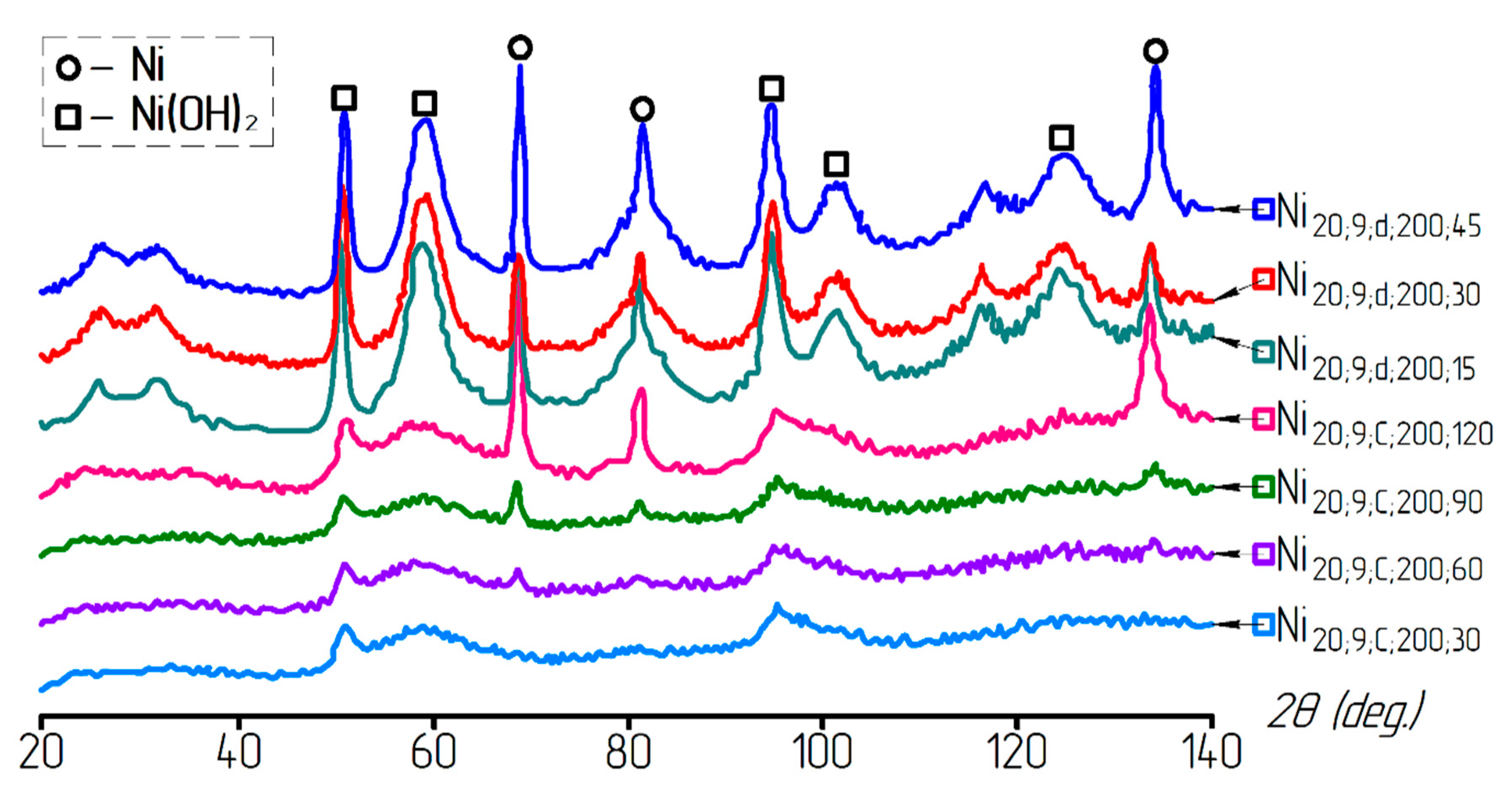
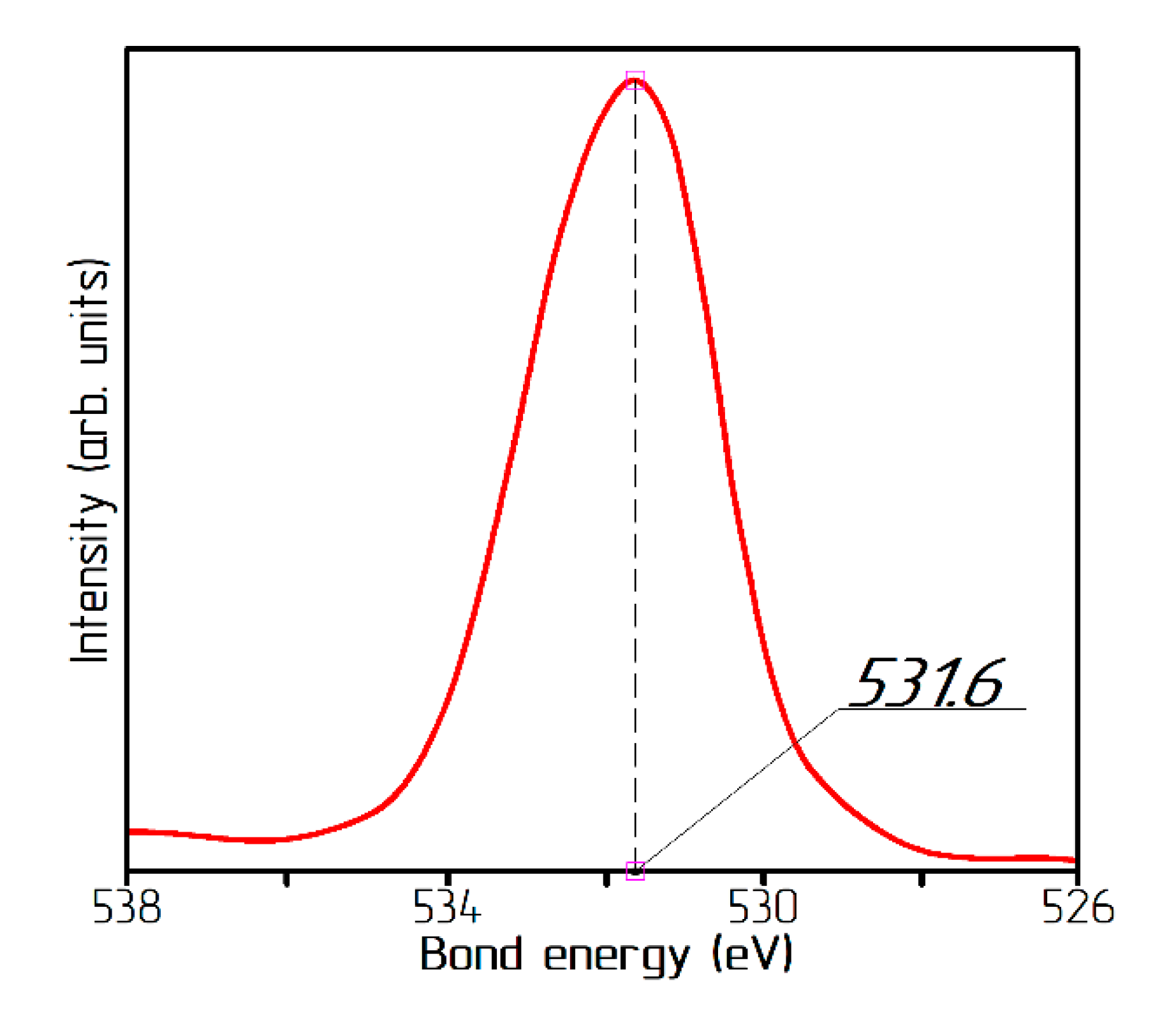


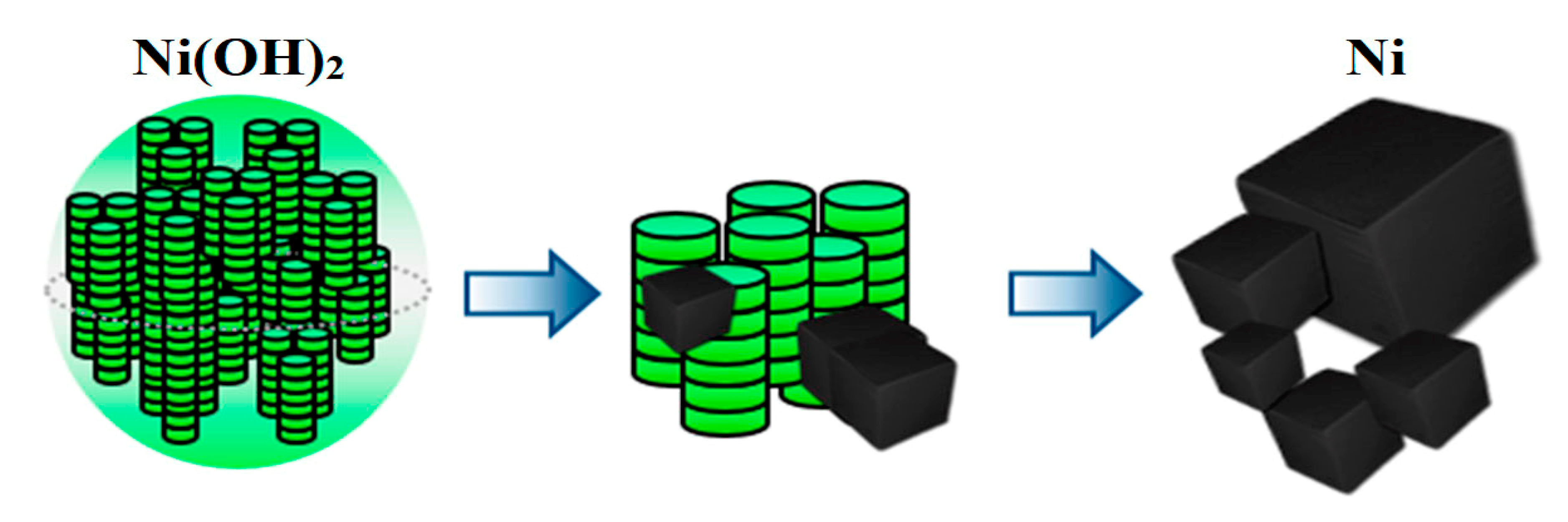
| Sample | T, °C | pH | Washing | Reduced Sample | T, °C | Time, min |
|---|---|---|---|---|---|---|
| Ni(OH)2_15;9;c | 15 | 9 | centrifuge | - | - | - |
| Ni(OH)2_20;9;c | 20 | 9 | centrifuge | Ni20;9;c;200;30 | 200 | 30 |
| Ni20;9;c;200;60 | 200 | 60 | ||||
| Ni20;9;c;200;90 | 200 | 90 | ||||
| Ni20;9;c;200;120 | 200 | 120 | ||||
| Ni(OH)2_30;9;c | 30 | 9 | centrifuge | - | - | - |
| Ni(OH)2_45;9;c | 45 | 9 | centrifuge | - | - | - |
| Ni(OH)2_20;8;c | 20 | 8 | centrifuge | - | - | - |
| Ni(OH)2_20;10;c | 20 | 10 | centrifuge | - | - | - |
| Ni(OH)2_20;9;d | 20 | 9 | decantation + centrifuge | Ni20;9;d;200;15 | 200 | 15 |
| Ni20;9;d;200;30 | 200 | 30 | ||||
| Ni20;9;d;200;45 | 200 | 45 | ||||
| Ni20;9;d;220;30 | 220 | 30 | ||||
| Ni(OH)2_20;9;u | 20 | 9 | ultrasound + centrifuge | - | - | - |
| Sample | a, nm | c, nm |
|---|---|---|
| Reference values | 0.3117 | 0.4595 |
| Ni(OH)2_15;9;c | 0.3071 | 0.4790 |
| Ni(OH)2_20;9;c | 0.3082 | 0.4762 |
| Ni(OH)2_30;9;c | 0.3077 | 0.4731 |
| Ni(OH)2_45;9;c | 0.3091 | 0.4816 |
| Ni(OH)2_20;8;c | 0.3088 | 0.4737 |
| Ni(OH)2_20;10;c | 0.3094 | 0.4786 |
| Ni(OH)2_20;9;d | 0.3089 | 0.4762 |
| Ni(OH)2_20;9;u | 0.3095 | 0.4796 |
| Sample | Ssp, m2/g | Average Aggregate Diameter, nm | Plate Diameter, nm | Number of Plates in One Aggregate, N |
|---|---|---|---|---|
| Ni(OH)2_15;9;c | 8 | 183 | 25 | 5230 |
| Ni(OH)2_20;9;c | 3 | 472 | 17 | 312,073 |
| Ni(OH)2_30;9;c | 13 | 109 | 17 | 2985 |
| Ni(OH)2_45;9;c | 52 | 27 | 24 | 28 |
| Ni(OH)2_20;8;c | 5 | 264 | 15 | 37,697 |
| Ni(OH)2_20;10;c | 35 | 41 | 18 | 164 |
| Ni(OH)2_20;9;d | 39 | 36 | 25 | 61 |
| Ni(OH)2_20;9;u | 4 | 400 | 21 | 80,481 |
| Sample | CSR Sizes, nm | Base Diameter TEM, nm | |
|---|---|---|---|
| (100) | (101) | ||
| Ni(OH)2_15;9;c | 39 | 4 | 28 |
| Ni(OH)2_20;9;c | 15 | 5 | 15 |
| Ni(OH)2_30;9;c | 34 | 8 | 17 |
| Ni(OH)2_45;9;c | 30 | 6 | 22 |
| Ni(OH)2_20;8;c | 25 | 5 | 18 |
| Ni(OH)2_20;10;c | 29 | 5 | 17 |
| Ni(OH)2_20;9;d | 36 | 5 | 23 |
| Ni(OH)2_20;9;u | 19 | 4 | 23 |
| Sample | Ni(OH)2 | Ni |
|---|---|---|
| Average size, nm | base diameter 38.5 | cube edge 28 |
| Volume, nm3 | 29,880 | 22,222 |
| Nickel atoms number | 12,944 | 2,020,224 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sidorova, E.N.; Dzidziguri, E.L.; Vinichenko, Y.P.; Ozherelkov, D.Y.; Shinkaryov, A.S.; Gromov, A.A.; Nalivaiko, A.Y. Metal Nanoparticles Formation from Nickel Hydroxide. Materials 2020, 13, 4689. https://doi.org/10.3390/ma13204689
Sidorova EN, Dzidziguri EL, Vinichenko YP, Ozherelkov DY, Shinkaryov AS, Gromov AA, Nalivaiko AY. Metal Nanoparticles Formation from Nickel Hydroxide. Materials. 2020; 13(20):4689. https://doi.org/10.3390/ma13204689
Chicago/Turabian StyleSidorova, Elena N., Ella L. Dzidziguri, Yulia P. Vinichenko, Dmitriy Yu. Ozherelkov, Alexander S. Shinkaryov, Alexander A. Gromov, and Anton Yu. Nalivaiko. 2020. "Metal Nanoparticles Formation from Nickel Hydroxide" Materials 13, no. 20: 4689. https://doi.org/10.3390/ma13204689
APA StyleSidorova, E. N., Dzidziguri, E. L., Vinichenko, Y. P., Ozherelkov, D. Y., Shinkaryov, A. S., Gromov, A. A., & Nalivaiko, A. Y. (2020). Metal Nanoparticles Formation from Nickel Hydroxide. Materials, 13(20), 4689. https://doi.org/10.3390/ma13204689







