Evaluation of 1-Ethyl-3-(3-Dimethylaminopropyl) Carbodiimide Cross-Linked Collagen Membranes for Guided Bone Regeneration in Beagle Dogs
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials and Their Characterization
2.1.1. Experimental Materials
2.1.2. Field Emission Scanning Electron Microscopy (FE-SEM)
2.1.3. Energy-Dispersive X-ray Spectroscopy (EDS) Analysis
2.1.4. Tensile Strength Test
2.1.5. Degradation Test
2.2. In Vitro Study
2.2.1. Cell Cultures
2.2.2. Proliferation Assay
2.2.3. Immunocytochemistry
2.3. In Vivo Study
2.3.1. Surgical Procedures
2.3.2. Micro-Computed Tomography (µCT) Analysis
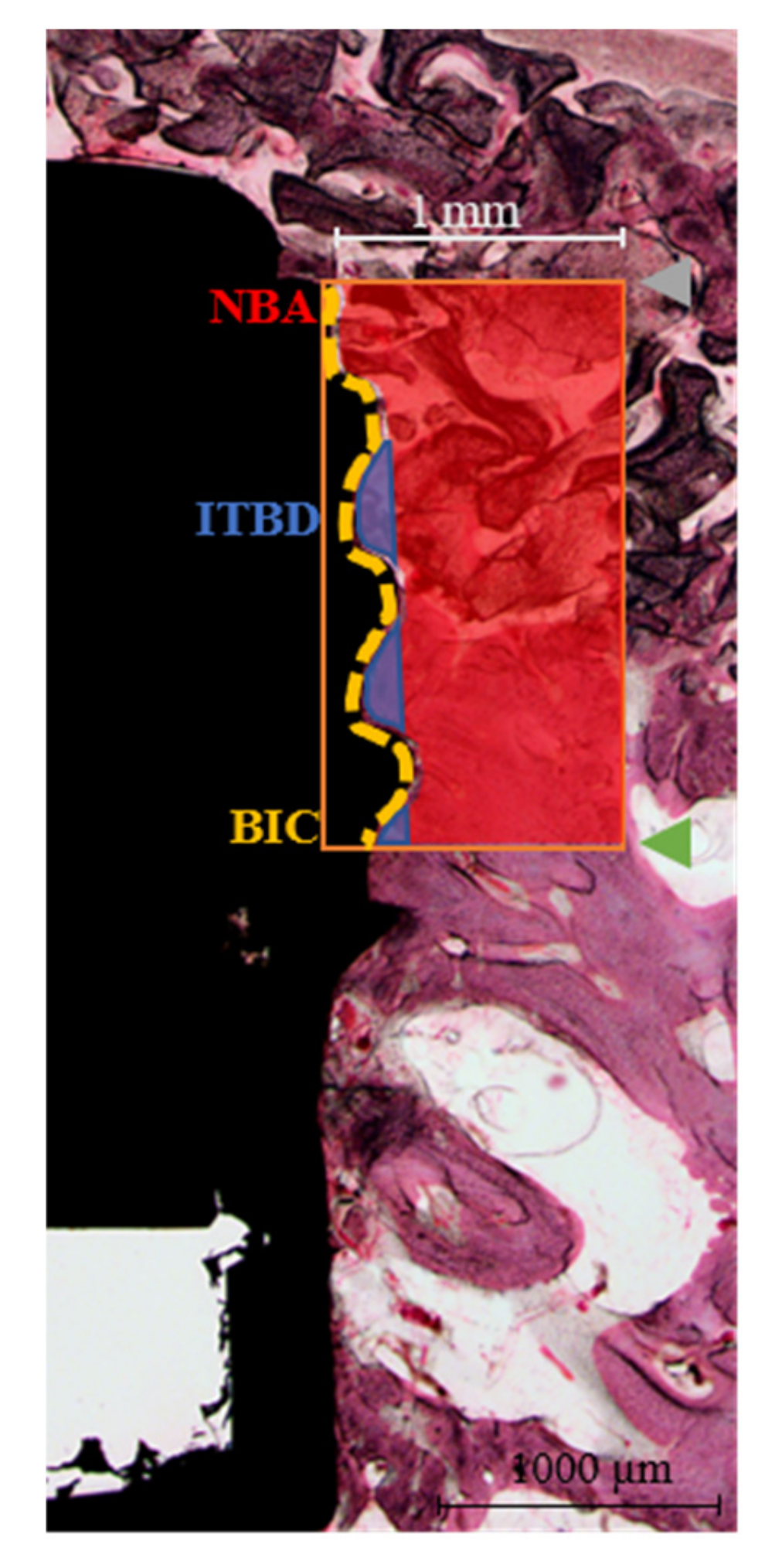
2.3.3. Histological and Histomorphometric Analysis
- NBA (%) = New bone area in the 1 mm-wide rectangle from the top of the implant fixture platform to the third thread (mm2)/total area of the 1 mm-wide rectangle (mm2) × 100
- ITBD (%) = New bone area between the first to third threads of the implant fixture (mm2)/total area between the first to third threads (mm2) × 100
- BIC (%) = Length of contact with new bone (mm)/total length from the platform to the third implant thread (mm) × 100
2.4. Statistical Analysis
3. Results
3.1. Characterization Results
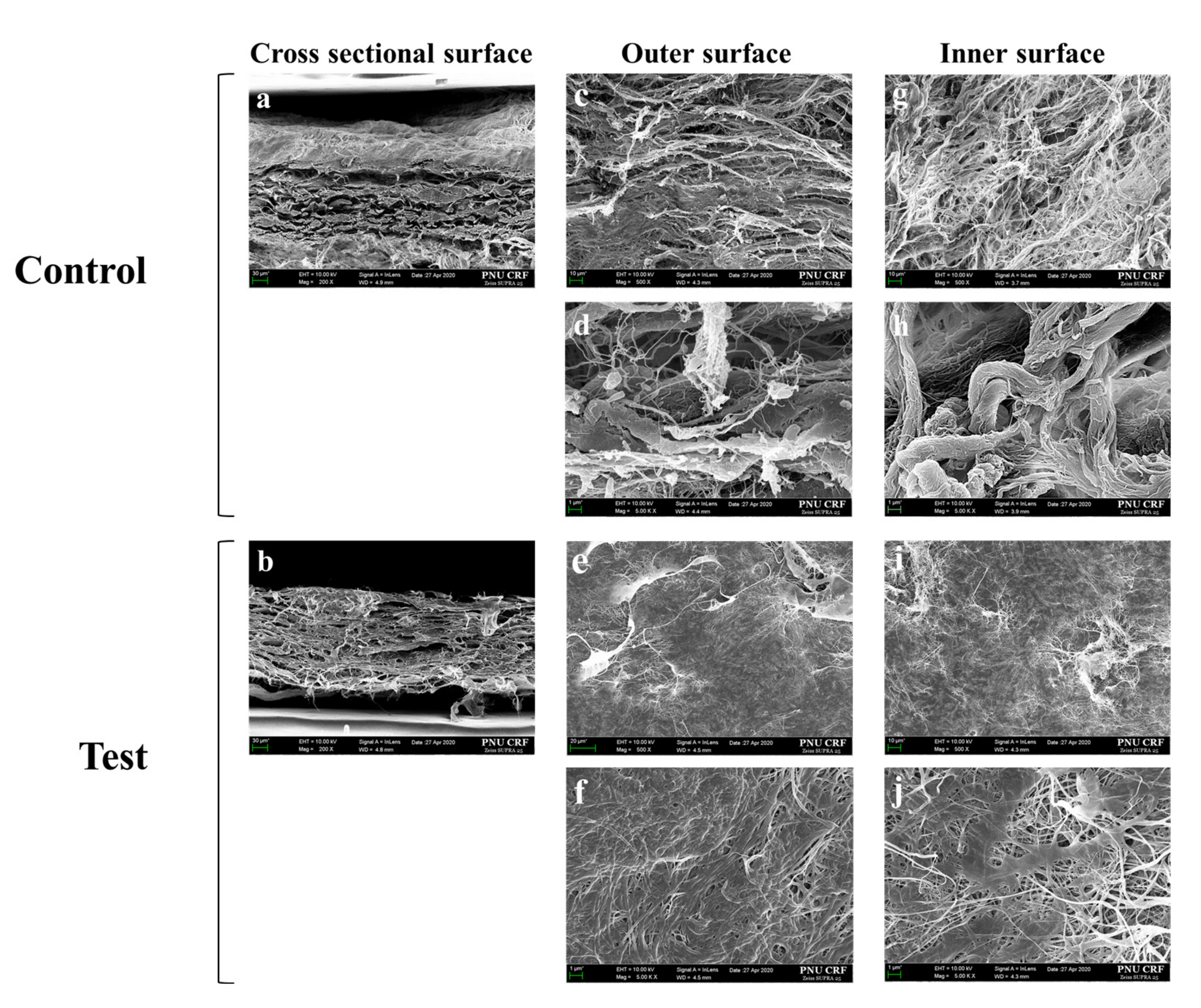
3.1.1. Morphological Findings
3.1.2. EDS Analysis
3.1.3. Tensile Strength Test
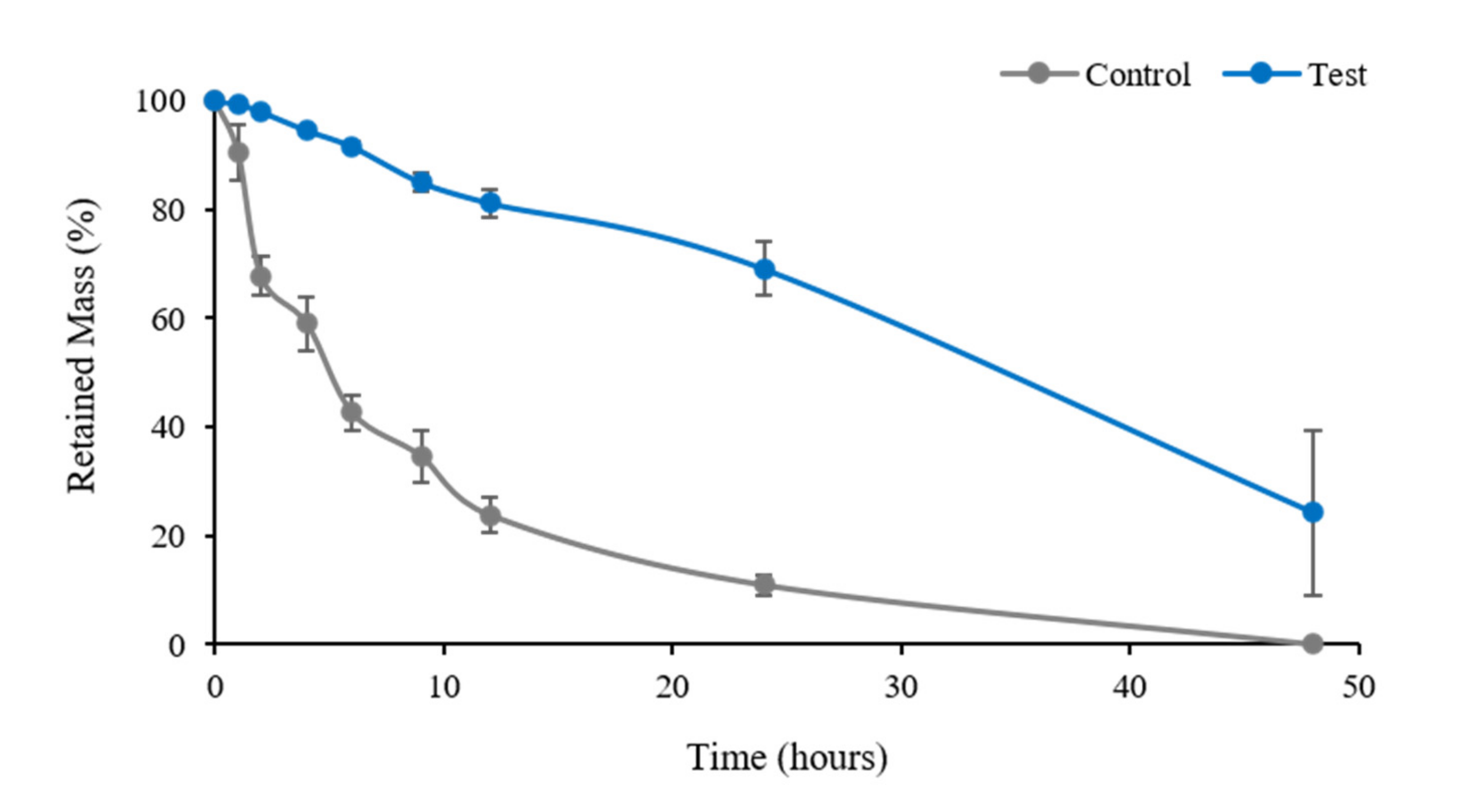
3.1.4. Enzymatic Degradation Test
3.2. In Vitro Results
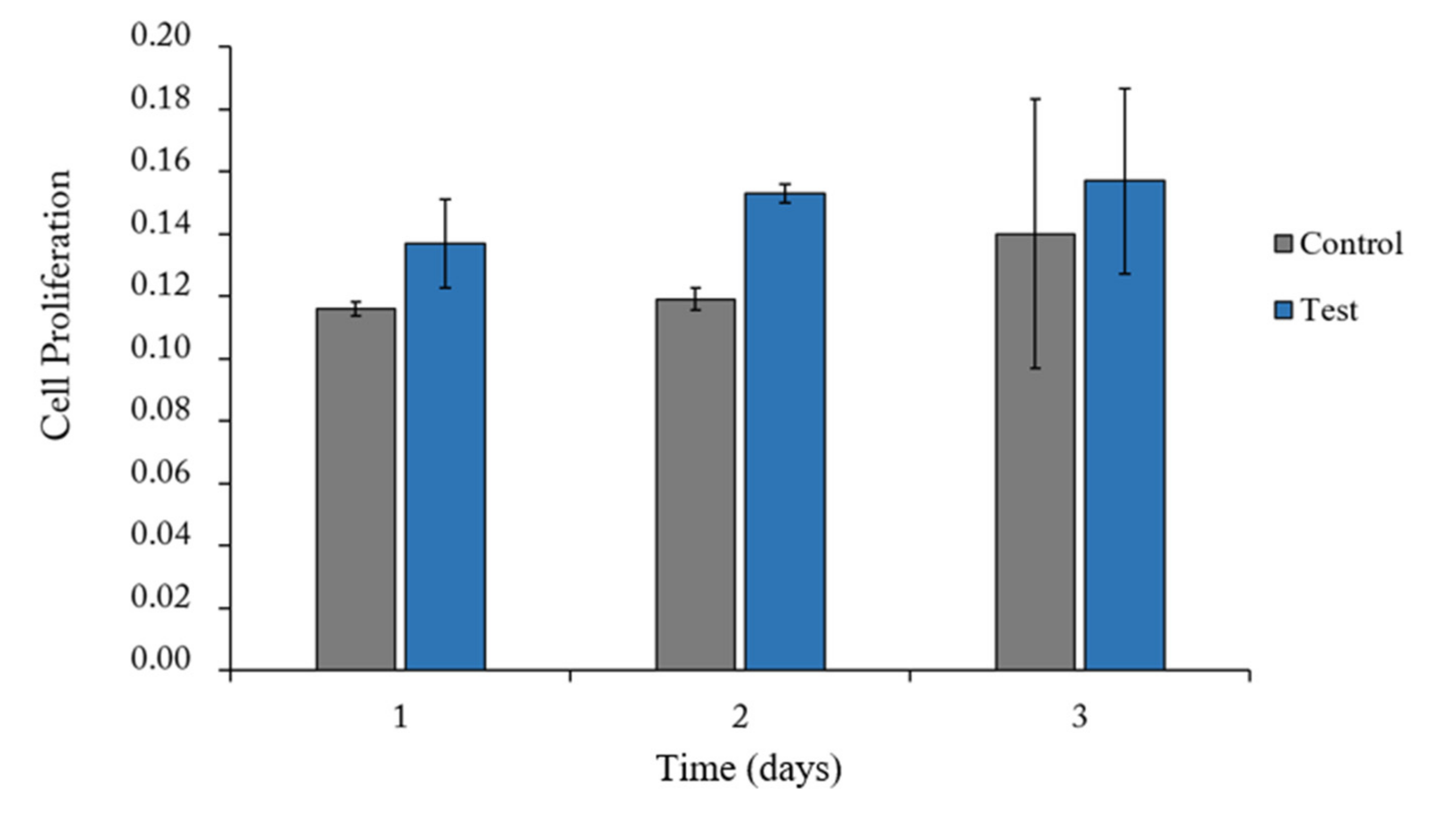
3.2.1. Proliferation Assay
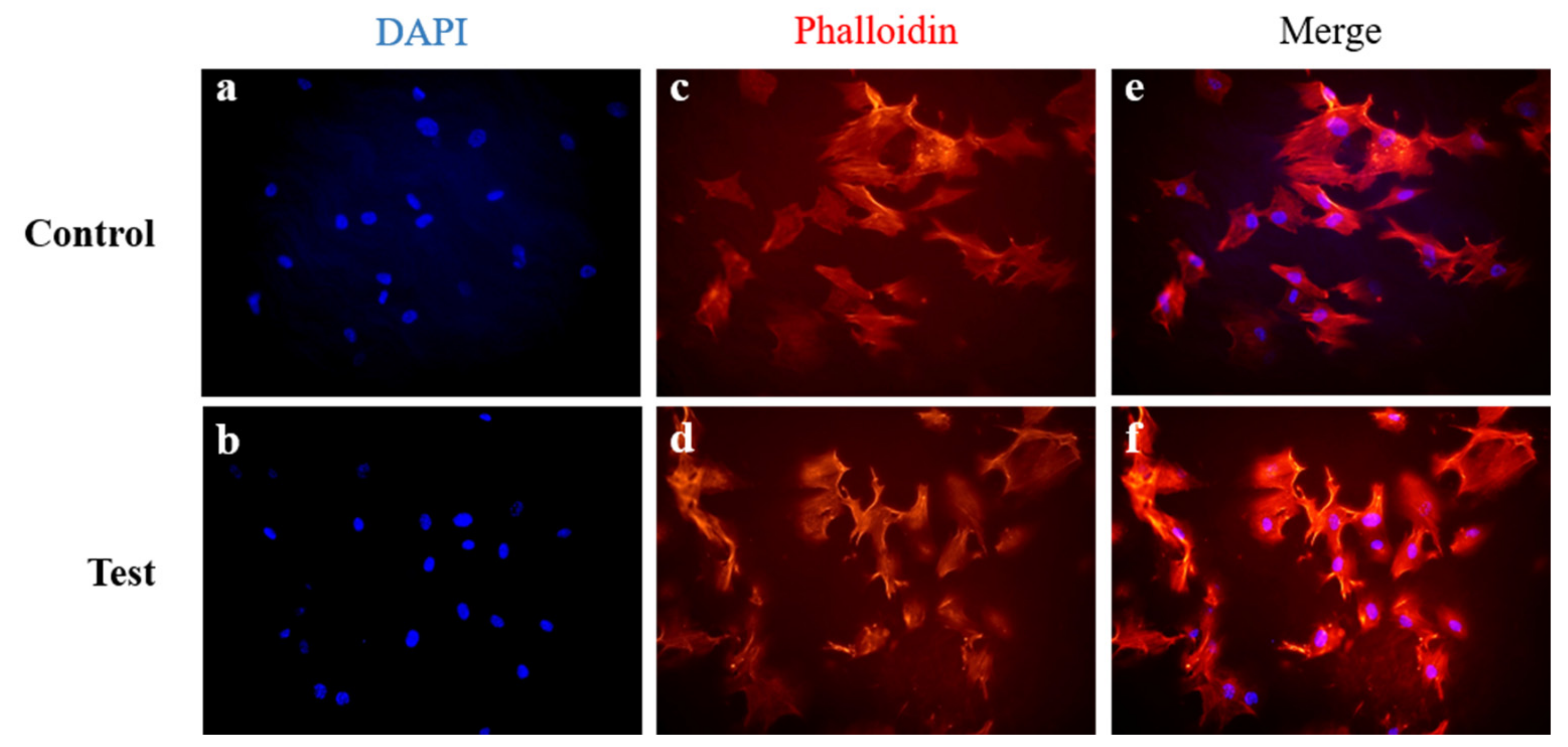
3.2.2. Immunofluorescent Staining
3.3. In Vivo Results
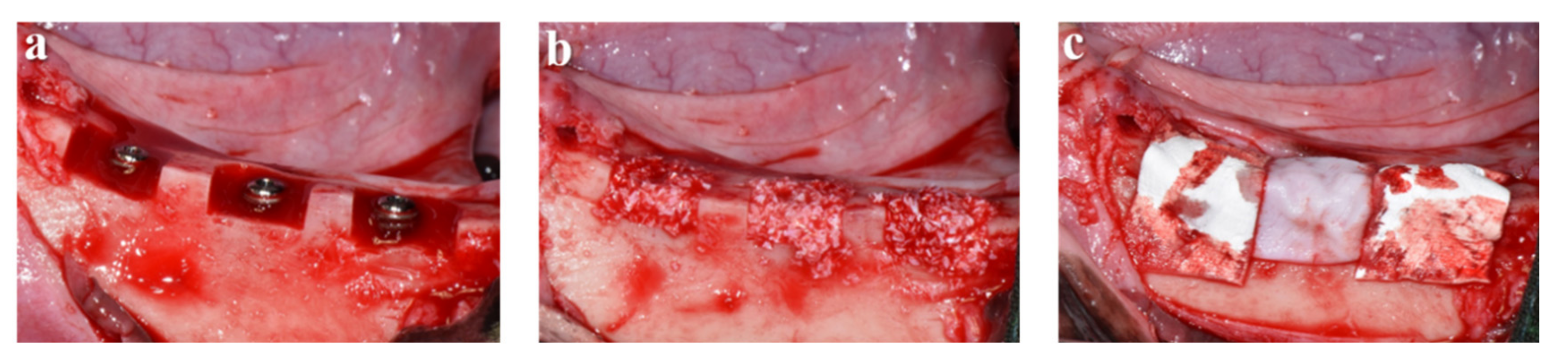
3.3.1. Clinical Findings
3.3.2. µCT Findings
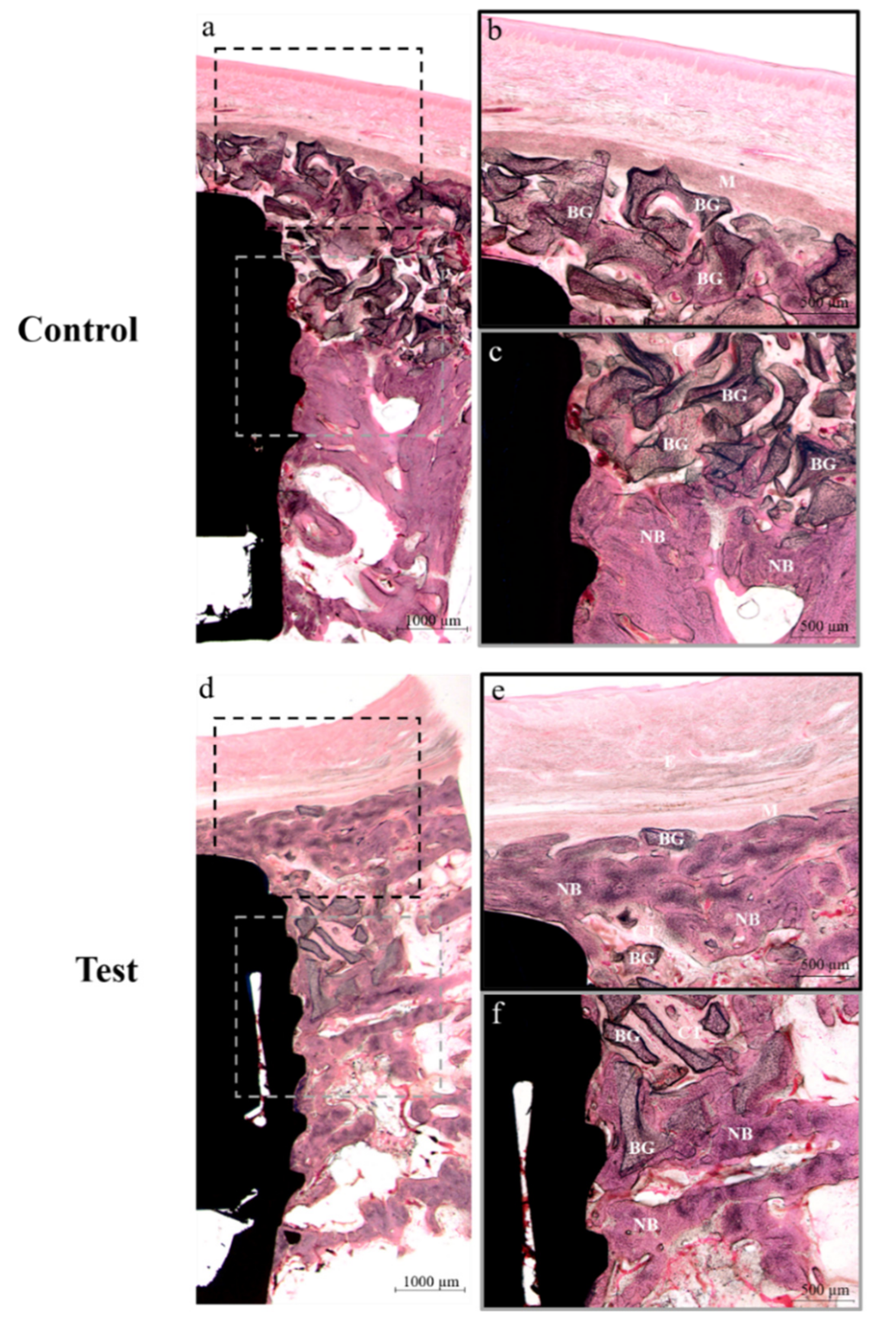
3.3.3. Histological Findings
3.3.4. Histomorphometric Findings
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Tal, H.; Kozlovsky, A.; Artzi, Z.; Nemcovsky, C.E.; Moses, O. Cross-linked and non-cross-linked collagen barrier membranes disintegrate following surgical exposure to the oral environment: A histological study in the cat. Clin. Oral Implant. Res. 2008, 19, 760–766. [Google Scholar] [CrossRef]
- Hammerle, C.H.; Karring, T. Guided bone regeneration at oral implant sites. Periodontol. 2000 1998, 17, 151–175. [Google Scholar] [CrossRef]
- Hammerle, C.H.F.; Lang, N.P. Single stage surgery combining transmucosal implant placement with guided bone regeneration and bioresorbable materials. Clin. Oral Implant Res. 2001, 12, 9–18. [Google Scholar] [CrossRef]
- Saghiri, M.A.; Asatourian, A.; Garcia-Godoy, F.; Sheibani, N. The role of angiogenesis in implant dentistry part II: The effect of bone-grafting and barrier membrane materials on angiogenesis. Med. Oral Patol. Oral Cir. Bucal. 2016, 21, e526–e537. [Google Scholar] [CrossRef] [PubMed]
- Buser, D.; Hoffmann, B.; Bernard, J.P.; Lussi, A.; Mettler, D.; Schenk, R.K. Evaluation of filling materials in membrane—protected bone defects. A comparative histomorphometric study in the mandible of miniature pigs. Clin. Oral Implant. Res. 1998, 9, 137–150. [Google Scholar] [CrossRef] [PubMed]
- Dahlin, C.; Linde, A.; Gottlow, J.; Nyman, S. Healing of bone defects by guided tissue regeneration. Plast. Reconstr. Surg. 1988, 81, 672–676. [Google Scholar] [CrossRef]
- Hammerle, C.H.F.; Jung, R.E. Bone augmentation by means of barrier membranes. Periodontol. 2000 2003, 33, 36–53. [Google Scholar] [CrossRef]
- Retzepi, M.; Donos, N. Guided Bone Regeneration: Biological principle and therapeutic applications. Clin. Oral Implant. Res. 2010, 21, 567–576. [Google Scholar] [CrossRef]
- Wessing, B.; Lettner, S.; Zechner, W. Guided Bone Regeneration with Collagen Membranes and Particulate Graft Materials: A Systematic Review and Meta-Analysis. Int. J. Oral Maxillofac Implant. 2018, 33, 87–100. [Google Scholar] [CrossRef]
- Wiltfang, J.; Merten, H.A.; Peters, J.H. Comparative study of guided bone regeneration using absorbable and permanent barrier membranes: A histologic report. Int. J. Oral Maxillofac Implant. 1998, 13, 416–421. [Google Scholar]
- Jiménez García, J.; Berghezan, S.; Caramês, J.M.M.; Dard, M.; Marques, D.J.J. Effect of cross-linked vs non-cross-linked collagen membranes on bone: A systematic review. J. Periodontal. Res. 2017, 52, 955–964. [Google Scholar] [CrossRef]
- Lang, N.P.; Hammerle, C.H.; Bragger, U.; Lehmann, B.; Nyman, S.R. Guided tissue regeneration in jawbone defects prior to implant placement. Clin. Oral Implant. Res. 1994, 5, 92–97. [Google Scholar] [CrossRef]
- Carpio, L.; Loza, J.; Lynch, S.; Genco, R. Guided bone regeneration around endosseous implants with anorganic bovine bone mineral. A randomized controlled trial comparing bioabsorbable versus non-resorbable barriers. J. Periodontol. 2000, 71, 1743–1749. [Google Scholar] [CrossRef]
- Becker, W.; Dahlin, C.; Becker, B.E.; Lekholm, U.; van Steenberghe, D.; Higuchi, K.; Kultje, C. The use of e-PTFE barrier membranes for bone promotion around titanium implants placed into extraction sockets: A prospective multicenter study. Int. J. Oral Maxillofac Implant. 1994, 9, 31–40. [Google Scholar]
- Hardwick, R.; Hayes, B.K.; Flynn, C. Devices for dentoalveolar regeneration: An up-to-date literature review. J. Periodontol. 1995, 66, 495–505. [Google Scholar] [CrossRef]
- Rowe, M.J.; Kamocki, K.; Pankajakshan, D.; Li, D.; Bruzzaniti, A.; Thomas, V.; Blanchard, S.B.; Bottino, M.C. Dimensionally stable and bioactive membrane for guided bone regeneration: An in vitro study. J. Biomed. Mater. Res. B Appl. Biomater. 2016, 104, 594–605. [Google Scholar] [CrossRef]
- Zitzmann, N.U.; Naef, R.; Scharer, P. Resorbable versus nonresorbable membranes in combination with Bio-Oss for guided bone regeneration. Int. J. Oral Maxillofac Implant. 1997, 12, 844–852. [Google Scholar]
- McAllister, B.S.; Haghighat, K. Bone augmentation techniques. J. Periodontol. 2007, 78, 377–396. [Google Scholar] [CrossRef]
- Aldemir Dikici, B.; Dikici, S.; Reilly, G.C.; MacNeil, S.; Claeyssens, F.A. Novel Bilayer Polycaprolactone Membrane for Guided Bone Regeneration: Combining Electrospinning and Emulsion Templating. Materials 2019, 12, 2643. [Google Scholar] [CrossRef]
- Park, J.H.; Park, C.K.; Kim, E.S.; Park, S.Y.; Jo, C.M.; Tak, W.Y.; Kweon, Y.O.; Kim, S.K.; Choi, Y.W. The diagnostic value of serum hyaluronic acid, 7S domain of type IV collagen and AST/ALT ratio as markers of hepatic fibrosis in chronic hepatitis B and cirrhosis patients. Korean J. Hepatol. 2003, 9, 79. [Google Scholar]
- Gentile, P.; Chiono, V.; Tonda-Turo, C.; Ferreira, A.M.; Ciardelli, G. Polymeric membranes for guided bone regeneration. Biotechnol. J. 2011, 6, 1187–1197. [Google Scholar] [CrossRef] [PubMed]
- Brunel, G.; Piantoni, P.; Elharar, F.; Benqué, E.; Marin, P.; Zahedi, S. Regeneration of rat calvarial defects using a bioabsorbable membrane technique: Influence of collagen cross-linking. J. Periodontol. 1996, 67, 1342–1348. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, F.; Rothamel, D.; Herten, M.; Sager, M.; Becker, J. Angiogenesis pattern of native and cross-linked collagen membranes: An immunohistochemical study in the rat. Clin. Oral Implant. Res. 2006, 17, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Singla, A.; Lee, Y. Biomedical applications of collagen. Int. J. Pharm. 2001, 221, 1–22. [Google Scholar] [CrossRef]
- Peinemann, K.-V.; Nunes, S.P. Membrane Technology. In Membranes for Life Sciences; John Wiley & Sons: Hoboken, NJ, USA, 2011; Volume 1, p. 239. [Google Scholar]
- Zahedi, S.; Legrand, R.; Brunel, G.; Albert, A.; Dewe, W.; Coumans, B.; Bernard, J.P. Evaluation of a diphenylphosphorylazide-crosslinked collagen membrane for guided bone regeneration in mandibular defects in rats. J. Periodontol. 1998, 69, 1238–1246. [Google Scholar] [CrossRef] [PubMed]
- Behring, J.; Junker, R.; Walboomers, X.F.; Chessnut, B.; Jansen, J.A. Toward guided tissue and bone regeneration: Morphology, attachment, proliferation, and migration of cells cultured on collagen barrier membranes. A systematic review. Odontology 2008, 96, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Bunyaratavej, P.; Wang, H.L. Collagen membranes: A review. J. Periodontol. 2001, 72, 215–229. [Google Scholar] [CrossRef] [PubMed]
- Moses, O.; Vitrial, D.; Aboodi, G.; Sculean, A.; Tal, H.; Kozlovsky, A.; Artzi, Z.; Weinreb, M.; Nemcovsky, C.E. Biodegradation of three different collagen membranes in the rat calvarium: A comparative study. J. Periodontol. 2008, 79, 905–911. [Google Scholar] [CrossRef]
- Bornstein, M.M.; Heynen, G.; Bosshardt, D.D.; Buser, D. Effect of two bioabsorbable barrier membranes on bone regeneration of standardized defects in calvarial bone: A comparative histomorphometric study in pigs. J. Periodontol. 2009, 80, 1289–1299. [Google Scholar] [CrossRef]
- Haugh, M.G.; Jaasma, M.J.; O’Brien, F.J. The effect of dehydrothermal treatment on the mechanical and structural properties of collagen-GAG scaffolds. J. Biomed. Mater. Res. A 2009, 89, 363–369. [Google Scholar] [CrossRef]
- Torres, D.S.; Freyman, T.M.; Yannas, I.V.; Spector, M. Tendon cell contraction of collagen-GAG matrices in vitro: Effect of cross-linking. Biomaterials 2000, 21, 1607–1619. [Google Scholar] [CrossRef]
- Moshnikova, A.B.; Afanasyev, V.N.; Proussakova, O.V.; Chernyshov, S.; Gogvadze, V.; Beletsky, I.P. Cytotoxic activity of 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide is underlain by DNA interchain cross-linking. Cell. Mol. Life Sci. 2006, 63, 229–234. [Google Scholar] [CrossRef]
- Hafemann, B.; Ghofrani, K.; Gattner, H.G.; Stieve, H.; Pallua, N. Cross-linking by 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide (EDC) of a collagen/elastin membrane meant to be used as a dermal substitute: Effects on physical, biochemical and biological features in vitro. J. Mater. Sci. Mater. Med. 2001, 12, 437–446. [Google Scholar] [CrossRef]
- Sallent, I.; Capella-Monsonís, H.; Zeugolis, D.I. Production and characterization of chemically cross-linked collagen scaffolds. In Collagen; Springer: Berlin/Heidelberg, Germany, 2019; pp. 23–38. [Google Scholar]
- Yamauchi, K.; Goda, T.; Takeuchi, N.; Einaga, H.; Tanabe, T. Preparation of collagen/calcium phosphate multilayer sheet using enzymatic mineralization. Biomaterials 2004, 25, 5481–5489. [Google Scholar] [CrossRef]
- Delgado, L.M.; Bayon, Y.; Pandit, A.; Zeugolis, D.I. To cross-link or not to cross-link? Cross-linking associated foreign body response of collagen-based devices. Tissue Eng. Part B 2015, 21, 298–313. [Google Scholar] [CrossRef] [PubMed]
- Rothamel, D.; Schwarz, F.; Sager, M.; Herten, M.; Sculean, A.; Becker, J. Biodegradation of differently cross-linked collagen membranes: An experimental study in the rat. Clin. Oral Implant. Res. 2005, 16, 369–378. [Google Scholar] [CrossRef]
- Park, S.N.; Park, J.C.; Kim, H.O.; Song, M.J.; Suh, H. Characterization of porous collagen/hyaluronic acid scaffold modified by 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide cross-linking. Biomaterials 2002, 23, 1205–1212. [Google Scholar] [CrossRef]
- Campiglio, C.E.; Contessi Negrini, N.; Fare, S.; Draghi, L. Cross-Linking Strategies for Electrospun Gelatin Scaffolds. Materials 2019, 12, 2476. [Google Scholar] [CrossRef] [PubMed]
- Bae, E.B.; Kim, H.J.; Ahn, J.J.; Bae, H.Y.; Kim, H.J.; Huh, J.B. Comparison of Bone Regeneration between Porcine-Derived and Bovine-Derived Xenografts in Rat Calvarial Defects: A Non-Inferiority Study. Materials 2019, 12, 3412. [Google Scholar] [CrossRef]
- Wang, K.; Abdala, A.; Hilal, N.; Khraisheh, M. Mechanical characterization of membranes. In Membrane Characterization; Elsevier: Amsterdam, The Netherlands, 2017; pp. 259–306. [Google Scholar]
- Schlegel, A.; Möhler, H.; Busch, F.; Mehl, A.J.B. Preclinical and clinical studies of a collagen membrane (Bio-Gide®). Biomaterials 1997, 18, 535–538. [Google Scholar] [CrossRef]
- Moses, O.; Pitaru, S.; Artzi, Z.; Nemcovsky, C.E. Healing of dehiscence-type defects in implants placed together with different barrier membranes: A comparative clinical study. Clin. Oral Implant. Res. 2005, 16, 210–219. [Google Scholar] [CrossRef] [PubMed]
- Rothamel, D.; Schwarz, F.; Sculean, A.; Herten, M.; Scherbaum, W.; Becker, J. Biocompatibility of various collagen membranes in cultures of human PDL fibroblasts and human osteoblast-like cells. Clin. Oral Implant. Res. 2004, 15, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, F.; Rothamel, D.; Herten, M.; Wüstefeld, M.; Sager, M.; Ferrari, D.; Becker, J. Immunohistochemical characterization of guided bone regeneration at a dehiscence-type defect using different barrier membranes: An experimental study in dogs. Biomaterials 2008, 19, 402–415. [Google Scholar] [CrossRef] [PubMed]
- Powell, H.M.; Boyce, S.T. EDC cross-linking improves skin substitute strength and stability. Biomaterials 2006, 27, 5821–5827. [Google Scholar] [CrossRef]
- Angele, P.; Abke, J.; Kujat, R.; Faltermeier, H.; Schumann, D.; Nerlich, M.; Kinner, B.; Englert, C.; Ruszczak, Z.; Mehrl, R.; et al. Influence of different collagen species on physico-chemical properties of crosslinked collagen matrices. Biomaterials 2004, 25, 2831–2841. [Google Scholar] [CrossRef]
- Ghodbane, S.A.; Dunn, M.G. Physical and mechanical properties of cross-linked type I collagen scaffolds derived from bovine, porcine, and ovine tendons. J. Biomed. Mater. Res. A 2016, 104, 2685–2692. [Google Scholar] [CrossRef]
- Speer, D.P.; Chvapil, M.; Eskelson, C.D.; Ulreich, J. Biological effects of residual glutaraldehyde in glutaraldehyde-tanned collagen biomaterials. J. Biomed. Mater. Res. 1980, 14, 753–764. [Google Scholar] [CrossRef]
- Vizarova, K.; Bakos, D. Modification of Layered Atelocollagen—Enzymatic Degradation and Cytotoxicity Evaluation. Biomaterials 1995, 16, 1217–1221. [Google Scholar] [CrossRef]
- Bozkurt, S.B.; Hakki, S.S.; Hakki, E.E.; Durak, Y.; Kantarci, A. Porphyromonas gingivalis Lipopolysaccharide Induces a Pro-inflammatory Human Gingival Fibroblast Phenotype. Inflammation 2017, 40, 144–153. [Google Scholar] [CrossRef]
- Qiu, Y.L.; Chen, X.; Hou, Y.L.; Hou, Y.J.; Tian, S.B.; Chen, Y.H.; Yu, L.; Nie, M.H.; Liu, X.Q. Characterization of different biodegradable scaffolds in tissue engineering. Mol. Med. Rep. 2019, 19, 4043–4056. [Google Scholar] [CrossRef]
- Warnke, P.H.; Douglas, T.; Sivananthan, S.; Wiltfang, J.; Springer, I.; Becker, S.T. Tissue engineering of periosteal cell membranes in vitro. Clin. Oral Implant. Res. 2009, 20, 761–766. [Google Scholar] [CrossRef] [PubMed]
- Park, S.N.; Kim, J.K.; Suh, H. Evaluation of antibiotic-loaded collagen-hyaluronic acid matrix as a skin substitute. Biomaterials 2004, 25, 3689–3698. [Google Scholar] [CrossRef]
- Li, H.; Zheng, J.; Zhang, S.; Yang, C.; Kwon, Y.D.; Kim, Y.J. Experiment of GBR for repair of peri-implant alveolar defects in beagle dogs. Sci. Rep. 2018, 8, 16532. [Google Scholar] [CrossRef]
- Gielkens, P.F.; Schortinghuis, J.; de Jong, J.R.; Raghoebar, G.M.; Stegenga, B.; Bos, R.R. Vivosorb®, Bio-Gide®, and Gore-Tex® as barrier membranes in rat mandibular defects: An evaluation by microradiography and micro-CT. Clin. Oral Implant. Res. 2008, 19, 516–521. [Google Scholar] [CrossRef]
| Elements | Group | |
|---|---|---|
| Control | Test | |
| C | 57.67 | 58.88 |
| O | 21.7 | 21.74 |
| N | 20.51 | 19.38 |
| Ca | 0.13 | 0 |
| Group | Mean | SD | p-Value |
|---|---|---|---|
| Control | 11.46 | 1.90 | 0.043 |
| Test | 16.70 | 2.43 |
| Groups | Mean | SD | p-Value |
|---|---|---|---|
| Control | 62.19 | 9.97 | 0.815 |
| Test | 60.98 | 10.02 |
| Group | Mean ± SD | ||
|---|---|---|---|
| NBA | ITBD | BIC | |
| Control | 24.78 ± 12.15 | 56.98 ± 12.68 | 50.44 ± 11.19 |
| Test | 23.46 ± 8.52 | 42.68 ± 21.89 | 48.24 ± 13.19 |
| p-value | 0.874 | 0.179 | 0.268 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahn, J.-J.; Kim, H.-J.; Bae, E.-B.; Cho, W.-T.; Choi, Y.; Hwang, S.-H.; Jeong, C.-M.; Huh, J.-B. Evaluation of 1-Ethyl-3-(3-Dimethylaminopropyl) Carbodiimide Cross-Linked Collagen Membranes for Guided Bone Regeneration in Beagle Dogs. Materials 2020, 13, 4599. https://doi.org/10.3390/ma13204599
Ahn J-J, Kim H-J, Bae E-B, Cho W-T, Choi Y, Hwang S-H, Jeong C-M, Huh J-B. Evaluation of 1-Ethyl-3-(3-Dimethylaminopropyl) Carbodiimide Cross-Linked Collagen Membranes for Guided Bone Regeneration in Beagle Dogs. Materials. 2020; 13(20):4599. https://doi.org/10.3390/ma13204599
Chicago/Turabian StyleAhn, Jong-Ju, Hyung-Joon Kim, Eun-Bin Bae, Won-Tak Cho, YunJeong Choi, Su-Hyun Hwang, Chang-Mo Jeong, and Jung-Bo Huh. 2020. "Evaluation of 1-Ethyl-3-(3-Dimethylaminopropyl) Carbodiimide Cross-Linked Collagen Membranes for Guided Bone Regeneration in Beagle Dogs" Materials 13, no. 20: 4599. https://doi.org/10.3390/ma13204599
APA StyleAhn, J.-J., Kim, H.-J., Bae, E.-B., Cho, W.-T., Choi, Y., Hwang, S.-H., Jeong, C.-M., & Huh, J.-B. (2020). Evaluation of 1-Ethyl-3-(3-Dimethylaminopropyl) Carbodiimide Cross-Linked Collagen Membranes for Guided Bone Regeneration in Beagle Dogs. Materials, 13(20), 4599. https://doi.org/10.3390/ma13204599





