On the Al–Al11Ce3 Eutectic Transformation in Aluminum–Cerium Binary Alloys
Abstract
1. Introduction
2. Material Synthesis and Experimental Details
2.1. Alloy Casting
2.2. Thermal Analysis
2.3. Structural Analysis
2.4. Mechanical Properties
3. Results
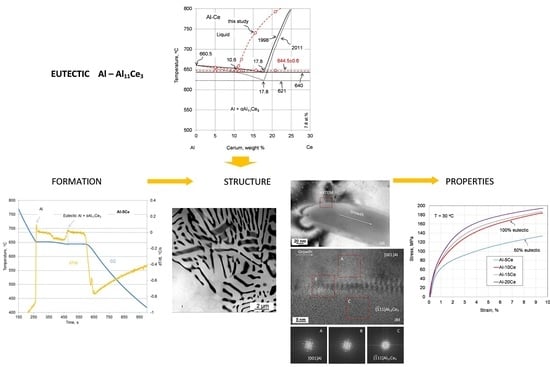
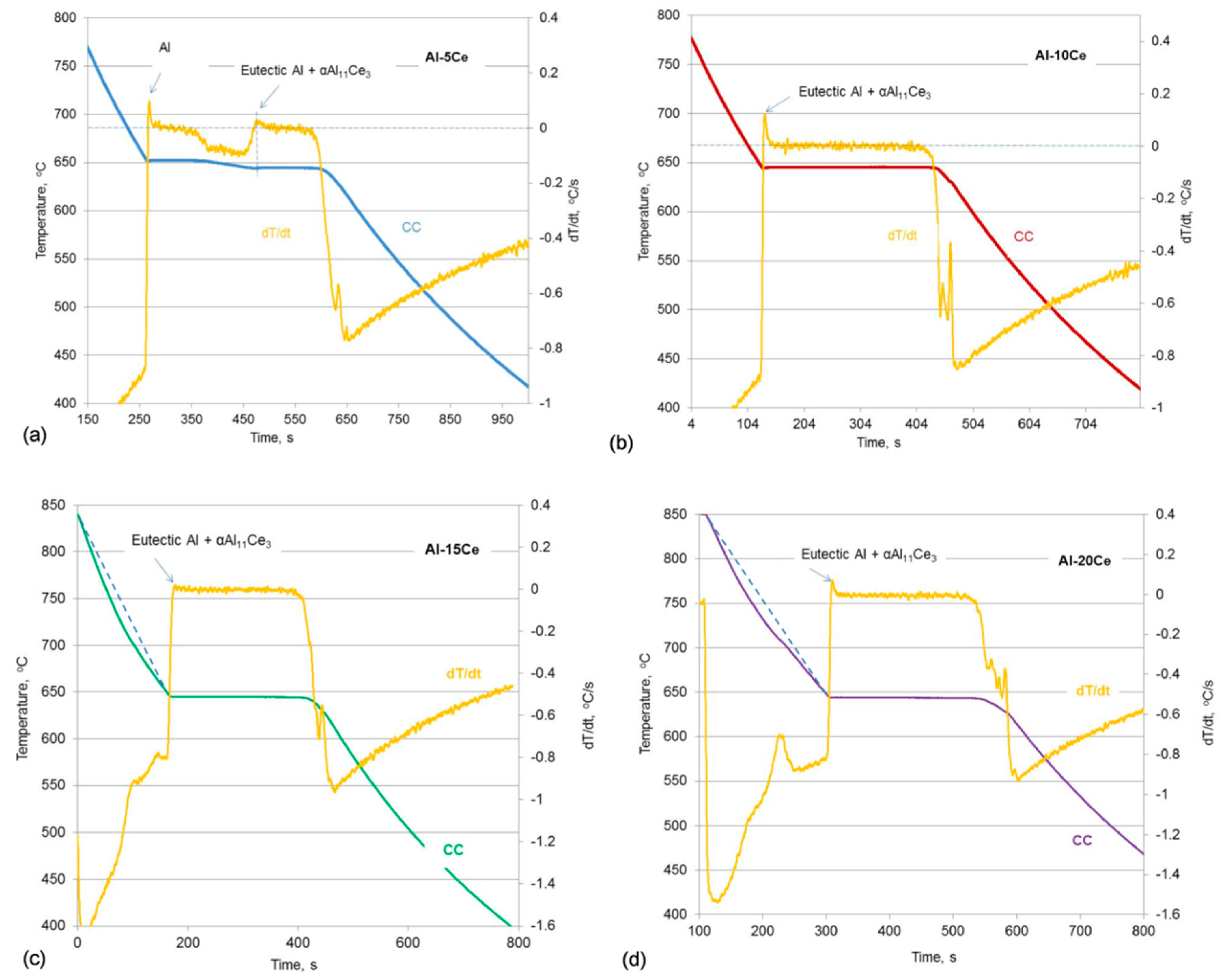
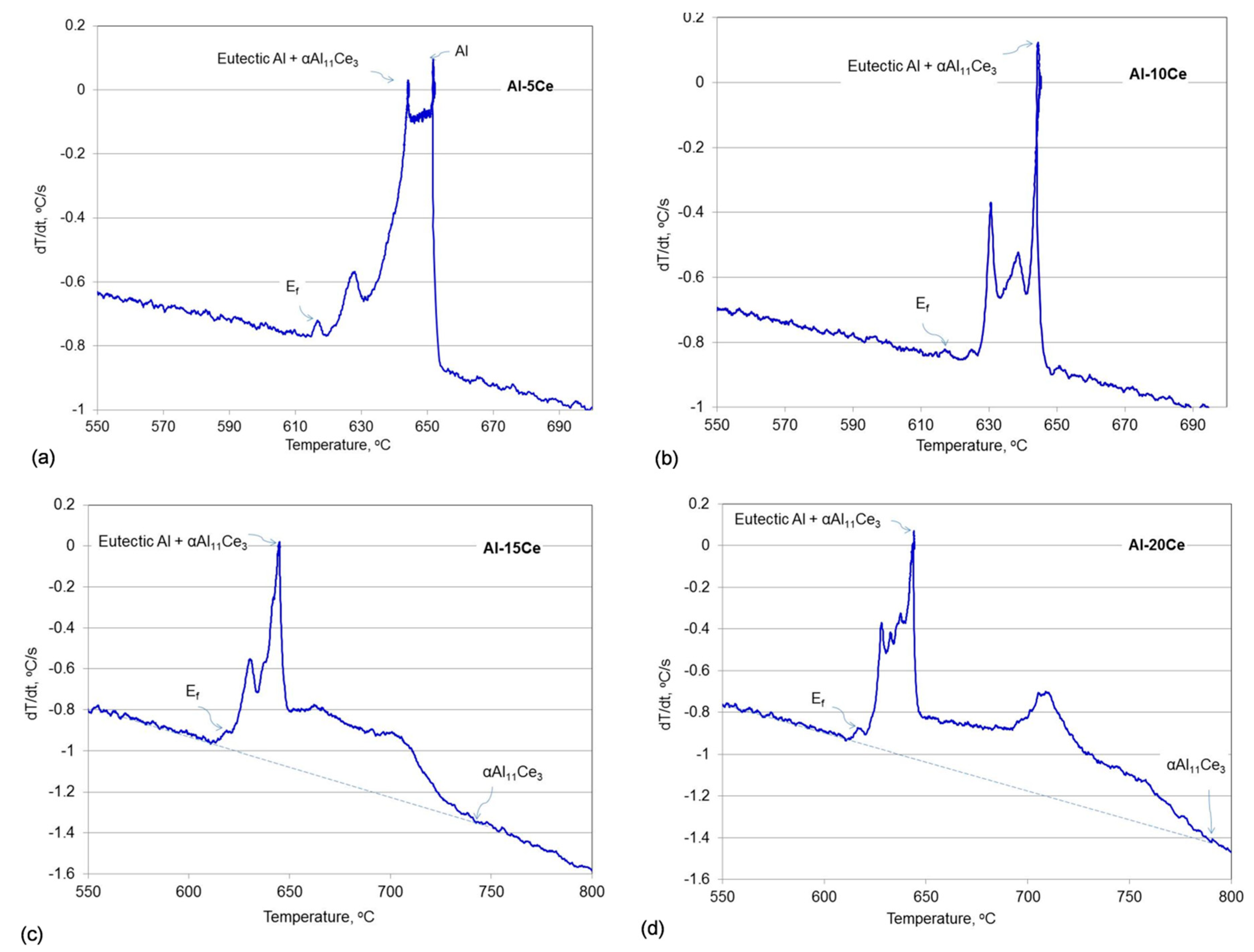
3.1. Thermal Events during Alloy Solidification
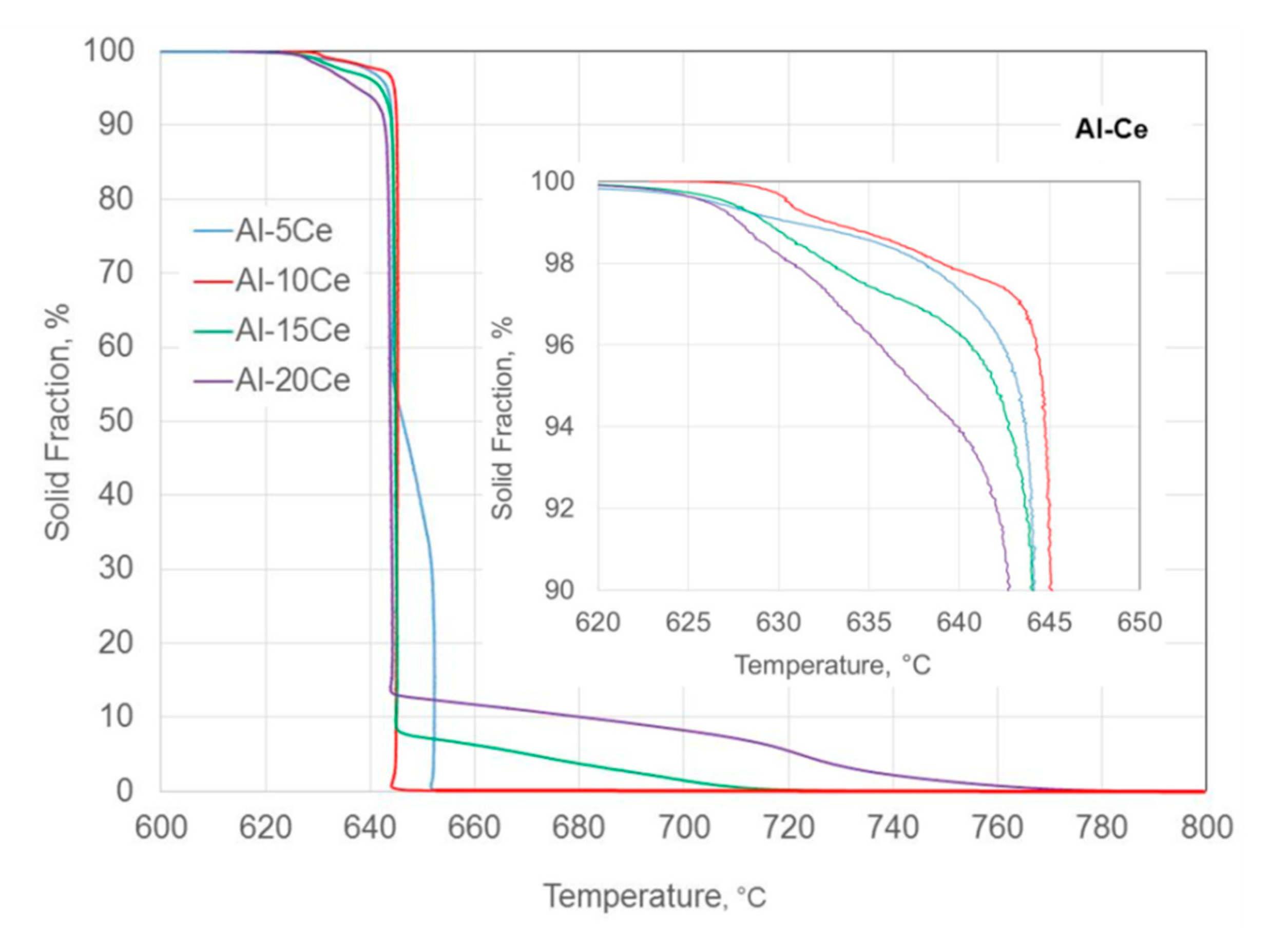
3.2. Solidification Sequence–Solid Fraction versus Temperature Plot
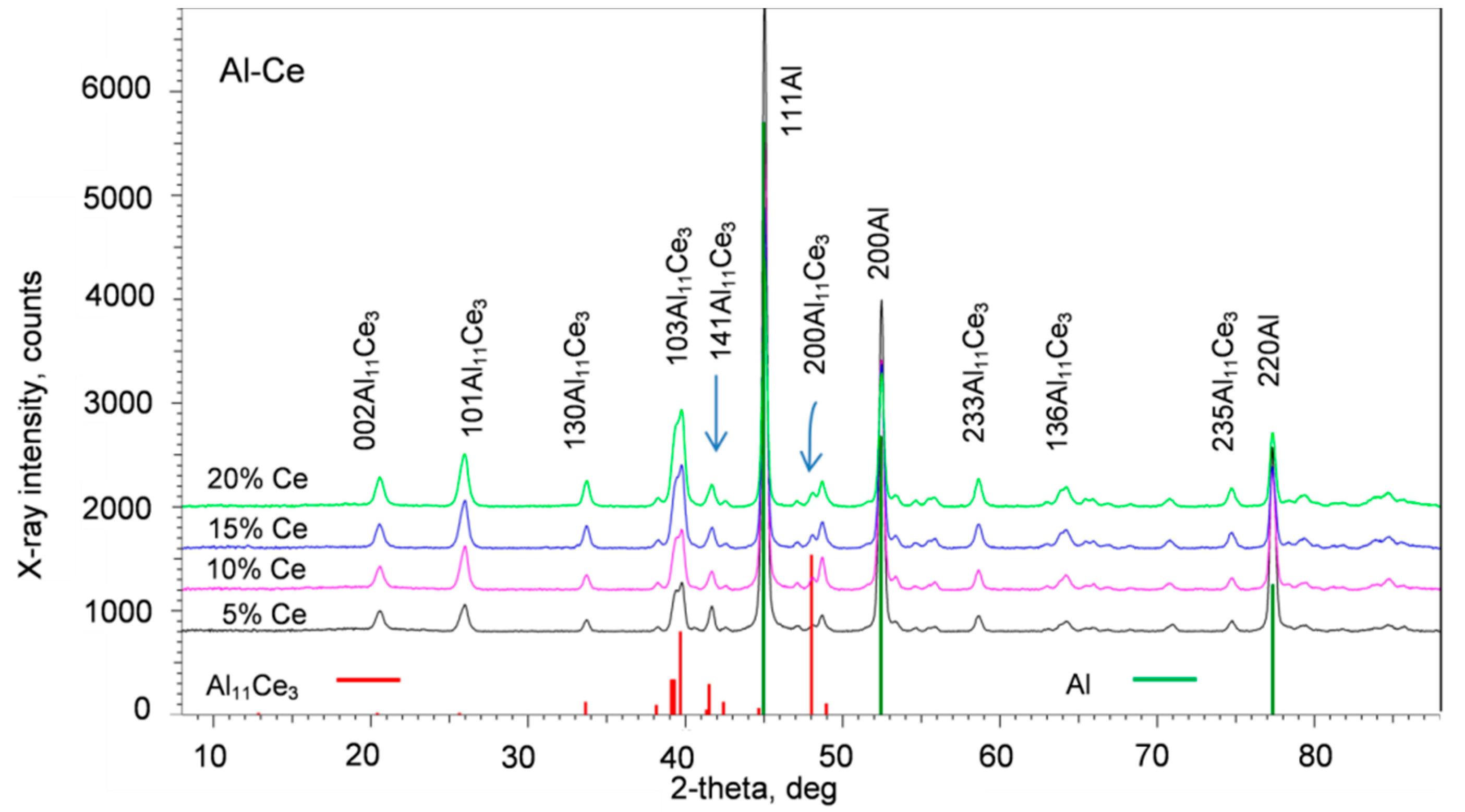
3.3. Bulk Phase Composition
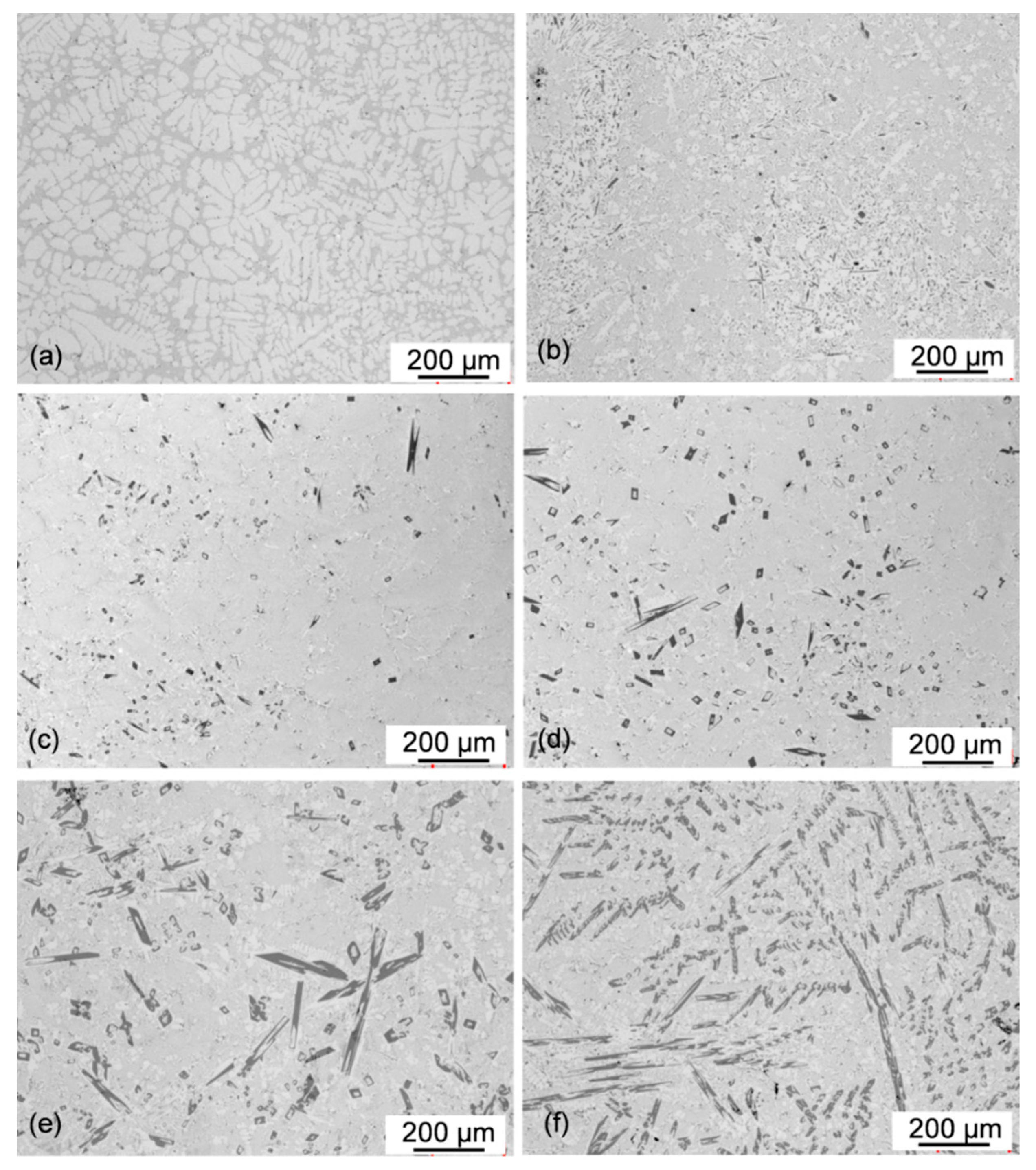
3.4. Effect of Chemical Composition on Alloy Microstructure
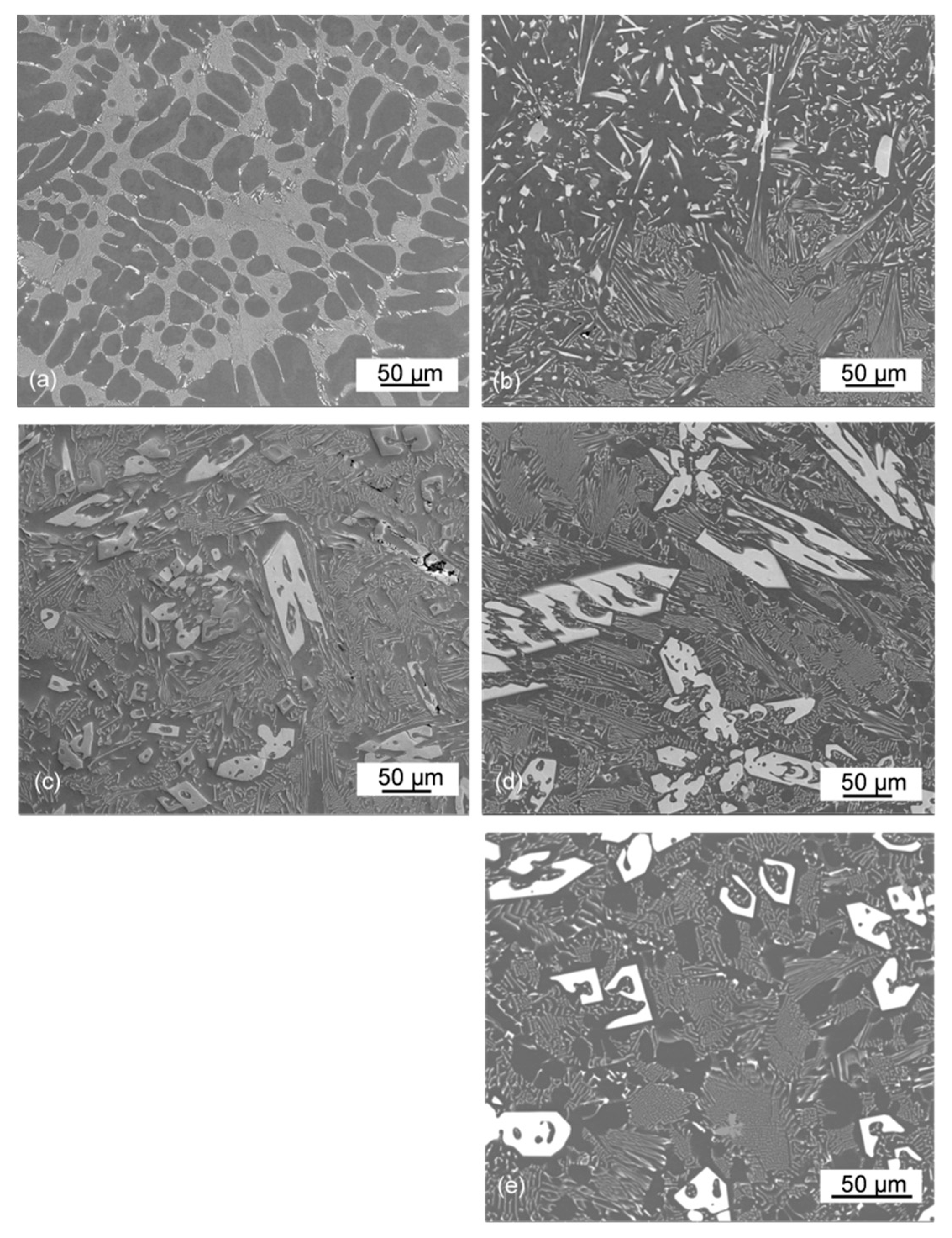
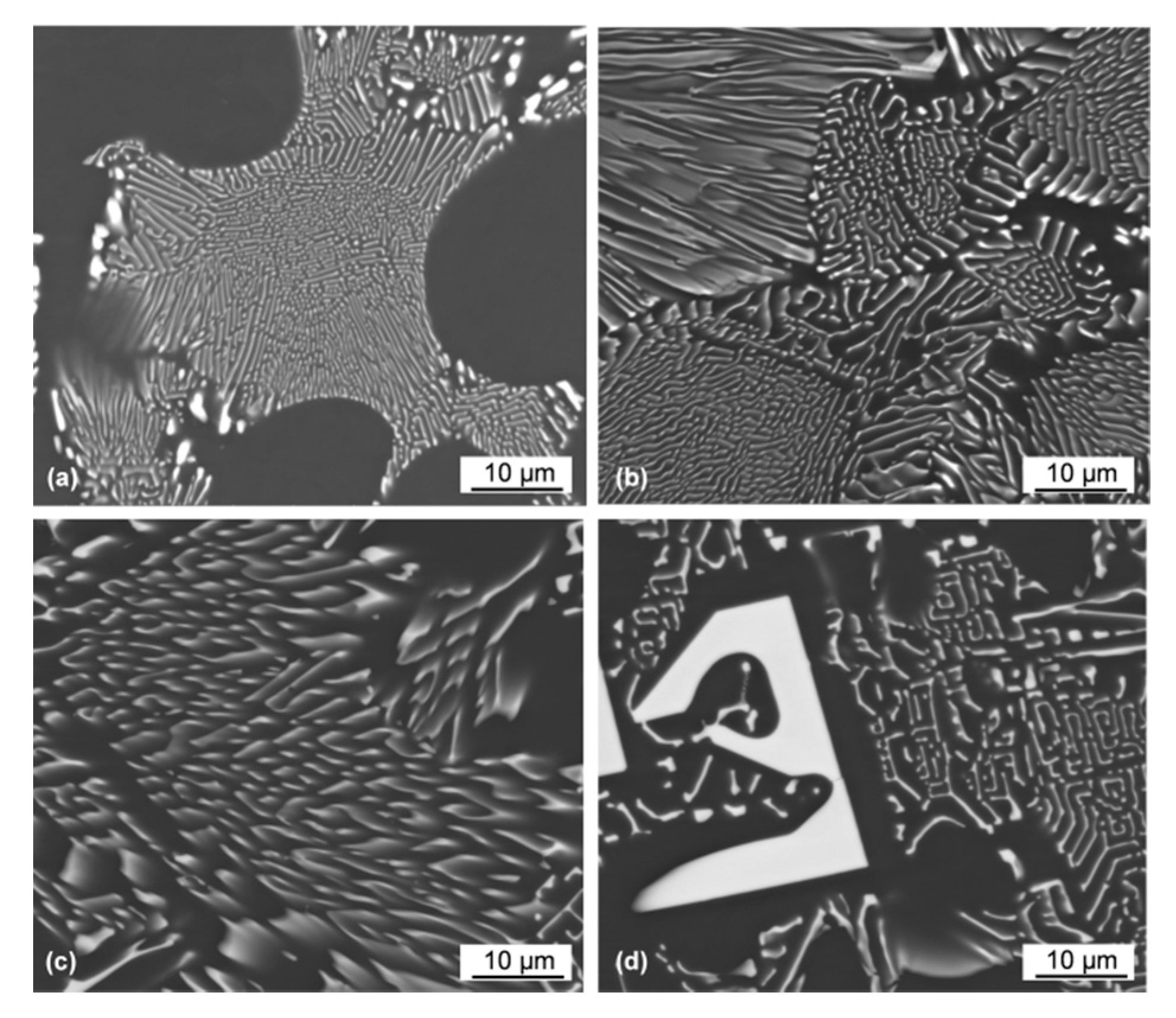
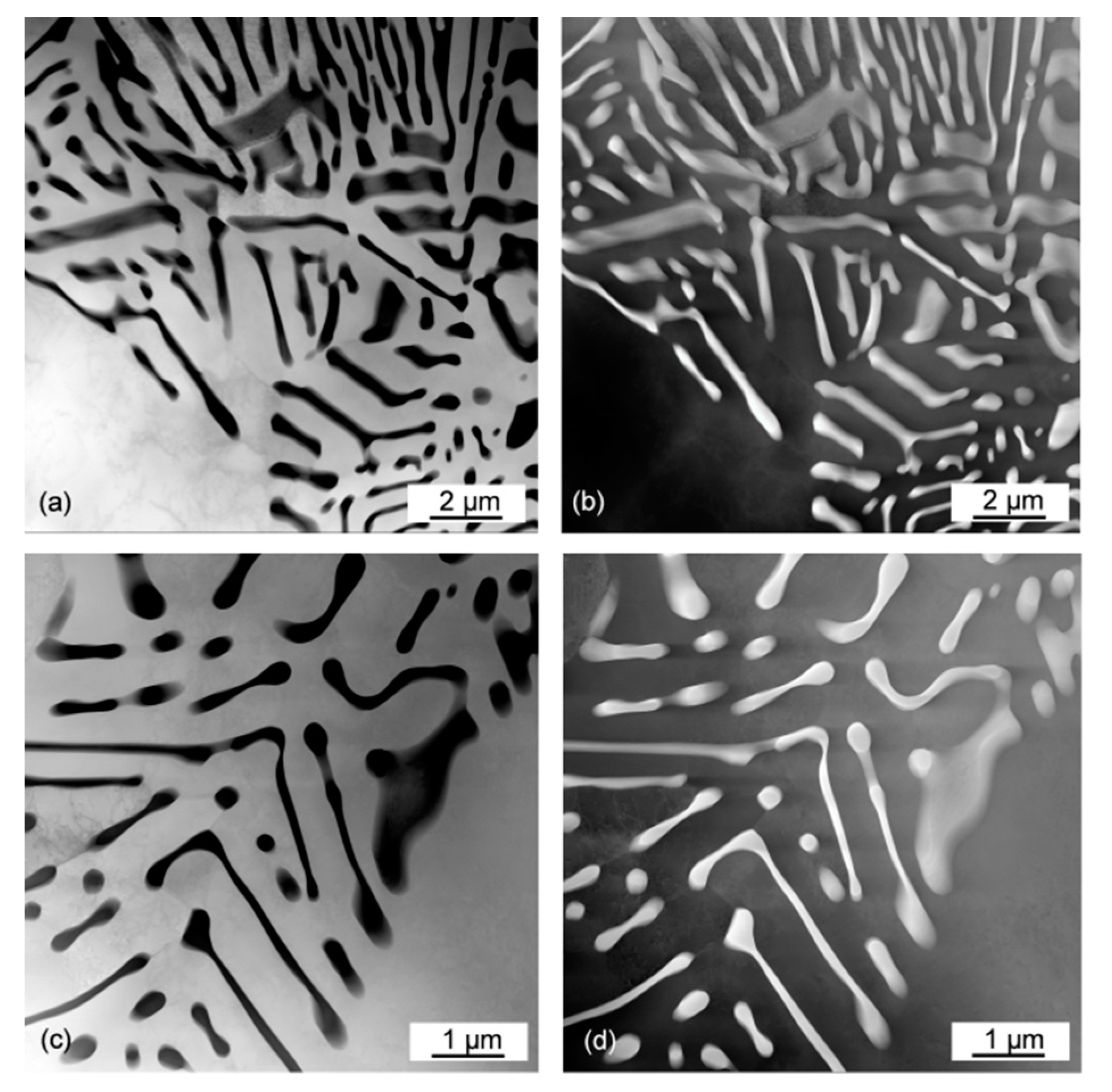
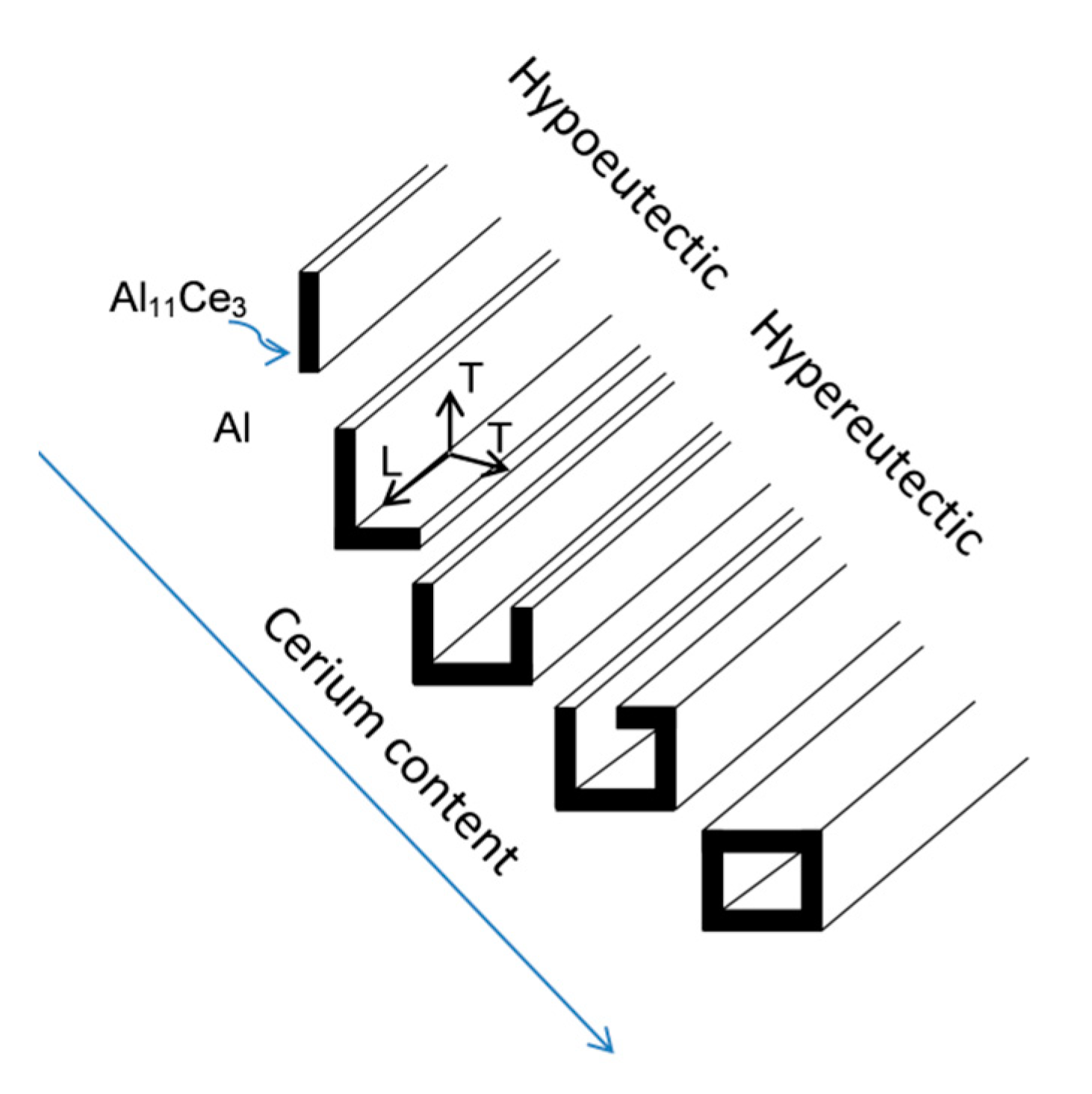
3.5. Morphology of As-Solidified Eutectic and Its Change with Ce Content
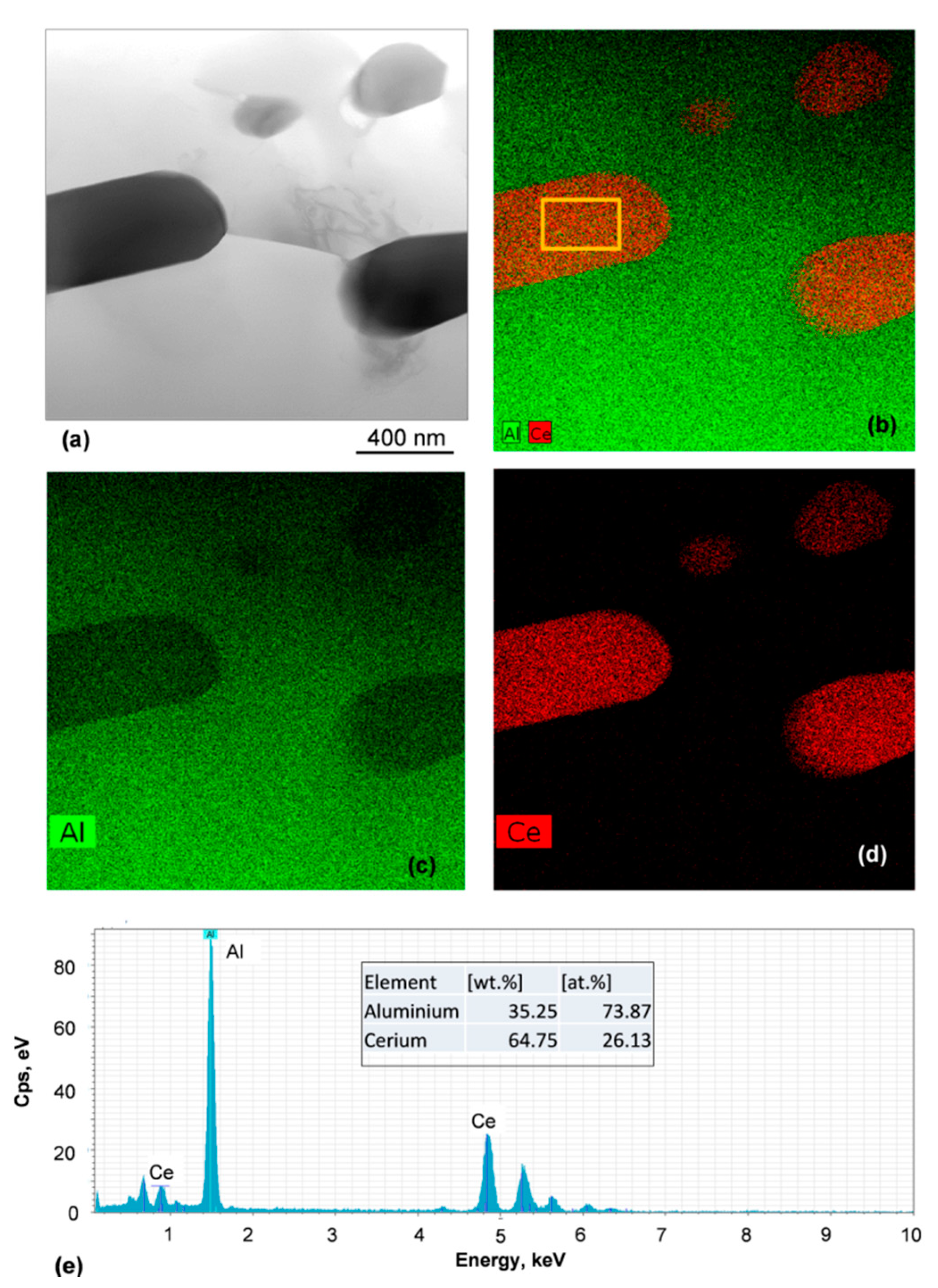
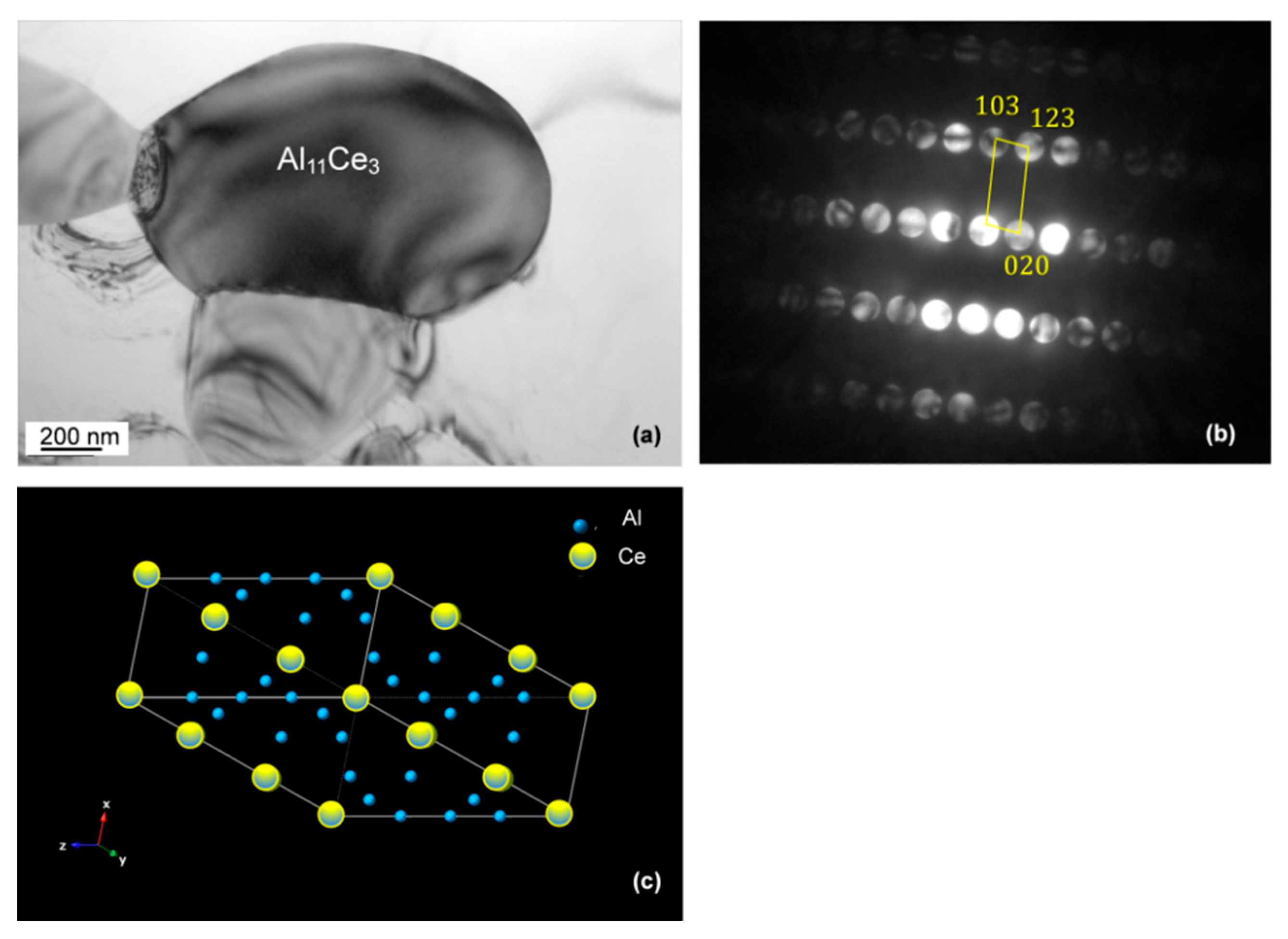
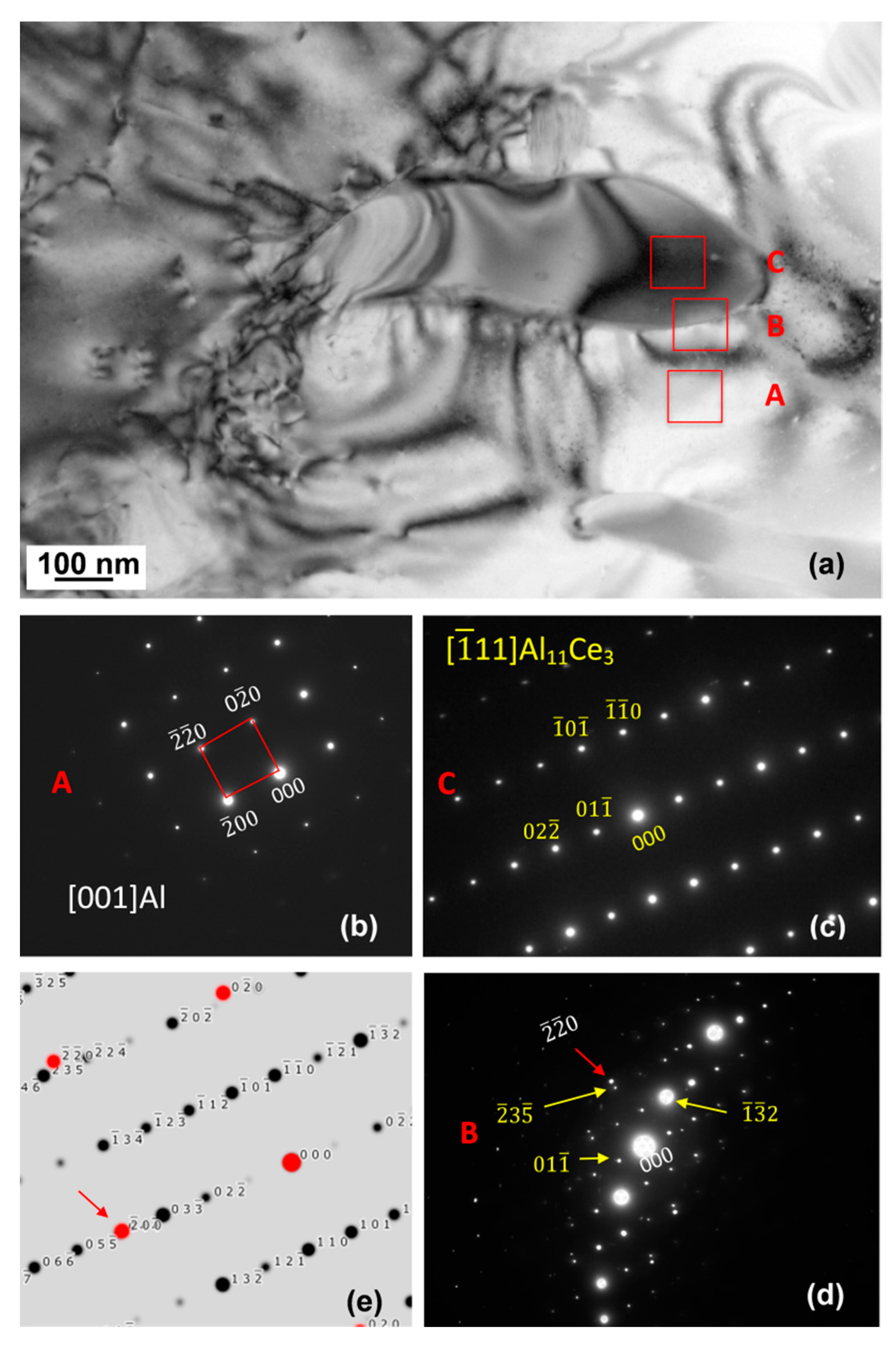
3.6. Chemistry and Crystallography of Eutectic Phases
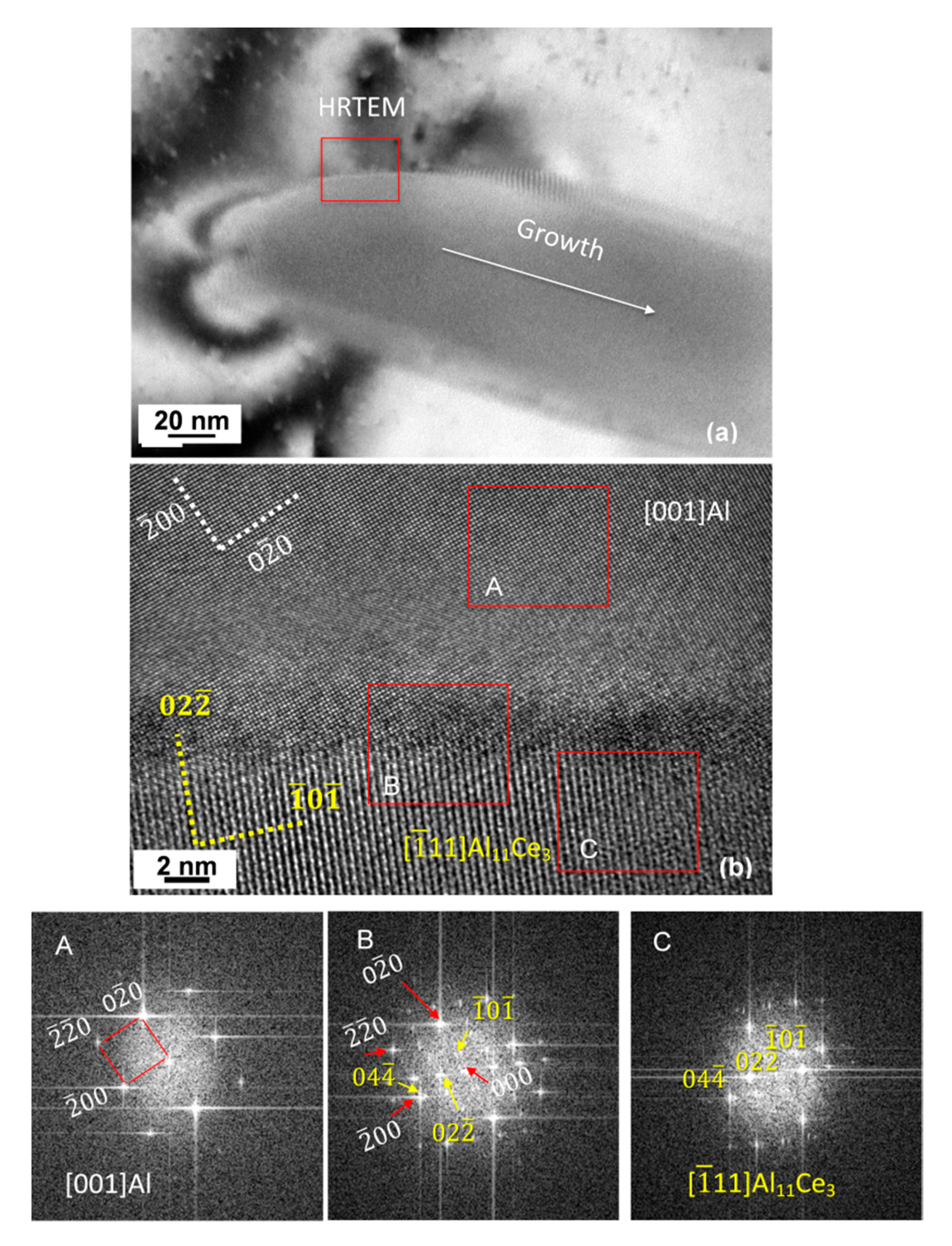
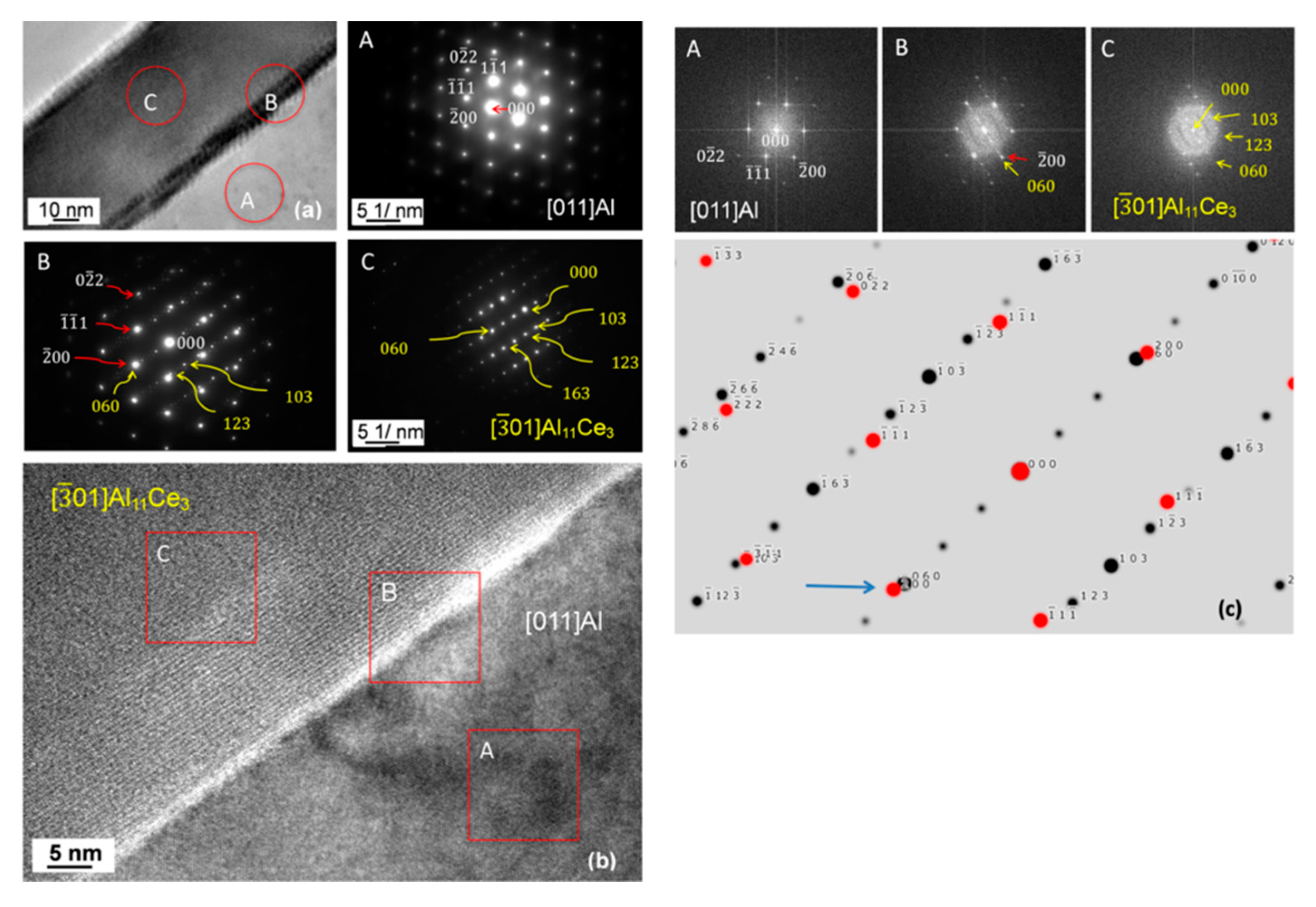
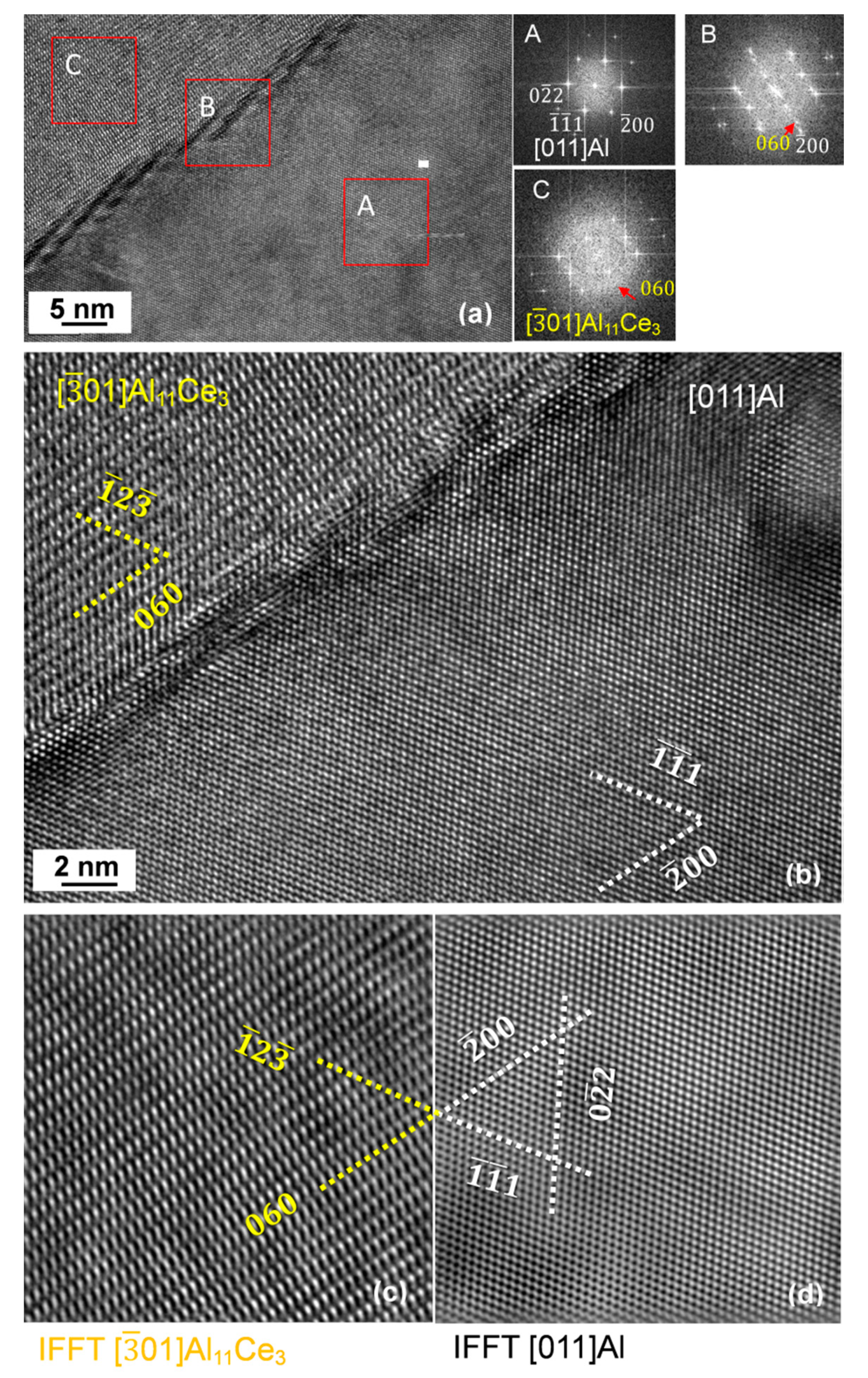
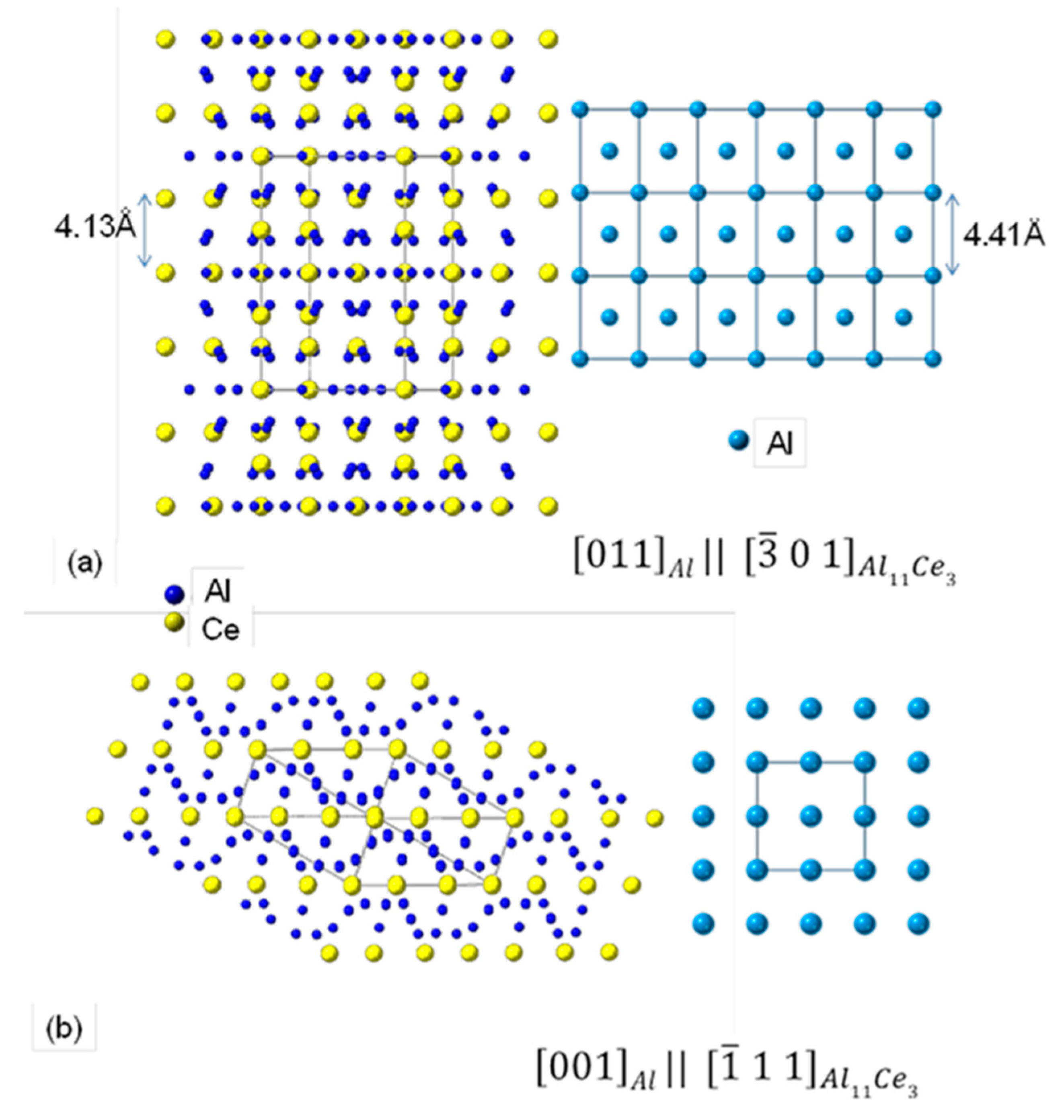
3.7. Orientation Relationship and Interface Structure between Al11Ce3 and Al within the Eutectic
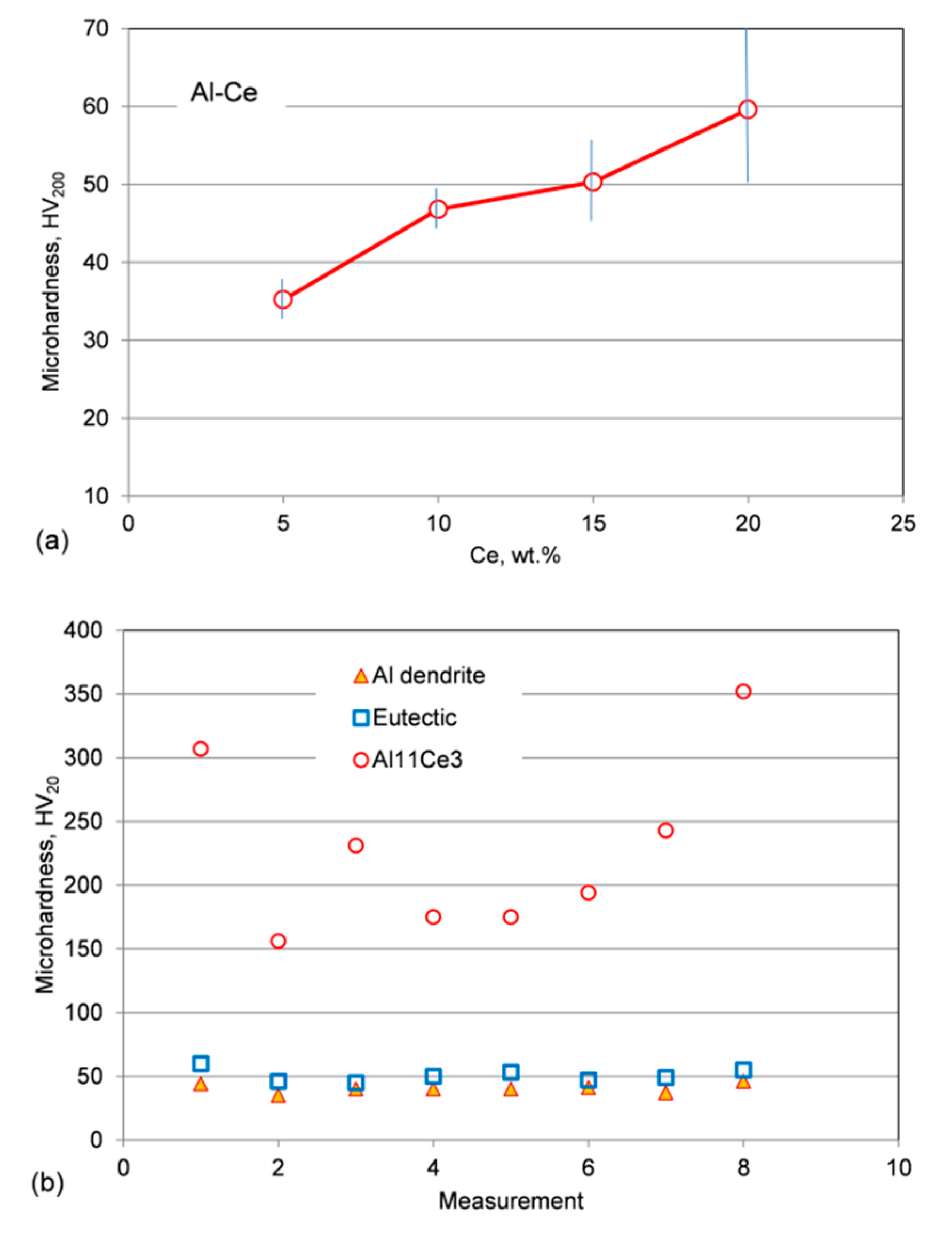
3.8. Hardness of Alloys, Eutectic and Individual Phases
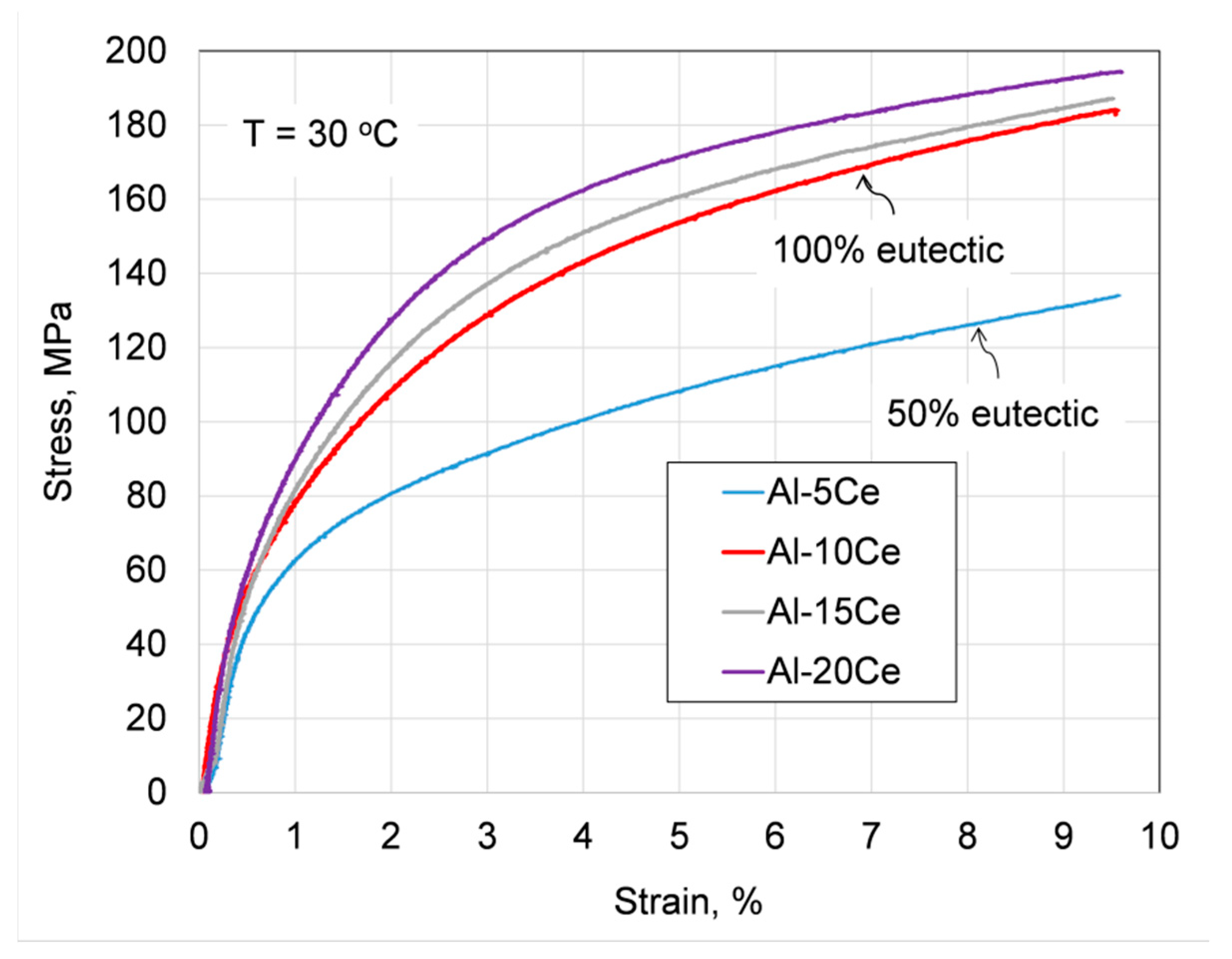
3.9. Assessing Alloy Strengthening by Ce and Eutectic
4. Discussion
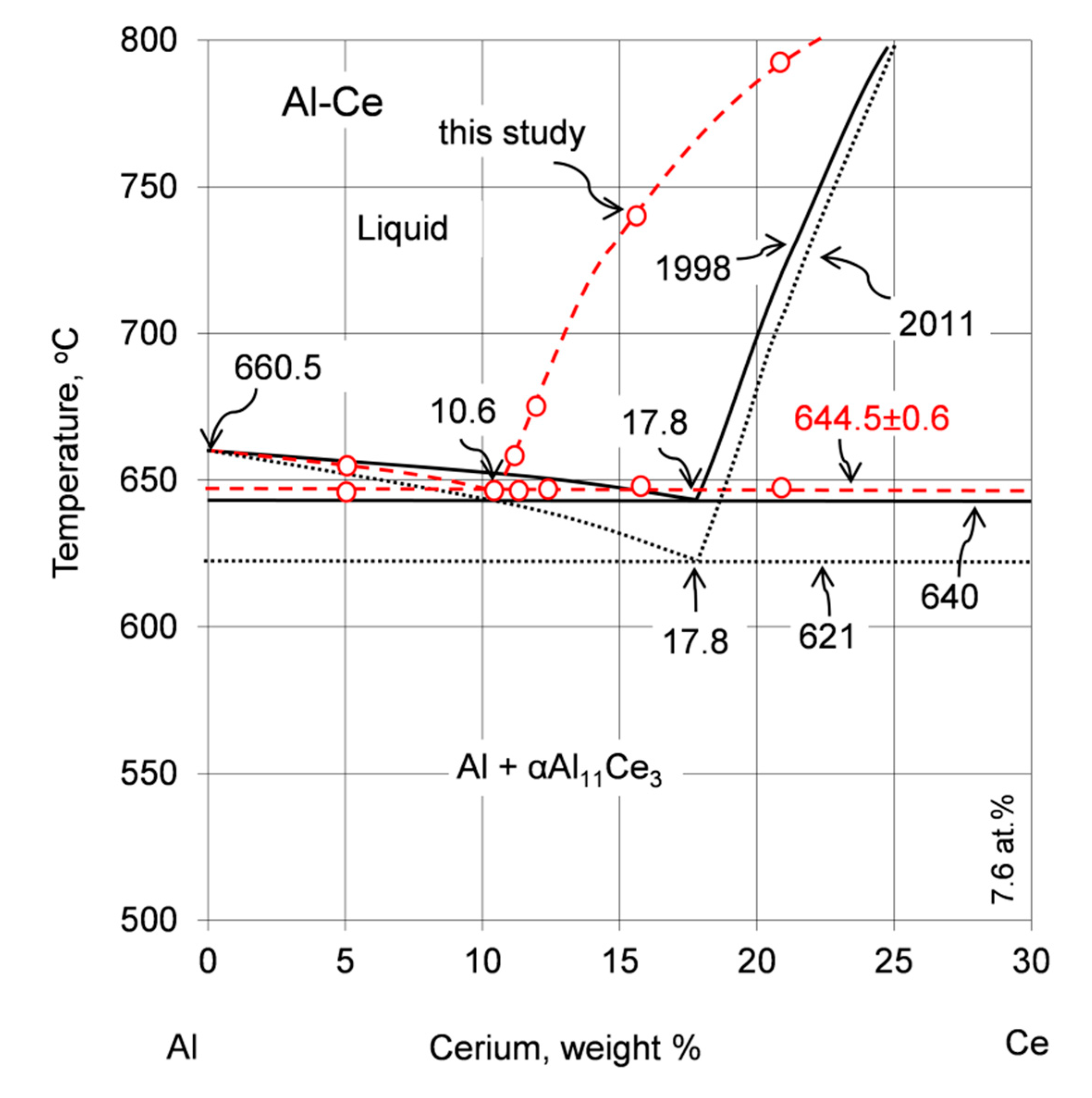
4.1. Coordinates of the Eutectic Reaction
4.2. Formation Mechanism, Morphology, and Crystallography of the Eutectic Structure
4.3. Contribution of the Al11Ce3 Phase to Eutectic and Alloy Strengthening
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Czerwinski, F.; Kasprzak, W.; Sediako, D.; Emadi, D.; Shaha, S.; Friedman, J.; Chen, D. High-Temperature Aluminum Alloys for Automotive Powertrains. Adv. Mater. Process. 2016, 174, 16–20. [Google Scholar]
- Czerwinski, F.; Shaha, S.; Kasprzak, W.; Friedman, J.; Chen, D. Effect of Transition Metals on Thermal Stability of Al‒Si Cast Alloys. In Frontiers in Materials Processing, Applications, Research and Technology; Springer: Singapore, 2017; pp. 287–296. [Google Scholar]
- Fan, Y.; Makhlouf, M. The Al-Al3Ni Eutectic Reaction: Crystallography and Mechanism of Formation. Metall. Mater. Trans. A 2015, 46, 3808–3812. [Google Scholar] [CrossRef]
- Suwanpreecha, C.; Pandee, P.; Patakham, U.; Limmaneevichitr, C. New Generation of Eutectic Al-Ni Casting Alloys for Elevated Temperature Services. Mater. Sci. Eng. A 2018, 709, 46–54. [Google Scholar] [CrossRef]
- Fujikawa, S.; Hirano, K.; Fukushima, Y. Diffusion of Silicon in Aluminum. Metall. Mater. Trans. A 1978, 9, 1811–1815. [Google Scholar] [CrossRef]
- Czerwinski, F. Cerium in Aluminum Alloys. J. Mater. Sci. 2020, 55, 24–72. [Google Scholar] [CrossRef]
- Czerwinski, F. Assessing Differences Between the Use of Cerium and Scandium in Aluminum Alloying. Mater. Sci. Technol. 2020, 36, 255–263. [Google Scholar] [CrossRef]
- Czerwinski, F. Thermal Stability of Aluminum Alloys. Materials 2020, 13, 3441. [Google Scholar] [CrossRef]
- Barth, O. Die Erhohung der Chemischen Widerstandsfahigkeit Mechanisch Noch gut Bearbeitbarer, fur Konstructionszwecke Verwendbarer Legierungen; Teil II: Uber den Einfluss des Cers auf die Mechanischen und Chemischen Eigenschaften des Aluminiums. Metall. Z. Gesamte Huttenkd. 1912, 8, 261–276. (In German) [Google Scholar]
- Gillett, H.; Schnee, V. Cerium in Aluminum Alloys. Ind. Eng. Chem. 1923, 15, 709–711. [Google Scholar] [CrossRef]
- Okamoto, H. Al-Ce (Aluminum-Cerium). J. Phase Equilibria Diffus. 2011, 32, 392–393. [Google Scholar] [CrossRef]
- Kononenko, V.; Golubev, S. Phase Diagrams of Binary Systems of Aluminum with Al-La, Ce, Pr, Nd, Sm, Eu, Yb, Sc and Y. Russ. Metall. 1990, 2, 193. [Google Scholar]
- Okamoto, H. Al-Ce (aluminum-cerium). J. Phase Equilibria 1998, 19, 396. [Google Scholar] [CrossRef]
- Cao, Z.; Kong, G.; Che, C.; Wang, Y.; Peng, H. Experimental Investigation of Eutectic Point in Al-Rich Al-La, Al-Ce, Al-Pr and Al-Nd Systems. J. Rare Earths 2017, 35, 1022–1028. [Google Scholar] [CrossRef]
- Gschneidner, K.; Calderwood, F. The Al−Ce (Aluminum-Cerium) System. Bull. Alloy Phase Diagr. 1988, 9, 669–672. [Google Scholar] [CrossRef]
- Saccone, A.; Cardinale, A.; Delfino, S.; Ferro, R. Phase Equilibria in the Rare Earth Metals(R)-Rich Regions of the R-Al Systems (R=La, Ce, Pr, Nd). Z. Met. 1996, 87, 82–87. [Google Scholar]
- Gao, M.; Ünlü, N.; Shiflet, G.; Mihalkovič, M. Reassessment of Al-Ce and Al-Nd Binary Systems Supported by Critical Experiments and First-Principles Energy Calculations. Metall. Mater. Trans. A 2005, 36, 3269–3279. [Google Scholar] [CrossRef]
- Buschow, K.; Van Vucht, J. The Binary Systems Cerium-Aluminum and Praseodymium-Aluminum. Z. Met. 1966, 57, 162–166. [Google Scholar]
- Kang, Y.; Pelton, A.; Chartrand, P.; Fuerst, C. Critical Evaluation and Thermodynamic Optimization of the Al–Ce, Al–Y, Al–SC and MG–SC Binary Systems. Calphad 2008, 32, 413–422. [Google Scholar] [CrossRef]
- Jin, L.; Kang, Y.; Chartrand, P.; Fuerst, C. Thermodynamic Evaluation and Optimization of Al–la, Al–Ce, Al–Pr, Al–ND and Al–SM Systems Using the Modified Quasichemical Model for Liquids. Calphad 2011, 35, 30–41. [Google Scholar] [CrossRef]
- Andersson, J.; Helander, T.; Höglund, L.; Shi, P.; Sundman, B. Thermo-Calc & DICTRA, Computational Tools for Materials Science. Calphad 2002, 26, 273–312. [Google Scholar] [CrossRef]
- Sims, Z.C.; Rios, O.R.; Weiss, D.; Turchi, P.E.A.; Perron, A.; Lee, J.R.I.; Li, T.T.; Hammons, J.A.; Bagge-Hansen, M.; Willey, T.M.; et al. High Performance Aluminum–Cerium Alloys for High-Temperature Applications. Mater. Horiz. 2017, 4, 1070–1078. [Google Scholar] [CrossRef]
- Plotkowski, A.; Rios, O.; Sridharan, N.; Sims, Z.; Unocic, K.; Ott, R.T.; Dehoff, R.R.; Babu, S.S. Evaluation of an Al-Ce Alloy for Laser Additive Manufacturing. Acta Mater. 2017, 126, 507–519. [Google Scholar] [CrossRef]
- Sokolowski, J.; Kierkus, W.; Kasprzak, M.; Kasprzak, W. Method and Apparatus for Universal Metallurgical Simulation and Analysis. U.S. Patent 7,354,491 B2, 8 April 2008. [Google Scholar]
- Buschow, K.; Van Vucht, J. Systematic Arrangement of the Binary Rare-Earth-Aluminium Systems. Philips Res. Rep. 1967, 22, 233–245. [Google Scholar]
- ASTM. Standard Test Methods of Compression Testing of Metallic Materials at Room Temperature, ASTM E9-09; ASTM: West Conshohocken, PA, USA, 2009. [Google Scholar]
- Sun, Y.; Hung, C.; Hebert, R.J.; Fennessy, C.; Tulyani, S.; Aindow, M. Eutectic Microstructures in Dilute Al-Ce and Al-Co Alloys. Mater. Charact. 2019, 154, 269–276. [Google Scholar] [CrossRef]
- Weiss, D. Improved High-Temperature Aluminum Alloys Containing Cerium. J. Mater. Eng. Perform. 2019, 28, 1903–1908. [Google Scholar] [CrossRef]
- Wang, L.; Qi, R.; Ye, B.; Bai, Y.; Huang, R.; Jiang, H.; Ding, W. Improved Tensile Strength of Al-5Ce Alloy by Permanent Magnet Stirring. Metall. Mater. Trans. A 2020, 51, 1972–1977. [Google Scholar] [CrossRef]
- Van Vucht, J. Contribution to the Knowledge of the System Cerium-Aluminum. Z. Met. 1957, 48, 252–258. [Google Scholar]
- Drits, M.; Kadaner, E.; Shoa, N. Solid Solubility of Rare Earth Metals in Aluminum. Izv. Akad. Nauk SSSR 1969, 1, 219–223. [Google Scholar]
- Van Vucht, J.; Buschow, K. On the Binary Aluminum-Rich Compounds of the Rare-Earth Elements. Philips Res. Rep. 1964, 19, 319–322. [Google Scholar]
- Czerwinski, F. Modern Aspects of Liquid Metal Engineering. Metall. Mater. Trans. B 2017, 48, 367–393. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, Y.; Bian, X. Microstructure Selection Map for Rapidly Solidified Al-Rich Al–Ce Alloys. J. Cryst. Growth 2004, 260, 557–565. [Google Scholar] [CrossRef]
- Croker, M.N.; Fidler, R.S.; Smith, R.W. The Characterization of Eutectic Structures. Proc. R. Soc. Lond. Ser. A 1973, 335, 15–37. [Google Scholar] [CrossRef]
- Jackson, K.; Hunt, J. Lamellar and Rod Eutectic Growth. AIME Metall. Soc. 1988, 236, 363–376. [Google Scholar] [CrossRef]
- Taylor, M.; Fidler, R.; Smith, R. Broken Lamellar Eutectic Growth; Structure of the Silver-Bismuth Eutectic. J. Cryst. Growth 1968, 3, 666–673. [Google Scholar] [CrossRef]
- Stefanescu, D.M. Solidification of two-phase alloys—micro-scale solidification. In Science and Engineering of Casting Solidification; Springer: Cham, Switzerland, 2015; pp. 197–250. [Google Scholar]
- Borzone, G.; Cacciamani, G.; Ferro, R. Heats of Formation of Aluminum-Cerium Intermetallic Compounds. Metall. Mater. Trans. A 1991, 22, 2119–2123. [Google Scholar] [CrossRef]
- Sommer, F.; Keita, M. Determination of the Enthalpies of Formation of Intermetallic Compounds of Aluminium with Cerium, Erbium and Gadolinium. J. Less Common Met. 1987, 136, 95–99. [Google Scholar] [CrossRef]
- Gao, M.C.; Rollett, A.D.; Widom, M. Lattice Stability of Aluminum-Rare Earth Binary Systems: A First-Principles Approach. Phys. Rev. B 2007, 75, 174120. [Google Scholar] [CrossRef]
- Shahani, A.J.; Xiao, X.; Voorhees, P.W. The Mechanism of Eutectic Growth in Highly Anisotropic Materials. Nat. Commun. 2016, 7, 12953. [Google Scholar] [CrossRef]
- Zhang, Z.; Bian, X.; Wang, Y.; Zhang, J. Solidification Microstructure Formation of an Al–Ce–SR Alloy Under Conventional and Rapid Solidification Conditions. J. Alloys Compd. 2002, 346, 134–141. [Google Scholar] [CrossRef]
- Day, M.; Hellawell, A. The Microstructure and Crystallography of Aluminum-Silicon Eutectic Alloys. Proc. R. Soc. A 1968, 305, 473–491. [Google Scholar]
- Medvedev, A.E.; Murashkin, M.Y.; Enikeev, N.; Valiev, R.Z.; Hodgson, P.D.; Lapovok, R.; Bikmukhametov, I. Effect of the Eutectic Al-(Ce,La) Phase Morphology on Microstructure, Mechanical Properties, Electrical Conductivity and Heat Resistance of Al-4.5(Ce,La) Alloy After SPD and Subsequent Annealing. J. Alloys Compd. 2019, 796, 321–330. [Google Scholar] [CrossRef]
- Wang, S.; Liu, G.; Wang, J.; Misra, A. Characteristic Orientation Relationships in Nanoscale Al-AlCu Eutectic. Mater. Charact. 2018, 142, 170–178. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, M.; Baker, I. Eutectic/Eutectoid Multi-Principle Component Alloys: A Review. Mater. Charact. 2019, 147, 545–557. [Google Scholar] [CrossRef]
- Weiss, D.; Rios, O.; Sims, Z.; McCall, S.K.; Ott, R.T. Casting Characteristics of High Cerium Content Aluminum Alloys. Light Met. 2017, 28, 205. Available online: https://e-reports-ext.llnl.gov/pdf/891293.pdf (accessed on 20 July 2019).
- Martínez-Villalobos, M.A.; Figueroa, I.A.; Suarez, M.A.; Rodríguez, G.; Ángel, L.; Peralta, O.N.; Reyes, G.G.; López, I.A.; Martínez, J.V.; Trujillo, C.D. Microstructural Evolution of Rapid Solidified Al-Ni Alloys. J. Mex. Chem. Soc. 2017, 60, 67–72. [Google Scholar] [CrossRef]
- Srivastava, N.; Chaudhari, G.P. Effect of Ultrasonic Treatment on the Mechanical Behaviour of Al–NI Alloys. Mater. Sci. Technol. 2019, 35, 1239–1247. [Google Scholar] [CrossRef]
- Lu, Y.; Dong, Y.; Sheng, G.; Li, J.; Kang, H.; Wang, T.; Wen, B.; Wang, Z.; Jie, J.; Cao, Z.; et al. A Promising New Class of High Temperature Alloys: Eutectic High-Entropy Alloys. Sci. Rep. 2014, 4, 6200. [Google Scholar] [CrossRef]
- Tiwary, C.S.; Kashiwar, A.; Bhowmick, S.; Kumar, K.H.; Chattopadhyay, K.; Banerjee, D. Engineering an Ultrafine Intermetallic Eutectic Ternary Alloy for High Strength and High Temperature Applications. Scr. Mater. 2018, 157, 67–71. [Google Scholar] [CrossRef]
- Shi, P.; Ren, W.; Zheng, T.; Ren, Z.; Hou, X.; Peng, J.; Hu, P.; Gao, Y.; Zhong, Y.; Liaw, P.K. Enhanced Strength–Ductility Synergy in Ultrafine-Grained Eutectic High-Entropy Alloys by Inheriting Microstructural Lamellae. Nat. Commun. 2019, 10, 1–8. [Google Scholar] [CrossRef]


| Year | Temperature | Cerium Content | Eutectic Phase | Determination Method | Reference | |
|---|---|---|---|---|---|---|
| °C | wt.% | at.% | ||||
| 1990 | 633 | 12.2 | 2.6 | αAl11Ce3 | Experimental | [12] |
| 1998 | 640 | 17.8 | 4 | αAl11Ce3 a) | Based on previous experimental data in References [15,16] | [13] |
| 2005 | 641 | 12.2 | 2.6 | Al11Ce3 b) | Experimental using DTA and calculated with CALPHAD | [17] |
| 2008, 2011 | 621 | Not specified | Al11Ce3 b) | Optimized with modified quasichemical model and previous experimental data in References [17,18] | [19,20] | |
| 2011 | 621 | 17.8 | 4 | αAl11Ce3 a) | Derived based on optimized diagram in References [19,20] | [11] |
| 2017 | 640, 642 | Approx. 10 | Approx. 2.1 | Al11Ce3 b) | Calculated with Thermo-Calc and diffusion module DICTRA in Reference [21] | [22,23] |
| 2017 | 645 | 12 | 2.6 | αAl11Ce3 | Experimental using DSC, X-ray diffraction, metallography | [14] |
| Alloy | Ce | Si | Mg | Ni | Ti | Mn | Cu |
|---|---|---|---|---|---|---|---|
| wt.% | |||||||
| Al–5Ce | 5.21 (1.05 at.%) | 0.016 | 0.042 | 0.013 | <0.001 | <0.001 | <0.005 |
| Al–10Ce | 10.61 (2.23 at.%) | 0.015 | 0.042 | 0.031 | <0.001 | <0.001 | <0.005 |
| Al–11Ce | 11.82 (2.49 at.%) | 0.055 | 0.010 | 0.038 | 0.038 | 0.019 | 0.004 |
| Al–12Ce | 12.51 (2.68 at.%) | 0.056 | 0.010 | 0.039 | 0.033 | 0.019 | 0.006 |
| Al–15Ce | 15.80 (3.49 at.%) | 0.018 | 0.080 | 0.058 | <0.024 | <0.001 | <0.005 |
| Al–20Ce | 20.76 (4.80 at.%) | 0.017 | 0.110 | 0.072 | <0.023 | <0.001 | <0.005 |
| Alloys | Liquidus, °C | Eutectic, °C | Ef, °C | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | Average | SDev. | 1 | 2 | 3 | Average | SDev. | Average | |
| Al–5Ce | 651.6 | 651.5 | 651.4 | 651.5 | 0.08 | 644.3 | 644.0 | 643.9 | 644.1 | 0.24 | 617.4 |
| Al–10Ce | Same as eutectic | 643.4 | 644.0 | 644.3 | 643.9 | 0.47 | 617.0 | ||||
| Al–11Ce | 660.0 | 661.0 | 659.2 | 660.1 | 0.74 | 644.3 | 644.3 | 644.2 | 644.3 | 0.05 | 617.0 |
| Al–12Ce | 670.0 | 672.3 | 671.0 | 671.1 | 0.94 | 645.1 | 645.0 | 645.0 | 645.0 | 0.05 | 617.3 |
| Al–15Ce | 741.3 | 742.6 | 742.4 | 742.1 | 0.57 | 645.2 | 645.1 | 645.2 | 645.2 | 0.05 | 618.8 |
| Al–20Ce | 791.3 | 792.1 | 790.2 | 791.2 | 0.78 | 644.9 | 644.9 | 644.9 | 644.9 | 0.02 | 617.2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Czerwinski, F.; Shalchi Amirkhiz, B. On the Al–Al11Ce3 Eutectic Transformation in Aluminum–Cerium Binary Alloys. Materials 2020, 13, 4549. https://doi.org/10.3390/ma13204549
Czerwinski F, Shalchi Amirkhiz B. On the Al–Al11Ce3 Eutectic Transformation in Aluminum–Cerium Binary Alloys. Materials. 2020; 13(20):4549. https://doi.org/10.3390/ma13204549
Chicago/Turabian StyleCzerwinski, Frank, and Babak Shalchi Amirkhiz. 2020. "On the Al–Al11Ce3 Eutectic Transformation in Aluminum–Cerium Binary Alloys" Materials 13, no. 20: 4549. https://doi.org/10.3390/ma13204549
APA StyleCzerwinski, F., & Shalchi Amirkhiz, B. (2020). On the Al–Al11Ce3 Eutectic Transformation in Aluminum–Cerium Binary Alloys. Materials, 13(20), 4549. https://doi.org/10.3390/ma13204549