Research on the Flame Retardancy Properties and Mechanism of Modified Asphalt with Halloysite Nanotubes and Conventional Flame Retardant
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. The Preparation of Flame-Retardant-Modified Asphalt
2.3. Testing Methods
2.3.1. Testing of Flame Retardancy Properties
2.3.2. Testing of the Flame-Retardant Mechanism
3. Results and Discussion
3.1. Test and Analysis of the Flame Retardant Property
3.1.1. Flammability: IT and LOI
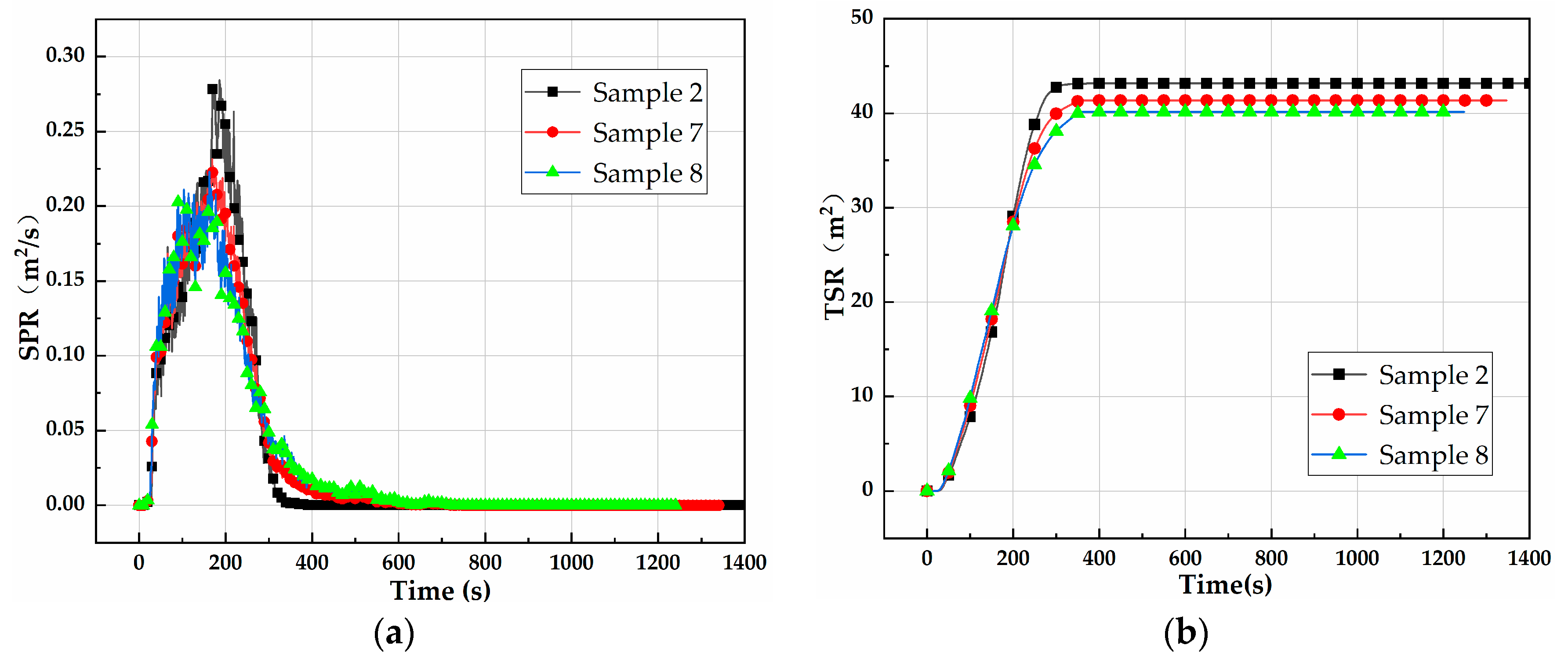
3.1.2. Dynamic Flammability: CCTs
3.2. Flame-Retardant Mechanism
3.2.1. Thermal Degradation Behavior of Asphalt Binder
3.2.2. FTIR Spectra of Residue for Asphalt Binders at Different Temperatures
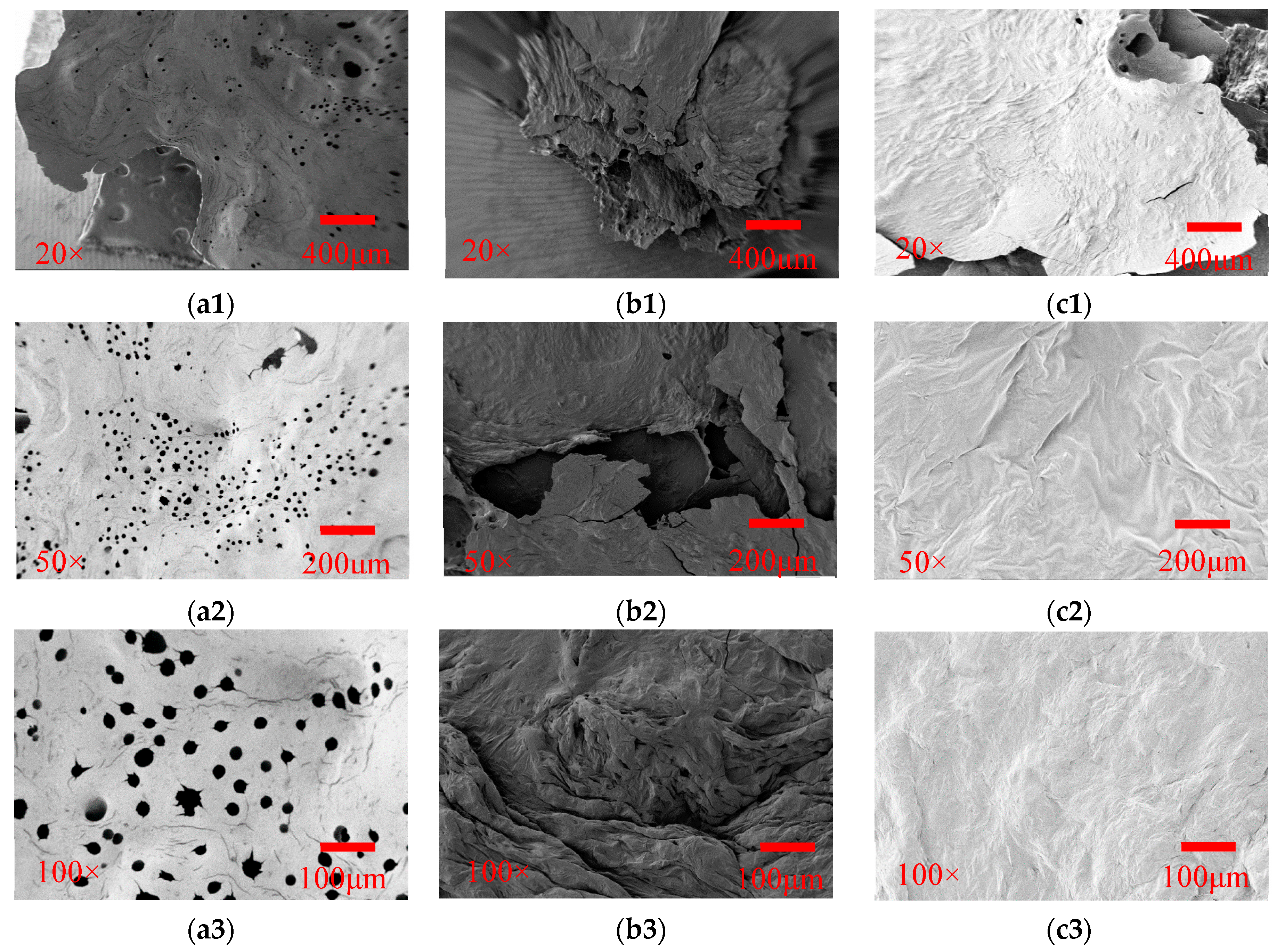
3.2.3. Char Residue Analysis
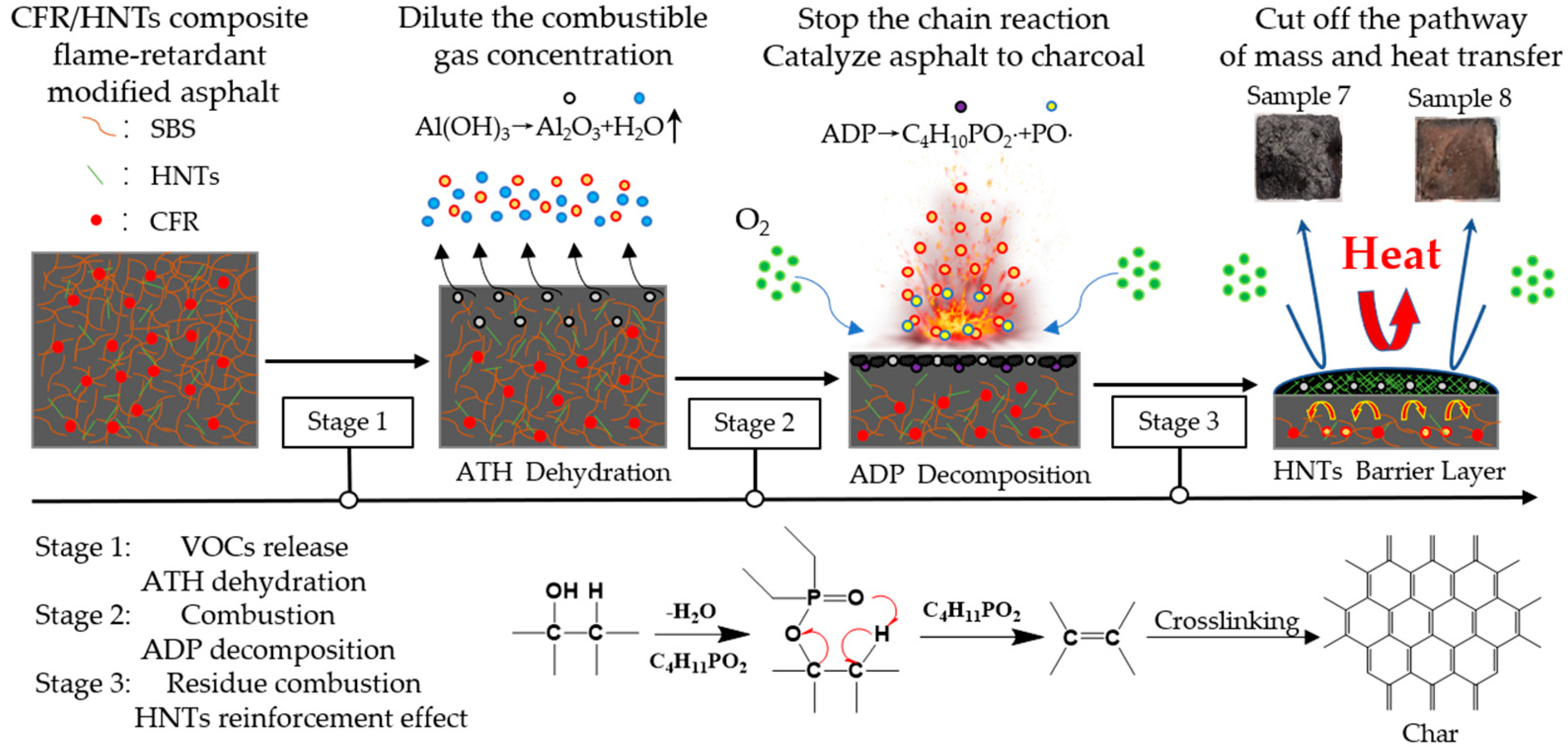
3.3. Flame Retardant Mechanism of HNTs Coefficient CFR Modified Asphalt
4. Conclusions
- Flame retardancy properties
- 2.
- Flame retardancy mechanism
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| HNTs | halloysite nanotubes |
| CFR | conventional flame retardants |
| COC | Cleveland open cup method |
| LOI | Limiting oxygen index |
| CCTs | Cone calorimeter tests |
| TGA | Thermogravimetric Analyzer |
| FTIR | Fourier-transform Infrared |
| SEM | Scanning Electron Microscopy |
| ATH | Aluminum hydroxide |
| ADP | Aluminum diethyl phosphate |
| Al2O3 | Aluminum oxide |
| OLSs | organic layered silicates |
| OMMT | organic montmorillonite |
| VOCs | Volatile organic compounds |
| OEVMT | organic expanded vermiculite |
| LDHs | nano-layered hydroxides |
| EG | expanded graphite |
| CEPPA | 2-carboxy ethyl phenyl phosphonic acid |
| IT | ignition temperature |
| TTI | time to ignition |
| PHRR | the peak of heat release rate |
| PSPR | the peak of smoke produce rate |
| THR | total heat release |
| TSR | total smoke production |
| ML | mass loss |
References
- Qiu, J.L.; Yang, T.; Wang, X.L.; Wang, L.X.; Zhang, G.L. Review of the Flame retardancy on highway tunnel asphalt pavement. Constr. Build. Mater. 2019, 195, 468–482. [Google Scholar] [CrossRef]
- Zhang, Y.G.; Zhang, Z.; Xue, S.; Wang, R.J.; Xiao, M. Stability analysis of a typical landslide mass in the Three Gorges Reservoir under varying reservoir water levels. Environ. Earth Sci. 2020, 79, 42. [Google Scholar] [CrossRef]
- Li, L.H.; Zou, X.L.; Chen, C.Y. Study on oxygen index and road performance of flame retardant asphalt with combined additives added. J. Build. Mater. 2013, 16, 76–80. [Google Scholar] [CrossRef]
- Xiao, F.P.; Guo, R.; Wang, J.G. Flame retardant and its influence on the performance of asphalt-A review. Constr. Build. Mater. 2019, 212, 841–861. [Google Scholar] [CrossRef]
- Bonati, A.; Merusi, F.; Polacco, G.; Filippi, S.; Felice, G. Ignitability and thermal stability of asphalt binders and mastics for flexible pavements in highway tunnels. Constr. Build. Mater. 2012, 37, 660–668. [Google Scholar] [CrossRef]
- Li, B.; Liu, J.X.; Han, F.; Li, X.L.; Li, L.Y.; Li, Y.; Duan, X.F. Preparation of Flame Retardant Modified with Titanate for Asphalt Binder. Adv. Mater. Sci. Eng. 2014, 510958. [Google Scholar] [CrossRef]
- Qian, G.P.; Yu, H.N.; ASCE, M.; Gong, X.B.; Zheng, W.F. Effect of Phosphorus Slag Powder on Flammability Properties of Asphalt. J. Mater. Civ. Eng. 2019, 31, 04019280. [Google Scholar] [CrossRef]
- Fu, Q.L.; Wei, J.G.; Peng, W.J.; Jin, L. Performance and Flame Retardant Mechanism of Coordinated Flame Retardant Asphalt with DBDPE and Sb2O3. China J. Highw. Transp. 2020, 33, 44–55. Available online: http://kns.cnki.net/kcms/detail/61.1313.U.20200222.1133.002.html (accessed on 23 February 2020).
- Altarawneh, M.; Dlugogorski, B.Z. Formation of polybrominated dibenzofurans from polybrominated biphenyls. Chemosphere 2015, 119, 1048–1053. [Google Scholar] [CrossRef]
- Wu, K.; Zhu, K.; Kang, C.; Wu, B.; Huang, Z.Y. An experimental investigation of flame retardant mechanism of hydrated lime in asphalt mastics. Mater. Des. 2016, 103, 223–229. [Google Scholar] [CrossRef]
- Xu, T.; Huang, X.M.; Zhao, Y.L. Investigation into the Properties of Asphalt Mixtures Containing Magnesium Hydroxide Flame Retardant. Fire Saf. J. 2011, 46, 330–334. [Google Scholar] [CrossRef]
- Wu, B.; Huang, Z.Y.; Zhu, K. Investigation on the Combustion Process and Flame Retardant Performance of Asphalt with Metal Hydroxides. Adv. Mater. Res. 2014, 1065–1069, 749–754. [Google Scholar] [CrossRef]
- Li, L.H.; Zou, X.L.; Chen, C.Y. Study on flame retardancy and smoke suppression and road performance of flame retardant asphalt mixture. J. Build. Mater. 2012, 15, 648–653. [Google Scholar] [CrossRef]
- Xu, T.; Wang, Y.; Xia, W.J.; Hu, Z.H. Effects of Flame Retardants on Thermal Decomposition of Sara Fractions Separated from Asphalt Binder. Constr. Build. Mater. 2018, 173, 209–219. [Google Scholar] [CrossRef]
- Li, X.L.; Zhou, Z.G.; Deng, X.; You, Z.P. Flame Resistance of Asphalt Mixtures with Flame Retardants through a Comprehensive Testing Program. J. Mater. Civ. Eng. 2017, 29, 04016266. [Google Scholar] [CrossRef]
- Zhang, Y.G.; Tang, J.; He, Z.Y.; Tan, J.K.; Li, C. A novel displacement prediction method using gated recurrent unit model with time series analysis in the Erdaohe landslide. Nat. Hazards. 2020. [CrossRef]
- Xu, G.J.; Chen, X.; Zhu, S.C.; Kong, L.D.; Huang, X.M.; Zhao, J.W.; Ma, T. Evaluation of Asphalt with Different Combinations of Fire Retardants. Materials 2019, 12, 1283. [Google Scholar] [CrossRef]
- Yang, X.L.; Shen, A.Q.; Guo, Y.C.; Wu, H.S.; Wang, H. A review of nano layered silicate technologies applied to asphalt materials. Road Mater. Pavement Des. 2020. [CrossRef]
- Liu, Y.; Gao, Y.S.; Wang, Q.; Lin, W.R. The Synergistic Effect of Layered Double Hydroxides with Other Flame Retardant Additives for Polymer Nanocomposites: A Critical Review. Dalton Trans. 2018, 47, 14827–14840. [Google Scholar] [CrossRef]
- Zhang, H.L.; Shi, C.J.; Han, J.; Yu, J.Y. Effect of Organic Layered Silicates on Flame Retardancy and Aging Properties of Bitumen. Constr. Build. Mater. 2013, 40, 1151–1155. [Google Scholar] [CrossRef]
- Bonati, A.; Merusi, F.; Bochicchio, G.; Tessadri, B.; Polacco, G.; Filippi, S.; Giuliani, F. Effect of nanoclay and conventional flame retardants on asphalt mixtures fire reaction. Constr. Build. Mater. 2013, 47, 990–1000. [Google Scholar] [CrossRef]
- Pei, J.Z.; Wen, Y.; Li, Y.W.; Shi, X.; Zhang, J.P.; Li, R.; Du, Q.L. Flame-retarding effects and combustion properties of asphalt binder blended with organo montmorillonite and alumina trihydrate. Constr. Build. Mater. 2014, 72, 41–47. [Google Scholar] [CrossRef]
- Yang, X.L.; Shen, A.Q.; Su, Y.X.; Zhao, W.D. Effects of Alumina Trihydrate (ATH) and Organic Montmorillonite (OMMT) on Asphalt Fume Emission and Flame Retardancy Properties of SBS-Modified Asphalt. Constr. Build. Mater. 2020, 236, 117576. [Google Scholar] [CrossRef]
- Liang, Y.S.; Yu, J.Y.; Feng, Z.G.; Ai, P.S. Flammability and Thermal Properties of Bitumen with Aluminium Trihydroxide and Expanded Vermiculite. Constr. Build. Mater. 2013, 48, 1114–1119. [Google Scholar] [CrossRef]
- Zhu, K.; Wang, Y.H.; Tang, D.Q.; Wang, Q.; Li, H.H.; Huang, Y.D.; Huang, Z.Y.; Wu, K. Flame-Retardant Mechanism of Layered Double Hydroxides in Asphalt Binder. Materials 2019, 12, 801. [Google Scholar] [CrossRef]
- Li, M.L.; Pang, L.; Chen, M.Z.; Xie, J.; Liu, Q.T. Effects of Aluminum Hydroxide and Layered Double Hydroxide on Asphalt Fire Resistance. Materials 2018, 11, 1939. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.F.; Zhu, Q.L.; Li, J.; Tao, K.; Xue, L.X.; Yan, Q. Synergistic Effect between Expandable Graphite and Ammonium Polyphosphate on Flame Retarded Polylactide. Polym. Degrad. Stab. 2011, 96, 183–189. [Google Scholar] [CrossRef]
- Sheng, Y.P.; Wu, Y.C.; Yan, Y.; Jia, H.C.; Qiao, Y.Y.; Underwood, B.S.; Niu, D.Y.; Kim, Y.R. Development of Environmentally Friendly Flame Retardant to Achieve Low Flammability for Asphalt Binder Used in Tunnel Pavements. J. Clean. Prod. 2020, 257, 120487. [Google Scholar] [CrossRef]
- Li, L.L.; Wu, Z.H.; Jiang, S.S.; Zhang, S.D.; Lu, S.Y.; Chen, W.P.; Sun, B.; Zhu, M.F. Effect of halloysite nanotubes on thermal and flame retardant properties of polyamide 6/melamine cyanurate composites. Polym. Compos. 2015, 36, 892–896. [Google Scholar] [CrossRef]
- Garcia-Garcia, D.; Garcia-Sanoguera, D.; Fombuena, V.; Lopez-Martinez, J.; Balart, R. Improvement of mechanical and thermal properties of poly(3-hydroxybutyrate) (PHB) blends with surface-modified halloysite nanotubes (HNT). Appl. Clay Sci. 2018, 162, 487–498. [Google Scholar] [CrossRef]
- Yuan, P.; Tan, D.Y.; Faïza, A. Properties and Applications of Halloysite Nanotubes: Recent Research Advances and Future Prospects. Appl. Clay Sci. 2015, 112–113, 75–93. [Google Scholar] [CrossRef]
- Liu, L.; Han, S.J.; Zhang, S.; Jin, H.Y.; Zhou, L. Preparation and properties of synergistic flame retardant nylon 66 with aluminium diethylphosphinate and halloysite nanotubes. Acta Mater. Compos. Sin. 2020. [CrossRef]
- Lyu, J.S.N.; Di, K.Y.; Cai, P.L.; Chen, X.T. Effects of halloysite nanotubes and 2-carboxyethyl phenylphosphonic acid on the flame retardant and mechanical properties of epoxy resin. Acta Mater. Compositae Sin. 2020. [CrossRef]
- Shi, H.Q.; Xu, T.; Zhou, P.; Jiang, R.L. Combustion Properties of Saturates, Aromatics, Resins, and Asphaltenes in Asphalt Binder. Constr. Build. Mater. 2017, 136, 515–523. [Google Scholar] [CrossRef]
- Xu, T.; Wang, H.C.; Huang, X.M.; Li, G.F. Inhibitory action of flame retardant on the dynamic evolution of asphalt pyrolysis volatiles. Fuel 2013, 105, 757–763. [Google Scholar] [CrossRef]
- Filippi, S.; Cappello, M.; Polacco, G. Limiting oxygen index reduction in bitumen modified with nanoclays. Fire Saf. J. 2020, 111, 102929. [Google Scholar] [CrossRef]
- Schartel, B.; Hull, T.R. Development of fire-retarded materials—Interpretation of cone calorimeter data. Fire Mater. 2007, 31, 327–354. [Google Scholar] [CrossRef]


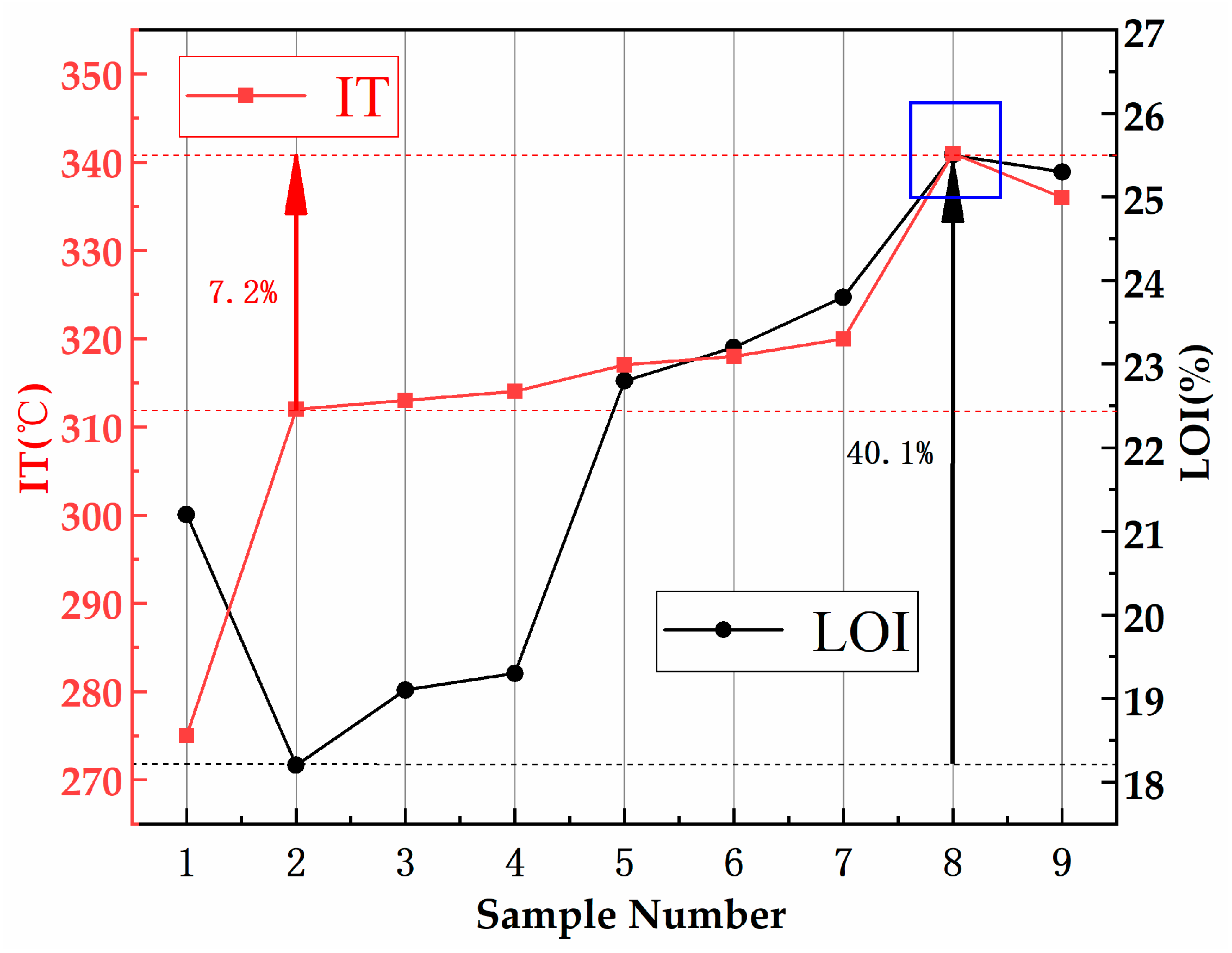




| No. | Matrix Asphalt | SBS wt % | HNTs wt % | ATH wt % | ADP wt % | Penetration/0.1 mm | Softening Point/°C | Ductility/cm |
|---|---|---|---|---|---|---|---|---|
| Sample 1 | 100 | - | - | - | - | 67.5 | 49.8 | >100 (15 °C) |
| Sample 2 | 100 | 5 | - | - | - | 51.9 | 71.5 | 30 (±5) |
| Sample 3 | 100 | 5 | 1 | - | - | 50.8 | 78.7 | 35 (±4) |
| Sample 4 | 100 | 5 | 2 | - | - | 48.3 | 82.3 | 37 (±2) |
| Sample 5 | 100 | 5 | - | 5 | 3 | 42.3 | 83.6 | 18 (±3) |
| Sample 6 | 100 | 5 | - | 4 | 4 | 45.6 | 81.3 | 24 (±5) |
| Sample 7 | 100 | 5 | - | 3 | 5 | 48.3 | 80.5 | 28 (±2) |
| Sample 8 1 | 100 | 5 | 1 | 3 | 5 | 45.6 | 87.3 | 30 (±3) |
| Sample 9 | 100 | 5 | 2 | 3 | 5 | 42.0 | 91.2 | 28 (±2) |
| Sample 10 2 | 100 | - | - | - | - | 64.3 | 50.6 | >100 (15 °C) |
| Standard | GB/T 0604 | GB/T 0606 | GB/T 0605 | |||||
| No. | TTI (s) | PHRR (kw/m2) | THR (MJ/m2) | PSPR (m2/s) | TSR (m2) | ML (%) |
|---|---|---|---|---|---|---|
| Sample 2 | 21 | 993.1 | 239.1 | 0.279 | 43.2 | 96.30 |
| Sample 7 | 28 | 666.6 | 170.3 | 0.231 | 41.3 | 92.04 |
| Sample 8 | 30 | 495.4 | 128.1 | 0.211 | 40.1 | 89.20 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, Y.; He, Z.; Li, X.; Jiang, B.; Li, J.; Zhang, Y. Research on the Flame Retardancy Properties and Mechanism of Modified Asphalt with Halloysite Nanotubes and Conventional Flame Retardant. Materials 2020, 13, 4509. https://doi.org/10.3390/ma13204509
Tan Y, He Z, Li X, Jiang B, Li J, Zhang Y. Research on the Flame Retardancy Properties and Mechanism of Modified Asphalt with Halloysite Nanotubes and Conventional Flame Retardant. Materials. 2020; 13(20):4509. https://doi.org/10.3390/ma13204509
Chicago/Turabian StyleTan, Yangwei, Zhaoyi He, Xiang Li, Bin Jiang, Jiaqi Li, and Yonggang Zhang. 2020. "Research on the Flame Retardancy Properties and Mechanism of Modified Asphalt with Halloysite Nanotubes and Conventional Flame Retardant" Materials 13, no. 20: 4509. https://doi.org/10.3390/ma13204509
APA StyleTan, Y., He, Z., Li, X., Jiang, B., Li, J., & Zhang, Y. (2020). Research on the Flame Retardancy Properties and Mechanism of Modified Asphalt with Halloysite Nanotubes and Conventional Flame Retardant. Materials, 13(20), 4509. https://doi.org/10.3390/ma13204509






