Cytotoxicity of Self-Etch Versus Etch-and-Rinse Dentin Adhesives: A Screening Study
Abstract
1. Introduction
- -
- Self-etch adhesives show no different cytotoxicity in relation to etch-and-rinse adhesives regarding the triangulated evaluation.
- -
- Sequentially applied substances have different cytotoxic effects other than single applied substances regarding the triangulated evaluation.
- -
- Self-etch one step and self-etch two steps adhesives show no different cytotoxicity regarding the triangulated evaluation.
2. Materials and Methods
2.1. Materials and Cells
2.2. The Triangulated Parameters Tested
2.2.1. Quantitative Evaluation
2.2.2. Qualitative Evaluation
2.2.3. Reactivity Index Evaluation
2.3. Statistical Analysis
3. Results
3.1. Quantitative Result
3.2. Qualitative Result
3.3. Reactivity Index Result
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Van Meerbeek, B.; Yoshihara, K.; Yoshida, Y.; Mine, A.; de Munck, J.; van Landuyt, K.L. State of the art of self-etch adhesives. Dent. Mater. 2011, 27, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Giannini, M.; Makishi, P.; Ayres, A.P.A.; Vermelho, P.M.; Fronza, B.M.; Nikaido, T.; Tagami, J. Self-Etch Adhesive Systems: A Literature Review. Braz. Dent. J. 2015, 26, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Szep, S.; Kunkel, A.; Ronge, K.; Heidemann, D. Cytotoxicity of modern dentin adhesives--in vitro testing on gingival fibroblasts. J. Biomed. Mater. Res. 2002, 63, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Sangwichit, K.; Kingkaew, R.; Pongprueksa, P.; Senawongse, P. Effect of thermocycling on the durability of etch-and-rinse and self-etch adhesives on dentin. Dent. Mater. J. 2016, 35, 360–368. [Google Scholar] [CrossRef] [PubMed]
- Caldas, I.P.; Alves, G.G.; Barbosa, I.B.; Scelza, P.; De Noronha, F.; Scelza, M.Z. In Vitro cytotoxicity of dental adhesives: A systematic review. Dent. Mater. 2019, 35, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Masarwa, N.; Mohamed, A.; Abou-Rabii, I.; Abu Zaghlan, R.; Steier, L. Longevity of Self-etch Dentin Bonding Adhesives Compared to Etch-and-rinse Dentin Bonding Adhesives: A Systematic Review. J. Evid. Based Dent. Pract. 2016, 16, 96–106. [Google Scholar] [CrossRef]
- Ergün, G.; Egilmez, F.; Üçtasli, M.B.; Yilmaz, S. Effect of light curing type on cytotoxicity of dentine-bonding agents. Int. Endod. J. 2007, 40, 216–223. [Google Scholar] [CrossRef]
- Caughman, W.; Caughman, G.B.; Shiflett, R.A.; Rueggeberg, F.; Schuster, G.S. Correlation of cytotoxicity, filler loading and curing time of dental composites. Biomaterials 1991, 12, 737–740. [Google Scholar] [CrossRef]
- Hanks, C.; Strawn, S.; Watahai, J.; Craig, R. Cytotoxic Effects of Resin Components on Cultured Mammalian Fibroblasts. J. Dent. Res. 1991, 70, 1450–1455. [Google Scholar] [CrossRef]
- Gerhardt-Szép, S.; Kastratovic, M.; Zahn, T.; Zahn, B.; Ottl, P.; Ronge, K. Zelluläre Verträglichkeit xylometazolinhydrochloridhaltiger gingivaler Retraktionsmedien. Dtsch. Zahnarztl. Z. 2016, 38–51. [Google Scholar] [CrossRef]
- Demirci, M.; Hiller, K.; Bosl, C.; Galler, K.; Schmalz, G.; Schweikl, H. The induction of oxidative stress, cytotoxicity, and genotoxicity by dental adhesives. Dent. Mater. 2008, 24, 362–371. [Google Scholar] [CrossRef]
- DIN Deutsches Institut für Normung e.V. Biologische Beurteilung von Medizinproduktion—Teil 5: Prüfungen auf In-Vitro-Zytotoxizität (ISO 10993-5:2009); Deutsche Fassung EN ISO 10993-5:2009; Beuth Verlag GmbH: Berlin, Germany, 2009. [Google Scholar]
- Murray, P.E.; García Godoy, C.; García Godoy, F. How is the biocompatibilty of dental biomaterials evaluated? Med. Oral Patol. Oral Cir. Bucal 2007, 12, 258–266. [Google Scholar]
- Alghanem, A.; Fernandes, G.; Visser, M.; Dziak, R.; Renné, W.G.; Sabatini, C. Biocompatibility and bond degradation of poly-acrylic acid coated copper iodide-adhesives. Dent. Mater. 2017, 33, e336–e347. [Google Scholar] [CrossRef] [PubMed]
- Cal, E.; Guneri, P.; Atay, A.; Cetintas, V.B. Cytotoxicity of dentin bonding agents. Gen. Dent. 2014, 62, 11–14. [Google Scholar]
- Elias, S.T.; Dos Santos, A.F.; Garcia, F.C.; Pereira, P.N.; Hilgert, L.; Fonseca-Bazzo, Y.M.; Guerra, E.N.S.; Ribeiro, A.P.D. Cytotoxicity of Universal, Self-Etching and Etch-and-Rinse Adhesive Systems According to the Polymerization Time. Braz. Dent. J. 2015, 26, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.S.; Jan, Y.D.; Huang, G.S.; Huang, C.H.; Chou, H.Y.; Wang, J.S.; Tseng, W.Y. Effect of dentin bonding agent diffusing through dentin slices on the reactive oxygen species production and apoptosis of pulpal cells. J. Formos. Med. Assoc. 2015, 114, 339–346. [Google Scholar] [CrossRef]
- Mirmotalebi, F.; Nazari, S. Comparison of Cytotoxicity of Three Dentin Bonding Systems with Two Thicknesses of Dentin Barrier on L929 Cell Line. Iran. Endod. J. 2006, 1, 109–113. [Google Scholar]
- Wegehaupt, F.J.; Lunghi, N.; Belibasakis, G.N.; Attin, T. Influence of light-curing distance on degree of conversion and cytotoxicity of etch-and-rinse and self-etch adhesives. BMC Oral Health 2016, 17, 12. [Google Scholar] [CrossRef]
- Galler, K.; Hiller, K.A.; Ettl, T.; Schmalz, G. Selective influence of dentin thickness upon cytotoxicity of dentin contacting materials. J. Endod. 2005, 31, 396–399. [Google Scholar] [CrossRef]
- Huang, F.M.; Chang, Y.C. Cytotoxicity of dentine-bonding agents on human pulp cells in vitro. Int. Endod. J. 2002, 35, 905–909. [Google Scholar] [CrossRef]
- Koulaouzidou, E.A.; Helvatjoglu-Antoniades, M.; Palaghias, G.; Karanika-Kouma, A.; Antoniades, D. Cytotoxicity of Dental Adhesives In Vitro. Eur. J. Dent. 2009, 3, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Özen, J.; Atay, A.; Toksoy Topcu, F.; Ural, A.U.; Dalkiz, M.; Tunca, Y.M. Analysis of the Cytotoxicity of Four Dentin Bonding Agents on Gingival Fibroblasts. Turk. J. Med. Sci. 2005, 35, 395–399. [Google Scholar]
- Porto, I.C.; Oliveira, D.C.; Raele, R.A.; Ribas, K.H.; Montes, M.A.; De Castro, C.M. Cytotoxicity of current adhesive systems: In vitro testing on cell cultures of primary murine macrophages. Dent. Mater. 2011, 27, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Vajrabhaya, L.O.; Pasasuk, A.; Harnirattisai, C. Cytotoxicity evaluation of single component dentin bonding agents. Oper. Dent. 2003, 28, 440–444. [Google Scholar]
- Lee, Y.; An, S.Y.; Park, Y.J.; Yu, F.H.; Park, J.C.; Seo, D.G. Cytotoxic effects of one-step self-etching adhesives on an odontoblast cell line. Scanning 2016, 38, 36–42. [Google Scholar] [CrossRef]
- Porenczuk, A.; Grzeczkowicz, A.; Maciejewska, I.; Gołaś, M.; Piskorska, K.; Kolenda, A.; Gozdowski, D.; Kopeć-Swoboda, E.; Granicka, L.; Olczak-Kowalczyk, D. An initial evaluation of cytotoxicity, genotoxicity and antibacterial effectiveness of a disinfection liquid containing silver nanoparticles alone and combined with a glass-ionomer cement and dentin bonding systems. Adv. Clin. Exp. Med. 2019, 28, 75–83. [Google Scholar] [CrossRef]
- Schmalz, G.; Schuster, U.; Koch, A.; Schweikl, H. Cytotoxicity of Low pH Dentin-Bonding Agents in a Dentin Barrier Test In Vitro. J. Endod. 2002, 28, 188–192. [Google Scholar] [CrossRef]
- Tu, M.G.; Liang, W.M.; Wu, T.C.; Chen, S.Y. Evaluation of cytotoxicity of resin bonding materials toward human oral epithlial cells using three assay systems. J. Dent. Sci. 2009, 4, 178–186. [Google Scholar] [CrossRef]
- Reddy, U.J. Determination of cytotoxicity of dentine bonding agents, hydroxyethyl methacrylate and bisphenol alpha. Int. Dent. Med. J. Adv. Res. 2017, 3. [Google Scholar] [CrossRef]
- Vajrabhaya, L.O.; Korsuwannawong, S.; Bosl, C.; Schmalz, G. The cytotoxicity of self-etching primer bonding agents in vitro. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2009, 107, e86–e90. [Google Scholar] [CrossRef]
- Huang, F.M.; Li, Y.C.; Lee, S.S.; Chang, Y.C. Cytotoxicity of dentine bonding agents on human pulp cells is related to intracellular glutathione levels. Int. Endod. J. 2010, 43, 1091–1097. [Google Scholar] [CrossRef]
- Koliniotou-Koubia, E.; Dionysopoulos, P.; Koulaouzidou, E.A.; Kortsaris, A.H.; Papadogiannis, Y. In vitro cytotoxicity of six dentin bonding agents. J. Oral Rehabil. 2001, 28, 971–975. [Google Scholar] [CrossRef]
- Prica, D.; Galic, N.; Želježić, D.; Prica, A. Genotoxicity evaluation of five different dentin bonding agents by chromosomal aberration analysis. J. Oral Rehabil. 2006, 33, 462–471. [Google Scholar] [CrossRef]
- Sigusch, B.W.; Pflaum, T.; Völpel, A.; Schinkel, M.; Jandt, K.D. The influence of various light curing units on the cytotoxicity of dental adhesives. Dent. Mater. 2009, 25, 1446–1452. [Google Scholar] [CrossRef]
- Süsgün Yıldırım, Z.; Bakır, Ş.; Bakır, E.; Foto, E. Qualitative and Quantitative Evaluation of Cytotoxicity of Five Different One-Step Self-Etching Adhesives. Oral Health Prev. Dent. 2018, 16, 525–532. [Google Scholar]
- Linke, M.; Rüttermann, S.; Gerhardt-Szep, S. Zytotoxizität von zwölf Bleachingpräparaten und drei Gingivaprotektoren. Dtsch. Zahnarztl. Z. 2018, 73, 7. [Google Scholar]
- Heidemann, D.; Lampert, F. Menschliche Gingiva in der Zellkultur. Dtsch. Zahnärztl. Z. 1980, 35, 430–433. [Google Scholar] [PubMed]
- Al-Nazhan, S.; Spångberg, L. Morphological cell changes due to chemical toxicity of a dental material: An electron microscopic study on human periodontal ligament fibroblasts and L929 cells. J. Endod. 1990, 16, 129–134. [Google Scholar] [CrossRef]
- Stanley, H.R.; Going, R.E.; Chauncey, H.H. Human pulp response to acid pretreatment of dentin and to composite restoration. J. Am. Dent. Assoc. 1975, 91, 817–825. [Google Scholar] [CrossRef] [PubMed]
- White, K.C.; Cox, C.F.; Kanka, J.; Dixon, D.L.; Farmer, J.B.; Snuggs, H.M. Pulpal response to adhesive resin systems applied to acid-etched vital dentin: Damp versus dry primer application. Quintessence Int. 1994, 25, 259–268. [Google Scholar]
- Moharamzadeh, K.; Van Noort, R.; Brook, I.M.; Scutt, A.M. Cytotoxicity of resin monomers on human gingival fibroblasts and HaCaT keratinocytes. Dent. Mater. 2007, 23, 40–44. [Google Scholar] [CrossRef] [PubMed]
- Ratanasathien, S.; Wataha, J.; Hanks, C.; Dennison, J. Cytotoxic Interactive Effects of Dentin Bonding Components on Mouse Fibroblasts. J. Dent. Res. 1995, 74, 1602–1606. [Google Scholar] [CrossRef] [PubMed]
- Kusdemir, M.; Gunal, S.; Özer, F.; Imazato, S.; Izutani, N.; Ebisu, S.; Blatz, M.B. Evaluation of cytotoxic effects of six self-etching adhesives with direct and indirect contact tests. Dent. Mater. J. 2011, 30, 799–805. [Google Scholar] [CrossRef] [PubMed]
- Cortés, O.; Alcaina, A.; Bernabé, A. Biocompatibility Evaluation of Four Dentin Adhesives Used as Indirect Pulp Capping Materials. Acta Stomatol. Croat. 2017, 51, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Yoshii, E. Cytotoxic effects of acrylates and methacrylates: Relationships of monomer structures and cytotoxicity. J. Biomed. Mater. Res. 1997, 37, 517–524. [Google Scholar] [CrossRef]
- Geurtsen, W.; Lehmann, F.; Spahl, W.; Leyhausen, G. Cytotoxicity of 35 dental resin composite monomers/additives in permanent 3T3 and three human primary fibroblast cultures. J. Biomed. Mater. Res. 1998, 41, 474–480. [Google Scholar] [CrossRef]
- Moszner, N.; Salz, U.; Zimmermann, J. Chemical aspects of self-etching enamel–dentin adhesives: A systematic review. Dent. Mater. 2005, 21, 895–910. [Google Scholar] [CrossRef]
- Schweikl, H.; Schmalz, G.; Göttke, C. Mutagenic activity of various dentine bonding agents. Biomaterials 1996, 17, 1451–1456. [Google Scholar] [CrossRef]
- CASY. Cell Counter-CellCounting. Available online: https://cellcounting.de/wp-content/uploads/2018/10/CASY_2018.pdf (accessed on 5 October 2019).




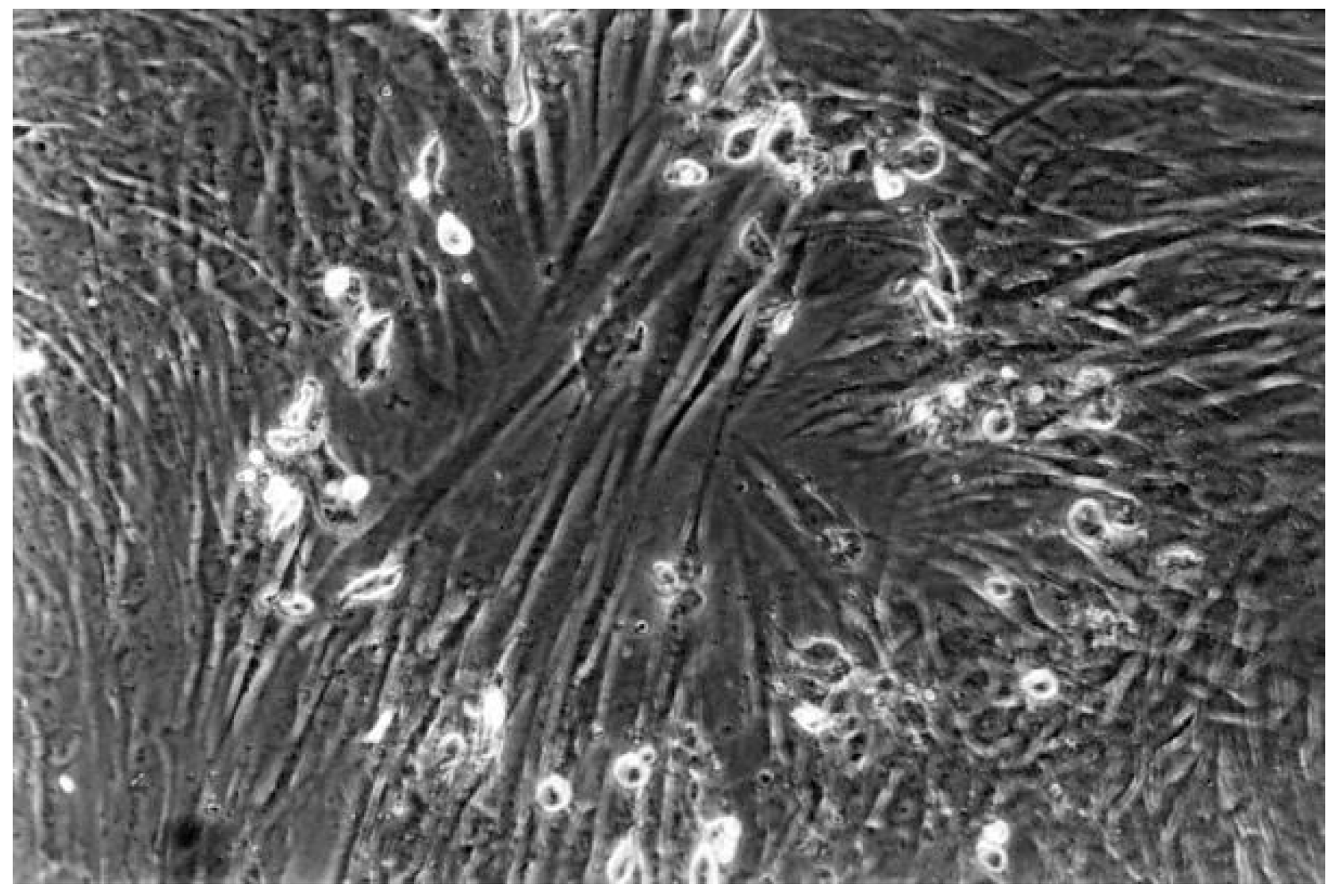
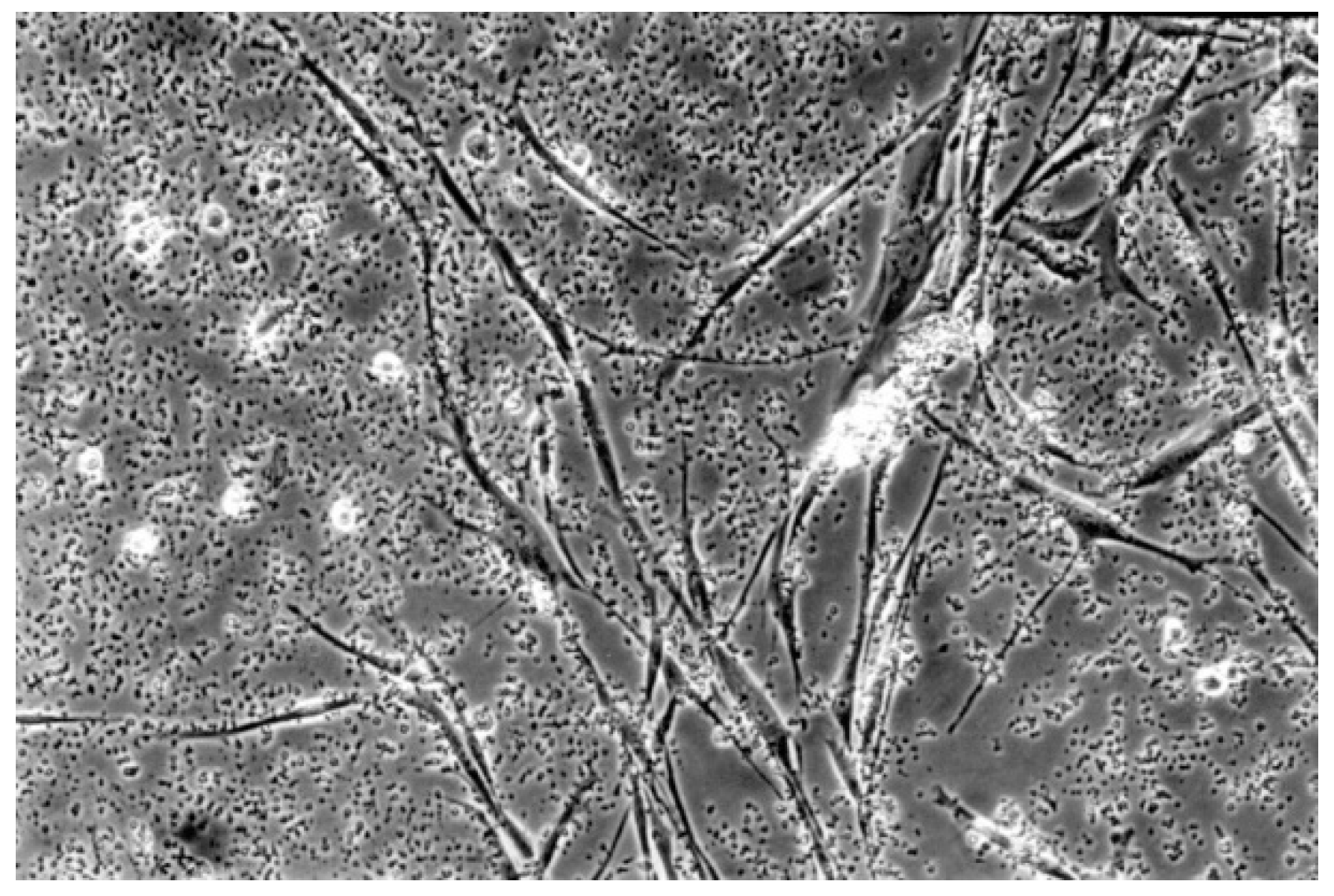
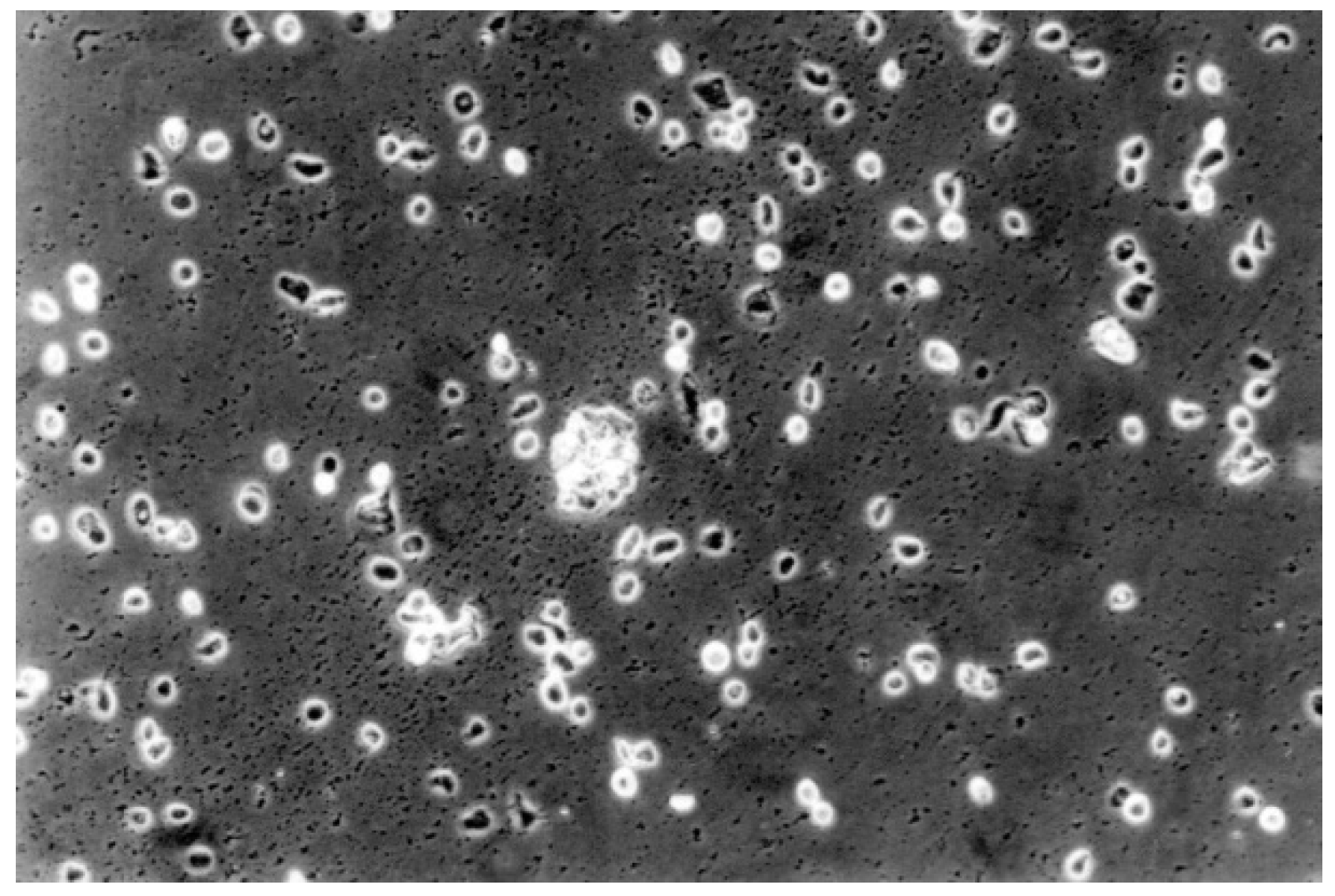
| No | Group | Dentin Adhesives | Manufactures | pH | Components | Curing Time |
| 1 | Self-etch, 1 step | Hybrid Bond | Sun medical, Moriyama, Japan | 1.0 | Part 1 Hybrid Base: Monomethacrylate, META, polyfunctional acrylate, water, acetone, photoinitiators, stabiliser Part 2 Hybrid Brushes: Sodium p-toluenesulfinate, aromatic amine | 10 s |
| 2 | Self-etch, 1 step | One-up Bond F Plus | Tokuyama, Tokyo, Japan | 1.17–1.26 | Part 1 Bonding Agent A: Dimethacrylate, MMA, MAC-10, water Part 2 Bonding Agent B: DMAEMA, HEMA, MMA | 10 s |
| 3 | Self-etch, 2 steps | AdheSE | Ivoclar Vivadent, Schaan, Liechtenstein | 2.9 | Part 1 Primer: Dimethacrylate, phosphoric acid acrylate, water, initiators, stabilisers Part 2 Bond: Dimethacrylate, HEMA, silicon dioxide, initiators, stabilisers | 10 s |
| 4 | Self-etch, 2 steps | Clearfil SE Bond | Kuraray, Okayama, Japan | 2.0 | Part 1 Primer: HEMA, MDP, hydrophilic dimethacrylate, N,N-diethanol-p-toluidine, water, dl-camphorquinone Part 2 Bond: HEMA, MDP, Bis-GMA, hydrophobic dimethacrylate, N,N-diethanol-p-toluidine, silanated colloidal silica, dl-camphorquinone | 10 s |
| 5 | Etch-and-rinse, 4 steps | Syntac | Ivoclar Vivadent, Schaan, Liechtenstein | 2.5 | Part 1 Primer: TEGDMA, PEGDMA, maleic acid, water, acetone, stabilisers Part 2 Adhesive: PEGDMA, glutaraldehyde, maleic acid, water Part 3 Heliobond: Bis-GMA, TEGDMA, stabilisers, catalysts | 20 s |
| 6 | Etch-and-rinse, 2 steps | Optibond Solo Plus | Kerr, West Collins Orange, USA | 2.4 | Bis-GMA, HEMA, GPDM, water, ethanol, barium aluminoborosilicate glass, fumed silicia, sodium hexafluorosilicate, photoinitiator | 20 s |
| 7 | Control: cell control | 7.44 |
| Grading | Reactivity | Condition of All Cultures |
|---|---|---|
| 0 | none | Discrete intracytoplasmatic granules, no cell lysis, no reduction of cell growth |
| 1 | slight | Not more than 20% of the cells are round, loosely attached and without intracytoplasmatic granules, or show changes in morphology; occasional lysedcells are present; only slight growth inhibition observable |
| 2 | mild | Not more than 50% of the cells are round, devoid of intracytoplasmatic granules, no extensive cell lysis; not more than 50% growth inhibition observable |
| 3 | moderate | Not more than 70% of the cell layers contain rounded cells or are lysed; cell layers not completely destroyed, but more than 50% growth inhibition observable |
| 4 | severe | Nearly complete or complete destruction of the cell layers |
| No. | Dental Adhesive (1–6) | Mean | SD | Min. | Max. | Median | Significance in Rel. to No. * |
|---|---|---|---|---|---|---|---|
| 1 | Hybrid Bond | 104,719,500 | 81,693,327 | 41,730,000 | 426,100,000 | 89,210,000 | 2 |
| 2 | One-up Bond F Plus | 55,844,444 | 27,706,721 | 9,969,000 | 96,830,000 | 64,050,000 | 1, 5, 7 |
| 3 | AdheSE | 76,550,000 | 37,983,471 | 29,010,000 | 170,300,000 | 72,060,000 | 7 |
| 4 | Clearfil SE Bond | 65,363,333 | 35,215,867 | 9,440,000 | 156,600,000 | 55,865,000 | 7 |
| 5 | Syntac | 90,745,000 | 38,932,657 | 39,580,000 | 206,700,000 | 83,495,000 | 2 |
| 6 | Optibond Solo Plus | 76,741,666 | 34,392,463 | 46,080,000 | 134,900,000 | 65,000,000 | - |
| 7 | Cell Control | 198,500,00 | 112,026,113 | 122,800,000 | 394,200,000 | 168,000,000 | 2, 3, 4 |
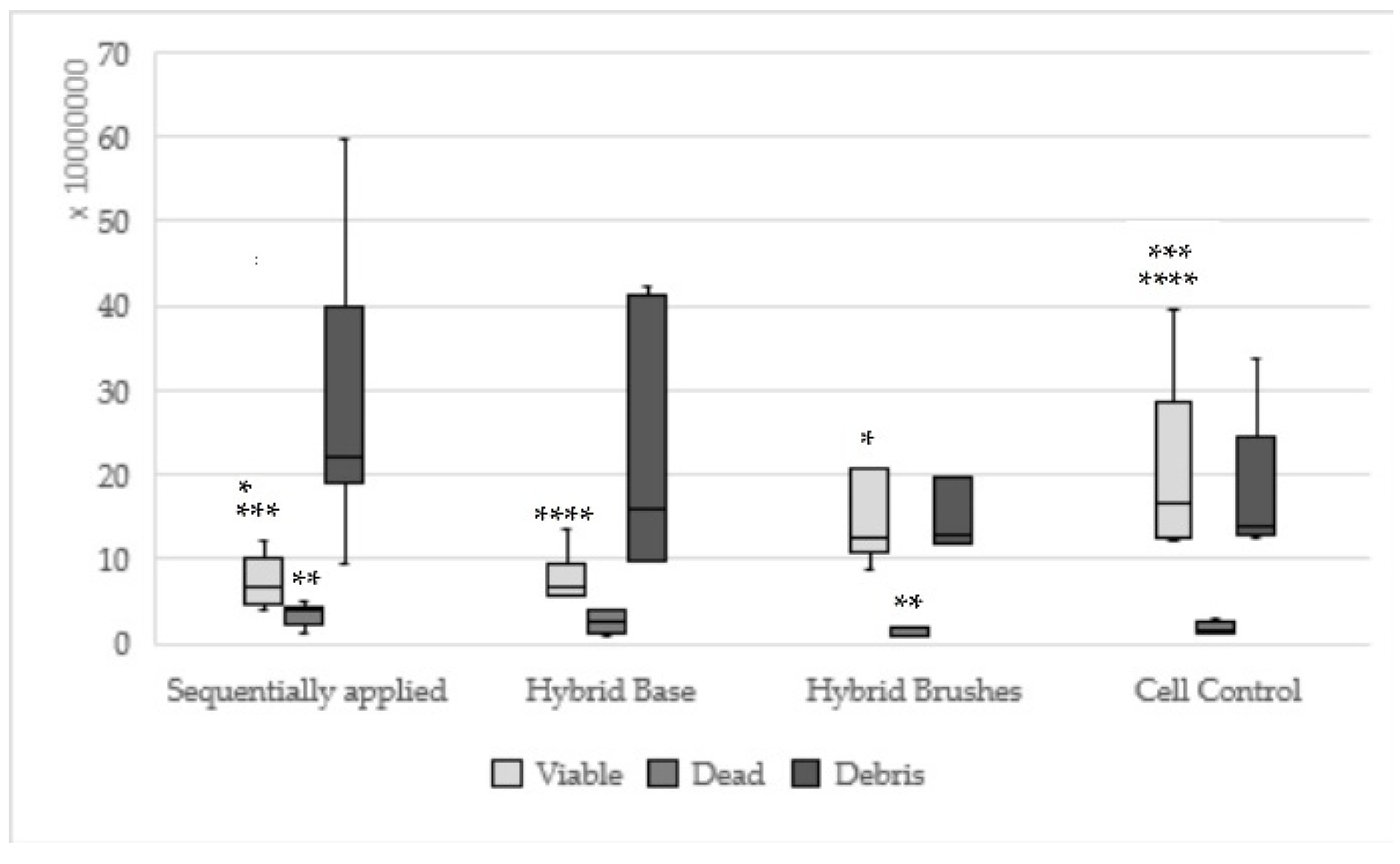
| Concentration | Components Sequentially Applied | Components Single Applied | |
|---|---|---|---|
| Hybrid Base | Hybrid Brushes | ||
| I | 0.1–2.0 µL: the most fibroblasts rounded off, single vital fibroblasts | 0.04–2.0 µL: almost all fibroblasts rounded off, few vital fibroblasts, 98%–100% cell death | 0.1–1.0 mg: partly not so dense fibroblast grass, mitoses present, few retractions, similar to cell control |
| II | 2.5–5.0 µL: 100% cell death | 3.0–5.0 µL: 100% cell death | 1.1–3.4 mg: similar to concentration I |
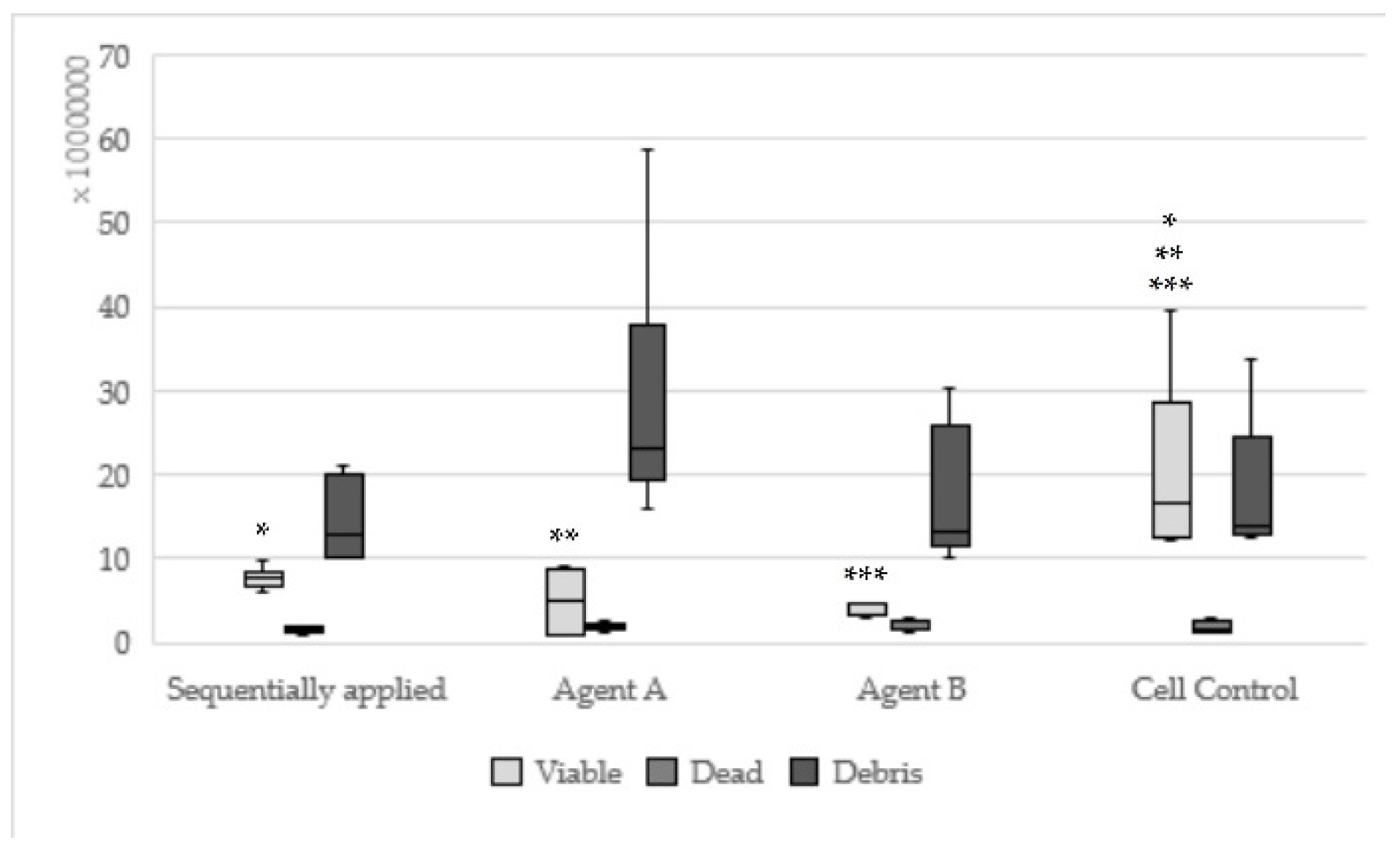
| Concentration | Components Sequentially Applied | Components Single Applied | |
|---|---|---|---|
| Agent A | Agent B | ||
| I | 1.0–6.0 µL: contact cells to the material all dead, many dead and rounded cells, fibroblast lawn less dense than cell control | 1.0–4.0 µL: contact cells to the material all dead, remaining fibroblast lawn appearing normal | 1.0–5.0 µL: material intensely distributed, fibroblast lawn less dense than cell control |
| II | 7.0–14.0 µL: many rounded cells, few mitoses, fibroblast lawn much less than cell control | 5.0–8.0 µL: fibroblast lawn less dense than cell control, rarely mitoses, cell death between 80 and 100% | 6.0–10.0 µL: few small vital cells, many rounded cells, no mitoses, isolated vital fibroblasts, up to 100% cell death |
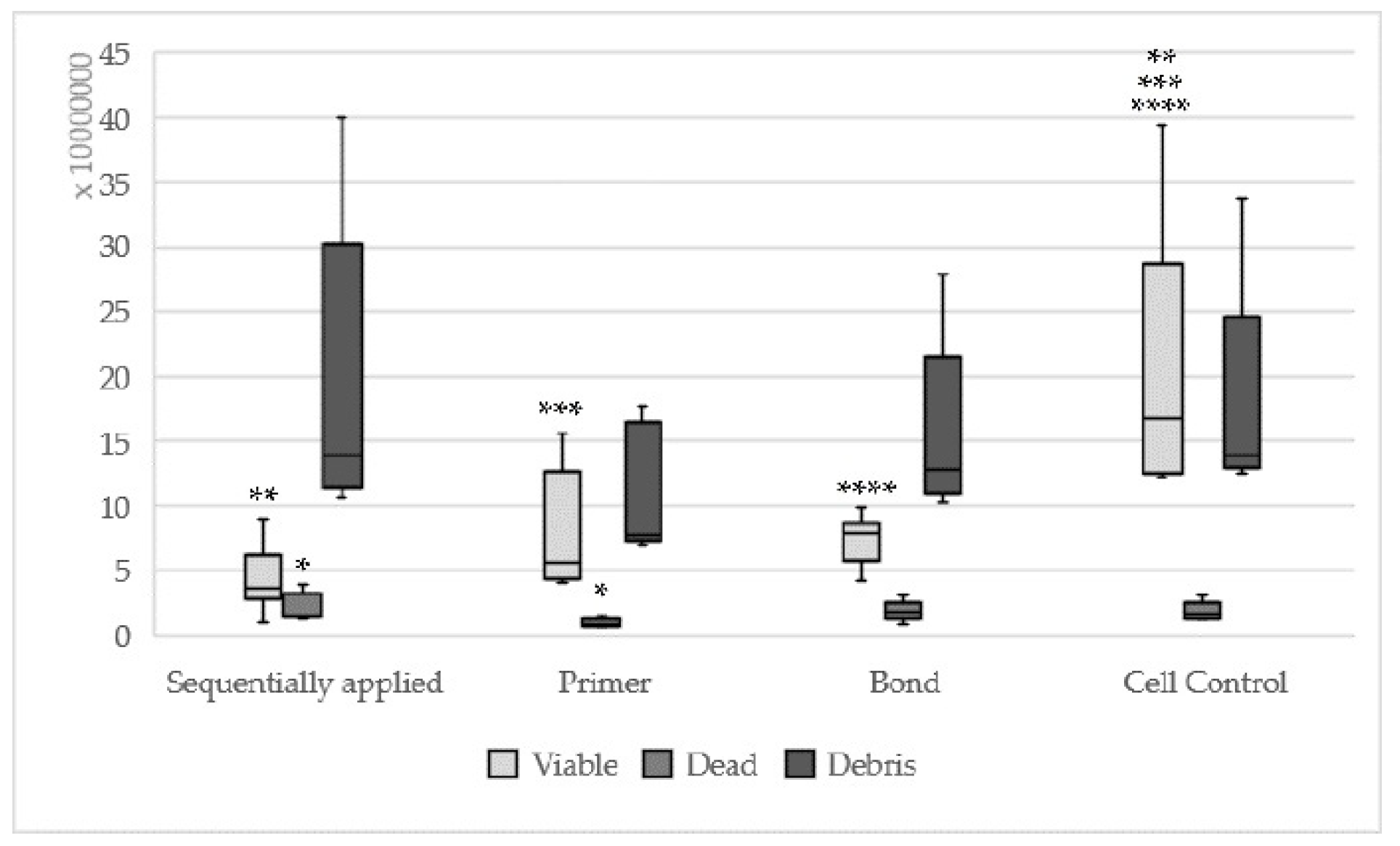
| Concentration | Components Sequentially Applied | Components Single Applied | |
|---|---|---|---|
| Primer | Bond | ||
| I | 2.5–6.0 µL: vital fibroblasts, mitoses present, many rounded cells, fibroblast lawn less dense than cell control | 5.0–9.0 µL: rounded cells, fibroblasts intensely vacuolated, mitoses present, fibroblast lawn less dense than cell control | 3.0–4.0 µL: rounded cells present, few vital fibroblasts, dense fibroblast lawn on the Petri dishes margin |
| II | 7.0–12.0 µL: dead cells, rounded fibroblast up to the petri dishes margin, material intensely distributed | 10.0–14.0 µL: similar to concentration I | 5.0–7.0 µL: fibroblasts vacuolated, dead cells, few small vital fibroblasts, no mitoses, cell dead between 75 and 100% |
| Concentration | Components Sequentially Applied | Components Single Applied | |
|---|---|---|---|
| Primer | Bond | ||
| I | 2.0–3.0 µL: small vital fibroblasts, no mitoses, dead cells, fibroblasts lawn less than cell control, material intensely distributed | 4.0–5.0 µL: few small vital fibroblasts, no mitoses, rounded cells, dead cells present, material intensely distributed | 3.0–4.0 µL: small vital fibroblasts, rounded cells, fibroblast lawn less dense than cell control |
| II | 4.0–5.0 µL: the most cells rounded, few small vital fibroblasts, cell death between 80 and 100% | 6.0–8.0 µL: rounded cells present, many dead fibroblasts, fibroblasts lawn much less than cell control, material intensely distributed | 5.0–6.0 µL: small vital fibroblasts, fibroblasts vacuolated, rounded and dead cells present, fibroblasts lawn much less than cell control |
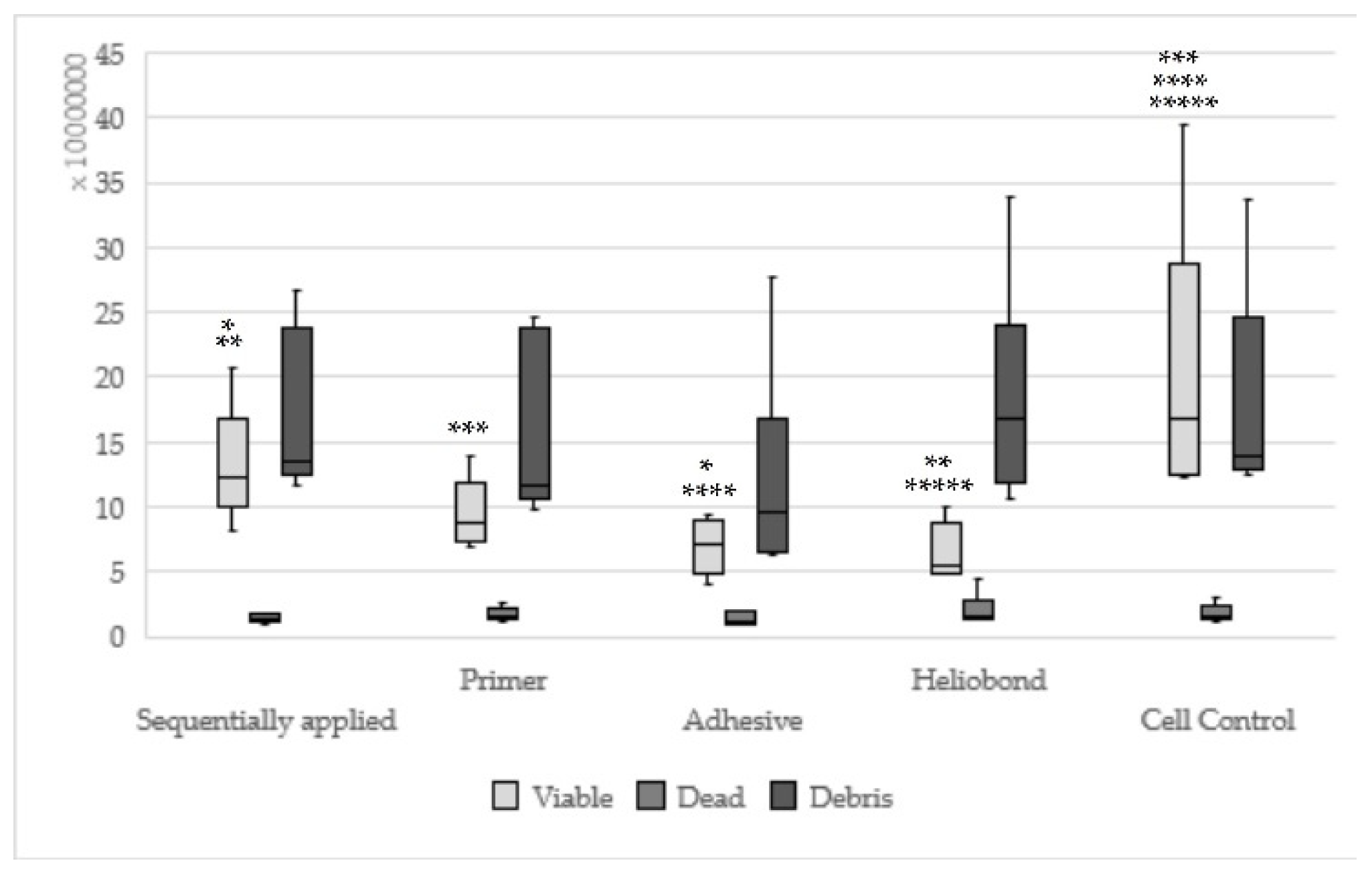
| Concentration | Components Sequentially Applied | Components Single Applied | ||
|---|---|---|---|---|
| Syntac Primer | Syntac Adhesive | Syntac Heliobond | ||
| I | 0.1–1.0 µL: rounded cells present, vital fibroblasts, fibroblast lawn less dense than cell control | 1.0–6.0 µL: rounded and dead fibroblasts, many normal fibroblasts | 0.2–2.0 µL: whole petri dish with few small vital fibroblasts and rounded cells, between 95 and 100% cell death | 1.0–5.0 µL: dead cells and rounded cells present, fibroblast lawn much less dense than cell control |
| II | 2.0–2.5 µL: the most fibroblast rounded, 100% cell dead | 6.0–12.0 µL: only in the transition zone fibroblast lawn much less dense, normal appeared cells, material zone sequentially grown | 3.0–5.0 µL: 100% cell death | 6.0–10.0 µL: at the Petri dishes margin normal vital fibroblasts, many rounded cells, fibroblast lawn much less dense than cell control |
| Concentration | Solo Plus |
|---|---|
| I | 1.0–4.0 µL: small vital fibroblasts, many rounded cells, remaining fibroblast lawn less dense or as dense as the cell control |
| II | 5.0–8.0 µL: rounded cells on the Petri dishes bottom, few vital fibroblasts at the Petri dishes margin, 95–100% cell dead |
| No. | Dental Adhesive (1–6) | Mean | SD | Min. | Max. | Median | Significance in Rel. to No. * |
|---|---|---|---|---|---|---|---|
| 1 | Hybrid Bond | 2.74 | 1.57 | 0.00 | 4.00 | 3.50 | 7 |
| 2 | One-up Bond F Plus | 2.98 | 0.86 | 1.00 | 4.00 | 3.00 | 7 |
| 3 | AdheSE | 2.96 | 0.87 | 1.00 | 4.00 | 3.00 | 7 |
| 4 | Clearfil SE Bond | 2.74 | 0.83 | 1.00 | 4.00 | 3.00 | 7 |
| 5 | Syntac | 3.02 | 0.83 | 1.00 | 4.00 | 3.00 | 7 |
| 6 | Optibond Solo Plus | 3.23 | 0.86 | 2.00 | 4.00 | 3.50 | 7 |
| 7 | Cell Control | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 1, 2, 3, 4, 5, 6 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fröb, L.; Rüttermann, S.; Romanos, G.E.; Herrmann, E.; Gerhardt-Szép, S. Cytotoxicity of Self-Etch Versus Etch-and-Rinse Dentin Adhesives: A Screening Study. Materials 2020, 13, 452. https://doi.org/10.3390/ma13020452
Fröb L, Rüttermann S, Romanos GE, Herrmann E, Gerhardt-Szép S. Cytotoxicity of Self-Etch Versus Etch-and-Rinse Dentin Adhesives: A Screening Study. Materials. 2020; 13(2):452. https://doi.org/10.3390/ma13020452
Chicago/Turabian StyleFröb, Luisa, Stefan Rüttermann, Georgios E. Romanos, Eva Herrmann, and Susanne Gerhardt-Szép. 2020. "Cytotoxicity of Self-Etch Versus Etch-and-Rinse Dentin Adhesives: A Screening Study" Materials 13, no. 2: 452. https://doi.org/10.3390/ma13020452
APA StyleFröb, L., Rüttermann, S., Romanos, G. E., Herrmann, E., & Gerhardt-Szép, S. (2020). Cytotoxicity of Self-Etch Versus Etch-and-Rinse Dentin Adhesives: A Screening Study. Materials, 13(2), 452. https://doi.org/10.3390/ma13020452






