Thermal Upgrade of Enzymatically Synthesized Aliphatic and Aromatic Oligoesters
Abstract
1. Introduction
2. Results and Discussion
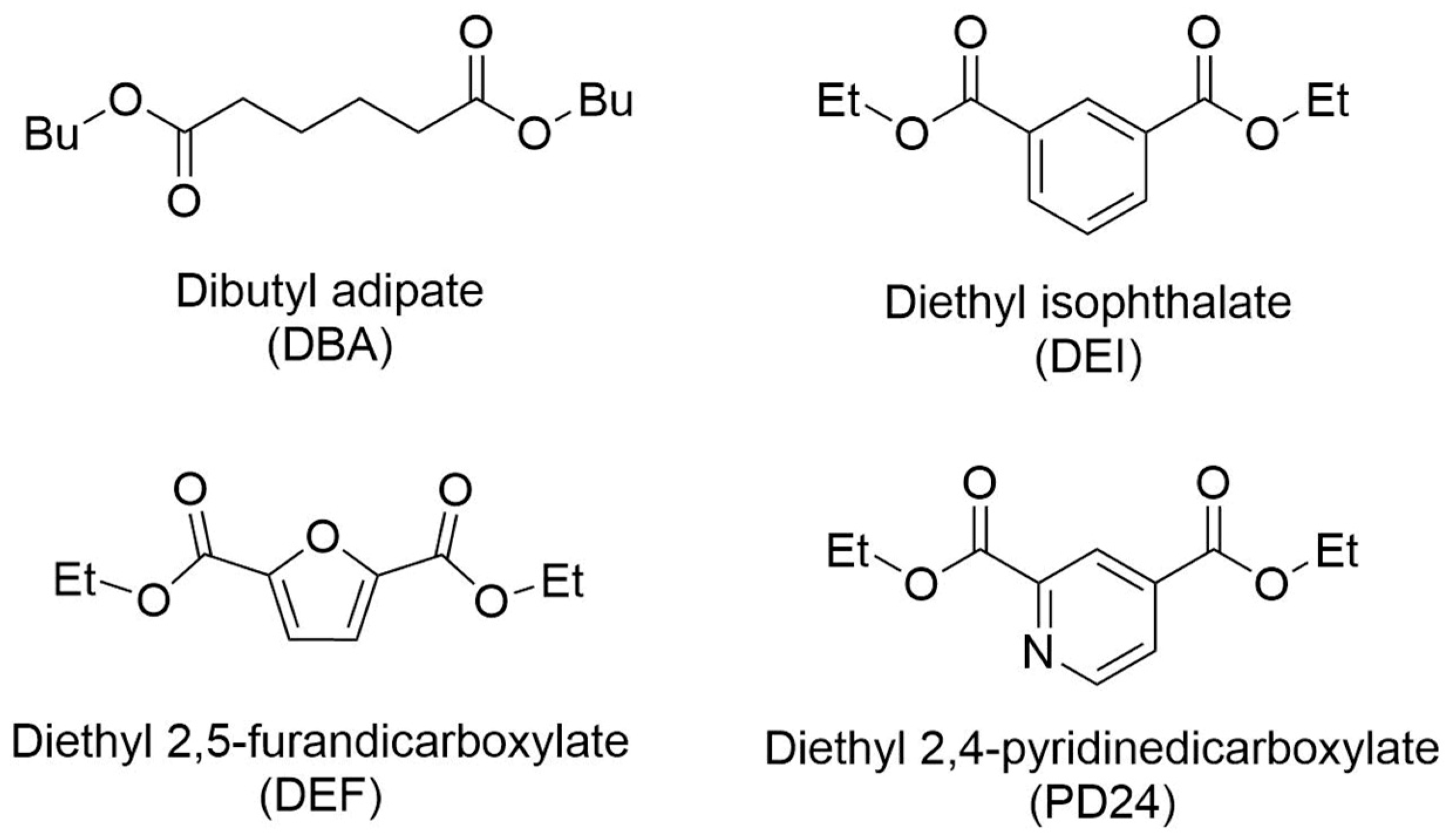
2.1. Enzymatic Synthesis of Aliphatic and Aromatic Oligoesters
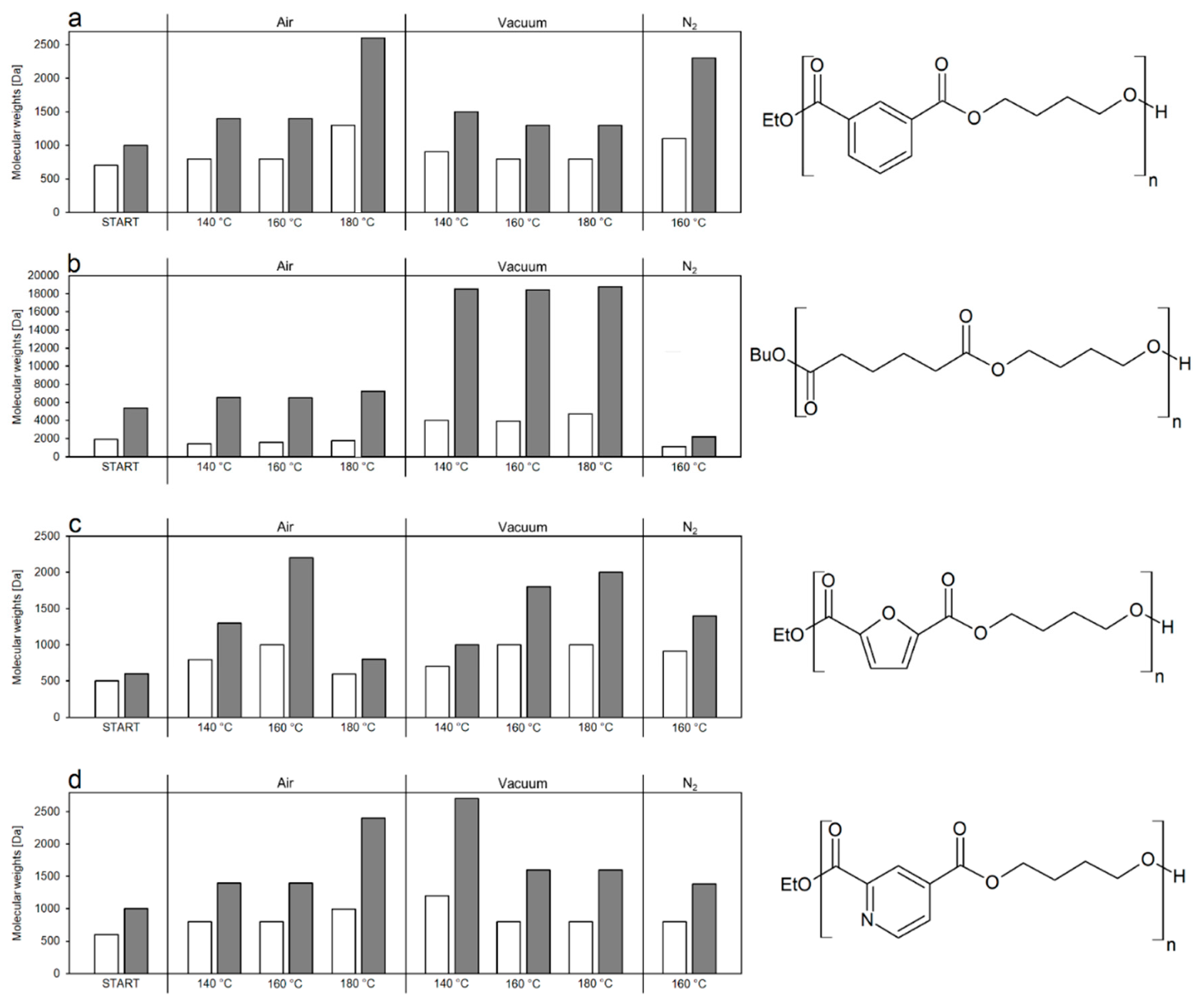
2.2. Catalyst-Free Thermal Upgrade of the Enzymatically Synthesized Oligoesters
3. Materials and Methods
3.1. Materials and Enzymes
3.2. Enzymatic Synthesis of Aliphatic and Aromatic Oligoesters
3.3. Catalyst-Free Thermal Upgrade of Oligomers
3.4. Nuclear Magnetic Resonance (NMR) Spectroscopy
3.5. Gel Permeation Chromatography (GPC)
3.6. Matrix-Assisted Laser Desorption Ionization (MALDI)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pellis, A.; Byrne, F.P.; Sherwood, J.; Vastano, M.; Comerford, J.W.; Farmer, T.J. Safer bio-based solvents to replace toluene and tetrahydrofuran for the biocatalyzed synthesis of polyesters. Green Chem. 2019, 21, 1686–1694. [Google Scholar] [CrossRef]
- Pellis, A.; Comerford, J.W.; Weinberger, S.; Guebitz, G.M.; Clark, J.H.; Farmer, T.J. Enzymatic synthesis of lignin derivable pyridine based polyesters for the substitution of petroleum derived plastics. Nat. Commun. 2019, 10, 1762. [Google Scholar] [CrossRef] [PubMed]
- Stavila, E.; Alberda van Ekenstein, G.O.R.; Loos, K. Enzyme-catalyzed synthesis of aliphatic—Aromatic oligoamides. Biomacromolecules 2013, 14, 1600–1606. [Google Scholar] [CrossRef] [PubMed]
- Gustini, L.; Noordover, B.A.J.; Gehrels, C.; Dietz, C.; Koning, C.E. Enzymatic synthesis and preliminary evaluation as coating of sorbitol-based, hydroxy-functional polyesters with controlled molecular weights. Eur. Polym. J. 2015, 67, 459–475. [Google Scholar] [CrossRef]
- Gustini, L.; Lavilla, C.; Finzel, L.; Noordover, B.A.J.; Hendrix, M.M.R.M.; Koning, C.E. Sustainable coatings from bio-based, enzymatically synthesized polyesters with enhanced functionalities. Polym. Chem. 2016, 7, 6586–6597. [Google Scholar] [CrossRef]
- Tsujimoto, T.; Uyama, H.; Kobayashi, S. Enzymatic synthesis of cross-linkable polyesters from renewable resources. Biomacromolecules 2001, 2, 29–31. [Google Scholar] [CrossRef] [PubMed]
- Uyama, H.; Kuwabara, M.; Tsujimoto, T.; Kobayashi, S. Enzymatic synthesis and curing of biodegradable epoxide-containing polyesters from renewable resources. Biomacromolecules 2003, 4, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Pellis, A.; Hanson, P.A.; Comerford, J.W.; Clark, J.H.; Farmer, T.J. Enzymatic synthesis of unsaturated polyesters: Functionalization and reversibility of the aza-Michael addition of pendants. Polym. Chem. 2019, 10, 843–851. [Google Scholar] [CrossRef]
- Jiang, Y.; Woortman, A.J.J.; Alberda Van Ekenstein, G.O.R.; Loos, K. Enzyme-catalyzed synthesis of unsaturated aliphatic polyesters based on green monomers from renewable resources. Biomolecules 2013, 3, 461–480. [Google Scholar] [CrossRef]
- Guarneri, A.; Cutifani, V.; Cespugli, M.; Pellis, A.; Vassallo, R.; Asaro, F.; Ebert, C.; Gardossi, L. Functionalization of enzymatically-synthesized rigid poly(itaconates) via post-polymerization aza-Michael addition of primary amines. Adv. Synth. Catal. 2019, 361, 2559–2573. [Google Scholar]
- Gustini, L.; Lavilla, C.; Janssen, W.W.T.J.; Martínez de Ilarduya, A.; Muñoz-Guerra, S.; Koning, C.E. Green and selective polycondensation methods toward linear sorbitol-based polyesters: Enzymatic versus organic and metal-based catalysis. Chem. Sus. Chem. 2016, 9, 2250–2260. [Google Scholar] [CrossRef] [PubMed]
- Pellis, A.; Corici, L.; Sinigoi, L.; D’Amelio, N.; Fattor, D.; Ferrario, V.; Ebert, C.; Gardossi, L. Towards feasible and scalable solvent-free enzymatic polycondensations: Integrating robust biocatalysts with thin film reactions. Green Chem. 2015, 17, 1756–1766. [Google Scholar] [CrossRef]
- Kricheldorf, H.R.; Behnken, G.; Schwarz, G. Telechelic polyesters of ethane diol and adipic or sebacic acid by means of bismuth carboxylates as non-toxic catalysts. Polymer 2005, 46, 11219–11224. [Google Scholar] [CrossRef]
- Ishii, M.; Okazaki, M.; Shibasaki, Y.; Ueda, M.; Teranishi, T. Convenient synthesis of aliphatic polyesters by distannoxane-catalyzed polycondensation. Biomacromolecules 2001, 2, 1267–1270. [Google Scholar] [CrossRef] [PubMed]
- Kricheldorf, H.R.; Rabenstein, M.; Maskos, M.; Schmidt, M. Macrocycles. 15. The role of cyclization in kinetically controlled polycondensations. 1. Polyester syntheses. Macromolecules 2001, 34, 713–722. [Google Scholar] [CrossRef]
- Duda, A.; Penczek, S. Mechanisms of Aliphatic Polyester Formation; Wiley-VCH: Weinheim, Germany, 2001; p. 378. [Google Scholar]
- Cerea, G.; Gardossi, L.; Sinigoi, L.; Fattor, D. Process for the Production of Polyesters through Synthesis Catalyzed by Enzyme. WO2013110446A1, 1 August 2013. [Google Scholar]
- Salmi, T.; Paatero, E.; Nyholm, P. Kinetic model for the increase of reaction order during polyesterification. Chem. Eng. Process. 2004, 43, 1487–1493. [Google Scholar] [CrossRef]
- Flory, P.J. Kinetics of polyesterification: A study of the effects of molecular weight and viscosity on reaction rate. J. Am. Chem. Soc. 1939, 61, 3334–3340. [Google Scholar] [CrossRef]
- Tang, A.U.; Yao, K.S. Mechanism of hydrogen ion catalysis in esterification. II. Studies on the kinetics of polyesterification reactions between dibasic acids and glycols. J. Polym. Sci. 1959, 35, 219–233. [Google Scholar]
- Pellis, A.; Vastano, M.; Quartinello, F.; Herrero Acero, E.; Guebitz, G.M. His-tag immobilization of cutinase 1 from thermobifida cellulosilytica for solvent-free synthesis of polyesters. Biotechnol. J. 2017, 12, 1700322. [Google Scholar] [CrossRef]
- Weinberger, S.; Pellis, A.; Comerford, J.W.; Farmer, T.J.; Guebitz, G.M. Efficient physisorption of Candida Antarctica Lipase B on Polypropylene Beads and application for polyester synthesis. Catalysts 2018, 8, 369. [Google Scholar] [CrossRef]
- Pellis, A.; Comerford, J.W.; Maneffa, A.J.; Sipponen, M.H.; Clark, J.H.; Farmer, T.J. Elucidating enzymatic polymerisations: Chain-length selectivity of Candida antarctica lipase B towards various aliphatic diols and dicarboxylic acid diesters. Eur. Polym. J. 2018, 106, 79–84. [Google Scholar] [CrossRef]
- Pellis, A.; Cantone, S.; Ebert, C.; Gardossi, L. Evolving biocatalysis to meet bioeconomy challenges and opportunities. New Biotechnol. 2018, 40, 154–169. [Google Scholar] [CrossRef] [PubMed]
- Quintana, R.; Martínez de Ilarduya, A.; Rudé, E.; Kint, D.P.R.; Alla, A.; Galbis, J.A.; Muñoz-Guerra, S. Poly(ethylene isophthalate)s: Effect of the tert-butyl substituent on structure and properties. Polymer 2004, 45, 5005–5012. [Google Scholar] [CrossRef]
- Akat, H.; Balcan, M. Polyesterification kinetics between adipic acid and hexamethylene glycol using diphenylammonium triflate as catalyst. Iran. Polym. J. 2006, 15, 921–928. [Google Scholar]

| Polymer | Synthesis t (°C) | Monomers Conversion * (%) | Mn + (Da) | Mw + (Da) | Đ + | Reference |
|---|---|---|---|---|---|---|
| PBA a | 50 | 96 | 1900 | 5400 | 2.89 | This work |
| 85 | 96 | 4200 | 6400 | 1.53 | [23] | |
| PBI | 50 | 81 | 700 | 1000 | 1.41 | This work |
| 85 | 84 | 900 | 1500 | 1.62 | [2] | |
| PBF | 50 | 78 | 500 | 600 | 1.19 | This work |
| 85 | 79 | 600 | 900 | 1.34 | [2] | |
| PBP | 50 | 80 | 600 | 1000 | 1.52 | This work |
| 85 | 82 | 800 | 1400 | 1.65 | [2] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Comerford, J.W.; Byrne, F.P.; Weinberger, S.; Farmer, T.J.; Guebitz, G.M.; Gardossi, L.; Pellis, A. Thermal Upgrade of Enzymatically Synthesized Aliphatic and Aromatic Oligoesters. Materials 2020, 13, 368. https://doi.org/10.3390/ma13020368
Comerford JW, Byrne FP, Weinberger S, Farmer TJ, Guebitz GM, Gardossi L, Pellis A. Thermal Upgrade of Enzymatically Synthesized Aliphatic and Aromatic Oligoesters. Materials. 2020; 13(2):368. https://doi.org/10.3390/ma13020368
Chicago/Turabian StyleComerford, James W., Fergal P. Byrne, Simone Weinberger, Thomas J. Farmer, Georg M. Guebitz, Lucia Gardossi, and Alessandro Pellis. 2020. "Thermal Upgrade of Enzymatically Synthesized Aliphatic and Aromatic Oligoesters" Materials 13, no. 2: 368. https://doi.org/10.3390/ma13020368
APA StyleComerford, J. W., Byrne, F. P., Weinberger, S., Farmer, T. J., Guebitz, G. M., Gardossi, L., & Pellis, A. (2020). Thermal Upgrade of Enzymatically Synthesized Aliphatic and Aromatic Oligoesters. Materials, 13(2), 368. https://doi.org/10.3390/ma13020368







