XRD and TG-DTA Study of New Alkali Activated Materials Based on Fly Ash with Sand and Glass Powder
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
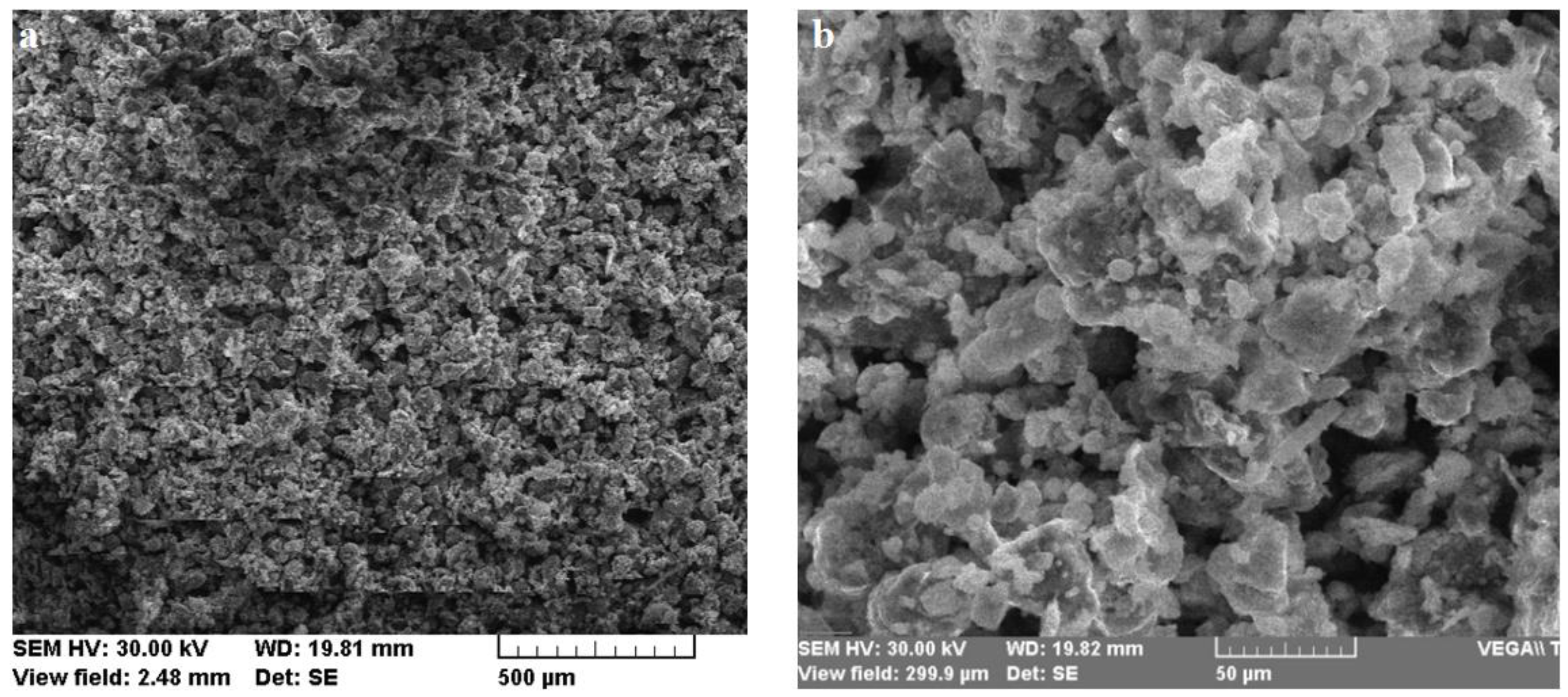
2.1.1. Indigenous Fly Ash
2.1.2. Glass Powder
2.1.3. Natural Aggregates
2.1.4. Sodium Silicate
2.1.5. Sodium Hydroxide
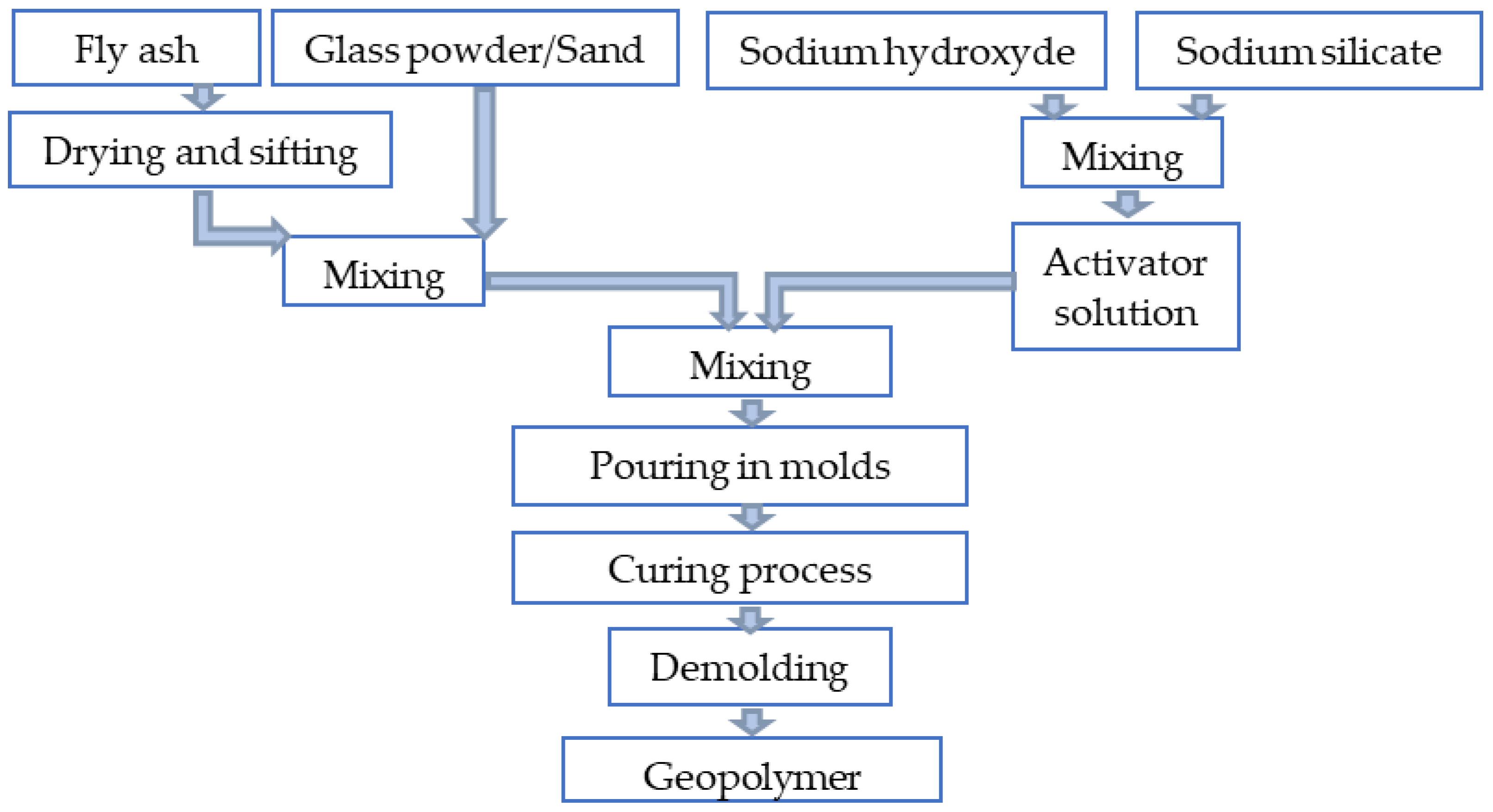
2.1.6. Sample Preparation
- -
- a raw materials relative humidity of 0;
- -
- fly ash particles lower than 80 µm;
- -
- glass powder particles lower than 100 µm;
- -
- sand particles lower than 4 mm;
- -
- curing temperature of 70 °C;
- -
- curing time of 8 h.
2.2. Methods
2.2.1. Simultaneous Thermal Analysis
2.2.2. X-ray Diffraction
3. Results and Discussion
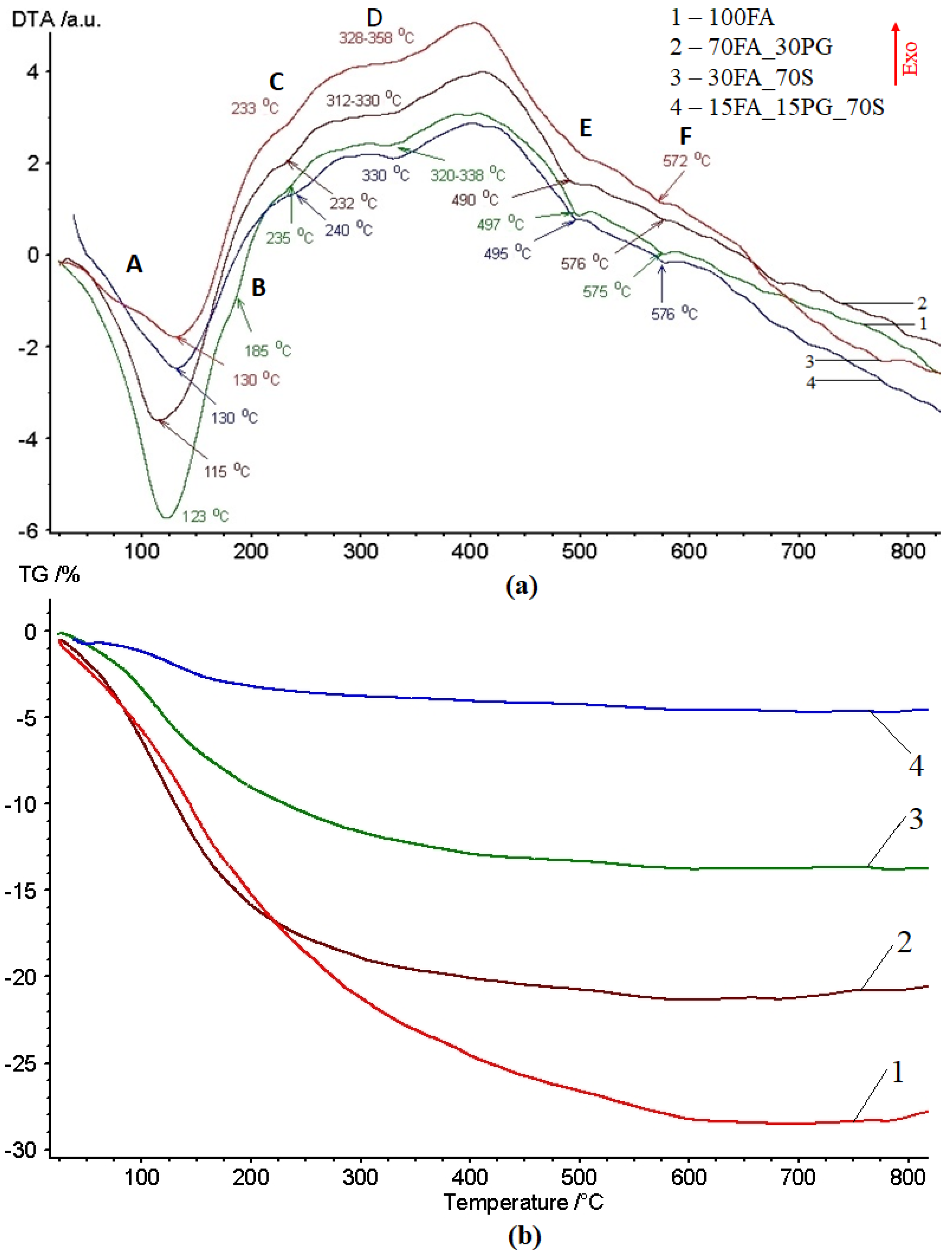
3.1. Thermal Behavior Evaluation
- -
- crystallization water (anionic and cationic or coordinative) which is removed from the structure in the 120–200 °C temperature range. This sub-type of water molecules are bonded in the structure during the formation of crystals from aqueous solution [38].
- -
- water from hydrogels that can be intercrystalline and network types that interact with the crystallization water. This sub-type of water is removed during heating in the 180–300 °C temperature range [39].
- -
- -
- Acids: M-O−H+ (Si(IV), Ti(IV), Fe(III))
- -
- Basics: M+HO− hydroxide (Na, Ca (II), K, Mg (II))
- -
- Neutral: M-OH hydroxyl (Al (III), Mn (III)).
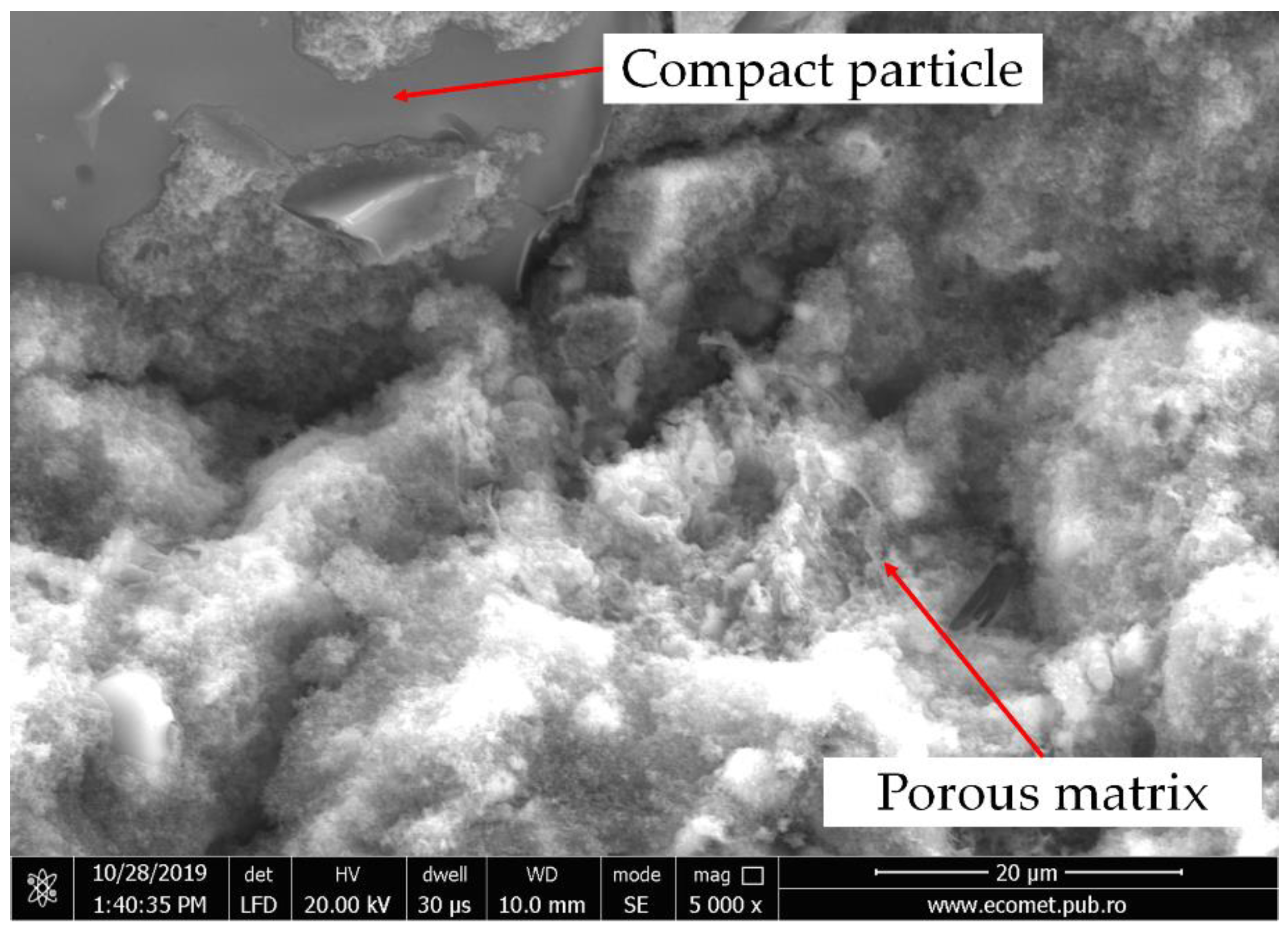
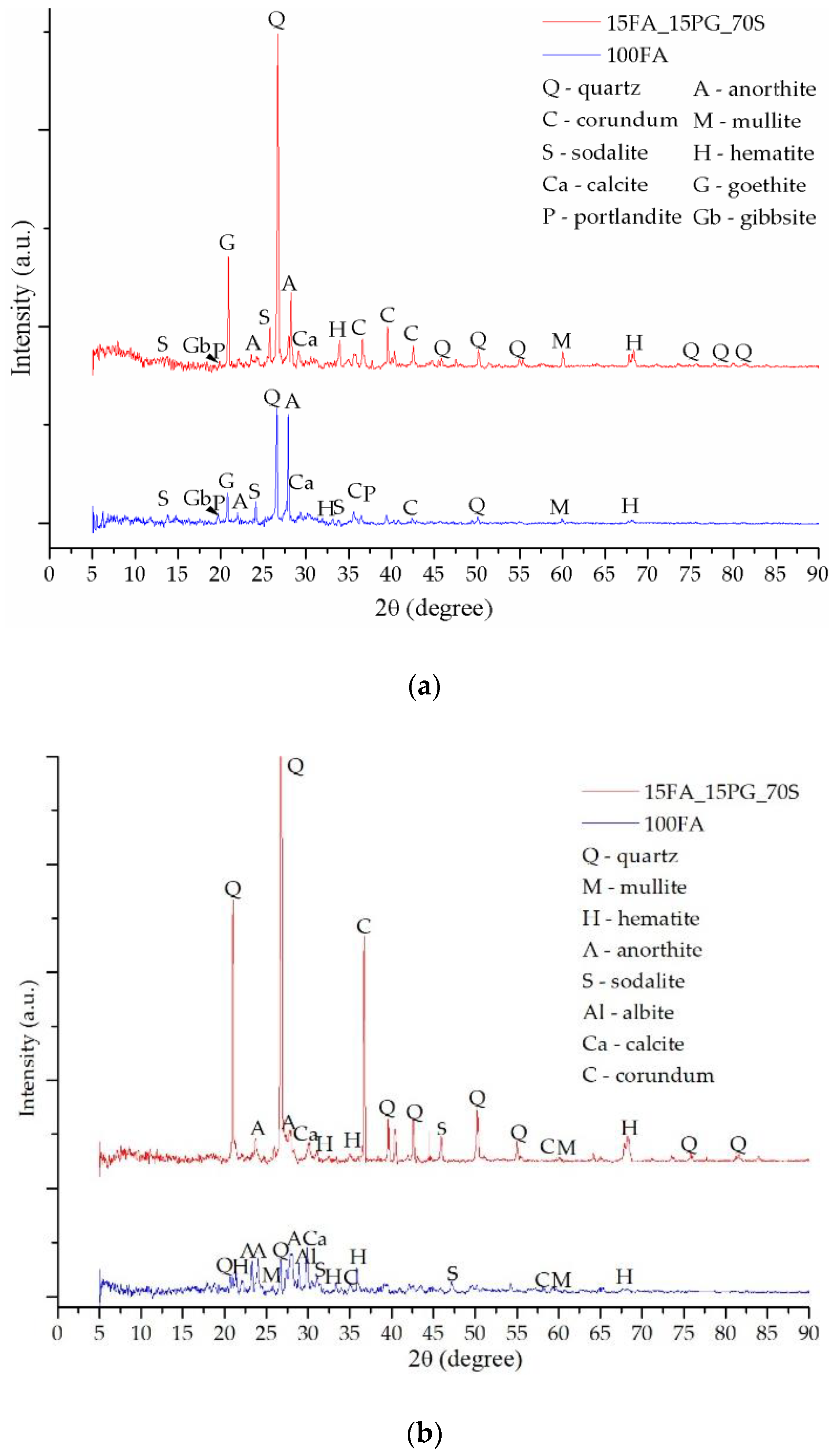
3.2. Mineralogical Evaluation
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Nergis, D.D.B.; Abdullah, M.M.A.B.; Vizureanu, P.; Tahir, M.F.M. Geopolymers and Their Uses: Review. IOP Conf. Ser. Mater. Sci. Eng. 2018, 374, 12019. [Google Scholar] [CrossRef]
- Vogt, O.; Ukrainczyk, N.; Ballschmiede, C.; Koenders, E. Reactivity and Microstructure of Metakaolin Based Geopolymers: Effect of Fly Ash and Liquid/Solid Contents. Materials 2019, 12, 3485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papa, E.; Medri, V.; Amari, S.; Manaud, J.; Benito, P.; Vaccari, A.; Landi, E. Zeolite-geopolymer composite materials: Production and characterization. J. Clean. Prod. 2018, 171, 76–84. [Google Scholar] [CrossRef]
- North, M.R.; Swaddle, T.W. Kinetics of Silicate Exchange in Alkaline Aluminosilicate Solutions. Inorg. Chem. 2000, 39, 2661–2665. [Google Scholar] [CrossRef] [PubMed]
- Duxson, P.; Fernández-Jiménez, A.; Provis, J.L.; Lukey, G.C.; Palomo, A.; van Deventer, J.S.J. Geopolymer technology: The current state of the art. J. Mater. Sci. 2006, 42, 2917–2933. [Google Scholar] [CrossRef]
- Farhana, Z.F.; Kamarudin, H.; Rahmat, A.; Al Bakri, A.M.M. A Study on Relationship between Porosity and Compressive Strength for Geopolymer Paste. Key Eng. Mater. 2013, 594–595, 1112–1116. [Google Scholar] [CrossRef]
- Ding, Y.; Dai, J.-G.; Shi, C.-J. Mechanical properties of alkali-activated concrete: A state-of-the-art review. Constr. Build. Mater. 2016, 127, 68–79. [Google Scholar] [CrossRef] [Green Version]
- Rowles, M.; O’Connor, B. Chemical optimisation of the compressive strength of aluminosilicate geopolymers synthesised by sodium silicate activation of metakaolinite. J. Mater. Chem. 2003, 13, 1161–1165. [Google Scholar] [CrossRef]
- Ukrainczyk, N.; Muthu, M.; Vogt, O.; Koenders, E. Geopolymer, Calcium Aluminate, and Portland Cement-Based Mortars: Comparing Degradation Using Acetic Acid. Materials 2019, 12, 3115. [Google Scholar] [CrossRef] [Green Version]
- Rangan, B.V. Design, Properties, and Applications of Low-Calcium Fly Ash-Based Geopolymer Concrete. In Developments in Porous, Biological and Geopolymer Ceramics; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2007; pp. 347–362. [Google Scholar]
- Ismail, N.; El-Hassan, H. Development and Characterization of Fly AshSlag Blended Geopolymer Mortar and Lightweight Concrete. J. Mater. Civ. Eng. 2018, 30, 4018029. [Google Scholar] [CrossRef]
- Reig, L.; Soriano, L.; Borrachero, M.V.; Monzó, J.; Payá, J. Influence of calcium aluminate cement (CAC) on alkaline activation of red clay brick waste (RCBW). Cem. Concr. Compos. 2016, 65, 177–185. [Google Scholar] [CrossRef]
- Scrivener, K.L.; Capmas, A. Calcium Aluminate Cements. In Leas Chemistry of Cement and Concrete; Elsevier: Amsterdam, The Netherlands, 1998; pp. 713–782. [Google Scholar]
- Vafaei, M.; Allahverdi, A. Influence of calcium aluminate cement on geopolymerization of natural pozzolan. Constr. Build. Mater. 2016, 114, 290–296. [Google Scholar] [CrossRef]
- Nath, P.; Sarker, P.K. Use of OPC to improve setting and early strength properties of low calcium fly ash geopolymer concrete cured at room temperature. Cem. Concr. Compos. 2015, 55, 205–214. [Google Scholar] [CrossRef]
- Azmi, A.A.; Abdullah, M.M.A.B.; Ghazali, C.M.R.; Sandu, A.V.; Hussin, K.; Sumarto, D.A. A Review on Fly Ash Based Geopolymer Rubberized Concrete. Key Eng. Mater. 2016, 700, 183–196. [Google Scholar] [CrossRef]
- Burduhos Nergis, D.D.; Vizureanu, P.; Corbu, O. Synthesis and characteristics of local fly ash based geopolymers mixed with natural aggregates. Rev. Chim. 2019, 70, 1262–1267. [Google Scholar]
- Ma, H.; Qian, S.; Li, V.C. Influence of Fly Ash Type on Mechanical Properties and Self-Healing Behavior of Engineered Cementitious Composite (ECC). In Proceedings of the 9th International Conference on Fracture Mechanics of Concrete and Concrete Structures, Berkeley, CA, USA, 28 May–1 June 2016. [Google Scholar]
- Ibrahim, W.M.W.; Hussin, K.; Abdullah, M.M.A.B.; Kadir, A.A.; Binhussain, M. A Review of Fly Ash-Based Geopolymer Lightweight Bricks. Appl. Mech. Mater. 2015, 754–755, 452–456. [Google Scholar] [CrossRef]
- Davidovits, J. Geopolymers Based on Natural and Synthetic Metakaolin a Critical Review. In Proceedings of the 41st International Conference on Advanced Ceramics and Composites, Daytona Beach, FL, USA, 22–27 January 2017; pp. 201–214. [Google Scholar]
- Available online: http://www.anpm.ro/documents/839616/33839940/Raport_Amplasament_CET2+Holboca+Rev.+Sep2017.pdf/7931f8ce-1e21-4af7-9abf-a16c602acc5b (accessed on 12 December 2019).
- Zhang, S.; Keulen, A.; Arbi, K.; Ye, G. Waste glass as partial mineral precursor in alkali-activated slag/fly ash system. Cement Concrete Res. 2017, 102, 29–40. [Google Scholar] [CrossRef] [Green Version]
- Shaikh, F.U.A. Mechanical and durability properties of fly ash geopolymer concrete containing recycled coarse aggregates. Int. J. Sustain. Built Environ. 2016, 5, 277–287. [Google Scholar] [CrossRef] [Green Version]
- Embong, R.; Kusbiantoro, A.; Shafiq, N.; Nuruddin, M.F. Strength and microstructural properties of fly ash based geopolymer concrete containing high-calcium and water-absorptive aggregate. J. Clean. Prod. 2016, 112, 816–822. [Google Scholar] [CrossRef]
- Joseph, B.; Mathew, G. Influence of aggregate content on the behavior of fly ash based geopolymer concrete. Sci. Iran. 2012, 19, 1188–1194. [Google Scholar] [CrossRef] [Green Version]
- Dave, S.; Bhogayata, A.; Arora, D.N. Impact Resistance of Geopolymer Concrete Containing Recycled Plastic Aggregates. EasyChair 2017, 1, 137–143. [Google Scholar]
- Provis, J.L. Alkali-activated Binders and Concretes: The Path to Standardization. In Geopolymer Binder Systems; ASTM International: West Conshohocken, PA, USA, 2013; pp. 185–195. [Google Scholar]
- Nordin, N.; Abdullah, M.M.A.B.; Fakri, W.M.N.R.W.; Tahir, M.F.M.; Sandu, A.V.; Hussin, K.; Zailani, W.W.A. Exploration on fly ash waste as global construction materials for dynamics marketability. In Proceedings of the Applied Physics of Condensed Matter (APCOM 2019), Pleso, Slovak, 19–21 June 2019; AIP Publishing: Melville, NY, USA, 2019. [Google Scholar]
- Xu, H.; van Deventer, J.S.J. Effect of source materials on geopolymerization. Ind. Eng. Chem. Res. 2003, 42, 1698–1706. [Google Scholar] [CrossRef]
- Mo, B.H.; Zhu, H.; Cui, X.M.; He, Y.; Gong, S.Y. Effect of curing temperature on geopolymerization of metakaolin-based geopolymers. Appl. Clay Sci. 2014, 99, 144–148. [Google Scholar] [CrossRef]
- Abdullah, M.M.A.B.; Yahya, Z.; Tahir, M.F.M.; Hussin, K.; Binhussain, M.; Sandhu, A.V. Fly Ash Based Lightweight Geopolymer Concrete Using Foaming Agent Technology. Appl. Mech. Mater. 2014, 679, 20–24. [Google Scholar] [CrossRef]
- Harja, M.; Barbuta, M.; Rusu, L. Obtaining and Characterization of the Polymer Concrete with Fly Ash. J. Appl. Sci. 2009, 9, 88–96. [Google Scholar] [CrossRef] [Green Version]
- Corbu, O.; Ioani, A.M.; Al Bakri Abdullah, M.M.; Meita, V.; Szilagyi, H.; Sandu, A.V. The pozzoolanic activity level of powder waste glass in comparisons with other powders. Key Eng. Mater. 2015, 660, 237–243. [Google Scholar] [CrossRef]
- Lee, W.K.W.; van Deventer, J.S.J. Chemical interactions between siliceous aggregates and low-Ca alkali-activated cements. Cem. Concr. Res. 2007, 37, 844–855. [Google Scholar] [CrossRef]
- Ismail, I.; Bernal, S.A.; Provis, J.L.; Hamdan, S.; van Deventer, J.S.J. Microstructural changes in alkali activated fly ash/slag geopolymers with sulfate exposure. Mater. Struct. 2012, 46, 361–373. [Google Scholar] [CrossRef]
- Wuddivira, M.N.; Robinson, D.A.; Lebron, I.; Bréchet, L.; Atwell, M.; de Caires, S.; Oatham, M.; Jones, S.B.; Abdu, H.; Verma, A.K.; et al. Estimation of soil clay content from hygroscopic water content measurements. Soil Sci. Soc. Am. J. 2012, 76, 1529–1535. [Google Scholar] [CrossRef] [Green Version]
- Rico, P.; Adriano, A.; Soriano, G.; Duque, J.V. Characterization of Water Absorption and Desorption properties of Natural Zeolites in Ecuador. In International Symposium on Energy; Puerto Rico Energy Center-Laccei: Puerto Rico, 2013. [Google Scholar]
- Longhi, M.A.; Zhang, Z.; Rodríguez, E.D.; Kirchheim, A.P.; Wang, H. Efflorescence of alkali-activated cements (geopolymers) and the impacts on material structures: A critical analysis. Front. Mater. 2019, 6. [Google Scholar] [CrossRef]
- Smart Nanoconcretes and Cement-Based Materials: Properties, Modelling and Applications. Available online: https://books.google.ro/books?id=oaq-DwAAQBAJ&pg=PA173&lpg=PA173&dq=strong+physically+bound+water&source=bl&ots=F2VLsZWYHy&sig=ACfU3U11W_kRxqwL2yooFg6auXynm-80ww&hl=ro&sa=X&ved=2ahUKEwjjptPehrLmAhV_wAIHHVmYCG8Q6AEwDnoECAoQAQ#v=onepage&q=strong%20physically%20bound%20water&f=false (accessed on 13 December 2019).
- Van Reeuwijk, L.P. The Thermal Dehydration of Natural Zeolites (with a Summary in Dutch); H. Veenman & Zonen: Wageningen, The Netherlands, 1974. [Google Scholar]
- Valdiviés-Cruz, K.; Lam, A.; Zicovich-Wilson, C.M. Chemical interaction of water molecules with framework Al in acid zeolites: A periodic ab initio study on H-clinoptilolite. Phys. Chem. Chem. Phys. 2015, 17, 23657–23666. [Google Scholar] [CrossRef] [PubMed]
- Calero, S.; Gómez-Álvarez, P. Hydrogen bonding of water confined in zeolites and their zeolitic imidazolate framework counterparts. RSC Adv. 2014, 4, 29571–29580. [Google Scholar] [CrossRef]
- Glad, B.E.; Kriven, W.M. Geopolymer with Hydrogel Characteristics via Silane Coupling Agent Additives. J. Am. Ceram. Soc. 2014, 97, 295–302. [Google Scholar] [CrossRef]
- Palomo, A.; Krivenko, P.; Garcia-Lodeiro, I.; Kavalerova, E.; Maltseva, O.; Fernández-Jiménez, A. A review on alkaline activation: New analytical perspectives. Mater. Constr. 2014, 64, 22. [Google Scholar] [CrossRef] [Green Version]
- Criado, M.; Aperador, W.; Sobrados, I. Microstructural and mechanical properties of alkali activated Colombian raw materials. Materials 2016, 9, 158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Musicá, S.M.; Krehula, S.; Popovićb, S.; Popovićb, P. Thermal decomposition of h-FeOOH. Mater. Lett. 2004, 58, 444–448. [Google Scholar]
- Derie, R.; Ghodsi, M.; Calvo-Roche, C. DTA study of the dehydration of synthetic goethite αFeOOH. J. Therm. Anal. 1976, 9, 435–440. [Google Scholar] [CrossRef]
- Rendon, J.L.; Cornejo, J.; de Arambarri, P.; Serna, C.J. Pore structure of thermally treated goethite (α-FeOOH). J. Colloid Interface Sci. 1983, 92, 508–516. [Google Scholar] [CrossRef] [Green Version]
- Giovanoli, R.; Brütsch, R. Kinetics and mechanism of the dehydration of γ-FeOOH. Thermochim. Acta 1975, 13, 15–36. [Google Scholar] [CrossRef]
- Walter, D.; Buxbaum, G.; Laqua, W. The mechanism of the thermal transformation from goethite to hematite*. J. Therm. Anal. Calorim. 2001, 63, 733–748. [Google Scholar] [CrossRef]
- Cheng-Yong, H.; Yun-Ming, L.; Abdullah, M.M.A.B.; Hussin, K. Thermal Resistance Variations of Fly Ash Geopolymers: Foaming Responses. Sci. Rep. 2017, 7, 45355. [Google Scholar] [CrossRef] [PubMed]
- Cornejo, M.H.; Togra, B.; Baykara, H.; Soriano, G.; Paredes, C.; Elsen, J. Effect of Calcium Hydroxide and Water to Solid Ratio on Compressive Strength of Mordenite-Based Geopolymer and the Evaluation of Its Thermal Transmission Property. In Proceedings of the ASME International Mechanical Engineering Congress and Exposition (IMECE), Pittsburgh, PA, USA, 9–15 November 2018; American Society of Mechanical Engineers (ASME): New York, NY, USA, 2018; Volume 12. [Google Scholar]
- Abdullah, M.M.A.B.; Ming, L.Y.; Yong, H.C.; Tahir, M.F.M. Clay-Based Materials in Geopolymer Technology. In Cement Based Materials; InTechOpen: London, UK, 2018. [Google Scholar] [CrossRef] [Green Version]
- Bajare, D.; Vitola, L.; Dembovska, L.; Bumanis, G. Waste stream porous alkali activated materials for high temperature application. Front. Mater. 2019, 6. [Google Scholar] [CrossRef] [Green Version]
- Dehydration Reactions and Kinetic Parameters of Gibbsite—Science Direct. Available online: https://www.sciencedirect.com/science/article/pii/S0272884210002592 (accessed on 13 December 2019).
- MacKenzie, K.J.D.; Temuujin, J.; Okada, K. Thermal decomposition of mechanically activated gibbsite. Thermochim. Acta 1999, 327, 103–108. [Google Scholar] [CrossRef]
- Zhu, B.; Fang, B.; Li, X. Dehydration reactions and kinetic parameters of gibbsite. Ceram. Int. 2010, 36, 2493–2498. [Google Scholar] [CrossRef]
- Redaoui, D.; Sahnoune, F.; Heraiz, M.; Raghdi, A. Mechanism and Kinetic Parameters of the Thermal Decomposition of Gibbsite Al(OH)3 by Thermogravimetric Analysis. Acta Phys. Pol. Ser. A 2017, 131, 562–565. [Google Scholar] [CrossRef]
- Hao, H.; Lin, K.-L.; Wang, D.; Chao, S.-J.; Shiu, H.-S.; Cheng, T.-W.; Hwang, C.-L. Elucidating Characteristics of Geopolymer with Solar Panel Waste Glass. Environ. Eng. Manag. J. 2015, 14, 79–87. [Google Scholar]
- Grawe, S.; Augustin-Bauditz, S.; Clemen, H.C.; Ebert, M.; Eriksen Hammer, S.; Lubitz, J.; Reicher, N.; Rudich, Y.; Schneider, J.; Staacke, R.; et al. Coal fly ash: Linking immersion freezing behavior and physicochemical particle properties. Atmos. Chem. Phys. 2018, 18, 13903–13923. [Google Scholar] [CrossRef] [Green Version]
- Duan, P.; Yan, C.; Zhou, W. Compressive strength and microstructure of fly ash based geopolymer blended with silica fume under thermal cycle. Cem. Concr. Compos. 2017, 78, 108–119. [Google Scholar] [CrossRef]
- Paunović, P.; Petrovski, A.; Načevski, G.; Grozdanov, A.; Marinkovski, M.; Andonović, B.; Makreski, P.; Popovski, O.; Dimitrov, A. Pathways for the production of non-stoichiometric titanium oxides. In Nanoscience Advances in CBRN Agents Detection, Information and Energy Security; Springer: Dordrecht, The Netherlands, 2015; pp. 239–253. ISBN 9789401796972. [Google Scholar]
- Anderson, P.J.; Horlock, R.F. Thermal decomposition of magnesium hydroxide. Trans. Faraday Soc. 1962, 58, 1993–2004. [Google Scholar] [CrossRef]
- Zulkifly, K.; Yong, H.C.; Abdullah, M.M.A.B.; Ming, L.Y.; Panias, D.; Sakkas, K. Review of Geopolymer Behaviour in Thermal Environment. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Bali, Indonesia, 26–27 July 2016; Institute of Physics Publishing: Bristol, UK, 2017; Volume 209. [Google Scholar]
- Alvarez-Ayuso, E.; Querol, X.; Plana, F.; Alastuey, A.; Moreno, N.; Izquierdo, M.; Font, O.; Moreno, T.; Diez, S.; Vázquez, E.; et al. Environmental, physical and structural characterisation of geopolymer matrixes synthesised from coal (co-)combustion fly ashes. J. Hazard. Mater. 2008, 154, 175–183. [Google Scholar] [CrossRef]

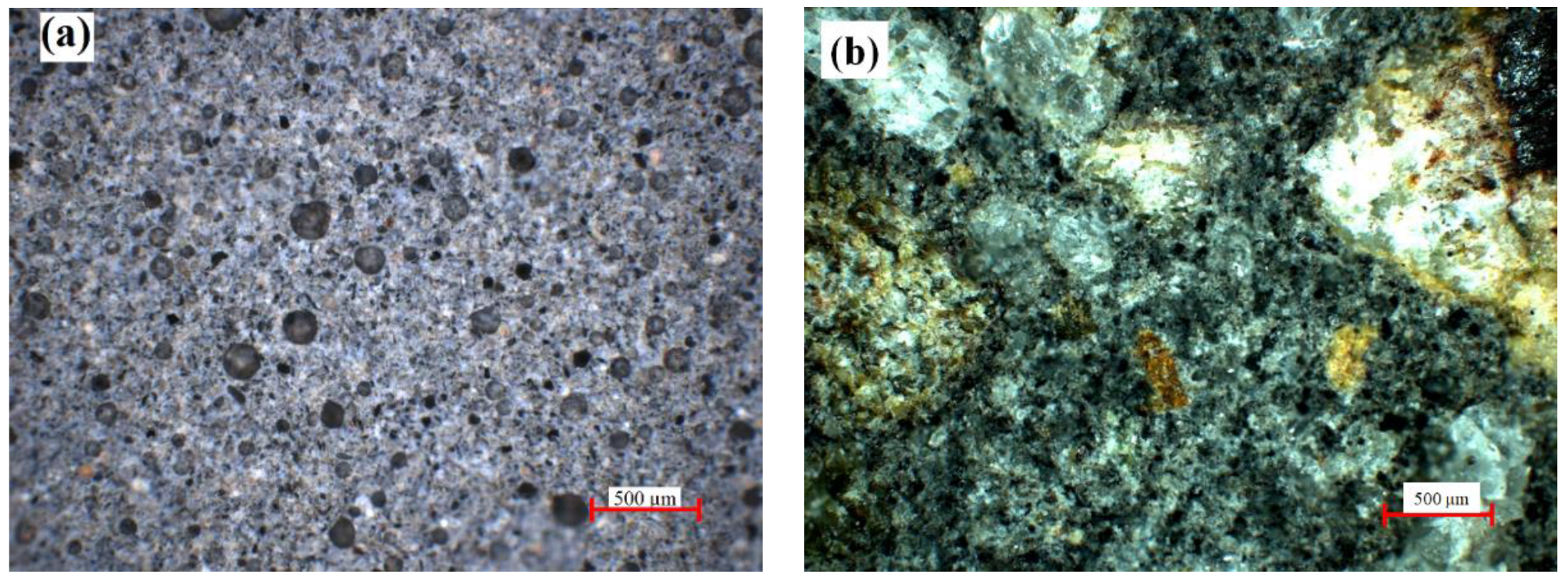
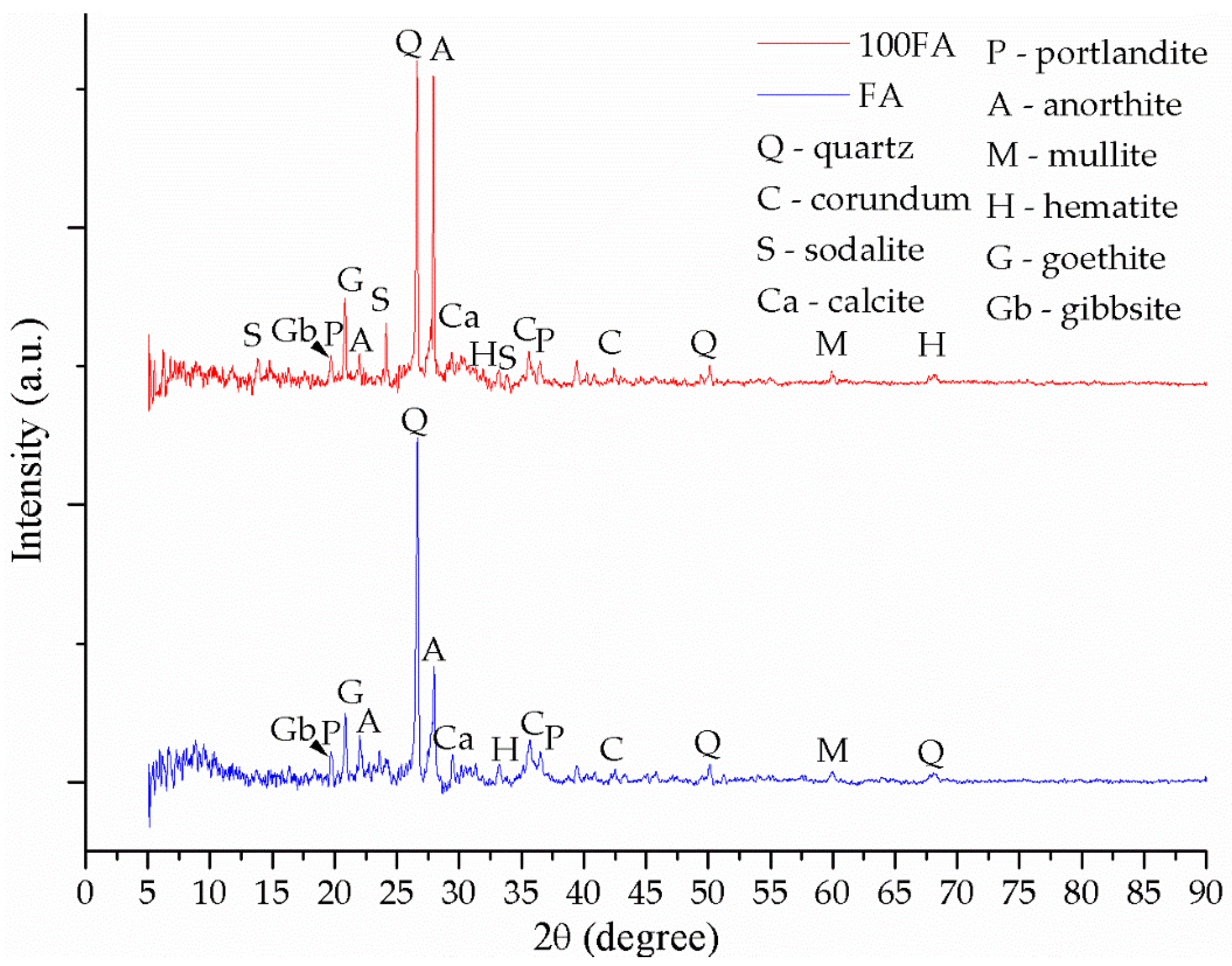
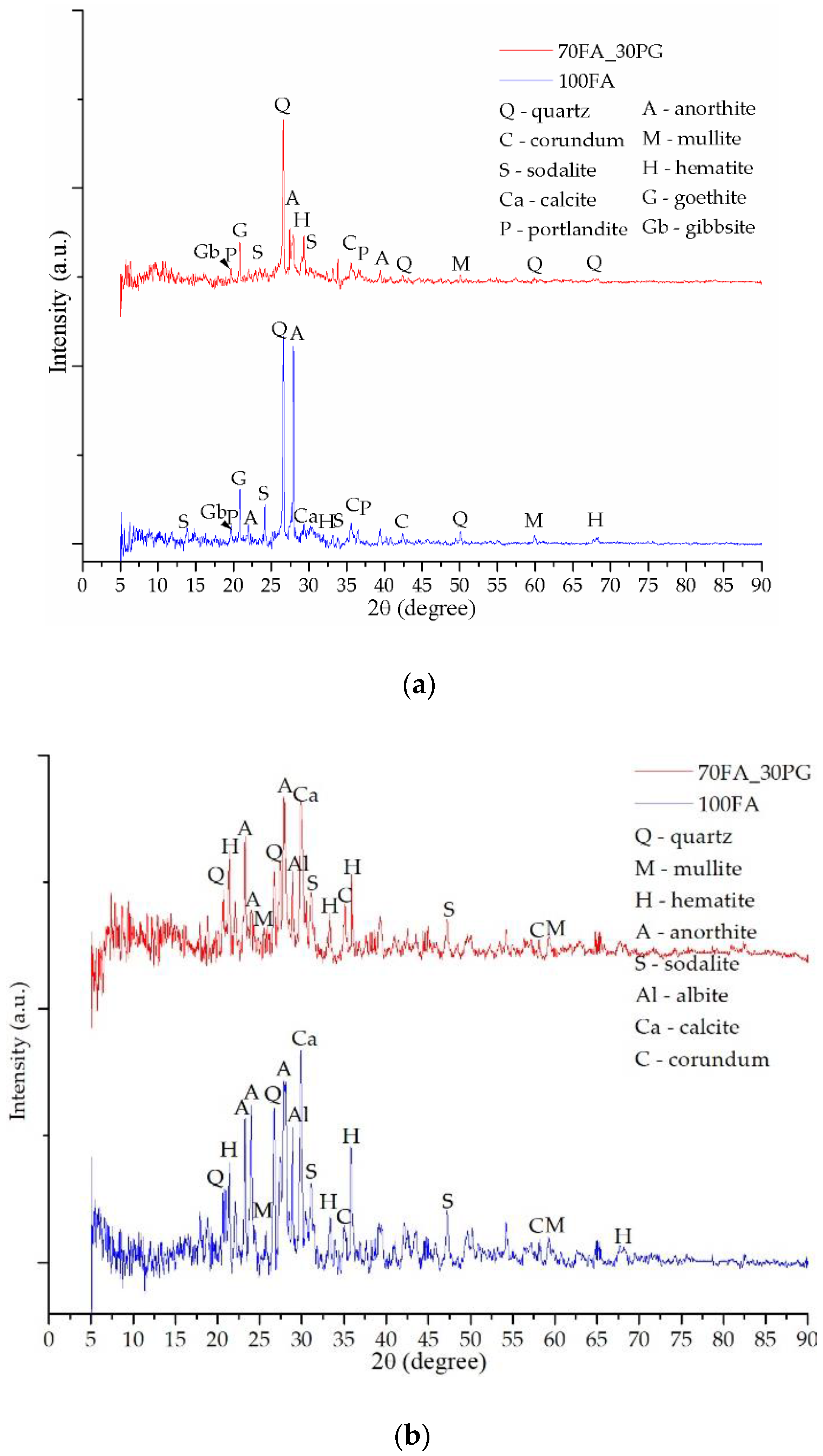
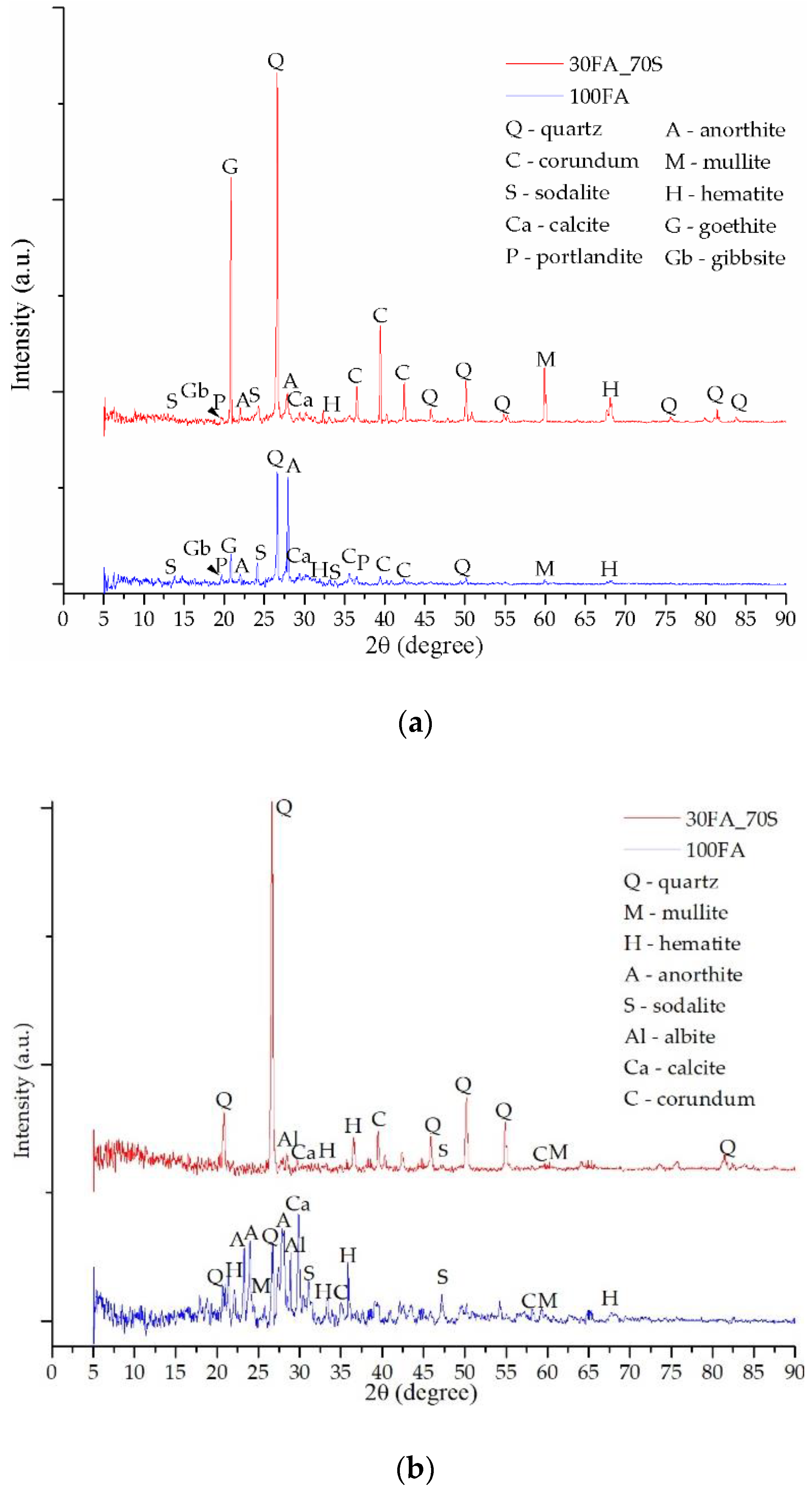
| Oxide | SiO2 | Al2O3 | FexOy | CaO | K2O | MgO | TiO2 | Na2O | P2O5 | Oth 1 |
|---|---|---|---|---|---|---|---|---|---|---|
| %, weight | 47.80 | 28.60 | 10.20 | 6.40 | 2.40 | 2.00 | 1.30 | 0.60 | 0.40 | 0.30 |
| Stat. error, % | 0.32 | 0.27 | 0.95 | 0.77 | 0.71 | 1.09 | 1.81 | 0.63 | 0.24 | - |
| Oxide | SiO2 | Al2O3 | FexOy | CaO | MgO | Na2O | Oth 1 |
|---|---|---|---|---|---|---|---|
| %, weight | 70–71 | 1.5–2 | 0.8–1 | 9–11 | 2–3 | 12–14 | <0.1 |
| Sample | Liquid Component, % Weight | Solid Component, % Weight | |||
|---|---|---|---|---|---|
| Na2SiO3 | NaOH | Fly Ash | Glass Powder | Sand | |
| 100FA | 60 | 40 | 100 | 0 | 0 |
| 70FA_30PG | 60 | 40 | 70 | 30 | 0 |
| 30FA_70S | 60 | 40 | 30 | 0 | 70 |
| 15FA_15PG_70S | 60 | 40 | 15 | 15 | 70 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burduhos Nergis, D.D.; Abdullah, M.M.A.B.; Sandu, A.V.; Vizureanu, P. XRD and TG-DTA Study of New Alkali Activated Materials Based on Fly Ash with Sand and Glass Powder. Materials 2020, 13, 343. https://doi.org/10.3390/ma13020343
Burduhos Nergis DD, Abdullah MMAB, Sandu AV, Vizureanu P. XRD and TG-DTA Study of New Alkali Activated Materials Based on Fly Ash with Sand and Glass Powder. Materials. 2020; 13(2):343. https://doi.org/10.3390/ma13020343
Chicago/Turabian StyleBurduhos Nergis, Dumitru Doru, Mohd Mustafa Al Bakri Abdullah, Andrei Victor Sandu, and Petrică Vizureanu. 2020. "XRD and TG-DTA Study of New Alkali Activated Materials Based on Fly Ash with Sand and Glass Powder" Materials 13, no. 2: 343. https://doi.org/10.3390/ma13020343
APA StyleBurduhos Nergis, D. D., Abdullah, M. M. A. B., Sandu, A. V., & Vizureanu, P. (2020). XRD and TG-DTA Study of New Alkali Activated Materials Based on Fly Ash with Sand and Glass Powder. Materials, 13(2), 343. https://doi.org/10.3390/ma13020343








