Limitation in Controlling the Morphology of Mammalian Vero Cells Induced by Cell Division on Asymmetric Tungsten-Silicon Oxide Nanocomposite
Abstract
1. Introduction
2. Materials and Methods
2.1. Asymmetric Parallel Line Comb Structure Surfaces
2.2. Cell Culture, Fixation, and Chemical Staining
2.3. Scanning Electron and Fluorescence Confocal Microscopy
3. Results
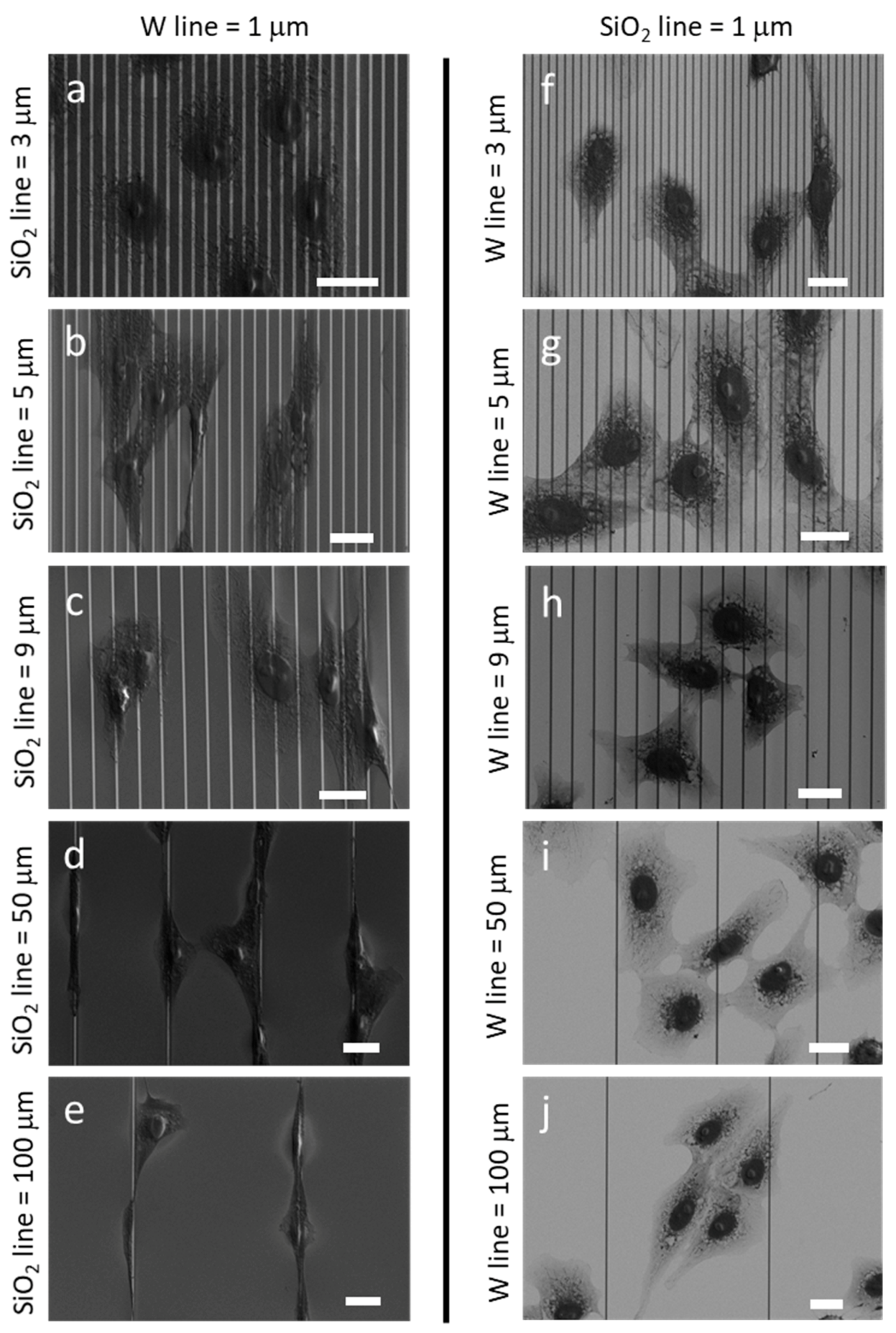
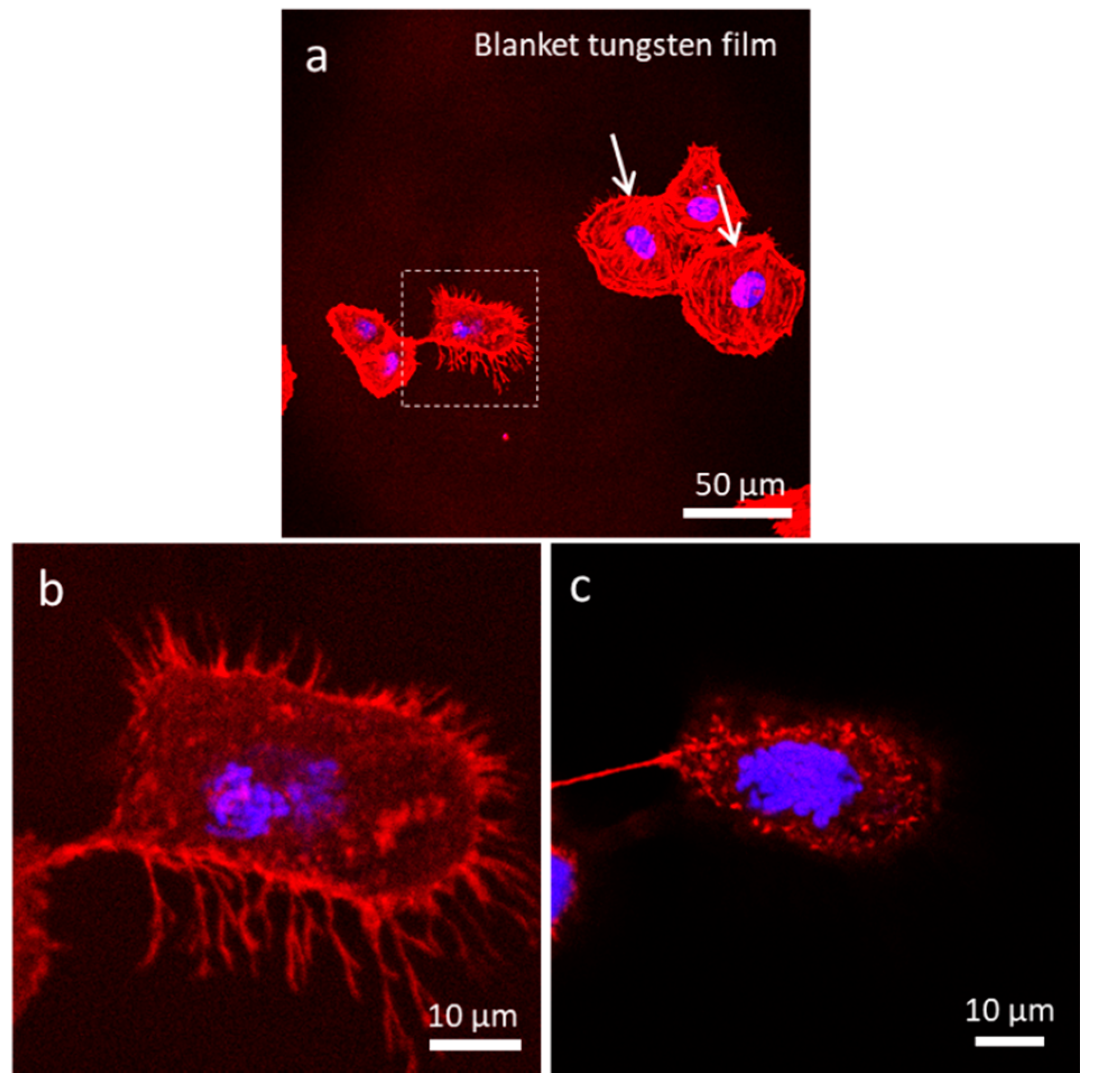
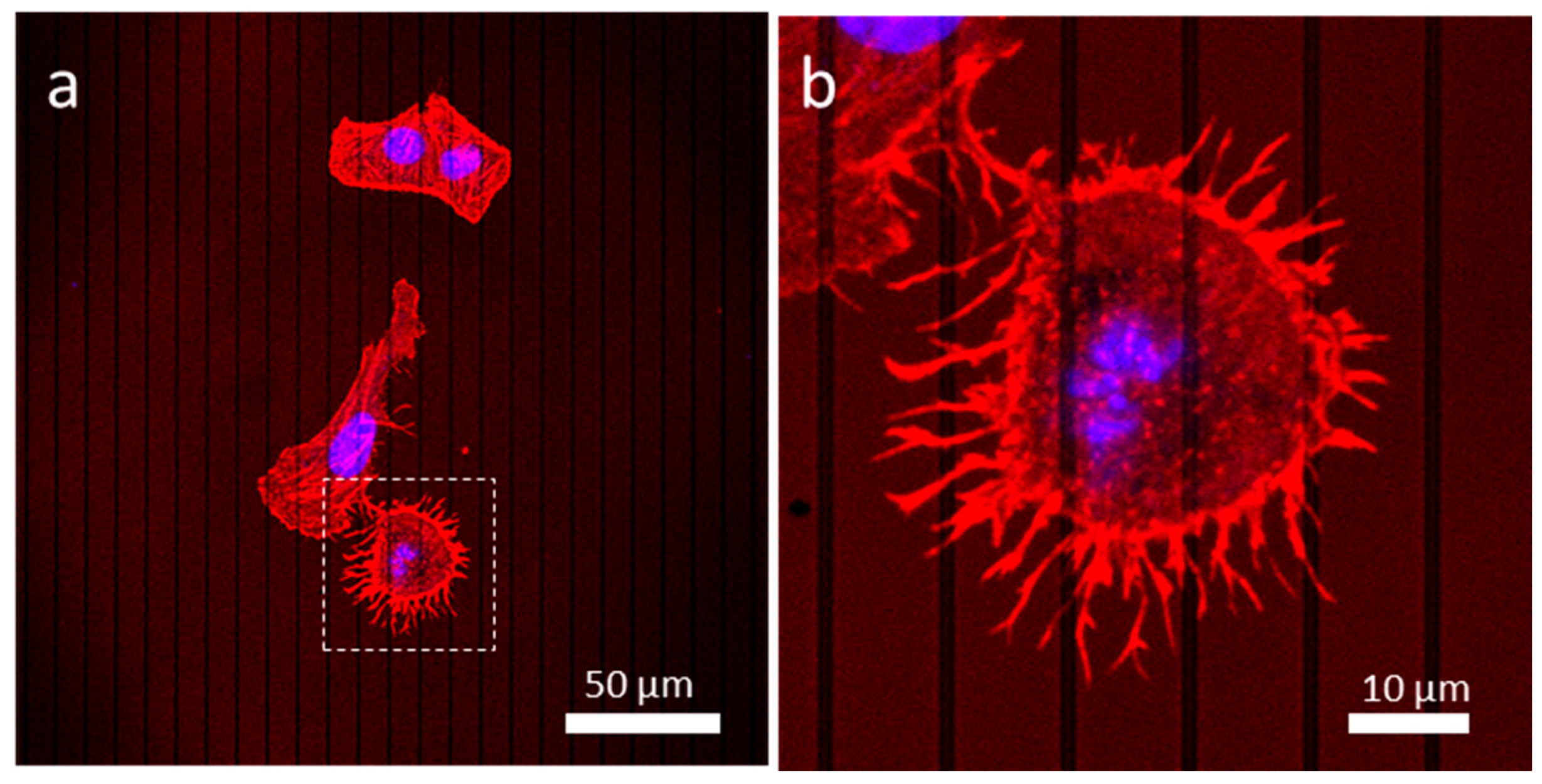
3.1. Morphology of Adherent Cells
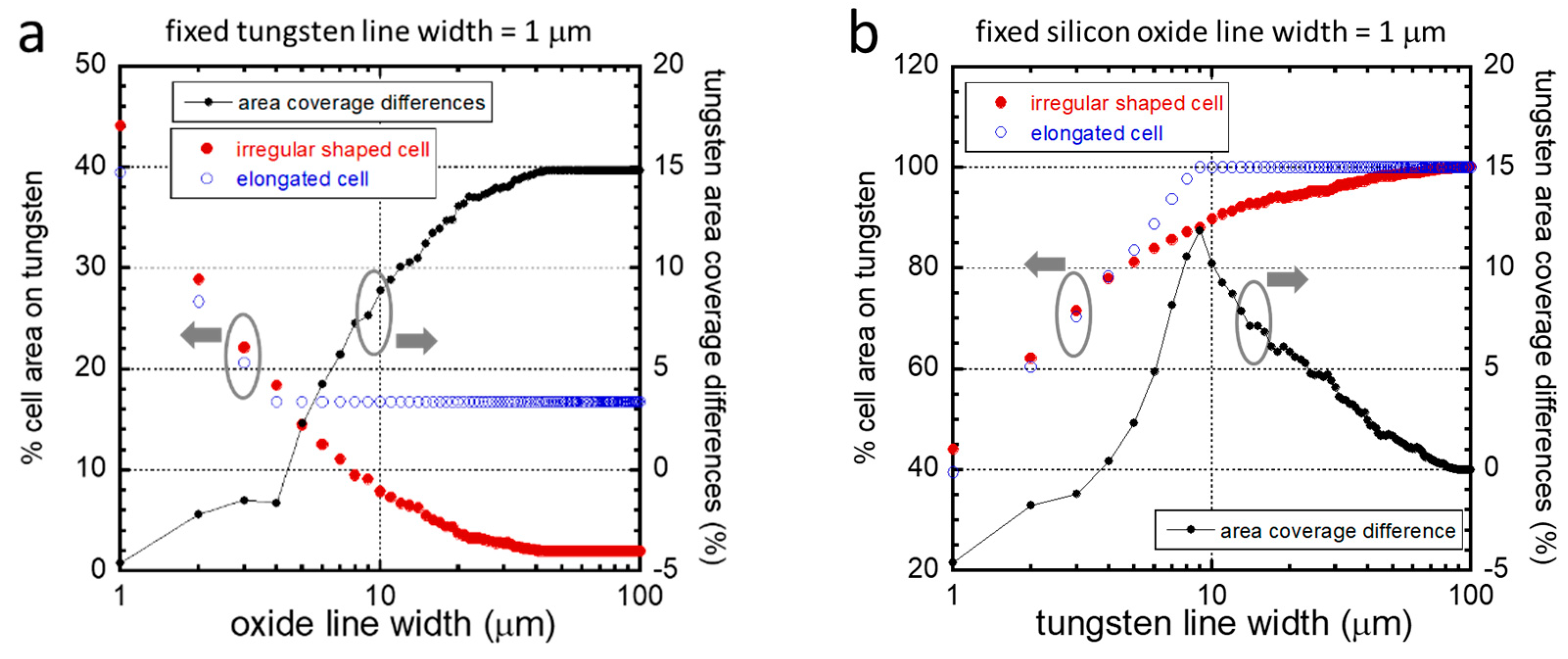
3.2. Quantitative Analyses of Cell Alignment
3.3. Agreement with the Established Model
Non-Aligned Cells
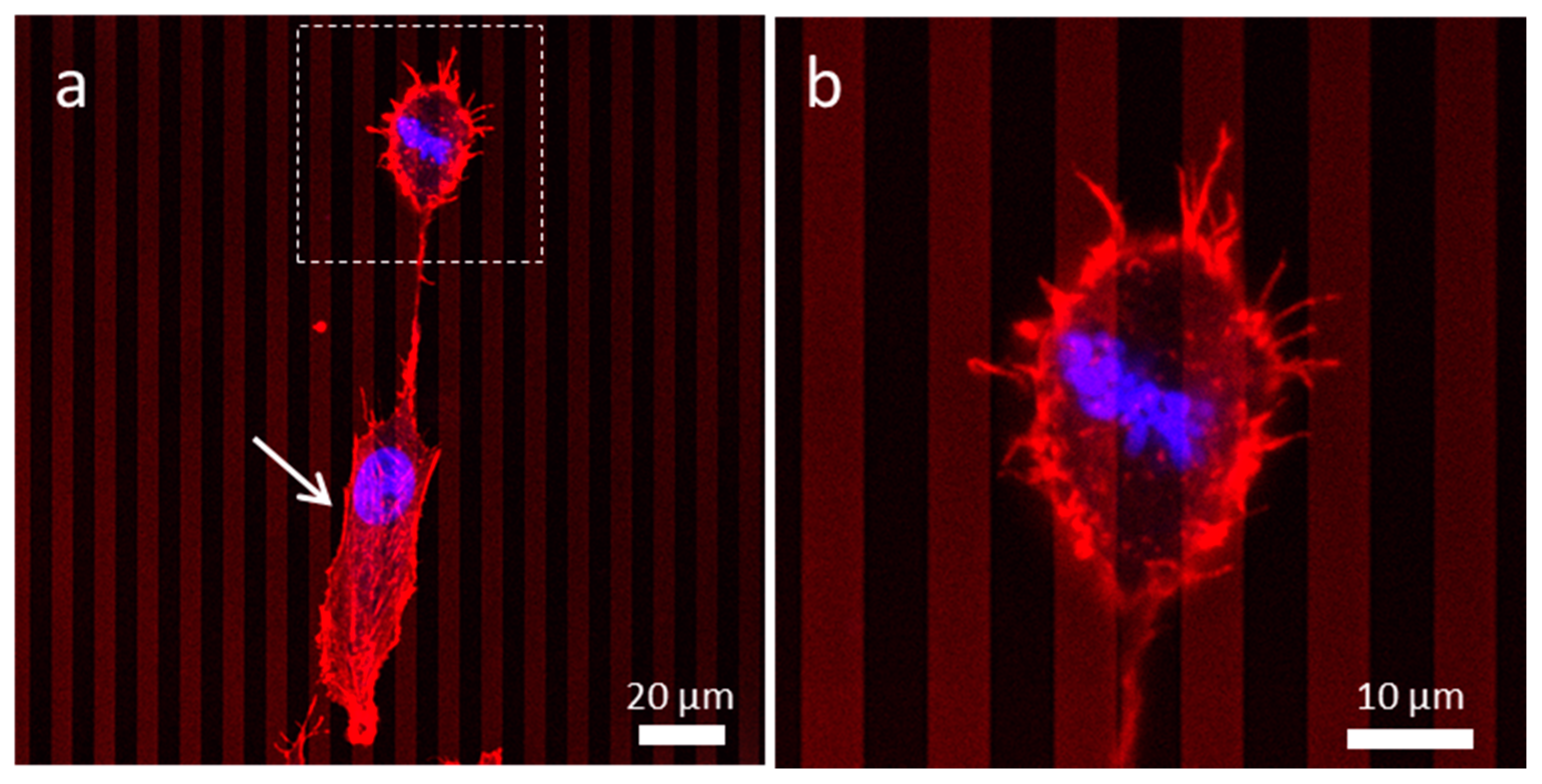
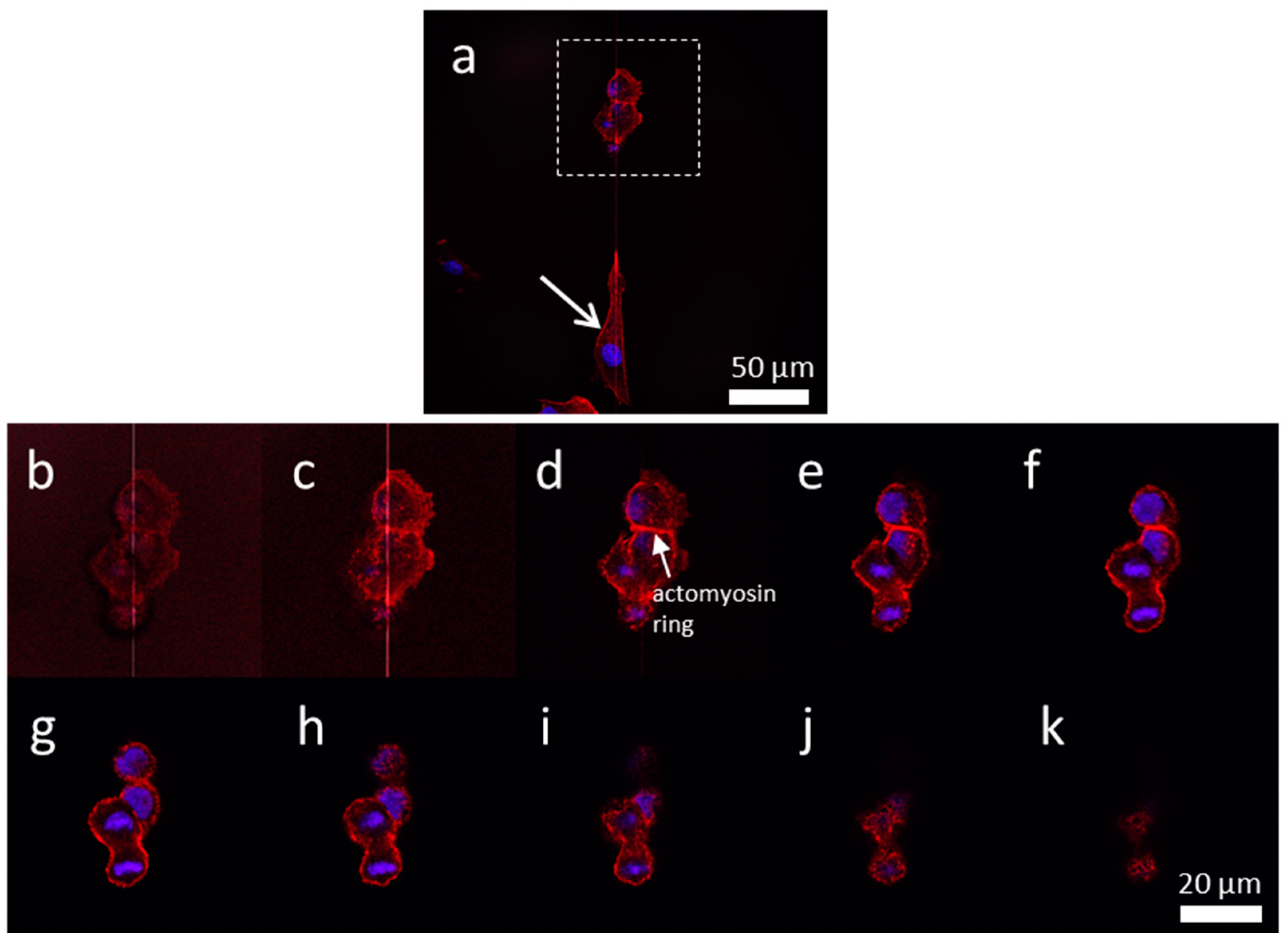
3.4. Influences of Cell Division on Cell Morphology and Alignment on Various Surfaces
3.4.1. Uniform Tungsten Surfaces
3.4.2. Patterned Comb Structures (Alternating Silicon Oxide (1 μm) and Tungsten Lines (9 μm))
3.4.3. Patterned Comb Structures (Alternating Silicon Oxide (5 μm) and Tungsten Lines (5 μm))
3.4.4. Patterned Comb Structures (Alternating Silicon Oxide (9 μm) and Tungsten Lines (1 μm))
3.4.5. Isolated Tungsten Lines (1 µm Tungsten Line Inlaid in a SiO2 Continuous Phase)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, Q.; Williams, G.; Tsui, T.; Aziz, H. Photochemical deterioration of the organic/metal contacts in organic optoelectronic devices. J. Appl. Phys. 2012, 112, 64502. [Google Scholar] [CrossRef]
- Mesguich, D.; Arnaud, C.; Lecouturier, F.; Ferreira, N.; Chevallier, G.; Estournès, C.; Weibel, A.; Josse, C.; Laurent, C. High strength-high conductivity carbon nanotube-copper wires with bimodal grain size distribution by spark plasma sintering and wire-drawing. Scr. Mater. 2017, 137, 78–82. [Google Scholar] [CrossRef]
- Han, B.; Guo, E.; Xue, X.; Zhao, Z.; Luo, L.; Qu, H.; Niu, T.; Xu, Y.; Hou, H. Fabrication and densification of high performance carbon nanotube/copper composite fibers. Carbon 2017, 123, 593–604. [Google Scholar] [CrossRef]
- Valipour, P.; Ghasemi, S.E.; Khosravani, M.R.; Ganji, D.D. Theoretical analysis on nonlinear vibration of fluid flow in single-walled carbon nanotube. J. Theor. Appl. Phys. 2016, 10, 211–218. [Google Scholar] [CrossRef]
- Tovide, O.; Jaheed, N.; Mohamed, N.; Nxusani, E.; Sunday, C.E.; Tsegaye, A.; Ajayi, R.F.; Njomo, N.; Makelane, H.; Bilibana, M.; et al. Graphenated polyaniline-doped tungsten oxide nanocomposite sensor for real time determination of phenanthrene. Electrochim. Acta 2014, 128, 138–148. [Google Scholar] [CrossRef]
- Yang, C.Y.; Liao, T.C.; Shuai, H.H.; Shen, T.L.; Yeh, J.A.; Cheng, C.M. Biomaterials Micropatterning of mammalian cells on inorganic-based nanosponges. Biomaterials 2012, 33, 4988–4997. [Google Scholar] [CrossRef]
- Chen, W.; Weng, S.; Zhang, F.; Allen, S.; Li, X.; Bao, L.; Lam, R.H.; Macoska, J.A.; Merajver, S.D.; Fu, J. Nanoroughened Surfaces for E ffi cient Capture of Circulating Tumor Cells without Using Capture Antibodies. ACS Nano 2013, 7, 566–575. [Google Scholar] [CrossRef]
- Moussa, H.I.; Logan, M.; Wong, K.; Rao, Z.; Aucoin, M.G.; Tsui, T.Y. Nanoscale-Textured Tantalum Surfaces for Mammalian Cell Alignment. Micromachines 2018, 9, 464. [Google Scholar] [CrossRef]
- Moussa, H.I.; Logan, M.; Chan, W.Y.; Wong, K.; Rao, Z.; Aucoin, M.G.; Tsui, T.Y. Pattern-Dependent Mammalian Cell (Vero) Morphology on Tantalum/Silicon Oxide 3D Nanocomposites. Materials 2018, 11, 1306. [Google Scholar] [CrossRef]
- Moussa, H.I.; Logan, M.; Siow, G.C.; Phann, D.L.; Rao, Z.; Aucoin, M.G.; Tsui, T.Y. Manipulating mammalian cell morphologies using chemical-mechanical polished integrated circuit chips. Sci. Technol. Adv. Mater. 2017, 18, 839–856. [Google Scholar] [CrossRef]
- Seo, B.B.; Jahed, Z.; Coggan, J.A.; Chau, Y.Y.; Rogowski, J.L.; Gu, F.X.; Wen, W.; Mofrad, M.R.K.; Tsui, T.Y. Mechanical Contact Characteristics of PC3 Human Prostate Cancer Cells on Complex-Shaped Silicon Micropillars. Materials 2017, 10, 892. [Google Scholar] [CrossRef] [PubMed]
- Nikkhah, M.; Eshak, N.; Zorlutuna, P.; Annabi, N.; Castello, M.; Kim, K.; Dolatshahi-Pirouz, A.; Edalat, F.; Bae, H.; Yang, Y.; et al. Biomaterials Directed endothelial cell morphogenesis in micropatterned gelatin methacrylate hydrogels. Biomaterials 2012, 33, 9009–9018. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, A.I.; McKie, G.A.; Foley, J.D.; Bertics, P.J.; Nealey, P.F.; Murphy, C.J. The effect of environmental factors on the response of human corneal epithelial cells to nanoscale substrate topography. Biomaterials 2006, 27, 3945–3954. [Google Scholar] [CrossRef] [PubMed]
- Jahed, Z.; Molladavoodi, S.; Seo, B.B.; Gorbet, M.; Tsui, T.Y.; Mofrad, M.R. Cell responses to metallic nanostructure arrays with complex geometries. Biomaterials 2014, 35, 9363–9371. [Google Scholar] [CrossRef] [PubMed]
- Moussa, H.I.; Kim, G.; Tong, J.; Glerum, D.M.; Tsui, T.Y. Influence of Antimycin A, a bacterial toxin, on human dermal fibroblast cell adhesion to tungsten-silicon oxide nanocomposites. J. Exp. Nanosci. 2019, 14, 69–88. [Google Scholar] [CrossRef]
- Lancaster, O.M.; Le Berre, M.; Dimitracopoulos, A.; Bonazzi, D.; Zlotek-Zlotkiewicz, E.; Picone, R.; Duke, T.; Piel, M.; Baum, B. Mitotic Rounding Alters Cell Geometry to Ensure Efficient Bipolar Spindle Formation. Dev. Cell 2013, 25, 270–283. [Google Scholar] [CrossRef] [PubMed]
- Dix, C.L.; Matthews, H.K.; Uroz, M.; McLaren, S.; Wolf, L.; Heatley, N.; Win, Z.; Almada, P.; Henriques, R.; Boutros, M.; et al. The Role of Mitotic Cell-Substrate Adhesion Re-modeling in Animal Cell Division. Dev. Cell 2018, 45, 132–145. [Google Scholar] [CrossRef]
- Lancaster, O.M.; Baum, B. Shaping up to divide: Coordinating actin and microtubule cytoskeletal remodelling during mitosis. Semin. Cell Dev. Boil. 2014, 34, 109–115. [Google Scholar] [CrossRef]
- Petridou, N.I.; Skourides, P.A. A ligand-independent integrin β1 mechanosensory complex guides spindle orientation. Nat. Commun. 2016, 7, 10899. [Google Scholar] [CrossRef]
- Poudel, I.; Lee, J.S.; Tan, L.; Lim, J.Y. Micropatterning–retinoic acid co-control of neuronal cell morphology and neurite outgrowth. Acta Biomater. 2013, 9, 4592–4598. [Google Scholar] [CrossRef]
- Aubin, H.; Nichol, J.W.; Hutson, C.B.; Bae, H.; Sieminski, A.L.; Cropek, N.M.; Akhyari, P.; Khademhosseini, A. Directed 3D cell alignment and elongation in microengineered hydrogels. Biomaterials 2010, 31, 6941–6951. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Xu, Y.; Zhang, Q.; Yang, F.; Yin, Z.; Wang, L.; Li, Q. Porcine epidemic diarrhea virus infections induce apoptosis in Vero cells via a reactive oxygen species (ROS)/p53, but not p38 MAPK and SAPK/JNK signalling pathways. Vet. Microbiol. 2019, 232, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Decotiis, J.L.; Ortiz, N.C.; Vega, B.A.; Lukac, D.M. An easily transfectable cell line that produces an infectious reporter virus for routine and robust quantitation of Kaposi’s sarcoma-associated herpesvirus reactivation. J. Virol. Methods 2017, 247, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Théry, M.; Bornens, M. Get round and stiff for mitosis. HFSP J. 2008, 2, 65–71. [Google Scholar] [CrossRef]
- Boucrot, E.; Kirchhausen, T. Mammalian Cells Change Volume during Mitosis. PLoS ONE 2008, 3, e1477. [Google Scholar] [CrossRef]
- Boucrot, E.; Kirchhausen, T. Endosomal recycling controls plasma membrane area during mitosis. Proc. Natl. Acad. Sci. USA 2007, 104, 7939–7944. [Google Scholar] [CrossRef]
- Quesney, S.; Marvel, J.; Marc, A.; Gerdil, C.; Meignier, B. Characterization of Vero cell growth and death in bioreactor with serum-containing and serum-free media. Cytotechnology 2001, 35, 115–125. [Google Scholar] [CrossRef]
- Raven, P.H.; Johnson, G.B. Biology, 3rd ed.; Mosby-Year Book Inc.: St. Louis, MO, USA, 1992. [Google Scholar]
- Joo, S.; Kim, J.Y.; Lee, E.; Hong, N.; Sun, W.; Nam, Y. Effects of ECM protein micropatterns on the migration and differentiation of adult neural stem cells. Sci. Rep. 2015, 5, 13043. [Google Scholar] [CrossRef]
- Nakamoto, T.; Wang, X.; Kawazoe, N.; Chen, G. Biointerfaces Influence of micropattern width on differentiation of human mesenchymal stem cells to vascular smooth muscle cells. Colloids Surf. B Biointerfaces 2014, 122, 316–323. [Google Scholar] [CrossRef]

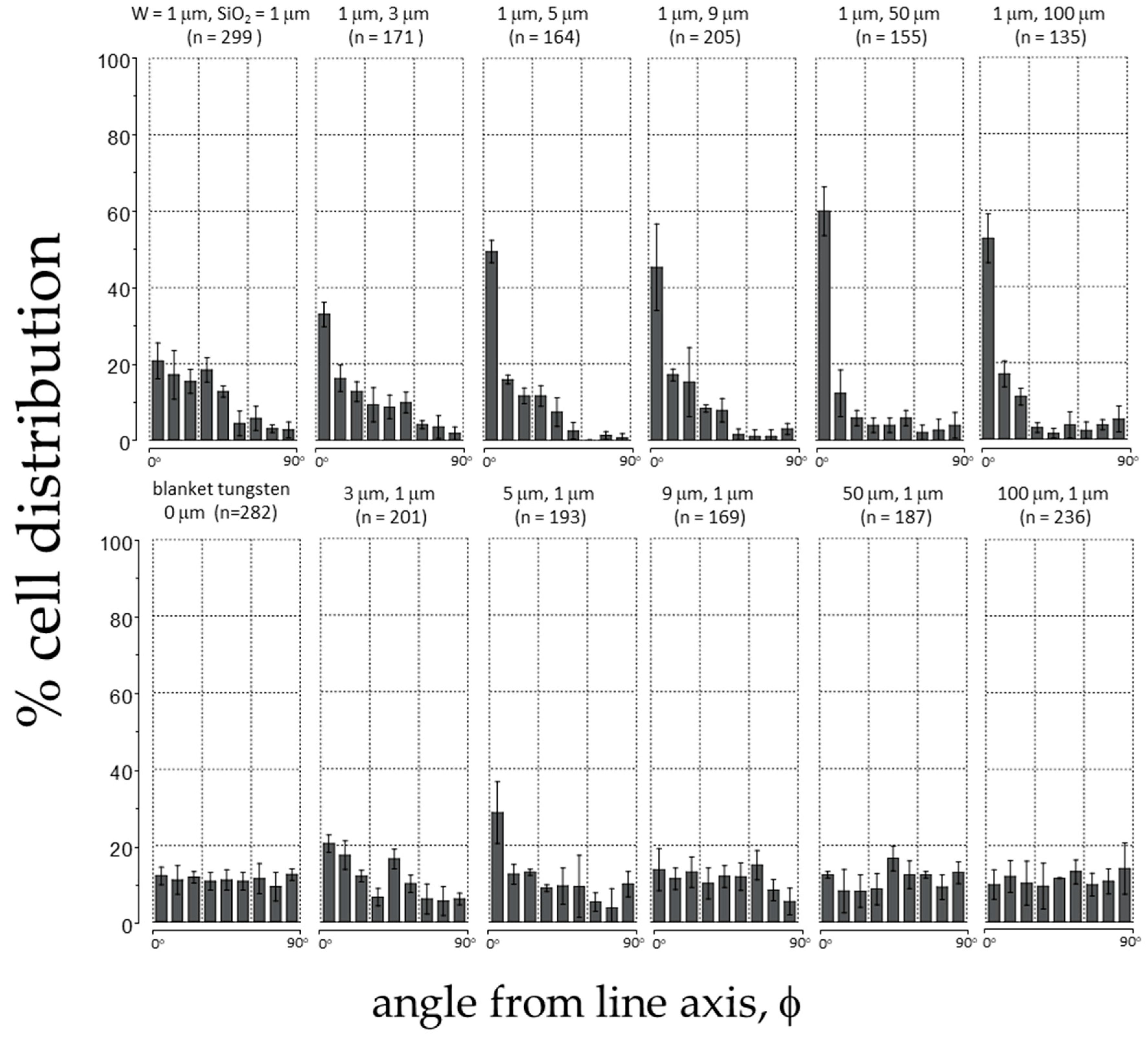


| Tungsten Line Width (μm) | SiO2 Line Width (μm) | Cells Counted (n) | Pattern Area (mm2) | Density (Cells/mm2) | % Cell Distribution ± 10° | % Distribution S.D. |
|---|---|---|---|---|---|---|
| 1 | 1 | 299 | 1.7 | 176 | 20.7 | 4.7 |
| 1 | 3 | 171 | 1.5 | 114 | 32.9 | 3.2 |
| 1 | 5 | 164 | 1.7 | 96 | 49.4 | 2.9 |
| 1 | 9 | 205 | 1.8 | 114 | 45.3 | 11.4 |
| 1 | 50 | 155 | 1.6 | 97 | 60.0 | 6.4 |
| 1 | 100 | 135 | 1.4 | 96 | 41.3 | 9.2 |
| 3 | 1 | 201 | 1.6 | 126 | 20.4 | 2.3 |
| 5 | 1 | 193 | 1.7 | 114 | 28.5 | 8.1 |
| 9 | 1 | 169 | 1.7 | 99 | 13.6 | 5.5 |
| 50 | 1 | 187 | 1.6 | 117 | 12.3 | 0.9 |
| 100 | 1 | 236 | 1.6 | 148 | 9.8 | 4.4 |
| Blanket W | N/A | 282 | 1.7 | 166 | 12.1 | 0.6 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moussa, H.I.; Chan, W.Y.; Logan, M.; Aucoin, M.G.; Tsui, T.Y. Limitation in Controlling the Morphology of Mammalian Vero Cells Induced by Cell Division on Asymmetric Tungsten-Silicon Oxide Nanocomposite. Materials 2020, 13, 335. https://doi.org/10.3390/ma13020335
Moussa HI, Chan WY, Logan M, Aucoin MG, Tsui TY. Limitation in Controlling the Morphology of Mammalian Vero Cells Induced by Cell Division on Asymmetric Tungsten-Silicon Oxide Nanocomposite. Materials. 2020; 13(2):335. https://doi.org/10.3390/ma13020335
Chicago/Turabian StyleMoussa, Hassan I., Wing Y. Chan, Megan Logan, Marc G. Aucoin, and Ting Y. Tsui. 2020. "Limitation in Controlling the Morphology of Mammalian Vero Cells Induced by Cell Division on Asymmetric Tungsten-Silicon Oxide Nanocomposite" Materials 13, no. 2: 335. https://doi.org/10.3390/ma13020335
APA StyleMoussa, H. I., Chan, W. Y., Logan, M., Aucoin, M. G., & Tsui, T. Y. (2020). Limitation in Controlling the Morphology of Mammalian Vero Cells Induced by Cell Division on Asymmetric Tungsten-Silicon Oxide Nanocomposite. Materials, 13(2), 335. https://doi.org/10.3390/ma13020335







