Carbon Nanotubes (CNTs)-Reinforced Magnesium-Based Matrix Composites: A Comprehensive Review
Abstract
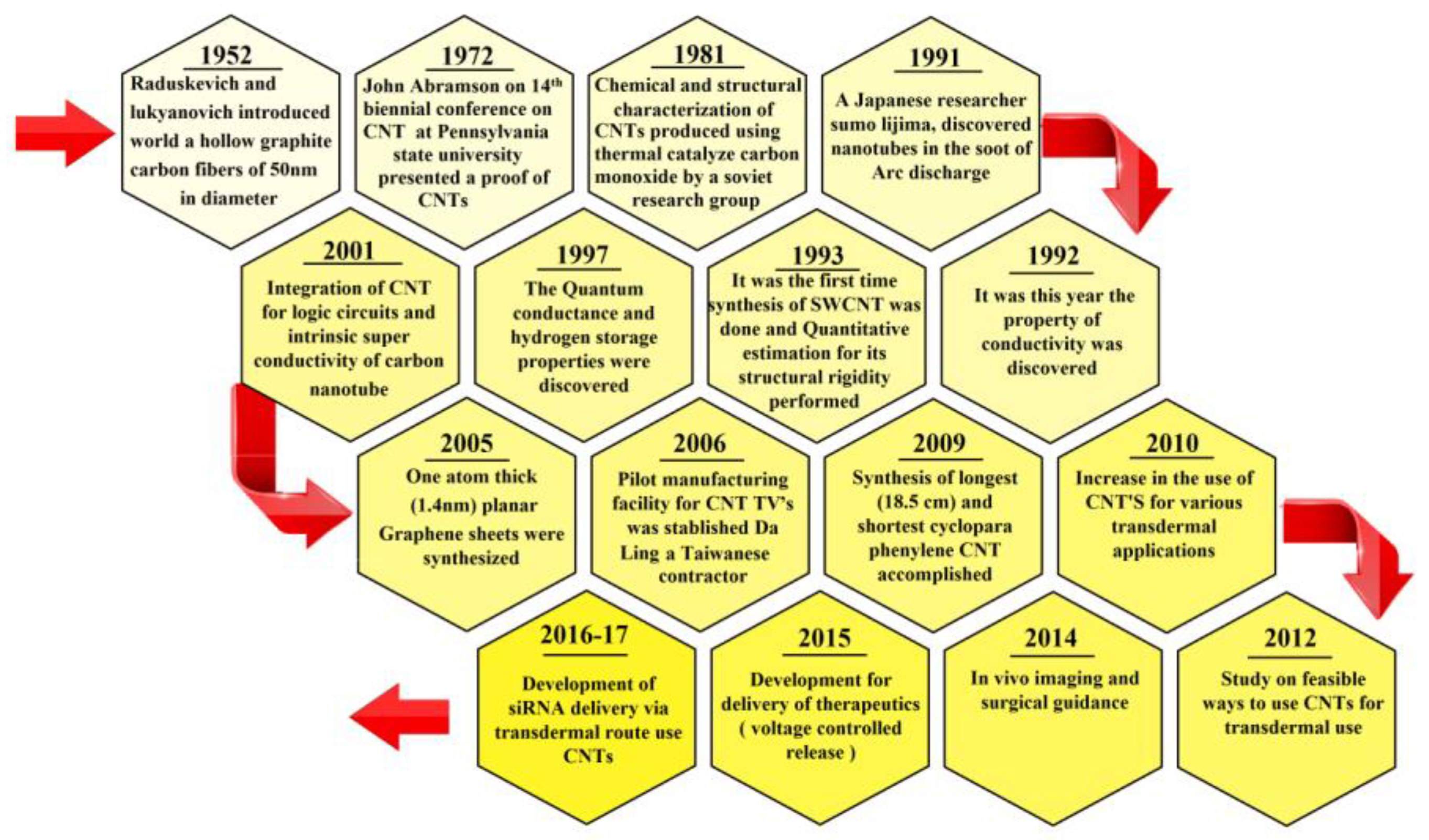
1. Introduction
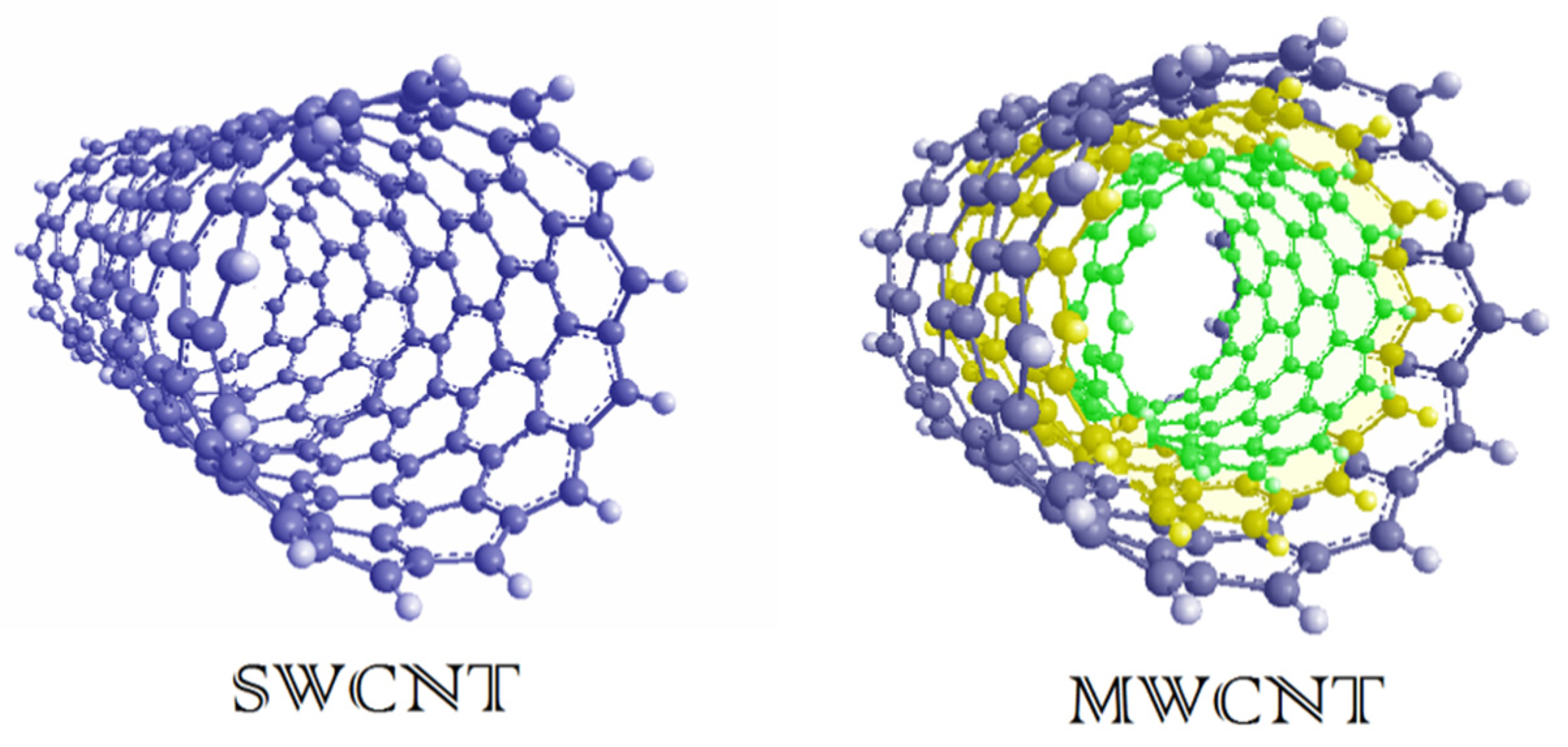
2. Carbon Nanotubes (CNTs)
3. Mg/CNT Nanocomposites
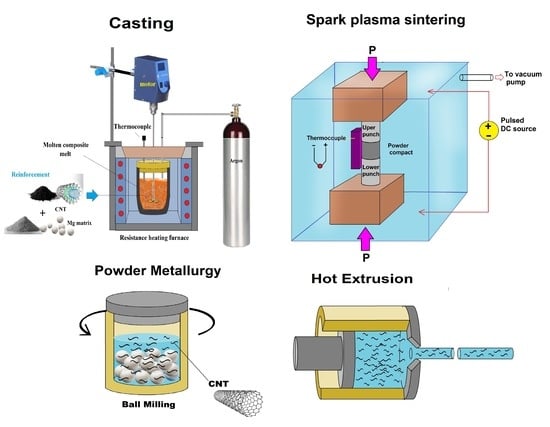
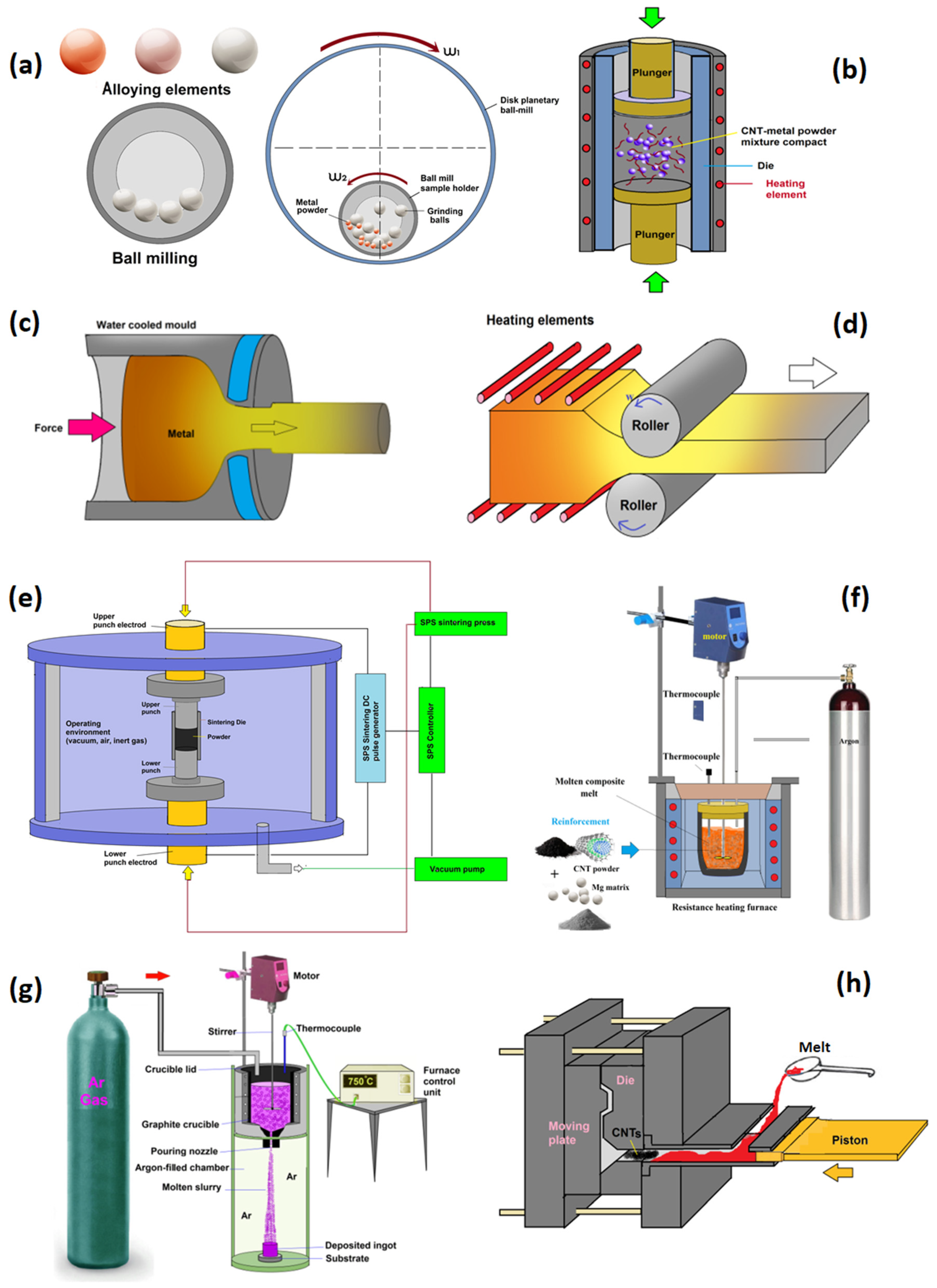
4. Fabrication of the Mg–CNTs Metal Matrix Nanocomposites (MMNCs)
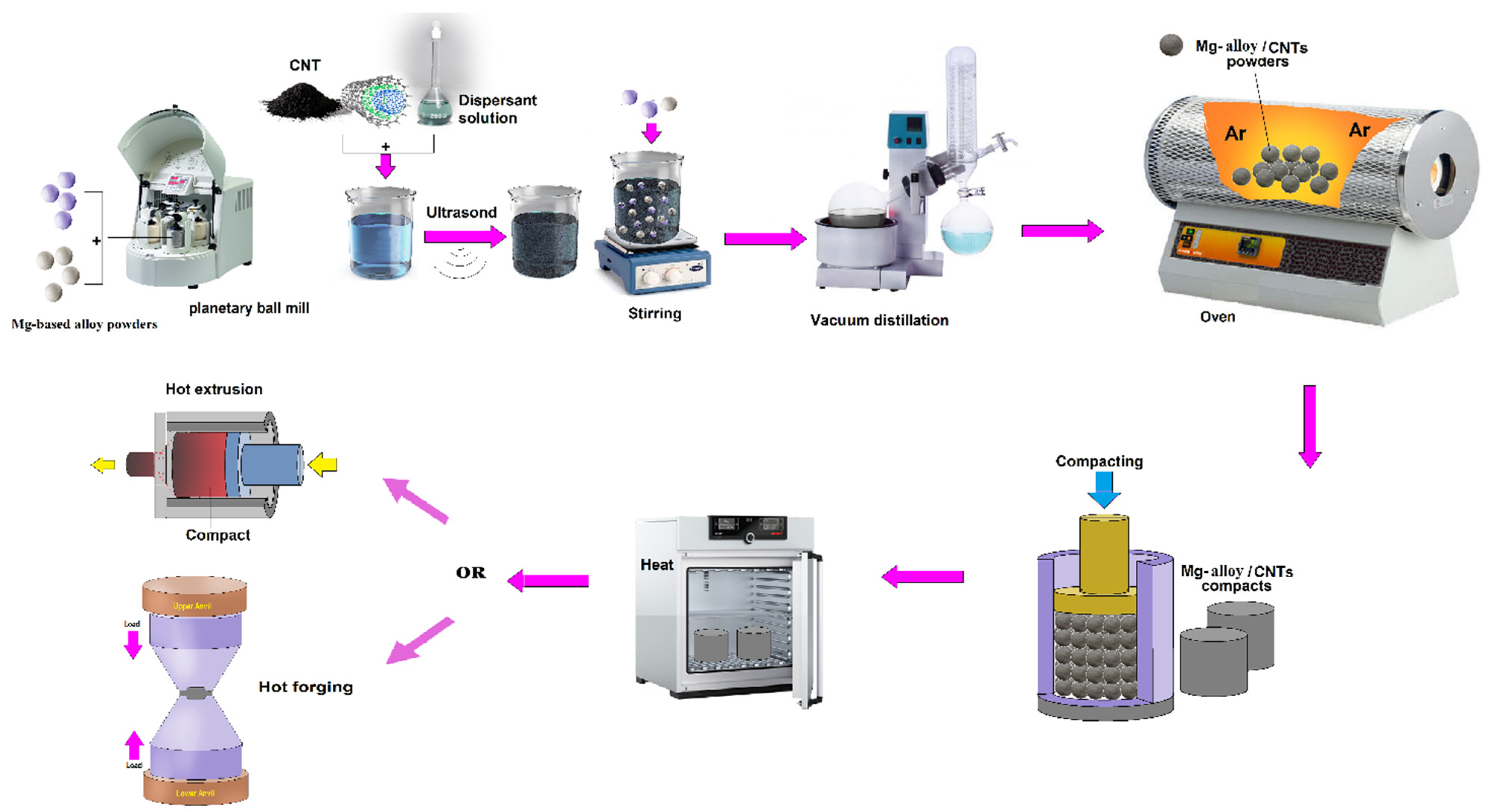
4.1. Powder Metallurgy (PM)
4.1.1. Ball Milling (BM) and Sintering
4.1.2. Ball Milling (BM) and Hot-Press Sintering (HPS)
4.1.3. Spark Plasma Sintering (SPS)
4.2. Semi-Powder Metallurgy (SPM)
4.2.1. Gemini Dispersant
4.2.2. Formation of Functional Groups at the CNTs’ Surface
4.3. Hot Extrusion
4.4. Melting and Solidification Technique
4.4.1. Stir Casting (SC)
4.4.2. Disintegrated Melt Deposition (DMD) Route
4.5. Friction Stir Processing (FSP)
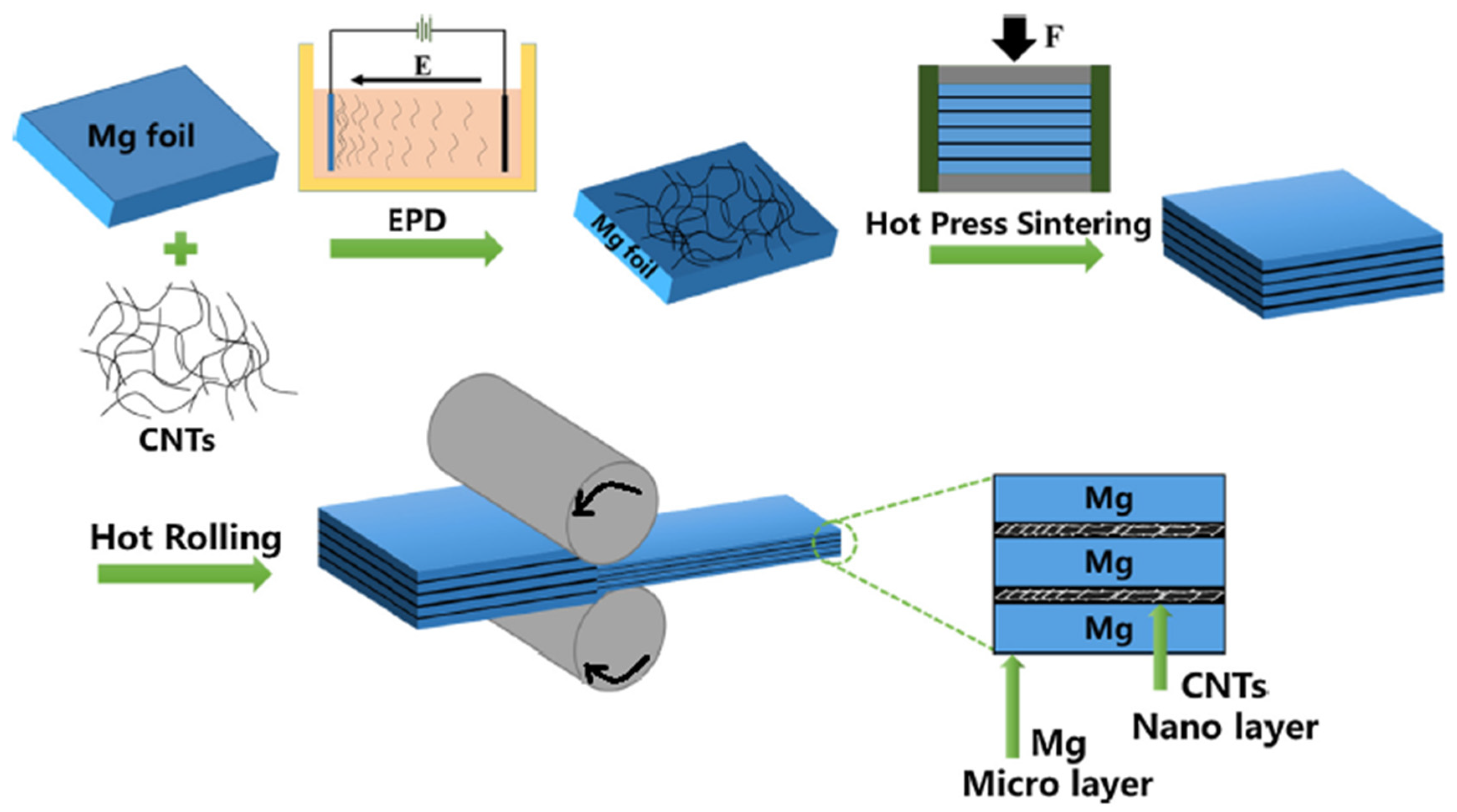
4.6. Spread Dispersion (SD)/Rolling Process (Micro-Nano Layered Structure)
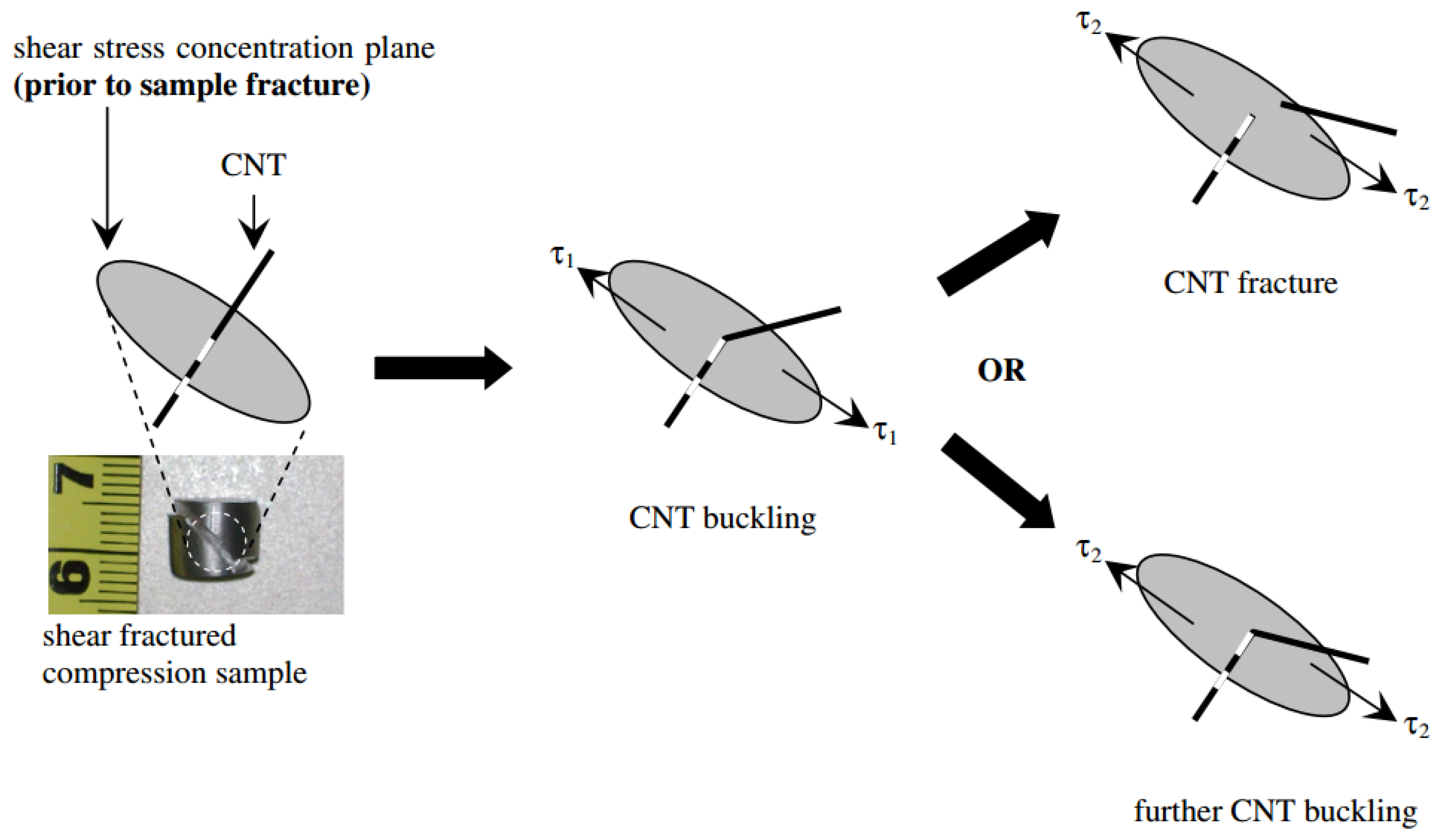
5. Strengthening Mechanisms and Mechanical Properties
| Sample(s) | Fabrication Method(s) | Young’s Modulus (GPa) | Tensile Properties | Compressive Properties | Hardness (HV) | Ref. | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0.2%TYS (MPa) | UTS (MPa) | Elongation (%) | CYS (MPa) | UCS (MPa) | Failure Strain (%) | |||||
| Pure Mg | BM + HPS | 136 | 163 | 72.1 | - | - | - | - | [71] | |
| Mg-(Ni-CNTs) | BM + HPS | 0.45 | 454 | 504 | 10.5 | 505 | - | - | - | [71] |
| Mg-1Al | SPM + VS + HTE | ET: 12.8 ± 0 EC: 5.0 ± 0.3 | 155 ± 3 | 202 ± 3 | 6.9 ± 0.5 | 100 ± 2 | 377 ± 8 | 18 ± 0.5 | 50 ± 4 | [28] |
| Mg-1Al-0.60GNPs | SPM + VS + HTE | ET: 17.2 ± 0.1 EC: 7.6 ± 0.5 | 204 ± 9 | 265 ± 8 | 4.0 ± 0.6 | 230 ± 5 | 407 ± 3 | 13 ± 0.3 | 63 ± 2 | [28] |
| Mg-1Al-0.60CNTs | SPM + VS + HTE | ET: 15.7 ± 0.3 EC: 6.7 ± 0.4 | 210 ± 5 | 287 ± 4 | 10 ± 0.3 | 237 ± 4 | 425 ± 5 | 12.6 ± 0.2 | 61 ± 5 | [28] |
| Mg-1Al-0.60 (1:5) (CNT+GNPs) | SPM + VS + HTE | ET: 15.0 ± 0.2 EC: 6.7 ± 0.2 | 185 ± 4 | 234 ± 3 | 16.4 ± 0.5 | 167 ± 6 | 397 ± 3 | 15 ± 0.4 | 56 ± 3 | [28] |
| Mg-6Zn | As-cast | - | 70 ± 3.3 | 129 ± 2.4 | 8.1 ± 2.1 | - | - | - | 55 ± 5.8 | [103] |
| Mg-6Zn | FSP | - | 134 ± 4.8 | 281 ± 4.3 | 18.9 ± 1.1 | - | - | - | 69 ± 3.8 | [103] |
| Mg-6Zn-1.0CNTs | MBM + SC + FSP | - | 171 ± 2.0 | 330 ± 5.5 | 15.2 ± 1.4 | - | - | - | 83 ± 7.2 | [103] |
| AZ31-0.1MWCNTs | SC + Aged | - | - | - | - | - | - | - | 47 | [35] |
| AZ31-0.5MWCNTs | SC + Aged | - | - | - | - | - | - | - | 50 | [35] |
| AZ31-1MWCNTs | SC + Aged | - | - | - | - | - | - | - | 52 | [35] |
| Mg-9Al | SPM + HTE | - | 235 ± 3 | 301 ±5 | 6 ± 2 | - | - | - | 80.6 ± 2.8 | [53] |
| Mg-9Al-0.2MWCNTs | SPM + HTE | - | 242 ± 3 | 346 ± 4 | 14 ± 1 | - | - | - | 91.8 ± 1.9 | [53] |
| Mg-9Al 0.4MWCNTs | SPM + HTE | - | 248 ± 5 | 355 ± 7 | 15 ± 3 | - | - | - | 94.2 ± 2.7 | [53] |
| Mg-9Al-0.6MWCNTs | SPM + HTE | - | 230 ± 2 | 329 ± 1 | 13 ± 1 | - | - | - | 89.6 ± 4.8 | [53] |
| AZ91 | SPM + HTE | - | 168 ± 5.0 | 215 ± 6.0 | 7.0 ± 0.2 | - | - | - | 72.4 ± 2.0 | [69] |
| AZ91-1CNT | SPM + HTE | - | 173 ± 4.0 | 228 ± 5.0 | 8.6 ± 0.1 | - | - | - | 79.2 ± 2.0 | [69] |
| AZ91-2CNT | SPM + HTE | - | 197 ± 4.5 | 263 ± 5.5 | 8.7 ± 0.2 | - | - | - | 87.1 ± 1.5 | [69] |
| AZ91-3CNT | SPM + HTE | - | 250 ± 3.8 | 301 ± 4.5 | 9.4 ± 0.1 | - | - | - | 94.1 ± 2.0 | [69] |
| AZ91-4CNT | SPM + HTE | - | 187 ± 3.5 | 248 ± 3.9 | 8.5 ± 0.1 | - | - | - | 84.3 ± 1.6 | [69] |
| AZ91-5CNT | SPM + HTE | - | 154 ± 4.4 | 228 ± 5.6 | 7.8 ± 0.2 | - | - | - | 80.2 ± 1.7 | [69] |
| AZ91-1(MgO-CNT) | SPM + HTE | - | 190 ± 3.6 | 260 ± 4.2 | 7.6 ± 0.1 | - | - | - | 80.2 ± 1.5 | [69] |
| AZ91-2(MgO-CNT) | SPM + HTE | - | 210 ± 5.0 | 294 ± 6.0 | 8.2 ± 0.2 | - | - | - | 89.5 ± 1.0 | [69] |
| AZ91-3(MgO-CNT) | SPM + HTE | - | 284 ± 4.6 | 331 ± 5.0 | 8.6 ± 0.1 | - | - | - | 96.4 ± 1.2 | [69] |
| AZ91-4(MgO-CNT) | SPM + HTE | - | 206 ± 3.7 | 272 ± 4.8 | 8.0 ± 0.1 | - | - | - | 86.5 ± 1.2 | [69] |
| AZ91-5(MgO-CNT) | SPM + HTE | - | 175 ± 5.5 | 255 ± 5.0 | 7.4 ± 0.2 | - | - | - | 83.6 ± 1.5 | [69] |
| AZ91D-2 CNT | SC | - | - | 290.4 | - | - | - | - | 80.23 | [99] |
| AZ91D- 3CNT | SC | - | - | 300.456 | - | - | - | - | 85.91 | [99] |
| AZ91D-4CNT | SC | - | - | 295.68 | - | - | - | - | 92.3 | [99] |
| Mg- 0.08CNTs | PM + SPS + HTE | - | 185 | 238 | 16.1 | - | - | - | - | [57] |
| AZ31-1CNTs | BM + Extrusion + Welding | - | 186 ± 5.6 | 272 ± 7.2 | 6 ± 1.5 | - | - | - | 67 ± 3.6 | [36] |
| AZ91D | SC | - | 202 | 264 | - | - | - | - | 71 | [98] |
| AZ91D-2CNT | SC | - | 216.14 | 289.23 | - | - | - | - | 79.49 | [98] |
| AZ91D-3CNT | SC | - | 228.26 | 296.47 | - | - | - | - | 83.78 | [98] |
| AZ91D-4CNT | SC | - | 222.20 | 293.35 | - | - | - | - | 90.88 | [98] |
| Mg-3Al-1Zn | SPM | 44.1 | 149 | 248 | 15.24 | - | - | - | 46.36 ± 3.6 | [126] |
| Mg-3Al-1Zn -0.5CNTs | SPM | 50.2 | 163 | 267 | 15.91 | - | - | - | 57.00 ± 4.0 | [126] |
| Mg-3Al-1Zn -1.0CNTs | SPM | 55.5 | 184 | 296 | 22.59 | - | - | - | 61.38 ± 2.2 | [126] |
| Mg-3Al-1Zn -1.5CNTs | SPM | 52.4 | 176 | 260 | 20.39 | - | - | - | 61.88 ± 3.3 | [126] |
| ZK60A | DMD | - | 163 ± 3 | 268 ± 3 | 6.6 ± 0.6 | 128 ± 11 | 522 ± 11 | 19.6 ± 0.9 | 138 ± 7 | [100] |
| ZK60A-1.0CNT | DMD | - | 180 ± 6 | 295 ± 8 | 15.0 ± 0.7 | 110 ± 7 | 547 ± 3 | 33.2 ± 6.2 | 114 ± 6 | [100] |
| Mg–6Zn-0.5 CNT | BM + Ultrasonic treatment + SC | - | 92 | 192 | - | - | - | - | - | [29] |
| AZ91-0.1 MWCNTs | SC | - | - | - | - | 161 ± 4.5 | 412 | 24.4 | - | [34] |
| AZ31 | PM + Extrusion | - | 195 ± 5.0 | 285 ± 2.9 | 14.5 ± 1.5 | 160 ± 6.0 | 363 ± 3.5 | 16.3 ± 1.5 | 58 ± 3.0 | [55] |
| AZ31-0.3GNP | PM + Extrusion | - | 173 ± 6.2 | 275 ± 5.7 | 21.7 ± 2.8 | 161 ± 4.5 | 397 ± 5.3 | 16.0 ± 1.8 | 71 ± 2.1 | [55] |
| AZ31-0.3 CNT | PM + Extrusion | - | 210 ± 2.8 | 310 ± 5.4 | 13.3 ± 3.0 | 242 ± 5.5 | 457 ± 6.0 | 14.0 ± 1.3 | 78 ± 2.8 | [55] |
| Mg-6Al-0.5CNT | MBM + CP + HTE | - | - | - | - | - | ~160 | - | ~40 | [92] |
| Mg-6Al-1CNT | MBM + CP + HTE | - | - | - | - | - | ~140 | - | ~36 | [92] |
| Mg-6Al-2CNT | MBM + CP + HTE | - | - | - | - | - | ~105 | - | ~34 | [92] |
| Mg-6Al-4CNT | MBM + CP + HTE | - | - | - | - | - | ~75 | - | ~28 | [92] |
| Mg-0.05CNT with 20% overall porosity | PM | - | 71.5 ± 19.5 | - | - | - | - | - | - | [72] |
| Mg-0.05CNT with 30% overall porosity | PM | - | 48 ± 18 | - | - | - | - | - | - | [72] |
| Mg-0.05CNT with 40% overall porosity | PM | - | 20 ± 8 | - | - | - | - | - | - | [72] |
| Mg-1CNT with 20% overall porosity | PM | - | 87.5 ± 25.5 | - | - | - | - | - | - | [72] |
| Mg-1CNT with 20% overall porosity | PM | - | 51.5 ± 19.5 | - | - | - | - | - | - | [72] |
| Mg-1CNT with 20% overall porosity | PM | - | 24.5 ± 10.5 | - | - | - | - | - | - | [72] |
| AZ81 | DMD + HTE | - | 225 | 336 | 7.9 | 157 ± 17 | 487 ± 14 | 17.0 ± 0.1 | 119± 2 | [61] |
| AZ81-1.5CNTs | DMD + HTE | - | 280 | 392 | 12.9 | 129 ± 19 | 488 ± 13 | 16.0 ± 1.8 | 114 ± 8 | [61] |
| Mg-0.05CNTs | EPD + HPS + HR | - | 115 ± 4.0 | 153 ± 4.5 | 4.6 ± 0.9 | - | - | - | - | [73] |
| Mg-0.10 CNTs | EPD + HPS + HR | - | 143 ± 7.8 | 172 ± 2.6 | 5.5 ± 0.9 | - | - | - | - | [73] |
| Mg (98.5% Purity) | MBM + CP + HTE | - | 127 ± 5 | 205 ± 4 9 | 9 ± 2 | - | - | - | 45 ± 0 | [30] |
| Mg-0.06 CNTs | MBM + CP + HTE | - | 133 ± 2 | 203 ± 1 | 12 ± 1 | - | - | - | 44 ± 0 | [30] |
| Mg-0.18 CNTs | MBM + CP + HTE | - | 138 ± 4 | 206 ± 7 | 11 ± 1 | - | - | - | 44 ± 1 | [30] |
| Mg-0.30 CNTs | MBM + CP + HTE | - | 146 ± 5 | 210 ± 6 | 8 ± 1 | - | - | - | 44 ± 0 | [30] |
| Mg-2 wt.% CNTs | BM + HPS | 38.6 ± 0.7 | 89 | 140 | 3 | - | - | - | - | [75] |
| Mg (98.5% Purity) | BM + MS + HTE | - | 126 ± 1 | 171 ± 2 | 7.9 ± 0.3 | - | - | - | 39 ± 3 | [104] |
| Mg-0.3CNTs | BM + MS + HTE | - | 119 ± 4 | 163 ± 7 | 5.7 ± 0.2 | - | - | - | 36 ± 1 | [104] |
| Mg-0.3 (Ni-CNTs) | BM + MS + HTE | - | 206 ± 2 | 237 ± 1 | 6.4 ± 0.3 | - | - | - | 55 ± 3 | [104] |
| AZ91 | SI | 5 ± 2 | 80 ± 5 | 205 ± 5 | - | - | - | - | 80 | [31] |
| AZ91-5MWCNTs | SI | 1.0 ± 2 | 210 ± 10 | 243 ± 10 | - | - | - | - | 150 | [31] |
| AZ91-5 (Si-MWCNTs) | SI | 1.3 ± 2 | 253 ± 10 | 296 ± 10 | - | - | - | - | 160 | [31] |
| Mg | PM + HTE | - | - | - | - | 106 ± 11 | 239 ± 15 | 19.8 ± 1.7 | 40 ± 2 | [70] |
| Mg-0.5Al-0.18CNT | PM + HTE | - | - | - | - | 120 ± 09 | 357 ± 13 | 11.0 ± 1.3 | 50 ± 4 | [70] |
| Mg-1Al-0.18CNT | PM + HTE | - | - | - | - | 132 ± 04 | 421 ± 15 | 12.5 ± 1.0 | 58 ± 3 | [70] |
| Mg-1.50Al-0.18CNT | PM + HTE | - | - | - | - | 144 ± 07 | 421 ± 11 | 11.3 ± 1.7 | 60 ± 4 | [70] |
6. Corrosion Properties
| Samples | Reinforcement | Processing Route | Reinforcement Particle Size | Corrosion Medium | icorr (μA·cm−2) | Ecorr (V vs. SCE) | Corrosion Rate (mm/year) | Rp (Ω·cm2) | Ref. | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Non Polarized | Polarized | ||||||||||
| Immersion Time (h) | HE or WL | PDP | |||||||||
| AZ31 (after 1, 24 and 336 h immersion time) | - | DMD | - | SBF | 94.70 | –1.545 | 1 | 2.176 mm/year | - | - | [144] |
| - | 44.22 | –1.441 | 24 | 1.016 mm/year | - | - | |||||
| - | 213.45 | –1.402 | 336 | 4.906 mm/year | - | - | |||||
| AZ31 (after 1, 24 and 336 h immersion time) | CNT (1wt.%) | - | 87. 62 | –1.502 | 1 | 2.013 mm/year | - | - | |||
| - | 17.42 | –1.408 | 24 | 0.400 mm/year | - | - | |||||
| - | 9.33 | –1.373 | 336 | 0.214 mm/year | - | - | |||||
| Mg–6Al | CNT (4 wt.%) | MBM + CP + HTE | average diameter: 9.5 nm average length: 1.5 μm | 3.5% NaCl | 5 | –1.55 | - | - | - | - | [92] |
| AZ91 | MWCNTs (1 wt.%) | MS | - | 3.5% NaCl | - | – | - | 5–9 gm−2day−1 | - | - | [132] |
| MWCNTs (5 wt.%) | - | - | – | - | 19–24 gm−2day−1 | - | - | [132] | |||
| Mg | MWCNTs (0.1 wt.%)-Dispersed during melt stirring process (0 h) | MS | - | 3.5% NaCl | 98 | –1.618 | - | 4.5 mm/year | 368 | [131] | |
| MWCNTs (0.1 wt.%)- Dispersed during melt stirring process (6 h) | - | 280 | –1.520 | - | 12.8 mm/year | 332 | |||||
| AZ91 | GNPs (0.5 wt.%) | SPM | diameter: 5 and 8 nm surface area: 750 m2/g | 3.5 wt.% NaCl | 326.902 μA | –1.449 | - | - | 4.13 mm/year | - | [145] |
| MWCNT (0.5 wt.%) | diameter: 8 nm surface area: 250 m2/g | 388.431 μA | –1.491 | - | - | 4.92 mm/year | - | ||||
| C60 (0.5 wt.%) | average thickness: 1–2 nm | 212.137 μA | –1.506 | - | - | 2.68 mm/year | - | ||||
| Mg | CNT (0.3wt.%) | DMD | average diameter: 20 nm and length: less than 100 μm | 3.5 wt.% NaCl | 56 | –1.57 | - | - | - | - | [63] |
| CNT (1.3wt.%) | 572 | –1.50 | - | - | - | - | |||||
| Mg | MWCNTs (0.5 wt.%) | SPM | - | 3.5 wt.% NaCl | 579.4 μA | –1.544 | - | - | 24.62 mm/year | - | [89] |
| Mg | MWCNTs (0.5 wt.%) | SPM + PEO | - | 130.4 μA | –1.401 | - | - | 14.78 mm/year | - | ||
| Mg-0.5 MWCNT | GNP | SPM + PEO (coating with graphene addition) | - | 101.0 μA | –1.424 | - | - | 14.46 mm/year | - | ||
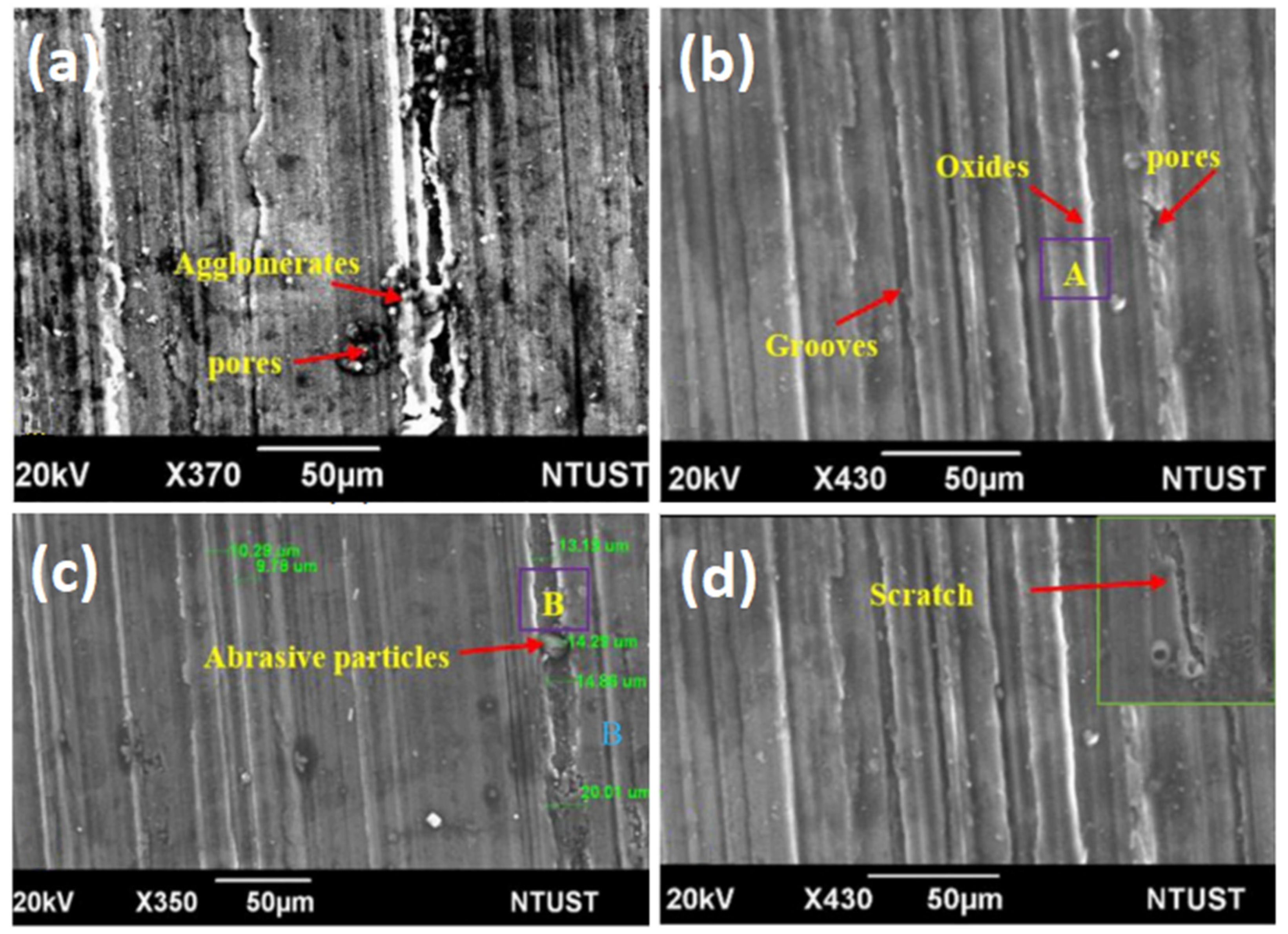
7. Wear and Friction Properties
8. Summary and Future Road Maps
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| Abbreviations | Abbreviations | ||
| BM | Ball milling | Icorr | Corrosion current density |
| CIP | Cold isostatic pressing | MBM | Mechanical ball milling |
| CNT | Carbon nanotube | MMCs | Metal matrix composite |
| COF | Coefficient of friction | MMNCs | Metal matrix nanocomposites |
| CP | Cold pressing | MS | Microwave sintering |
| CTE | Coefficient of thermal expansion | MWCNT | Multi-walled carbon nanotube |
| CVD | Chemical vapor deposition | PDP | Potentiodynamic polarization |
| CYS | Compressive yield strength | PEO | Plasma electrolytic oxidation |
| DMD | Disintegrated melt deposition | PM | Powder metallurgy |
| EC | Elastic modulus in compressive | Rp | Polarization resistance |
| ECAP | Equi-channel angular processing | SC | Stir Casting |
| Ecorr | Corrosion potentials | SHE | Standard hydrogen electrode |
| EPD | Electrophoretic deposition | SI | Squeeze casting infiltration |
| ET | Elastic modulus in tensile | SPM | Semi powder metallurgy |
| FSP | Friction stir processing | SPS | Spark plasma sintering |
| FSW | Friction stir welding | SWCNT | Single-walled carbon nanotube |
| GNP | Graphene nanoplatelet | TYS | Tensile yield strength |
| HCP | Hexagonal close-packed | UCS | Ultimate compressive strength |
| HE | Hydrogen evolution | UTS | Ultimate tensile strength |
| HEBM | High-energy ball milling | VS | Vacuum sintering |
| HIP | Hot isostatic pressing | WL | Weight loss |
| HPS | Hot-press sintering | YS | Yield strength |
| HR | Hot rolling | ||
| HTE | Hot extrusion | ||
References
- Ali, Y.; Qiu, D.; Jiang, B.; Pan, F.; Zhang, M.-X. Current research progress in grain refinement of cast magnesium alloys: A review article. J. Alloys Compd. 2015, 619, 639–651. [Google Scholar] [CrossRef]
- Saberi, A.; Bakhsheshi-Rad, H.R.; Karamian, E.; Kasiri-Asgarani, M.; Ghomi, H. Magnesium-graphene nano-platelet composites: Corrosion behavior, mechanical and biological properties. J. Alloys Compd. 2020, 821, 153379. [Google Scholar] [CrossRef]
- Chai, F.; Zhang, D.; Zhang, W.; Li, Y. Microstructure evolution during high strain rate tensile deformation of a fine-grained AZ91 magnesium alloy. Mater. Sci. Eng. A 2014, 590, 80–87. [Google Scholar] [CrossRef]
- Pollock, T.M. Weight loss with magnesium alloys. Science 2010, 328, 986–987. [Google Scholar] [CrossRef] [PubMed]
- Barnett, M.R.; Ghaderi, A.; Da Fonseca, J.Q.; Robson, J.D. Influence of orientation on twin nucleation and growth at low strains in a magnesium alloy. Acta Mater. 2014, 80, 380–391. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, C.-G.; Wang, H.-Y.; Nan, X.-L.; Liu, G.-J.; Jiang, Q.-C. Slip-induced texture evolution of rolled Mg–6Al–3Sn alloy during uniaxial tension along rolling and transverse directions. Mater. Sci. Eng. A 2014, 597, 376–380. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, K.; Wang, Z.; Li, S.; Du, W. Microstructure, texture and mechanical properties of as-extruded Mg–Zn–Er alloys containing W-phase. J. Alloys Compd. 2014, 602, 32–39. [Google Scholar] [CrossRef]
- Allen, M.J.; Tung, V.C.; Kaner, R.B. Honeycomb Carbon: A Review of Graphene. Chem. Rev. 2010, 110, 132–145. [Google Scholar] [CrossRef]
- Artiles, M.S.; Rout, C.S.; Fisher, T.S. Graphene-based hybrid materials and devices for biosensing. Adv. Drug Deliv. Rev. 2011, 63, 1352–1360. [Google Scholar] [CrossRef]
- Thostenson, E.T.; Ren, Z.; Chou, T.-W. Advances in the science and technology of carbon nanotubes and their composites: A review. Compos. Sci. Technol. 2001, 61, 1899–1912. [Google Scholar] [CrossRef]
- Tjong, S.C. Recent progress in the development and properties of novel metal matrix nanocomposites reinforced with carbon nanotubes and graphene nanosheets. Mater. Sci. Eng. R Rep. 2013, 74, 281–350. [Google Scholar] [CrossRef]
- Shahin, M.; Munir, K.; Wen, C.; Li, Y. Magnesium matrix nanocomposites for orthopedic applications: A review from mechanical, corrosion, and biological perspectives. Acta Biomater. 2019, 96, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Manchado, M.A.L.; Valentini, L.; Biagiotti, J.; Kenny, J.M. Thermal and mechanical properties of single-walled carbon nanotubes–polypropylene composites prepared by melt processing. Carbon 2005, 43, 1499–1505. [Google Scholar] [CrossRef]
- Bakshi, S.R.; Lahiri, D.; Agarwal, A. Carbon nanotube reinforced metal matrix composites—A review. Int. Mater. Rev. 2010, 55, 41–64. [Google Scholar] [CrossRef]
- Radhamani, A.V.; Lau, H.C.; Ramakrishna, S. CNT-reinforced metal and steel nanocomposites: A comprehensive assessment of progress and future directions. Compos. Part A Appl. Sci. Manuf. 2018, 114, 170–187. [Google Scholar] [CrossRef]
- De Vita, A.; Charlier, J.-C.; Blase, X.; Car, R. Electronic structure at carbon nanotube tips. Appl. Phys. A 1999, 68, 283–286. [Google Scholar] [CrossRef]
- Popov, V.N. Carbon nanotubes: Properties and application. Mater. Sci. Eng. R Rep. 2004, 43, 61–102. [Google Scholar] [CrossRef]
- Fan, W.; Zhang, L.; Liu, T. Graphene-Carbon Nanotube Hybrids for Energy and Environmental Applications; Springer: Berlin, Germany, 2017; ISBN 9811028036. [Google Scholar]
- Kuche, K.; Maheshwari, R.; Tambe, V.; Mak, K.-K.; Jogi, H.; Raval, N.; Pichika, M.R.; Kumar Tekade, R. Carbon nanotubes (CNTs) based advanced dermal therapeutics: Current trends and future potential. Nanoscale 2018, 10, 8911–8937. [Google Scholar] [CrossRef]
- Zhao, X.; Song, B.; Fan, W.; Zhang, Y.; Shi, Y. Selective laser melting of carbon/AlSi10Mg composites: Microstructure, mechanical and electronical properties. J. Alloys Compd. 2016, 665, 271–281. [Google Scholar] [CrossRef]
- Huang, Y.; Ouyang, Q.; Zhang, D.; Zhu, J.; Li, R.; Yu, H. Carbon materials reinforced aluminum composites: A review. Acta Metall. Sin. Engl. Lett. 2014, 27, 775–786. [Google Scholar] [CrossRef]
- Singh, A.; Prabhu, T.R.; Sanjay, A.R.; Koti, V. An overview of processing and properties of Cu/CNT nano composites. Mater. Today Proc. 2017, 4, 3872–3881. [Google Scholar] [CrossRef]
- Mittal, G.; Dhand, V.; Rhee, K.Y.; Park, S.-J.; Lee, W.R. A review on carbon nanotubes and graphene as fillers in reinforced polymer nanocomposites. J. Ind. Eng. Chem. 2015, 21, 11–25. [Google Scholar] [CrossRef]
- Cha, S.I.; Kim, K.T.; Arshad, S.N.; Mo, C.B.; Hong, S.H. Extraordinary strengthening effect of carbon nanotubes in metal-matrix nanocomposites processed by molecular-level mixing. Adv. Mater. 2005, 17, 1377–1381. [Google Scholar] [CrossRef]
- Prusty, R.K.; Rathore, D.K.; Ray, B.C. CNT/polymer interface in polymeric composites and its sensitivity study at different environments. Adv. Colloid Interface Sci. 2017, 240, 77–106. [Google Scholar] [CrossRef]
- Neubauer, E.; Kitzmantel, M.; Hulman, M.; Angerer, P. Potential and challenges of metal-matrix-composites reinforced with carbon nanofibers and carbon nanotubes. Compos. Sci. Technol. 2010, 70, 2228–2236. [Google Scholar] [CrossRef]
- Dorri Moghadam, A.; Omrani, E.; Menezes, P.L.; Rohatgi, P.K. Mechanical and tribological properties of self-lubricating metal matrix nanocomposites reinforced by carbon nanotubes (CNTs) and graphene—A review. Compos. Part B Eng. 2015, 77, 402–420. [Google Scholar] [CrossRef]
- Rashad, M.; Pan, F.; Tang, A.; Asif, M.; Aamir, M. Synergetic effect of graphene nanoplatelets (GNPs) and multi-walled carbon nanotube (MW-CNTs) on mechanical properties of pure magnesium. J. Alloys Compd. 2014, 603, 111–118. [Google Scholar] [CrossRef]
- Shi, H.; Wang, X.; Li, C.; Hu, X.; Ding, C.; Wu, K.; Huang, Y. A novel method to fabricate cNT/Mg–6Zn composites with high strengthening efficiency. Acta Metall. Sin. Engl. Lett. 2014, 27, 909–917. [Google Scholar] [CrossRef]
- Goh, C.S.; Wei, J.; Lee, L.C.; Gupta, M. Development of novel carbon nanotube reinforced magnesium nanocomposites using the powder metallurgy technique. Nanotechnology 2005, 17, 7. [Google Scholar] [CrossRef]
- Park, Y.; Cho, K.; Park, I.; Park, Y. Fabrication and mechanical properties of magnesium matrix composite reinforced with Si coated carbon nanotubes. Proc. Eng. 2011, 10, 1446–1450. [Google Scholar] [CrossRef]
- Morisada, Y.; Fujii, H.; Nagaoka, T.; Fukusumi, M. MWCNTs/AZ31 surface composites fabricated by friction stir processing. Mater. Sci. Eng. A 2006, 419, 344–348. [Google Scholar] [CrossRef]
- Dorri Moghadam, A.; Schultz, B.F.; Ferguson, J.B.; Omrani, E.; Rohatgi, P.K.; Gupta, N. Functional Metal Matrix Composites: Self-lubricating, Self-healing, and Nanocomposites-An Outlook. JOM 2014, 66, 872–881. [Google Scholar] [CrossRef]
- Li, Q.; Viereckl, A.; Rottmair, C.A.; Singer, R.F. Improved processing of carbon nanotube/magnesium alloy composites. Compos. Sci. Technol. 2009, 69, 1193–1199. [Google Scholar] [CrossRef]
- Abbas, A.; Huang, S.J.; Ballóková, B.; Sülleiová, K. Tribological effects of carbon nanotubes on magnesium alloy AZ31 and analyzing aging effects on CNTs/AZ31 composites fabricated by stir casting process. Tribol. Int. 2020, 142, 105982. [Google Scholar] [CrossRef]
- Sabetghadam-Isfahani, A.; Abbasi, M.; Sharifi, S.M.H.; Fattahi, M.; Amirkhanlou, S.; Fattahi, Y. Microstructure and mechanical properties of carbon nanotubes/AZ31 magnesium composite gas tungsten arc welding filler rods fabricated by powder metallurgy. Diam. Relat. Mater. 2016, 69, 160–165. [Google Scholar] [CrossRef]
- Ferguson, J.B.; Sheykh-Jaberi, F.; Kim, C.-S.; Rohatgi, P.K.; Cho, K. On the strength and strain to failure in particle-reinforced magnesium metal-matrix nanocomposites (Mg MMNCs). Mater. Sci. Eng. A 2012, 558, 193–204. [Google Scholar] [CrossRef]
- Nanostructured & Amorphous Materials, Inc. (NanoAmor). Available online: https://nanoamor.com/ (accessed on 4 October 2020).
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Hashimoto, S.; Iwamoto, T.; Kurachi, D.; Kayahara, E.; Yamago, S. Shortest Double-Walled Carbon Nanotubes Composed of Cycloparaphenylenes. Chempluschem 2017, 82, 1015–1020. [Google Scholar] [CrossRef]
- Iijima, S.; Ichihashi, T. Single-shell carbon nanotubes of 1-nm diameter. Nature 1993, 363, 603–605. [Google Scholar] [CrossRef]
- Liu, Z.; Tabakman, S.; Welsher, K.; Dai, H. Carbon nanotubes in biology and medicine: In vitro and in vivo detection, imaging and drug delivery. Nano Res. 2009, 2, 85–120. [Google Scholar] [CrossRef]
- Guo, J.; Zhang, Q.; Gao, L.; Zhong, W.; Sui, G.; Yang, X. Significantly improved electrical and interlaminar mechanical properties of carbon fiber laminated composites by using special carbon nanotube pre-dispersion mixture. Compos. Part A Appl. Sci. Manuf. 2017, 95, 294–303. [Google Scholar] [CrossRef]
- Hu, D.; Xing, Y.; Chen, M.; Gu, B.; Sun, B.; Li, Q. Ultrastrong and excellent dynamic mechanical properties of carbon nanotube composites. Compos. Sci. Technol. 2017, 141, 137–144. [Google Scholar] [CrossRef]
- Kumar, S.; Rani, R.; Dilbaghi, N.; Tankeshwar, K.; Kim, K.-H. Carbon nanotubes: A novel material for multifaceted applications in human healthcare. Chem. Soc. Rev. 2017, 46, 158–196. [Google Scholar] [CrossRef]
- O’connell, M.J. Carbon Nanotubes: Properties and Applications; CRC Press: Boca Raton, FL, USA, 2006; ISBN 1420004212. [Google Scholar]
- Dong, Z.; Jiang, C.; Cheng, H.; Zhao, Y.; Shi, G.; Jiang, L.; Qu, L. Facile fabrication of light, flexible and multifunctional graphene fibers. Adv. Mater. 2012, 24, 1856–1861. [Google Scholar] [CrossRef] [PubMed]
- Ruoff, R.S.; Qian, D.; Liu, W.K. Mechanical properties of carbon nanotubes: Theoretical predictions and experimental measurements. Comptes Rendus Phys. 2003, 4, 993–1008. [Google Scholar] [CrossRef]
- Yu, Y.P. Preparation, Purification and Properties of Carbon Nanotubes. Adv. Mater. Res. 2012, 557, 472–477. [Google Scholar] [CrossRef]
- Terrones, M. Carbon nanotubes: Synthesis and properties, electronic devices and other emerging applications. Int. Mater. Rev. 2004, 49, 325–377. [Google Scholar] [CrossRef]
- Yang, S.; Yu, S.; Cho, M. Influence of Thrower–Stone–Wales defects on the interfacial properties of carbon nanotube/polypropylene composites by a molecular dynamics approach. Carbon 2013, 55, 133–143. [Google Scholar] [CrossRef]
- Ying, L.S.; bin Mohd Salleh, M.A.; Rashid, S.B.A. Continuous production of carbon nanotubes—A review. J. Ind. Eng. Chem. 2011, 17, 367–376. [Google Scholar] [CrossRef]
- Hou, J.; Du, W.; Parande, G.; Gupta, M.; Li, S. Significantly enhancing the strength + ductility combination of Mg-9Al alloy using multi-walled carbon nanotubes. J. Alloys Compd. 2019, 790, 974–982. [Google Scholar] [CrossRef]
- Wang, Q.; Du, W.; Liu, K.; Wang, Z.; Li, S.; Wen, K. Microstructure, texture and mechanical properties of as-extruded Mg–Zn–Er alloys. Mater. Sci. Eng. A 2013, 581, 31–38. [Google Scholar] [CrossRef]
- Rashad, M.; Pan, F.; Zhang, J.; Asif, M. Use of high energy ball milling to study the role of graphene nanoplatelets and carbon nanotubes reinforced magnesium alloy. J. Alloys Compd. 2015, 646, 223–232. [Google Scholar] [CrossRef]
- Rashad, M.; Pan, F.; Asif, M. Exploring mechanical behavior of Mg–6Zn alloy reinforced with graphene nanoplatelets. Mater. Sci. Eng. A 2016, 649, 263–269. [Google Scholar] [CrossRef]
- Han, G.Q.; Shen, J.H.; Ye, X.X.; Chen, B.; Imai, H.; Kondoh, K.; Du, W.B. The influence of CNTs on the microstructure and ductility of CNT/Mg composites. Mater. Lett. 2016, 181, 300–304. [Google Scholar] [CrossRef]
- Bakshi, S.R.; Agarwal, A. An analysis of the factors affecting strengthening in carbon nanotube reinforced aluminum composites. Carbon 2011, 49, 533–544. [Google Scholar] [CrossRef]
- Fukuda, H.; Kondoh, K.; Umeda, J.; Fugetsu, B. Interfacial analysis between Mg matrix and carbon nanotubes in Mg–6 wt. % Al alloy matrix composites reinforced with carbon nanotubes. Compos. Sci. Technol. 2011, 71, 705–709. [Google Scholar] [CrossRef]
- Han, G.; Wang, Z.; Liu, K.; Li, S.; Du, X.; Du, W. Synthesis of CNT-reinforced AZ31 magnesium alloy composites with uniformly distributed CNTs. Mater. Sci. Eng. A 2015, 628, 350–357. [Google Scholar] [CrossRef]
- Paramsothy, M.; Tan, X.H.; Chan, J.; Kwok, R.; Gupta, M. Carbon nanotube addition to concentrated magnesium alloy AZ81: Enhanced ductility with occasional significant increase in strength. Mater. Des. 2013, 45, 15–23. [Google Scholar] [CrossRef]
- Ghali, E.; Dietzel, W.; Kainer, K.-U. General and localized corrosion of magnesium alloys: A critical review. J. Mater. Eng. Perform. 2004, 13, 7–23. [Google Scholar] [CrossRef]
- Aung, N.N.; Zhou, W.; Goh, C.S.; Nai, S.M.L.; Wei, J. Effect of carbon nanotubes on corrosion of Mg–CNT composites. Corros. Sci. 2010, 52, 1551–1553. [Google Scholar] [CrossRef]
- Fukuda, H.; Szpunar, J.A.; Kondoh, K.; Chromik, R. The influence of carbon nanotubes on the corrosion behaviour of AZ31B magnesium alloy. Corros. Sci. 2010, 52, 3917–3923. [Google Scholar] [CrossRef]
- Stansbury, E.E.; Buchanan, R.A. Fundamentals of Electrochemical Corrosion; ASM International: Novelty, OH, USA, 2000; ISBN 1615030670. [Google Scholar]
- Li, C.D.; Wang, X.J.; Liu, W.Q.; Shi, H.L.; Ding, C.; Hu, X.S.; Zheng, M.Y.; Wu, K. Effect of solidification on microstructures and mechanical properties of carbon nanotubes reinforced magnesium matrix composite. Mater. Des. 2014, 58, 204–208. [Google Scholar] [CrossRef]
- Liang, J.; Li, H.; Qi, L.; Tian, W.; Li, X.; Zhou, J.; Wang, D.; Wei, J. Influence of Ni-CNTs additions on the microstructure and mechanical properties of extruded Mg-9Al alloy. Mater. Sci. Eng. A 2016, 678, 101–109. [Google Scholar] [CrossRef]
- Honma, T.; Nagai, K.; Katou, A.; Arai, K.; Suganuma, M.; Kamado, S. Synthesis of high-strength magnesium alloy composites reinforced with Si-coated carbon nanofibres. Scr. Mater. 2009, 60, 451–454. [Google Scholar] [CrossRef]
- Yuan, Q.; Zeng, X.; Liu, Y.; Luo, L.; Wu, J.; Wang, Y.; Zhou, G. Microstructure and mechanical properties of AZ91 alloy reinforced by carbon nanotubes coated with MgO. Carbon 2016, 96, 843–855. [Google Scholar] [CrossRef]
- Habibi, M.K.; Paramsothy, M.; Hamouda, A.M.S.; Gupta, M. Enhanced compressive response of hybrid Mg–CNT nano-composites. J. Mater. Sci. 2011, 46, 4588–4597. [Google Scholar] [CrossRef]
- Ding, Y.; Xu, J.; Hu, J.; Gao, Q.; Guo, X.; Zhang, R.; An, L. High performance carbon nanotube-reinforced magnesium nanocomposite. Mater. Sci. Eng. A 2020, 771, 138575. [Google Scholar] [CrossRef]
- Zou, N.; Li, Q. Compressive mechanical property of porous magnesium composites reinforced by carbon nanotubes. J. Mater. Sci. 2016, 51, 5232–5239. [Google Scholar] [CrossRef]
- Xiang, Y.; Wang, X.; Hu, X.; Meng, L.; Song, Z.; Li, X.; Sun, Z.; Zhang, Q.; Wu, K. Achieving ultra-high strengthening and toughening efficiency in carbon nanotubes/magnesium composites via constructing micro-nano layered structure. Compos. Part A Appl. Sci. Manuf. 2019, 119, 225–234. [Google Scholar] [CrossRef]
- Li, Q.; Tian, B. Compression behavior of magnesium/carbon nanotube composites. J. Mater. Res. 2013, 28, 1877–1884. [Google Scholar] [CrossRef]
- Carreño-Morelli, E.; Yang, J.; Couteau, E.; Hernadi, K.; Seo, J.W.; Bonjour, C.; Forro, L.; Schaller, R. Carbon nanotube/magnesium composites. Phys. Status Solidi 2004, 201, R53–R55. [Google Scholar] [CrossRef]
- Endo, M.; Hayashi, T.; Itoh, I.; Kim, Y.A.; Shimamoto, D.; Muramatsu, H.; Shimizu, Y.; Morimoto, S.; Terrones, M.; Iinou, S. An anticorrosive magnesium/carbon nanotube composite. Appl. Phys. Lett. 2008, 92, 63105. [Google Scholar] [CrossRef]
- Fukuda, H.; Kondoh, K.; Umeda, J.; Fugetsu, B. Fabrication of magnesium based composites reinforced with carbon nanotubes having superior mechanical properties. Mater. Chem. Phys. 2011, 127, 451–458. [Google Scholar] [CrossRef]
- Hulbert, D.M.; Anders, A.; Andersson, J.; Lavernia, E.J.; Mukherjee, A.K. A discussion on the absence of plasma in spark plasma sintering. Scr. Mater. 2009, 60, 835–838. [Google Scholar] [CrossRef]
- Rashad, M.; Pan, F.; Asif, M.; Tang, A. Powder metallurgy of Mg–1%Al–1%Sn alloy reinforced with low content of graphene nanoplatelets (GNPs). J. Ind. Eng. Chem. 2014, 20, 4250–4255. [Google Scholar] [CrossRef]
- Kondoh, K.; Fukuda, H.; Umeda, J.; Imai, H.; Fugetsu, B.; Endo, M. Microstructural and mechanical analysis of carbon nanotube reinforced magnesium alloy powder composites. Mater. Sci. Eng. A 2010, 527, 4103–4108. [Google Scholar] [CrossRef]
- Suslick, K.S.; Hammerton, D.A.; Cline, R.E. Sonochemical hot spot. J. Am. Chem. Soc. 1986, 108, 5641–5642. [Google Scholar] [CrossRef]
- Matsuda, T.; Minami, D.; Khoerunnisa, F.; Sunaga, M.; Nakamura, M.; Utsumi, S.; Itoh, T.; Fujimori, T.; Hayashi, T.; Hattori, Y. Aqueous Nanosilica dispersants for carbon nanotube. Langmuir 2015, 31, 3194–3202. [Google Scholar] [CrossRef]
- O’connell, M.J.; Bachilo, S.M.; Huffman, C.B.; Moore, V.C.; Strano, M.S.; Haroz, E.H.; Rialon, K.L.; Boul, P.J.; Noon, W.H.; Kittrell, C. Band gap fluorescence from individual single-walled carbon nanotubes. Science 2002, 297, 593–596. [Google Scholar] [CrossRef]
- Di Crescenzo, A.; Cambré, S.; Germani, R.; Di Profio, P.; Fontana, A. Dispersion of SWCNTs with imidazolium-rich surfactants. Langmuir 2014, 30, 3979–3987. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, L.; Zhang, S.; Yuan, J.; Shi, L.; Zheng, L. Dispersion of multiwalled carbon nanotubes by ionic liquid-type Gemini imidazolium surfactants in aqueous solution. Colloids Surfaces A Physicochem. Eng. Asp. 2010, 359, 66–70. [Google Scholar] [CrossRef]
- Chen, L.; Xie, H. Properties of carbon nanotube nanofluids stabilized by cationic gemini surfactant. Thermochim. Acta 2010, 506, 62–66. [Google Scholar] [CrossRef]
- Hou, J.; Du, W.; Meng, F.; Zhao, C.; Du, X. Effective dispersion of multi-walled carbon nanotubes in aqueous solution using an ionic-gemini dispersant. J. Colloid Interface Sci. 2018, 512, 750–757. [Google Scholar] [CrossRef] [PubMed]
- Goh, C.S.; Wei, J.; Lee, L.C.; Gupta, M. Simultaneous enhancement in strength and ductility by reinforcing magnesium with carbon nanotubes. Mater. Sci. Eng. A 2006, 423, 153–156. [Google Scholar] [CrossRef]
- Aydin, F.; Ayday, A.; Turan, M.E.; Zengin, H. Role of graphene additive on wear and electrochemical corrosion behaviour of plasma electrolytic oxidation (PEO) coatings on Mg–MWCNT nanocomposite. Surf. Eng. 2020, 36, 791–799. [Google Scholar] [CrossRef]
- Kumar, H.G.P.; Xavior, M.A. Graphene Reinforced Metal Matrix Composite (GRMMC): A Review. Proc. Eng. 2014, 97, 1033–1040. [Google Scholar] [CrossRef]
- Shimizu, Y.; Miki, S.; Soga, T.; Itoh, I.; Todoroki, H.; Hosono, T.; Sakaki, K.; Hayashi, T.; Kim, Y.A.; Endo, M. Multi-walled carbon nanotube-reinforced magnesium alloy composites. Scr. Mater. 2008, 58, 267–270. [Google Scholar] [CrossRef]
- Mindivan, H.; Efe, A.; Kosatepe, A.H.; Kayali, E.S. Fabrication and characterization of carbon nanotube reinforced magnesium matrix composites. Appl. Surf. Sci. 2014, 318, 234–243. [Google Scholar] [CrossRef]
- Goh, C.S.; Wei, J.; Lee, L.C.; Gupta, M. Ductility improvement and fatigue studies in Mg-CNT nanocomposites. Compos. Sci. Technol. 2008, 68, 1432–1439. [Google Scholar] [CrossRef]
- Li, Q.; Rottmair, C.A.; Singer, R.F. CNT reinforced light metal composites produced by melt stirring and by high pressure die casting. Compos. Sci. Technol. 2010, 70, 2242–2247. [Google Scholar] [CrossRef]
- Gupta, M.; Ling, S.N.M. Magnesium, Magnesium Alloys, and Magnesium Composites; John Wiley & Sons: Hoboken, NJ, USA, 2011; ISBN 1118102703. [Google Scholar]
- Tun, K.S.; Gupta, M. Improving mechanical properties of magnesium using nano-yttria reinforcement and microwave assisted powder metallurgy method. Compos. Sci. Technol. 2007, 67, 2657–2664. [Google Scholar] [CrossRef]
- Sherif, E.-S.M.; Latief, F.H.; Junaedi, H.; Almajid, A.A. Influence of exfoliated graphite nanoplatelets particles additions and sintering temperature on the mechanical properties of aluminum matrix composites. Int. J. Electrochem. Sci. 2012, 7, 4352–4361. [Google Scholar]
- Kumar, S.P.; Selvamani, S.T.; Vigneshwar, M.; Hariharan, S.J. Tensile, Microhardness, and Microstructural Analysis on Mg-CNT Nano Composites. Mater. Today Proc. 2018, 5, 7882–7888. [Google Scholar] [CrossRef]
- Selvamani, S.T.; Premkumar, S.; Vigneshwar, M.; Hariprasath, P.; Palanikumar, K. Influence of carbon nano tubes on mechanical, metallurgical and tribological behavior of magnesium nanocomposites. J. Magnes. Alloys 2017, 5, 326–335. [Google Scholar] [CrossRef]
- Paramsothy, M.; Chan, J.; Kwok, R.; Gupta, M. Addition of CNTs to enhance tensile/compressive response of magnesium alloy ZK60A. Compos. Part. A Appl. Sci. Manuf. 2011, 42, 180–188. [Google Scholar] [CrossRef]
- Mishra, R.S.; Ma, Z.Y. Friction stir welding and processing. Mater. Sci. Eng. R Rep. 2005, 50, 1–78. [Google Scholar] [CrossRef]
- Jamshidijam, M.; Akbari-Fakhrabadi, A.; Masoudpanah, S.M.; Hasani, G.H.; Mangalaraja, R.V. Wear behavior of multiwalled carbon nanotube/AZ31 composite obtained by friction stir processing. Tribol. Trans. 2013, 56, 827–832. [Google Scholar] [CrossRef]
- Huang, Y.; Li, J.; Wan, L.; Meng, X.; Xie, Y. Strengthening and toughening mechanisms of CNTs/Mg-6Zn composites via friction stir processing. Mater. Sci. Eng. A 2018, 732, 205–211. [Google Scholar] [CrossRef]
- Nai, M.H.; Wei, J.; Gupta, M. Interface tailoring to enhance mechanical properties of carbon nanotube reinforced magnesium composites. Mater. Des. 2014, 60, 490–495. [Google Scholar] [CrossRef]
- Clyne, T.W.; Withers, P.J. An Introduction to Metal Matrix Composites; Cambridge University Press: Cambridge, UK, 1995; ISBN 0521483573. [Google Scholar]
- Nardone, V.C.; Prewo, K.M. On the strength of discontinuous silicon carbide reinforced aluminum composites. Scr. Metall. 1986, 20, 43–48. [Google Scholar] [CrossRef]
- Chen, B.; Shen, J.; Ye, X.; Jia, L.; Li, S.; Umeda, J.; Takahashi, M.; Kondoh, K. Length effect of carbon nanotubes on the strengthening mechanisms in metal matrix composites. Acta Mater. 2017, 140, 317–325. [Google Scholar] [CrossRef]
- Chen, B.; Shen, J.; Ye, X.; Imai, H.; Umeda, J.; Takahashi, M.; Kondoh, K. Solid-state interfacial reaction and load transfer efficiency in carbon nanotubes (CNTs)-reinforced aluminum matrix composites. Carbon 2017, 114, 198–208. [Google Scholar] [CrossRef]
- Chen, B.; Li, S.; Imai, H.; Jia, L.; Umeda, J.; Takahashi, M.; Kondoh, K. Load transfer strengthening in carbon nanotubes reinforced metal matrix composites via in-situ tensile tests. Compos. Sci. Technol. 2015, 113, 1–8. [Google Scholar] [CrossRef]
- Xiang, S.; Wang, X.; Gupta, M.; Wu, K.; Hu, X.; Zheng, M. Graphene nanoplatelets induced heterogeneous bimodal structural magnesium matrix composites with enhanced mechanical properties. Sci. Rep. 2016, 6, 38824. [Google Scholar] [CrossRef]
- Landis, C.M.; McMeeking, R.M. A shear-lag model for a broken fiber embedded in a composite with a ductile matrix. Compos. Sci. Technol. 1999, 59, 447–457. [Google Scholar] [CrossRef]
- Park, J.G.; Keum, D.H.; Lee, Y.H. Strengthening mechanisms in carbon nanotube-reinforced aluminum composites. Carbon 2015, 95, 690–698. [Google Scholar] [CrossRef]
- Mirza, F.A.; Chen, D.L. A unified model for the prediction of yield strength in particulate-reinforced metal matrix nanocomposites. Materials 2015, 8, 5138–5153. [Google Scholar] [CrossRef]
- Ma, P.; Jia, Y.; Konda Gokuldoss, P.; Yu, Z.; Yang, S.; Zhao, J.; Li, C. Effect of Al2O3 nanoparticles as reinforcement on the tensile behavior of Al-12Si composites. Metals 2017, 7, 359. [Google Scholar] [CrossRef]
- Li, J.; Liu, B.; Fang, Q.; Huang, Z.; Liu, Y. Atomic-scale strengthening mechanism of dislocation-obstacle interaction in silicon carbide particle-reinforced copper matrix nanocomposites. Ceram. Int. 2017, 43, 3839–3846. [Google Scholar] [CrossRef]
- Zhao, X.; Lu, C.; Tieu, A.K.; Pei, L.; Zhang, L.; Cheng, K.; Huang, M. Strengthening mechanisms and dislocation processes in<111> textured nanotwinned copper. Mater. Sci. Eng. A 2016, 676, 474–486. [Google Scholar]
- Kim, K.T.; Eckert, J.; Menzel, S.B.; Gemming, T.; Hong, S.-H. Grain refinement assisted strengthening of carbon nanotube reinforced copper matrix nanocomposites. Appl. Phys. Lett. 2008, 92, 121901. [Google Scholar] [CrossRef]
- Wang, F.-C.; Zhang, Z.-H.; Sun, Y.-J.; Liu, Y.; Hu, Z.-Y.; Wang, H.; Korznikov, A.V.; Korznikova, E.; Liu, Z.-F.; Osamu, S. Rapid and low temperature spark plasma sintering synthesis of novel carbon nanotube reinforced titanium matrix composites. Carbon 2015, 95, 396–407. [Google Scholar] [CrossRef]
- George, R.; Kashyap, K.T.; Rahul, R.; Yamdagni, S. Strengthening in carbon nanotube/aluminium (CNT/Al) composites. Scr. Mater. 2005, 53, 1159–1163. [Google Scholar] [CrossRef]
- Zeng, X.; Zhou, G.; Xu, Q.; Xiong, Y.; Luo, C.; Wu, J. A new technique for dispersion of carbon nanotube in a metal melt. Mater. Sci. Eng. A 2010, 527, 5335–5340. [Google Scholar] [CrossRef]
- Liu, S.-Y.; Gao, F.-P.; Zhang, Q.-Y.; Xue, Z.; Li, W.-Z. Fabrication of carbon nanotubes reinforced AZ91D composites by ultrasonic processing. Trans. Nonferr. Met. Soc. China 2010, 20, 1222–1227. [Google Scholar] [CrossRef]
- Sun, F.; Shi, C.; Rhee, K.Y.; Zhao, N. In situ synthesis of CNTs in Mg powder at low temperature for fabricating reinforced Mg composites. J. Alloys Compd. 2013, 551, 496–501. [Google Scholar] [CrossRef]
- Thostenson, E.T.; Chou, T.-W. Nanotube buckling in aligned multi-wall carbon nanotube composites. Carbon 2004, 42, 3015–3018. [Google Scholar] [CrossRef]
- Namilae, S.; Chandra, N. Role of atomic scale interfaces in the compressive behavior of carbon nanotubes in composites. Compos. Sci. Technol. 2006, 66, 2030–2038. [Google Scholar] [CrossRef]
- Lu, J.-M.; Hwang, C.-C.; Kuo, Q.-Y.; Wang, Y.-C. Mechanical buckling of multi-walled carbon nanotubes: The effects of slenderness ratio. Phys. E Low Dimens. Syst. Nanostruct. 2008, 40, 1305–1308. [Google Scholar] [CrossRef]
- Wu, L.; Wu, R.; Hou, L.; Zhang, J.; Sun, J.; Zhang, M. Microstructure and mechanical properties of CNT-reinforced AZ31 matrix composites prepared using hot-press sintering. J. Mater. Eng. Perform. 2017, 26, 5495–5500. [Google Scholar] [CrossRef]
- Hamdy, A.S.; Alfosail, F.; Gasem, Z. Electrochemical behavior of a discontinuously A6092/SiC/17.5 p metal matrix composite in chloride containing solution. Electrochim. Acta 2013, 88, 129–134. [Google Scholar] [CrossRef]
- Tiwari, S.; Balasubramaniam, R.; Gupta, M. Corrosion behavior of SiC reinforced magnesium composites. Corros. Sci. 2007, 49, 711–725. [Google Scholar] [CrossRef]
- Bakkar, A.; Neubert, V. Corrosion characterisation of alumina–magnesium metal matrix composites. Corros. Sci. 2007, 49, 1110–1130. [Google Scholar] [CrossRef]
- Abhijeet, S.B.; Balasubramaniam, R.; Gupta, M. Corrosion behaviour of Mg–Cu and Mg–Mo composites in 3.5% NaCl. Corros. Sci. 2008, 50, 2423–2428. [Google Scholar] [CrossRef]
- Turhan, M.C.; Li, Q.; Jha, H.; Singer, R.F.; Virtanen, S. Corrosion behaviour of multiwall carbon nanotube/magnesium composites in 3.5% NaCl. Electrochim. Acta 2011, 56, 7141–7148. [Google Scholar] [CrossRef]
- Li, Q.; Turhan, M.C.; Rottmair, C.A.; Singer, R.F.; Virtanen, S. Influence of MWCNT dispersion on corrosion behaviour of their Mg composites. Mater. Corros. 2012, 63, 384–387. [Google Scholar] [CrossRef]
- Aung, N.N.; Zhou, W. Effect of grain size and twins on corrosion behaviour of AZ31B magnesium alloy. Corros. Sci. 2010, 52, 589–594. [Google Scholar] [CrossRef]
- Zhou, W.; Aung, N.N.; Sun, Y. Effect of antimony, bismuth and calcium addition on corrosion and electrochemical behaviour of AZ91 magnesium alloy. Corros. Sci. 2009, 51, 403–408. [Google Scholar] [CrossRef]
- Aung, N.N.; Zhou, W. Effect of heat treatment on corrosion and electrochemical behaviour of AZ91D magnesium alloy. J. Appl. Electrochem. 2002, 32, 1397–1401. [Google Scholar] [CrossRef]
- Jones, D.A. Principles and Prevention of Corrosion; Macmillan: Macmillan, NY, USA, 1992; ISBN 0029464390. [Google Scholar]
- Hi, J.; Ming, J.; Sun, W. Passivation and chloride-induced corrosion of a duplex alloy steel in alkali-activated slag extract solutions. Constr. Build. Mater. 2017, 155, 992–1002. [Google Scholar]
- Jia, J.X.; Atrens, A.; Song, G.; Muster, T.H. Simulation of galvanic corrosion of magnesium coupled to a steel fastener in NaCl solution. Mater. Corros. 2005, 56, 468–474. [Google Scholar] [CrossRef]
- Andreatta, F.; Apachitei, I.; Kodentsov, A.A.; Dzwonczyk, J.; Duszczyk, J. Volta potential of second phase particles in extruded AZ80 magnesium alloy. Electrochim. Acta 2006, 51, 3551–3557. [Google Scholar] [CrossRef]
- Ali, M.; Hussein, M.A.; Al-Aqeeli, N. Magnesium-based composites and alloys for medical applications: A review of mechanical and corrosion properties. J. Alloys Compd. 2019, 792, 1162–1190. [Google Scholar] [CrossRef]
- Zhang, T.; Shao, Y.; Meng, G.; Wang, F. Electrochemical noise analysis of the corrosion of AZ91D magnesium alloy in alkaline chloride solution. Electrochim. Acta 2007, 53, 561–568. [Google Scholar] [CrossRef]
- Biezma, M.V. The role of hydrogen in microbiologically influenced corrosion and stress corrosion cracking. Int. J. Hydrog. Energy 2001, 26, 515–520. [Google Scholar] [CrossRef]
- Funatsu, K.; Fukuda, H.; Takei, R.; Umeda, J.; Kondoh, K. Quantitative evaluation of initial galvanic corrosion behavior of CNTs reinforced Mg–Al alloy. Adv. Powder Technol. 2013, 24, 833–837. [Google Scholar] [CrossRef]
- Kumar, A.M.; Hassan, S.F.; Sorour, A.A.; Paramsothy, M.; Gupta, M. Investigation on the controlled degradation and invitro mineralization of carbon nanotube reinforced AZ31 nanocomposite in simulated body fluid. Met. Mater. Int. 2019, 25, 105–116. [Google Scholar] [CrossRef]
- Turan, M.E.; Sun, Y.; Aydin, F.; Zengin, H.; Turen, Y.; Ahlatci, H. Effects of carbonaceous reinforcements on microstructure and corrosion properties of magnesium matrix composites. Mater. Chem. Phys. 2018, 218, 182–188. [Google Scholar] [CrossRef]
- Al-Qutub, A.M.; Khalil, A.; Saheb, N.; Hakeem, A.S. Wear and friction behavior of Al6061 alloy reinforced with carbon nanotubes. Wear 2013, 297, 752–761. [Google Scholar] [CrossRef]
- Choi, H.J.; Lee, S.M.; Bae, D. Wear characteristic of aluminum-based composites containing multi-walled carbon nanotubes. Wear 2010, 270, 12–18. [Google Scholar] [CrossRef]
- Taltavull, C.; Rodrigo, P.; Torres, B.; López, A.J.; Rams, J. Dry sliding wear behavior of AM50B magnesium alloy. Mater. Des. 2014, 56, 549–556. [Google Scholar] [CrossRef]
- Wu, J.; Zeng, X.; Luo, L.; Yuan, Q. Friction and wear properties of Carbon Nanotubes/AZ91 composites. Mater. Mech. Eng. 2015, 11, 25. [Google Scholar]
- Habibnejad-Korayem, M.; Mahmudi, R.; Ghasemi, H.M.; Poole, W.J. Tribological behavior of pure Mg and AZ31 magnesium alloy strengthened by Al2O3 nano-particles. Wear 2010, 268, 405–412. [Google Scholar] [CrossRef]
- Zhang, J.; Alpas, A.T. Transition between mild and severe wear in aluminium alloys. Acta Mater. 1997, 45, 513–528. [Google Scholar] [CrossRef]
- Faruk, M. Wear behaviour of hot rolled AZ31B magnesium alloy as candidate for biodegradable implant material. Trans. Nonferr. Met. Soc. China 2017, 27, 2598–2606. [Google Scholar]
- Salahshoor, M.; Guo, Y. Biodegradable orthopedic magnesium-calcium (MgCa) alloys, processing, and corrosion performance. Materials 2012, 5, 135–155. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Hu, L.; Li, J.; An, Z.; Li, J.; Huang, Q. Achieving high strength and good ductility in as-extruded Mg–Gd–Y–Zn alloys by Ce micro-alloying. Materials 2018, 11, 102. [Google Scholar] [CrossRef] [PubMed]
- Raman, R.K.; Harandi, S.E. Resistance of magnesium alloys to corrosion fatigue for biodegradable implant applications: Current status and challenges. Materials 2017, 10, 1316. [Google Scholar] [CrossRef]
- Guo, S.; Liu, R.; Jiang, X.; Zhang, H.; Zhang, D.; Wang, J.; Pan, F. Statistical analysis on the mechanical properties of magnesium alloys. Materials 2017, 10, 1271. [Google Scholar] [CrossRef]
- Chiu, C.; Lu, C.-T.; Chen, S.-H.; Ou, K.-L. Effect of hydroxyapatite on the mechanical properties and corrosion behavior of Mg-Zn-Y alloy. Materials 2017, 10, 855. [Google Scholar] [CrossRef]
- Seetharaman, S.; Subramanian, J.; Tun, K.S.; Hamouda, A.S.; Gupta, M. Synthesis and characterization of nano boron nitride reinforced magnesium composites produced by the microwave sintering method. Materials 2013, 6, 1940–1955. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Liu, L.; Hu, L.; Zhou, T.; Yang, M.; Lian, Y.; Zhang, J. Effect of Final Rolling Temperature on Microstructures and Mechanical Properties of AZ31 Alloy Sheets Prepared by Equal Channel Angular Rolling and Continuous Bending. Materials 2020, 13, 3346. [Google Scholar] [CrossRef] [PubMed]
- Tun, K.S.; Padnuru Sripathy, A.; Tekumalla, S.; Gupta, M. Development of Novel Lightweight Metastable Metal–(Metal+ Ceramic) Composites Using a New Powder Metallurgy Approach. Materials 2020, 13, 3283. [Google Scholar] [CrossRef] [PubMed]
- Mordekovitz, Y.; Shoval, Y.; Froumin, N.; Hayun, S. Effect of Structure and Composition of Non-Stoichiometry Magnesium Aluminate Spinel on Water Adsorption. Materials 2020, 13, 3195. [Google Scholar] [CrossRef]
- Jiang, Z.; Zhang, L.; Geng, T.; Lai, Y.; Zheng, W.; Huang, M. Study on the Compressive Properties of Magnesium Phosphate Cement Mixing with Eco-Friendly Coir Fiber Considering Fiber Length. Materials 2020, 13, 3194. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, C.; Gao, Y.; Guo, X.; Wan, Y. Enhanced Mechanical and Corrosion Performance by Forming Micro Shear Bands in Cold Forged Mg-Gd-Y-Zr Alloy. Materials 2020, 13, 3181. [Google Scholar] [CrossRef]
- Ma, Z.; Hu, F.; Wang, Z.; Fu, K.; Wei, Z.; Wang, J.; Li, W. Constitutive Equation and Hot Processing Map of Mg-16Al Magnesium Alloy Bars. Materials 2020, 13, 3107. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, J.; Deng, B.; Ye, T.; Li, Q.; Zhou, X.; Zhang, H. Microstructure, Texture and Mechanical Properties of AZ31 Magnesium Alloy Fabricated by High Strain Rate Biaxial Forging. Materials 2020, 13, 3050. [Google Scholar] [CrossRef]
- Zamin, H.; Yabutsuka, T.; Takai, S.; Sakaguchi, H. Role of Magnesium and the Effect of Surface Roughness on the Hydroxyapatite-Forming Ability of Zirconia Induced by Biomimetic Aqueous Solution Treatment. Materials 2020, 13, 3045. [Google Scholar] [CrossRef]
- Chang, Y.-H.; Tseng, C.C.; Chao, C.-Y.; Chen, C.-H.; Lin, S.-Y.; Du, J.-K. Mg-Zn-Ca Alloys for Hemostasis Clips for Vessel Ligation: In Vitro and In Vivo Studies of Their Degradation and Response. Materials 2020, 13, 3039. [Google Scholar] [CrossRef]
- Cao, H.; Luo, Z.; Wang, C.; Wang, J.; Hu, T.; Xiao, L.; Che, J. The Stress Concentration Mechanism of Pores Affecting the Tensile Properties in Vacuum Die Casting Metals. Materials 2020, 13, 3019. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Sun, Y.; Lu, Y.; He, J.; Liu, X.; Wan, S. Effect of the Strain Rate on the Damping and Mechanical Properties of a ZK60 Magnesium Alloy. Materials 2020, 13, 2969. [Google Scholar] [CrossRef] [PubMed]
- Degli Esposti, L.; Ionescu, A.C.; Brambilla, E.; Tampieri, A.; Iafisco, M. Characterization of a Toothpaste Containing Bioactive Hydroxyapatites and In Vitro Evaluation of Its Efficacy to Remineralize Enamel and to Occlude Dentinal Tubules. Materials 2020, 13, 2928. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.; Huang, R.; Zhao, H.; Gong, X.; Chen, B.; Tan, C. Effect of Al Content in Magnesium Alloy on Microstructure and Mechanical Properties of Laser-Welded Mg/Ti Dissimilar Joints. Materials 2020, 13, 2743. [Google Scholar] [CrossRef]
- Masood Chaudry, U.; Hamad, K.; Kim, J.-G. A Further Improvement in the Room-Temperature Formability of Magnesium Alloy Sheets by Pre-Stretching. Materials 2020, 13, 2633. [Google Scholar] [CrossRef]
- You, J.; Huang, Y.; Liu, C.; Zhan, H.; Huang, L.; Zeng, G. Microstructural Study of a Mg–Zn–Zr Alloy Hot Compressed at a High Strain Rate. Materials 2020, 13, 2348. [Google Scholar] [CrossRef]
- Gao, H.; Ma, B.; Singh, R.P.; Yang, H. Areal Surface Roughness of AZ31B Magnesium Alloy Processed by Dry Face Turning: An Experimental Framework Combined with Regression Analysis. Materials 2020, 13, 2303. [Google Scholar] [CrossRef]
- Gui, Z.; Zhang, J.; Kang, Z. Characterization and Properties of Mg-xGd-1.5 Nd-0.5 Zn-0.5 Zr Alloys for Biodegradation Applications. Materials 2020, 13, 1421. [Google Scholar] [CrossRef]
- Wasserbauer, J.; Buchtík, M.; Tkacz, J.; Fintová, S.; Minda, J.; Doskočil, L. Improvement of AZ91 Alloy Corrosion Properties by Duplex NI-P Coating Deposition. Materials 2020, 13, 1357. [Google Scholar] [CrossRef]
- Razavi, M.; Fathi, M.; Savabi, O.; Tayebi, L.; Vashaee, D. Biodegradable Magnesium Bone Implants Coated with a Novel Bioceramic Nanocomposite. Materials 2020, 13, 1315. [Google Scholar] [CrossRef]
- Papenberg, N.P.; Gneiger, S.; Weißensteiner, I.; Uggowitzer, P.J.; Pogatscher, S. Mg-Alloys for Forging Applications—A Review. Materials 2020, 13, 985. [Google Scholar] [CrossRef] [PubMed]
- Zagórski, I.; Korpysa, J. Surface Quality Assessment after Milling AZ91D Magnesium Alloy Using PCD Tool. Materials 2020, 13, 617. [Google Scholar] [CrossRef] [PubMed]
- Bohlen, J.; Meyer, S.; Wiese, B.; Luthringer-Feyerabend, B.J.C.; Willumeit-Römer, R.; Letzig, D. Alloying and Processing Effects on the Microstructure, Mechanical Properties, and Degradation Behavior of Extruded Magnesium Alloys Containing Calcium, Cerium, or Silver. Materials 2020, 13, 391. [Google Scholar] [CrossRef] [PubMed]
- Dobroň, P.; Drozdenko, D.; Horváth Fekete, K.; Olejňák, J.; Bohlen, J. Grain Size-Related Strengthening and Softening of a Precompressed and Heat-Treated Mg–Zn–Ca Alloy. Materials 2020, 13, 351. [Google Scholar] [CrossRef] [PubMed]
- Kamrani, S.; Hübler, D.; Ghasemi, A.; Fleck, C. Enhanced strength and ductility in magnesium matrix composites reinforced by a high volume fraction of nano-and submicron-sized SiC particles produced by mechanical milling and hot extrusion. Materials 2019, 12, 3445. [Google Scholar] [CrossRef]
- Straumal, P.; Martynenko, N.; Kilmametov, A.; Nekrasov, A.; Baretzky, B. Microstructure, microhardness and corrosion resistance of WE43 alloy based composites after high-pressure torsion. Materials 2019, 12, 2980. [Google Scholar] [CrossRef]
- Kania, A.; Nowosielski, R.; Gawlas-Mucha, A.; Babilas, R. Mechanical and corrosion properties of Mg-based alloys with Gd addition. Materials 2019, 12, 1775. [Google Scholar] [CrossRef]
- Jiang, Z.; Feng, J.; Chen, Q.; Jiang, S.; Dai, J.; Jiang, B.; Pan, F. Preparation and Characterization of Magnesium Alloy Containing Al2Y Particles. Materials 2018, 11, 1748. [Google Scholar] [CrossRef]
- Chiu, C.; Huang, H.-M. Microstructure and properties of Mg-Zn-Y alloy powder compacted by equal channel angular pressing. Materials 2018, 11, 1678. [Google Scholar] [CrossRef]
- Prakash, C.; Singh, S.; Gupta, M.K.; Mia, M.; Królczyk, G.; Khanna, N. Synthesis, characterization, corrosion resistance and in-vitro bioactivity behavior of biodegradable Mg–Zn–Mn–(Si–HA) composite for orthopaedic applications. Materials 2018, 11, 1602. [Google Scholar] [CrossRef]
- Razzaghi, M.; Kasiri-Asgarani, M.; Bakhsheshi-Rad, H.R.; Ghayour, H. Microstructure, mechanical properties, and in-vitro biocompatibility of nano-NiTi reinforced Mg–3Zn-0.5Ag alloy: Prepared by mechanical alloying for implant applications. Compos. Part. B Eng. 2020, 190, 107947. [Google Scholar] [CrossRef]
- Bakhsheshi-Rad, H.R.; Hamzah, E.; Kasiri-Asgarani, M.; Saud, S.N.; Yaghoubidoust, F.; Akbari, E. Structure, corrosion behavior, and antibacterial properties of nano-silica/graphene oxide coating on biodegradable magnesium alloy for biomedical applications. Vacuum 2016, 131, 106–110. [Google Scholar] [CrossRef]
- Khalajabadi, S.Z.; Kadir, M.R.A.; Izman, S.; Bakhsheshi-Rad, H.R.; Farahany, S. Effect of mechanical alloying on the phase evolution, microstructure and bio-corrosion properties of a Mg/HA/TiO2/MgO nanocomposite. Ceram. Int. 2014, 40, 16743–16759. [Google Scholar] [CrossRef]
- Razzaghi, M.; Asgarani, M.K.; Bakhsheshi-Rad, H.R.; Ghayour, H. In vitro degradation, antibacterial activity and cytotoxicity of Mg-3Zn-xAg nanocomposites synthesized by mechanical alloying for implant applications. J. Mater. Eng. Perform. 2019, 28, 1441–1455. [Google Scholar] [CrossRef]
- Bakhsheshi-Rad, H.R.; Abdellahi, M.; Hamzah, E.; Daroonparvar, M.; Rafiei, M. Introducing a composite coating containing CNTs with good corrosion properties: Characterization and simulation. RSC Adv. 2016, 6, 108498–108512. [Google Scholar] [CrossRef]
- Bakhsheshi-Rad, H.R.; Hamzah, E.; Fereidouni-Lotfabadi, A.; Daroonparvar, M.; Yajid, M.A.M.; Mezbahul-Islam, M.; Kasiri-Asgarani, M.; Medraj, M. Microstructure and bio-corrosion behavior of Mg–Zn and Mg–Zn–Ca alloys for biomedical applications. Mater. Corros. 2014, 65, 1178–1187. [Google Scholar] [CrossRef]
- Bakhsheshi-Rad, H.R.; Idris, M.H.; Abdul-Kadir, M.R.; Ourdjini, A.; Medraj, M.; Daroonparvar, M.; Hamzah, E. Mechanical and bio-corrosion properties of quaternary Mg–Ca–Mn–Zn alloys compared with binary Mg–Ca alloys. Mater. Des. 2014, 53, 283–292. [Google Scholar] [CrossRef]
- Soleymani Eil Bakhtiari, S.; Bakhsheshi-Rad, H.R.; Karbasi, S.; Tavakoli, M.; Razzaghi, M.; Ismail, A.F.; RamaKrishna, S.; Berto, F. Polymethyl methacrylate-based bone cements containing carbon nanotubes and graphene oxide: An overview of physical, mechanical, and biological properties. Polymers 2020, 12, 1469. [Google Scholar] [CrossRef]
- Abazari, S.; Shamsipur, A.; Bakhsheshi-Rad, H.R.; Ramakrishna, S.; Berto, F. Graphene family nanomaterial reinforced magnesium-based matrix composites for biomedical application: A comprehensive review. Metals 2020, 10, 1002. [Google Scholar] [CrossRef]
- Bakhsheshi-Rad, H.R.; Idris, M.H.; Abdul Kadir, M.R.; Farahany, S. Microstructure analysis and corrosion behavior of biodegradable Mg–Ca implant alloys. Mater. Des. 2012, 33, 88–97. [Google Scholar]
- Bakhsheshi-Rad, H.R.; Abdul-Kadir, M.R.; Idris, M.H.; Farahany, S. Relationship between the corrosion behavior and the thermal characteristics and microstructure of Mg–0.5Ca–xZn alloys. Corros. Sci. 2012, 64, 184–197. [Google Scholar] [CrossRef]
- Farahany, S.; Bakhsheshi-Rad, H.R.; Idris, M.H.; Kadir, M.R.A.; Lotfabadi, A.F.; Ourdjini, A. In-situ thermal analysis and macroscopical characterization of Mg–xCa and Mg–0.5Ca–xZn alloy systems. Thermochim. Acta 2012, 527, 180–189. [Google Scholar] [CrossRef]
- Bakhsheshi-Rad, H.R.; Hamzah, E.; Farahany, S.; Staiger, M.P. The mechanical properties and corrosion behavior of quaternary Mg-6Zn-0.8Mn-xCa alloys. J. Mater. Eng. Perform. 2015, 24, 598–608. [Google Scholar] [CrossRef]
- Saberi, A.; Bakhsheshi-Rad, H.R.; Karamian, E.; Kasiri, M.; Ghomi, H. A study on the corrosion behavior and biological properties of polycaprolactone/bredigite composite coating on biodegradable Mg-Zn-Ca- GNP nanocomposite. Prog. Org. Coat. 2020, 147, 105822. [Google Scholar] [CrossRef]
- Saheban, M.; Bakhsheshi-Rad, H.R.; Hamzah, E.; Ismail, A.F.; Aziz, M.; Hamzah, E.; Najafinezhad, A. Effect of zeolite on the corrosion behavior, biocompatibility and antibacterial activity of porous magnesium/zeolite composite scaffolds. Mater. Technol. 2019, 34, 258–269. [Google Scholar] [CrossRef]
- Bakhsheshi-Rad, H.R.; Hamzah, E.; Tok, H.Y.; Kasiri-Asgarani, M.; Jabbarzare, S.; Medraj, M. Microstructure, In Vitro corrosion behavior and cytotoxicity of biodegradable Mg-Ca-Zn and Mg-Ca-Zn-Bi alloys. J. Mater. Eng. Perform. 2017, 26, 653–666. [Google Scholar] [CrossRef]
- Tok, H.Y.; Hamzah, E.; Bakhsheshi-Rad, H.R. The role of bismuth on the microstructure and corrosion behavior of ternary Mg-1.2Ca-xBi alloys for biomedical application. J. Alloys Compd. 2015, 640, 335–346. [Google Scholar] [CrossRef]
- Rad, H.R.B.; Najafinezhad, A.; Hamzah, E.; Ismail, A.F.; Berto, F.; Chen, X. Clinoenstatite/Tantalum Coating for Enhancement of Biocompatibility and Corrosion Protection of Mg Alloy. J. Funct. Biomater. 2020, 11, 26. [Google Scholar]
- Iqbal, N.; Iqbal, S.; Iqbal, T.; Rad, H.R.B.; Alsakkaf, A.; Kamil, A.; Idris, M.H.; Raghav, H.B. Zinc-doped hydroxyapatite—zeolite/polycaprolactone composites coating on magnesium substrate for enhancing in-vitro corrosion and antibacterial performance. Trans. Nonferrous. Met. Soc. China 2020, 30, 123–133. [Google Scholar] [CrossRef]
- Daroonparvar, M.; YAJID, M.A.M.; Gupta, R.K.; YUSOF, N.M.; Rad, H.R.B.; Ghandvar, H.; Ghasemi, E. Antibacterial activities and corrosion behavior of novel PEO/nanostructured ZrO2 coating on Mg alloy. Trans. Nonferrous. Met. Soc. China 2018, 28, 1571–1581. [Google Scholar] [CrossRef]
- Rad, H.R.B.; Hamzah, E.; Ismail, A.F.; Aziz, M.; Asgarani, M.K.; Ghayour, H.; Razzaghi, M.; Hadisi, Z. In vitro corrosion behavior, bioactivity, and antibacterial performance of the silver-doped zinc oxide coating on magnesium alloy. Mater. Corros. 2017, 68, 1228–1236. [Google Scholar]
- Rad, H.R.B.; Hamzah, E.; Dias, G.J.; Saud, S.N.; Yaghoubidoust, F.; Hadisi, Z. Fabrication and characterisation of novel ZnO/MWCNT duplex coating deposited on Mg alloy by PVD coupled with dip-coating techniques. J. Alloys Compd. 2017, 728, 159–168. [Google Scholar]
- Daroonparvar, M.; Yajid, M.A.M.; Yusof, N.M.; Rad, H.R.B. Preparation and corrosion resistance of a nanocomposite plasma electrolytic oxidation coating on Mg-1% Ca alloy formed in aluminate electrolyte containing titania nano-additives. J. Alloys Compd. 2016, 688, 841–857. [Google Scholar] [CrossRef]
- Peron, M.; Bertolini, R.; Ghiotti, A.; Torgersen, J.; Bruschi, S.; Berto, F. Enhancement of stress corrosion cracking of AZ31 magnesium alloy in simulated body fluid thanks to cryogenic machining. J. Mech. Behav. Biomed. Mater. 2020, 101, 103429. [Google Scholar] [CrossRef] [PubMed]
- Safari, N.; Toroghinejad, M.R.; Kharaziha, M. Influence of copper on the structural, mechanical, and biological characteristics of Mg–1Al–Cu alloy. Mater. Chem. Phys. 2019, 237, 121838. [Google Scholar]
- Golshirazi, A.; Kharaziha, M.; Golozar, M.A. Polyethylenimine/kappa carrageenan: Micro-arc oxidation coating for passivation of magnesium alloy. Carbohydr. Polym. 2017, 167, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Daroonparvar, M.; Yajid, M.A.M.; Yusof, N.M.; Rad, H.R.B.; Hamzah, E.; Mardanikivi, T. Deposition of duplex MAO layer/nanostructured titanium dioxide composite coatings on Mg–1% Ca alloy using a combined technique of air plasma spraying and micro arc oxidation. J. Alloys Compd. 2015, 649, 591–605. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abazari, S.; Shamsipur, A.; Bakhsheshi-Rad, H.R.; Ismail, A.F.; Sharif, S.; Razzaghi, M.; Ramakrishna, S.; Berto, F. Carbon Nanotubes (CNTs)-Reinforced Magnesium-Based Matrix Composites: A Comprehensive Review. Materials 2020, 13, 4421. https://doi.org/10.3390/ma13194421
Abazari S, Shamsipur A, Bakhsheshi-Rad HR, Ismail AF, Sharif S, Razzaghi M, Ramakrishna S, Berto F. Carbon Nanotubes (CNTs)-Reinforced Magnesium-Based Matrix Composites: A Comprehensive Review. Materials. 2020; 13(19):4421. https://doi.org/10.3390/ma13194421
Chicago/Turabian StyleAbazari, Somayeh, Ali Shamsipur, Hamid Reza Bakhsheshi-Rad, Ahmad Fauzi Ismail, Safian Sharif, Mahmood Razzaghi, Seeram Ramakrishna, and Filippo Berto. 2020. "Carbon Nanotubes (CNTs)-Reinforced Magnesium-Based Matrix Composites: A Comprehensive Review" Materials 13, no. 19: 4421. https://doi.org/10.3390/ma13194421
APA StyleAbazari, S., Shamsipur, A., Bakhsheshi-Rad, H. R., Ismail, A. F., Sharif, S., Razzaghi, M., Ramakrishna, S., & Berto, F. (2020). Carbon Nanotubes (CNTs)-Reinforced Magnesium-Based Matrix Composites: A Comprehensive Review. Materials, 13(19), 4421. https://doi.org/10.3390/ma13194421