Effects of CeO2 on the Si Precipitation Mechanism of SiCp/Al-Si Composite Prepared by Powder Metallurgy
Abstract
1. Introduction
2. Experimental Process
3. Results and Discussion
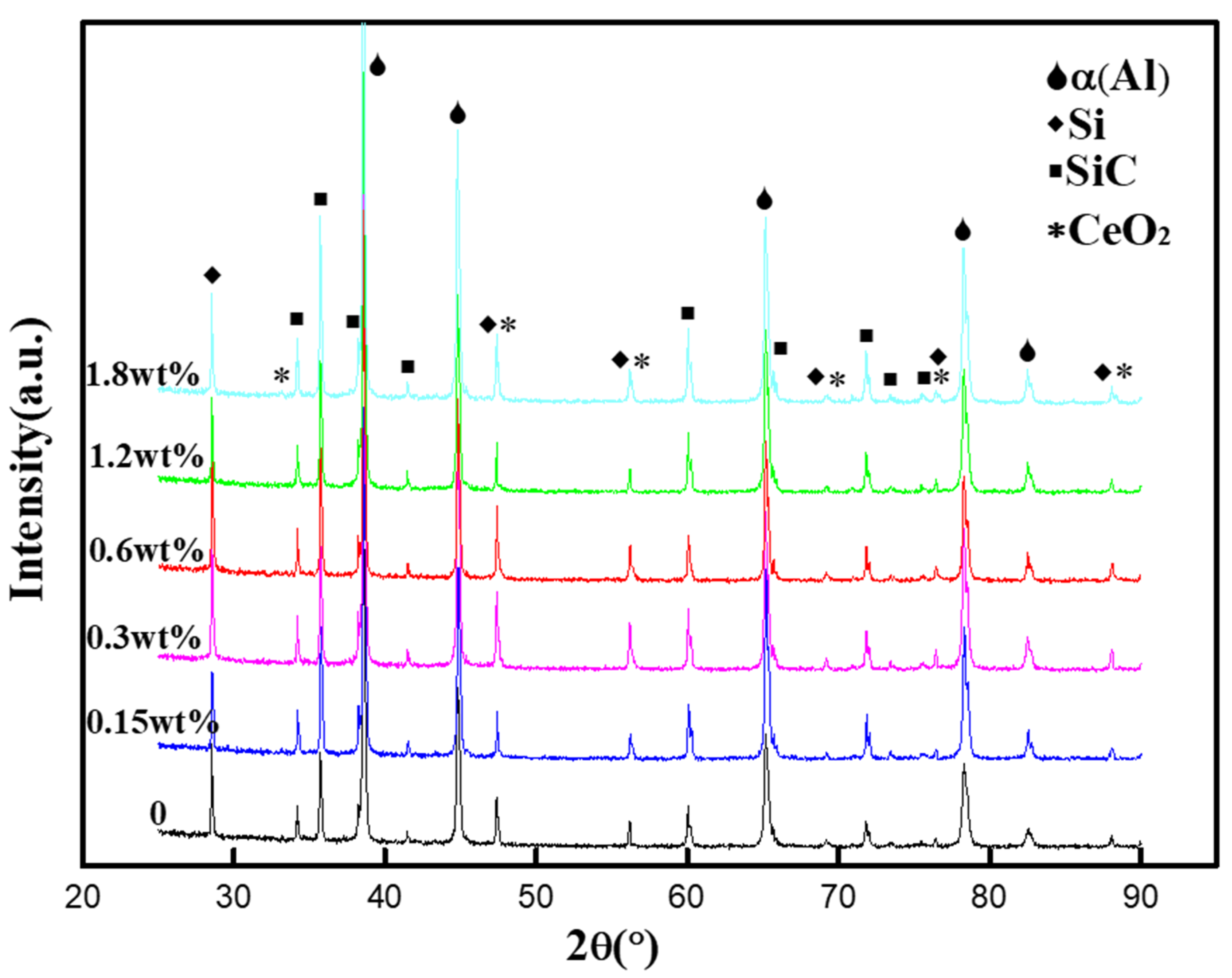
3.1. The XRD Analysis of SiCp/Al-Si Composites with Different CeO2 Contents
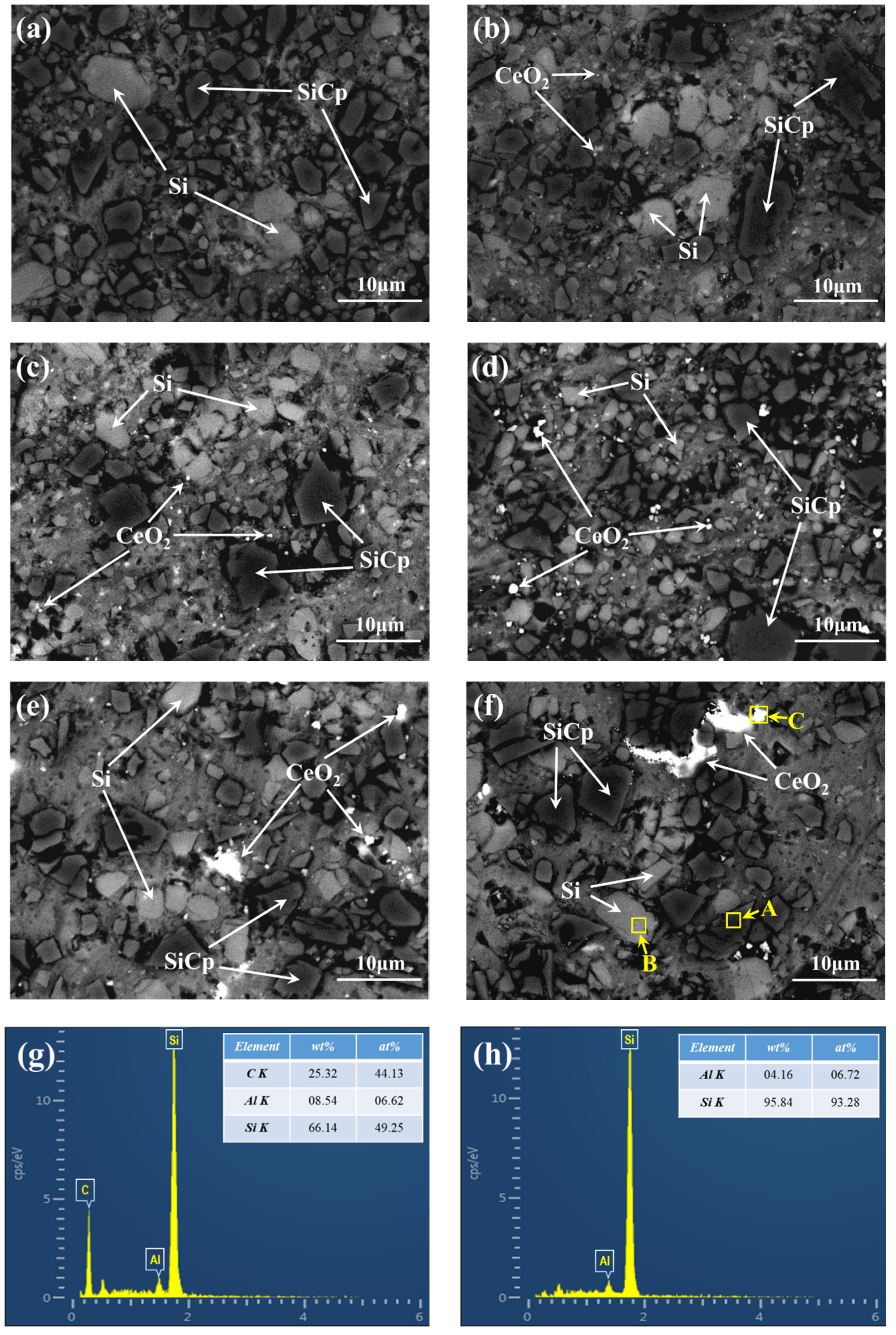
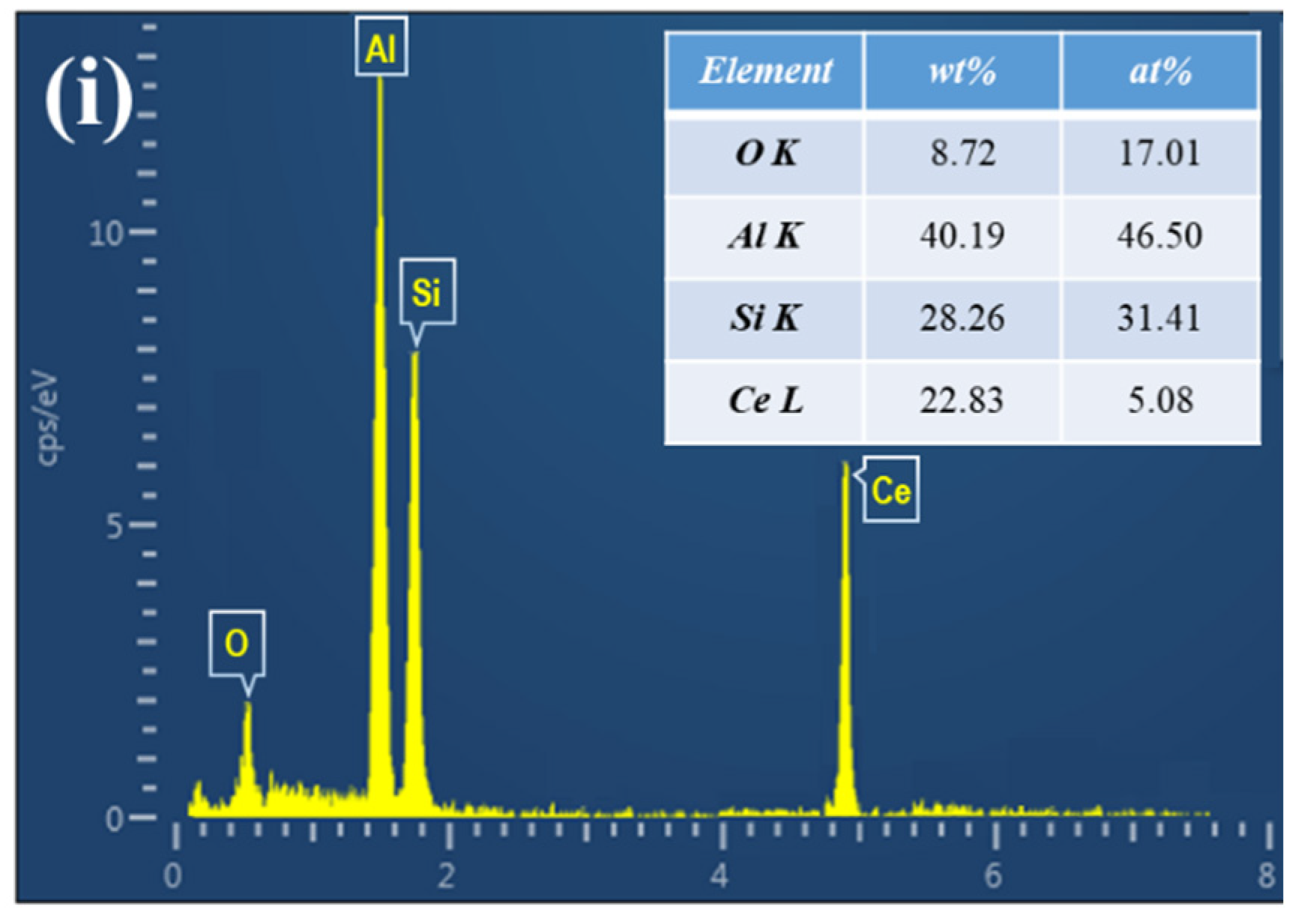
3.2. Effect of CeO2 Additions on the Microstructure of SiCp/Al-Si Composites
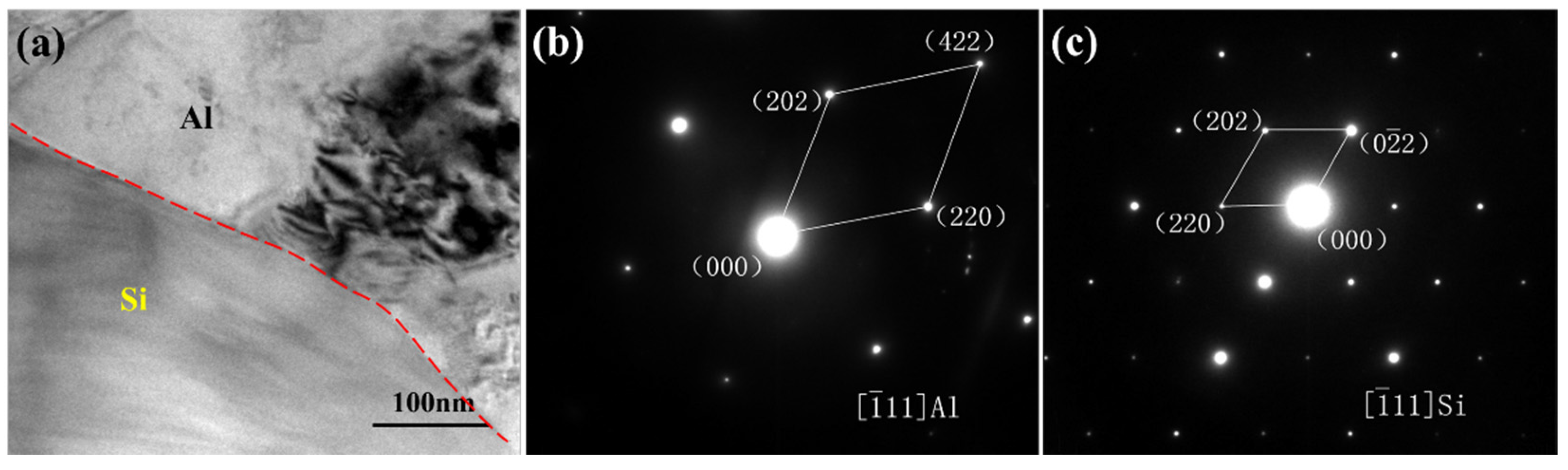
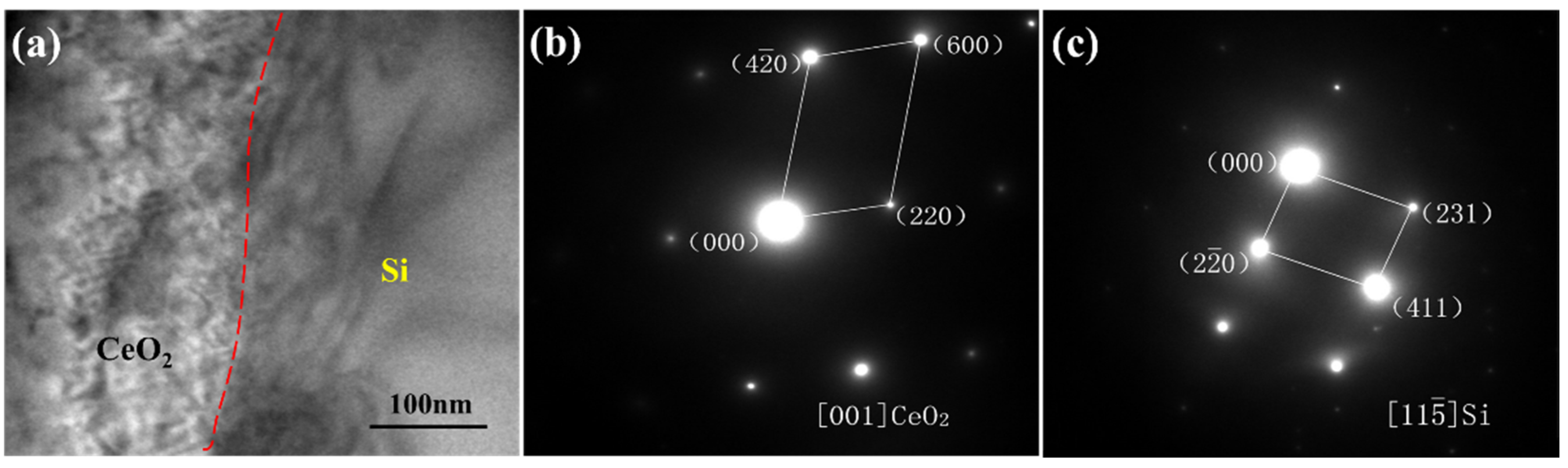
3.3. Influence Mechanism of CeO2 on the Microstructure of SiCp/Al-Si Composites
3.4. Calculation of Nucleation Rate of Precipitated Si in Composites
4. Conclusions
- (1)
- SiCp/Al-Si composites with different CeO2 contents were successfully prepared by a powder metallurgy method. When the content of CeO2 is less than 0.6 wt%, CeO2 mainly exists in the form of discrete particles and when the content of CeO2 is more than 0.6 wt%, the agglomeration of CeO2 increases.
- (2)
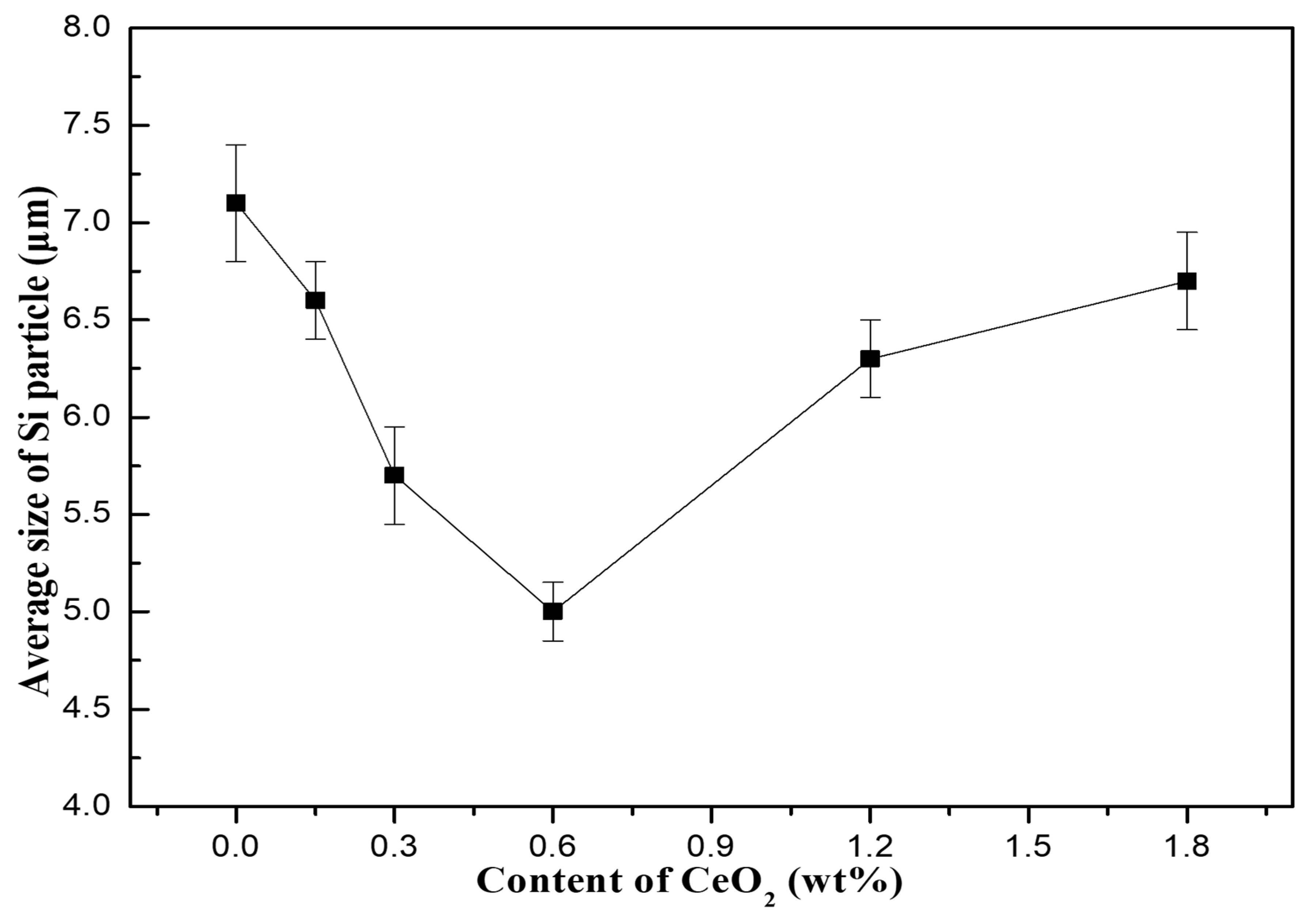
- An appropriate amount of CeO2 can obviously refine the size of precipitated Si particles and increase the amount of Si particles. With the increase of CeO2 content from 0 to 1.8 wt%, the number of precipitated Si particles first increases and then decreases, and the average size of precipitated Si particles first increases and then decreases, too. When the CeO2 content is 0.6 wt%, the number of Si particles precipitated in the composites is the largest and the average size is the smallest.
- (3)
- For the composite without CeO2, the nucleation of precipitated Si is mainly based on Al matrix. The addition of CeO2 can be used as the heterogeneous nucleation substrate for precipitated Si, which improves the nucleation rate of precipitated Si. Moreover, the nucleation rate of precipitated Si on CeO2 substrate is higher than that on Al substrate, which further improves the nucleation rate of precipitated Si, thus increasing the number of precipitated Si particles and refining the size of precipitated Si particles.
Author Contributions
Funding
Conflicts of Interest
References
- Ezugwu, R.A.; Bonney, V.; Yamane, Y. An overview of the machinability of aeroengine alloys. Mater. Chem. Phys. 2008, 108, 283–289. [Google Scholar] [CrossRef]
- Guo, X.; Wang, L.; Wang, M.; Qin, J.; Zhang, D.; Lu, W. Effects of degree of deformation on the microstructure, mechanical properties & texture of hybrid-reinforced titanium matrix composites. Acta Mater. 2012, 60, 2656–2667. [Google Scholar] [CrossRef]
- Luedtke, A. Thermal management materials for high-performance applications. Adv. Eng. Mater. 2004, 6, 142–144. [Google Scholar] [CrossRef]
- Crouch, I.G. Body armour—New materials, new systems. Def. Technol. 2019, 15, 241–253. [Google Scholar] [CrossRef]
- Tjong, S.C. Recent progress in the development and properties of novel metal matrix nanocomposites reinforced with carbon nanotubes and graphene nanosheets. Mater. Sci. Eng. R Rep. 2013, 74, 281–350. [Google Scholar] [CrossRef]
- Hooker, J.A.; Doorbar, P.J. Metal matrix composites for aeroengines. Mater. Sci. Technol. 2000, 16, 725–731. [Google Scholar] [CrossRef]
- Vasantha, K.P.; Sekar, K.; Venkatesh, K. Recent developments in powder metallurgy based aluminium alloy composite for aerospace applications. Mater. Today 2019, 18, 5400–5409. [Google Scholar] [CrossRef]
- Ghasali, E.; Yazdani-Rad, R.; Asadian, K.; Ebadzadeh, T. Production of Al-SiC hybrid composites using pure and 1056 aluminum powders prepared through microwave and conventional heating methods. J. Alloys Compd. 2017, 690, 512–518. [Google Scholar] [CrossRef]
- Veillère, A.; Kurita, H.; Kawasaki, A.; Lu, Y.; Heintz, J.-M.; Silvain, J.-F. Aluminum/Carbon Composites Materials Fabricated by the Powder Metallurgy Process. Materials 2019, 12, 4030. [Google Scholar] [CrossRef]
- Yang, Y.; Lan, J.; Li, X. Study on bulk aluminium matrix nano-composite fabricated by ultrasonic dispersion of nano-sized SiC particles in molten aluminium alloy. Mater. Sci. Eng. A 2004, 380, 378–383. [Google Scholar] [CrossRef]
- Singh, S.; Pal, K. Effect of texture evolution on mechanical and damping properties of SiC/ZnAl2O4 composite through friction stir processing. J. Mater. Res. Technol. 2018, 338–350. [Google Scholar] [CrossRef]
- Urena, A.; Martinez, E.E.; Rodrigo, P.; Gil, L. Oxidation treatments for SiC particles used as reinforcement in aluminium matrix composites. Comp. Sci. Technol. 2004, 64, 1843–1854. [Google Scholar] [CrossRef]
- Shin, S.; Cho, S.; Lee, D.; Kim, Y.; Lee, S.-B.; Lee, S.-K.; Jo, I. Microstructural Evolution and Strengthening Mechanism of SiC/Al Composites Fabricated by a Liquid-Pressing Process and Heat Treatment. Materials 2019, 12, 3374. [Google Scholar] [CrossRef] [PubMed]
- Zare, R.; Sharifi, H.; Reza Saeri, M.; Tayebi, M. Investigating the effect of SiC particles on the physical and thermal properties of Al6061/SiCp composite. J. Alloys Compd. 2019, 801, 520–528. [Google Scholar] [CrossRef]
- Mckimpson, M.G.; Scott, T.E. Processing and properties of metal matrix composites containing discontinuous reinforcement. Mater. Sci. Eng. A 1989, 107, 93–106. [Google Scholar] [CrossRef]
- Bai, M.; Xue, Q. Investigation of wear mechanism of SiC particulate-reinforced Al-20Si-3Cu-1Mg aluminium matrix composites under dry sliding and water lubrication. Tribol. Int. 1997, 30, 261–269. [Google Scholar] [CrossRef]
- Molina, J.M.; Narciso, J.; Weber, L.; Mortensen, A.; Louis, E. Thermal conductivity of Al-SiC composites with monomodal and bimodal particle size distribution. Mater. Sci. Eng. A 2008, 480, 483–488. [Google Scholar] [CrossRef]
- Selvam, D.R.; Robinson, J.; Dinaharan, D.S. Syntheses and characterization of Al6061-fly ash-SiC composites by stir casting and compocasting methods. Energy Procedia 2013, 34, 637–646. [Google Scholar] [CrossRef]
- Shi, C.; Zhang, L.; Wu, G.; Zhang, X.; Cheng, A.; Tao, J. Effects of Sc addition on the microstructure and mechanical properties of cast Al-3Li-1.5Cu-0.15Zr alloy. Mater. Sci. Eng. A 2017, 680, 232–238. [Google Scholar] [CrossRef]
- Mao, F.; Yan, G.; Xuan, Z.; Cao, Z.; Wang, T. Effect of Eu addition on the microstructures and mechanical properties of A356 aluminium alloys. J. Alloys Compd. 2015, 650, 896–906. [Google Scholar] [CrossRef]
- Tsai, Y.; Lee, S.; Lin, C.K. Effect of trace Ce addition on the microstructures and mechanical properties of A356 (Al–7Si–0.35 Mg) aluminum alloys. J. Chin. Inst. Eng. 2011, 34, 609–616. [Google Scholar] [CrossRef]
- Li, H.; Gao, Z.; Yin, H.; Jiang, H.; Su, X.; Bin, J. Effects of Er and Zr additions on precipitation and recrystallization of pure aluminium. Scr. Mater. 2013, 68, 59–62. [Google Scholar] [CrossRef]
- Li, Q.; Xia, T.; Lan, Y.; Zhao, W.; Fan, L.; Li, P. Effect of rare earth cerium addition on the microstructure and tensile properties of hypereutectic Al-20% Si alloy. J. Alloys Compd. 2013, 562, 25–32. [Google Scholar] [CrossRef]
- Xue, J.; Wang, J.; Han, Y.F.; Li, P.; Sun, B.D. Effects of CeO2 additive on the microstructure and mechanical properties of in situ TiB2/Al composite. J. Alloys Compd. 2011, 509, 1573–1578. [Google Scholar] [CrossRef]
- Wu, C.; Wang, Z.; Li, Q.G.; Shi, G.P.; Liu, M.J.; Li, Y.Y. Mechanical properties and microstructure evolution of Ti/Al2O3 cermet composite with CeO2 addition. J. Alloys Compd. 2014, 617, 729–733. [Google Scholar] [CrossRef]
- Turnbull, D.; Vonnegut, B. Nucleation Catalysis. Ind. Eng. Chem. 1952, 44, 1292–1298. [Google Scholar] [CrossRef]
- Liu, F.; Song, S.J.; Xu, J.F.; Wang, J. Determination of nucleation and growth modes from evaluation of transformed fraction in solid-state transformation. Acta Mater. 2008, 56, 6003–6012. [Google Scholar] [CrossRef]
| Phase | Melting Point (K) | Crystal Structure | Lattice Constant (Å) |
|---|---|---|---|
| Si | 1673 | FCC | 0.6636 |
| CeO2 | 2873 | FCC | 0.5411 |
| Al | 933 | FCC | 0.4050 |
| Parameter Value | Nucleation Substrates | ||
|---|---|---|---|
| Al Matrix | CeO2 | ||
| ΔGv | (J/cm3) | 104 | 104 |
| ΔGA | (J) | 2.19 × 10−19 | 2.19 × 10−19 |
| ΔGsi | (J/cm3) | 0 | 15 |
| N0 | ((s·cm3)−1) | 3.94 × 1021 | 3.94 × 1021 |
| γi | (J/cm2) | 4 × 10−6 | 6 × 10−6 |
| σi | (J/cm2) | 1 × 10−4 | 5 × 10−5 |
| k | (J/k) | 1.38 × 10−23 | 1.38 × 10−23 |
| h | (J·s) | 6.62 × 10−34 | 6.62 × 10−34 |
| T | (K) | 823 | 823 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, B.; Wang, A.; Liu, K.; Liu, C.; Xie, J.; Wang, G.; Wei, S. Effects of CeO2 on the Si Precipitation Mechanism of SiCp/Al-Si Composite Prepared by Powder Metallurgy. Materials 2020, 13, 4365. https://doi.org/10.3390/ma13194365
Yang B, Wang A, Liu K, Liu C, Xie J, Wang G, Wei S. Effects of CeO2 on the Si Precipitation Mechanism of SiCp/Al-Si Composite Prepared by Powder Metallurgy. Materials. 2020; 13(19):4365. https://doi.org/10.3390/ma13194365
Chicago/Turabian StyleYang, Bin, Aiqin Wang, Kunding Liu, Chenlu Liu, Jingpei Xie, Guangxin Wang, and Shizhong Wei. 2020. "Effects of CeO2 on the Si Precipitation Mechanism of SiCp/Al-Si Composite Prepared by Powder Metallurgy" Materials 13, no. 19: 4365. https://doi.org/10.3390/ma13194365
APA StyleYang, B., Wang, A., Liu, K., Liu, C., Xie, J., Wang, G., & Wei, S. (2020). Effects of CeO2 on the Si Precipitation Mechanism of SiCp/Al-Si Composite Prepared by Powder Metallurgy. Materials, 13(19), 4365. https://doi.org/10.3390/ma13194365





