Pore Filling Effect of Forced Carbonation Reactions Using Carbon Dioxide Nanobubbles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Specimen Preparation
2.2. Immersion Prodecure and Measurements
2.3. Microscopic Investigations
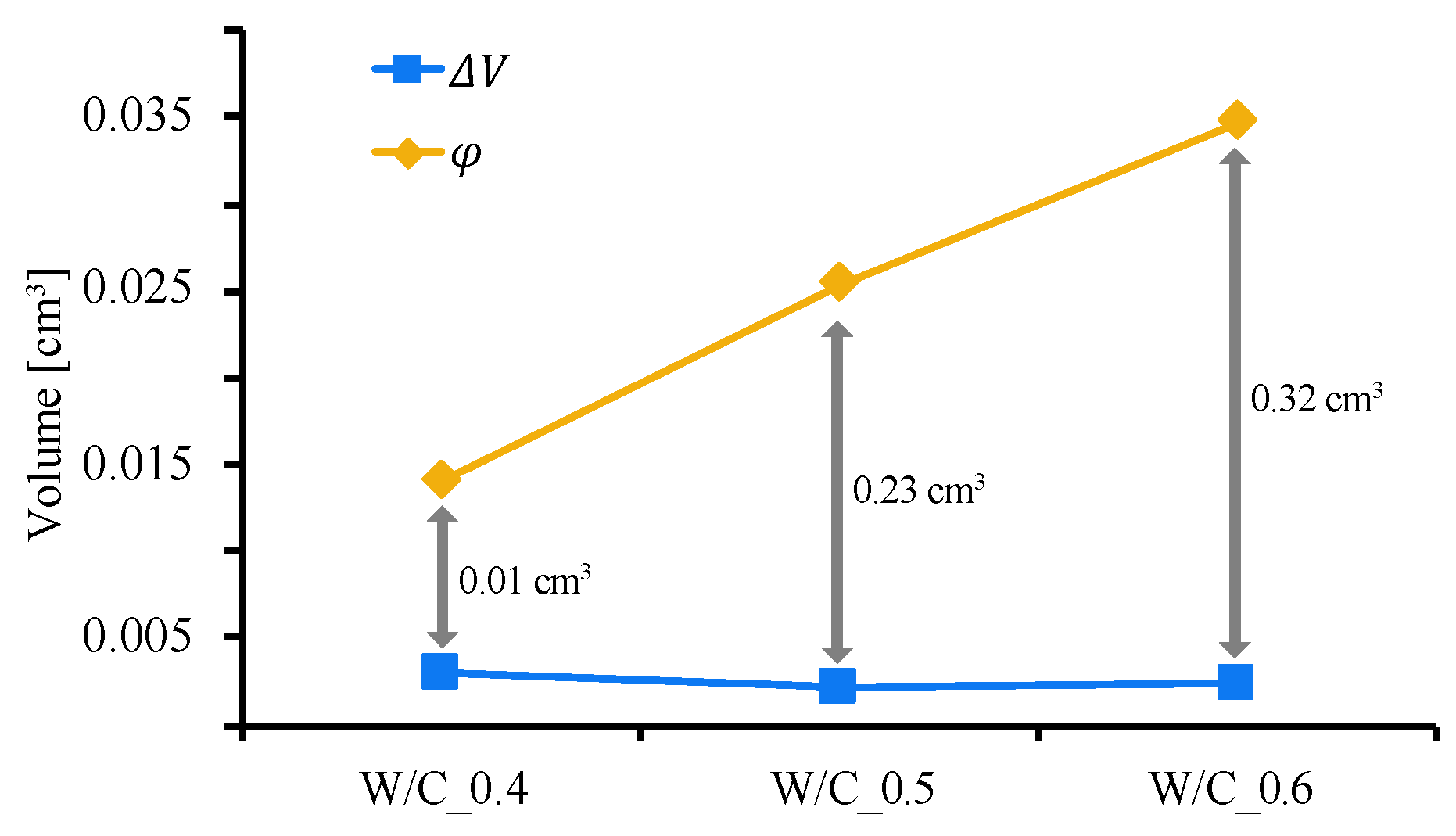
3. Results and Discussion
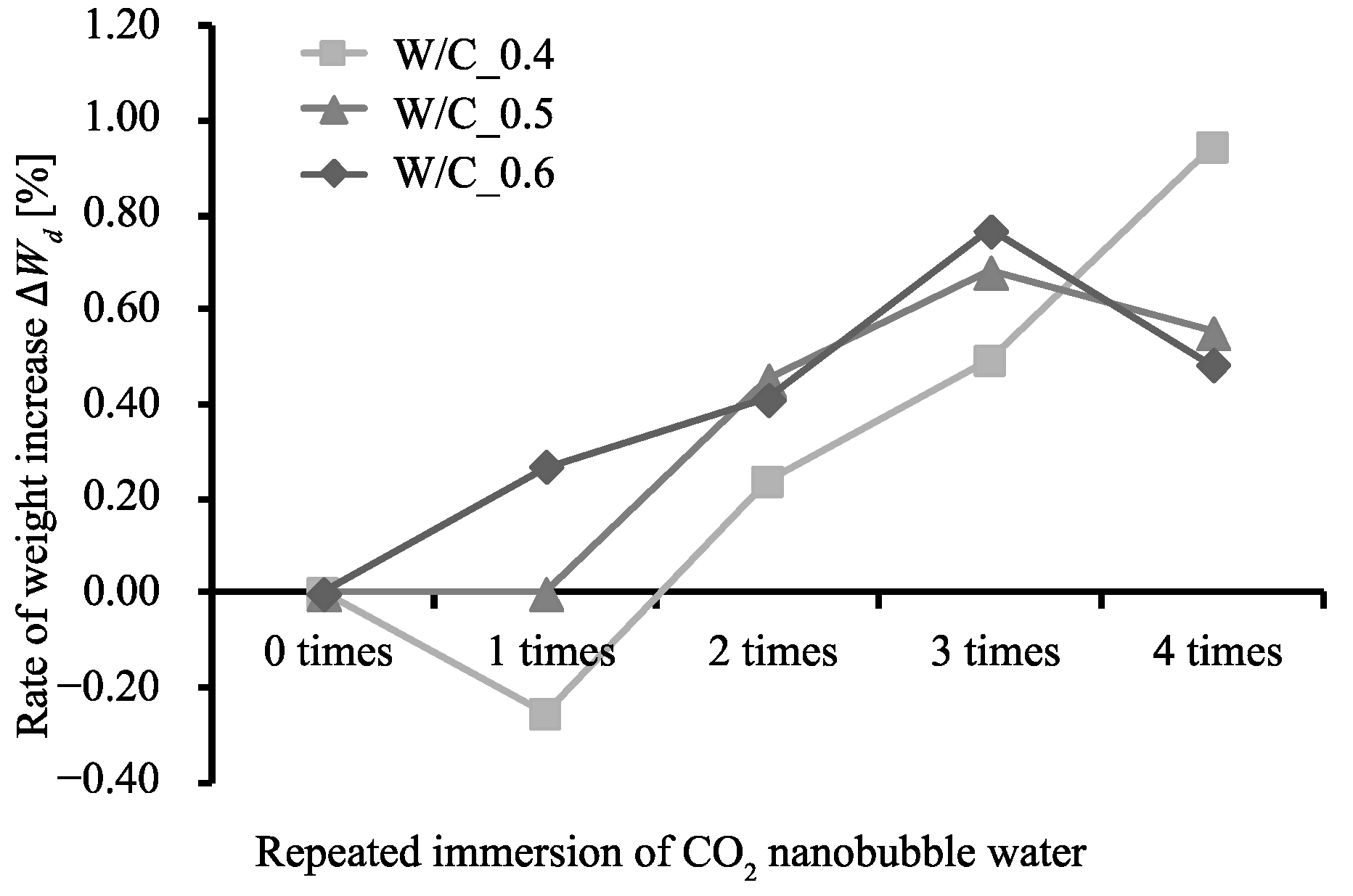
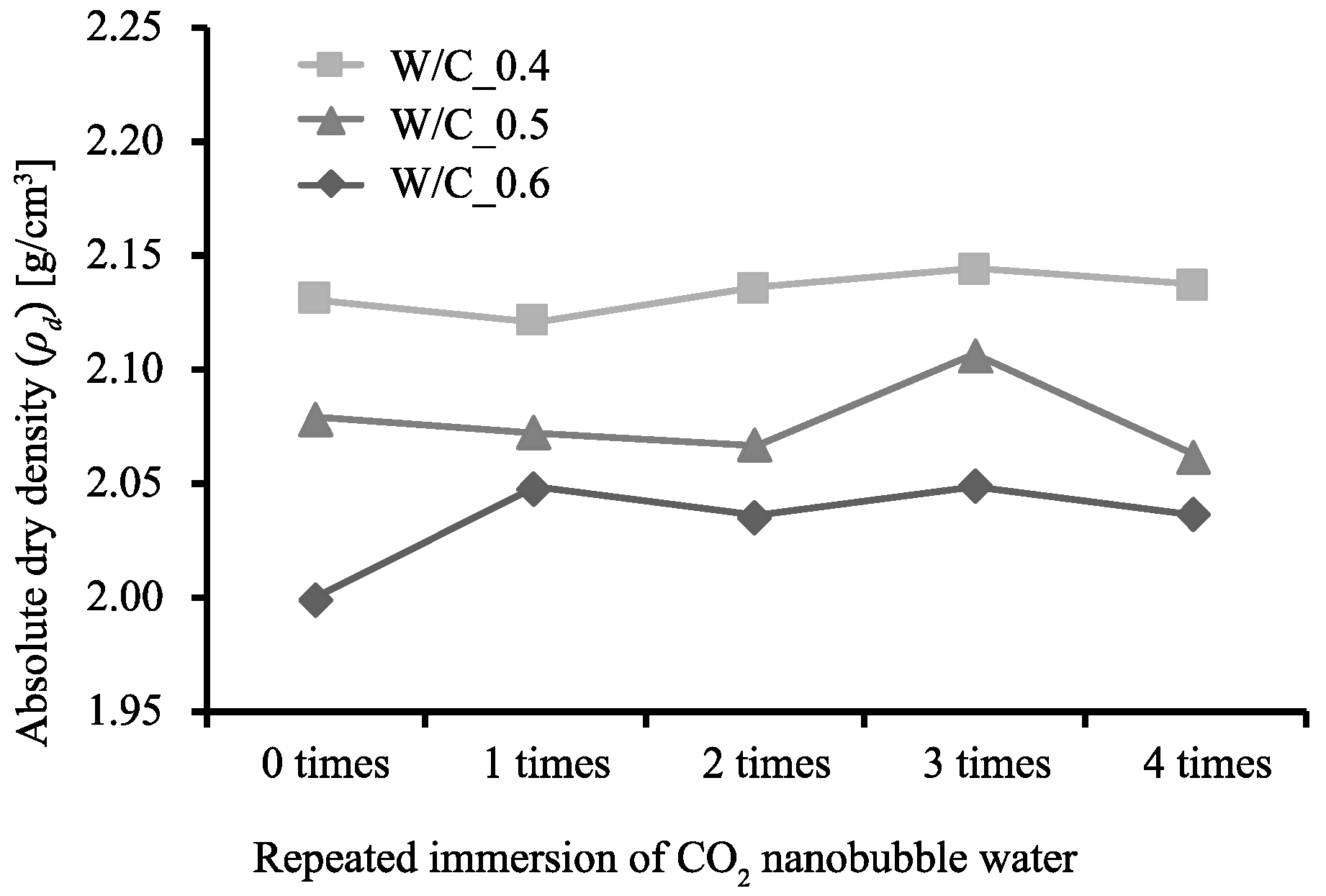
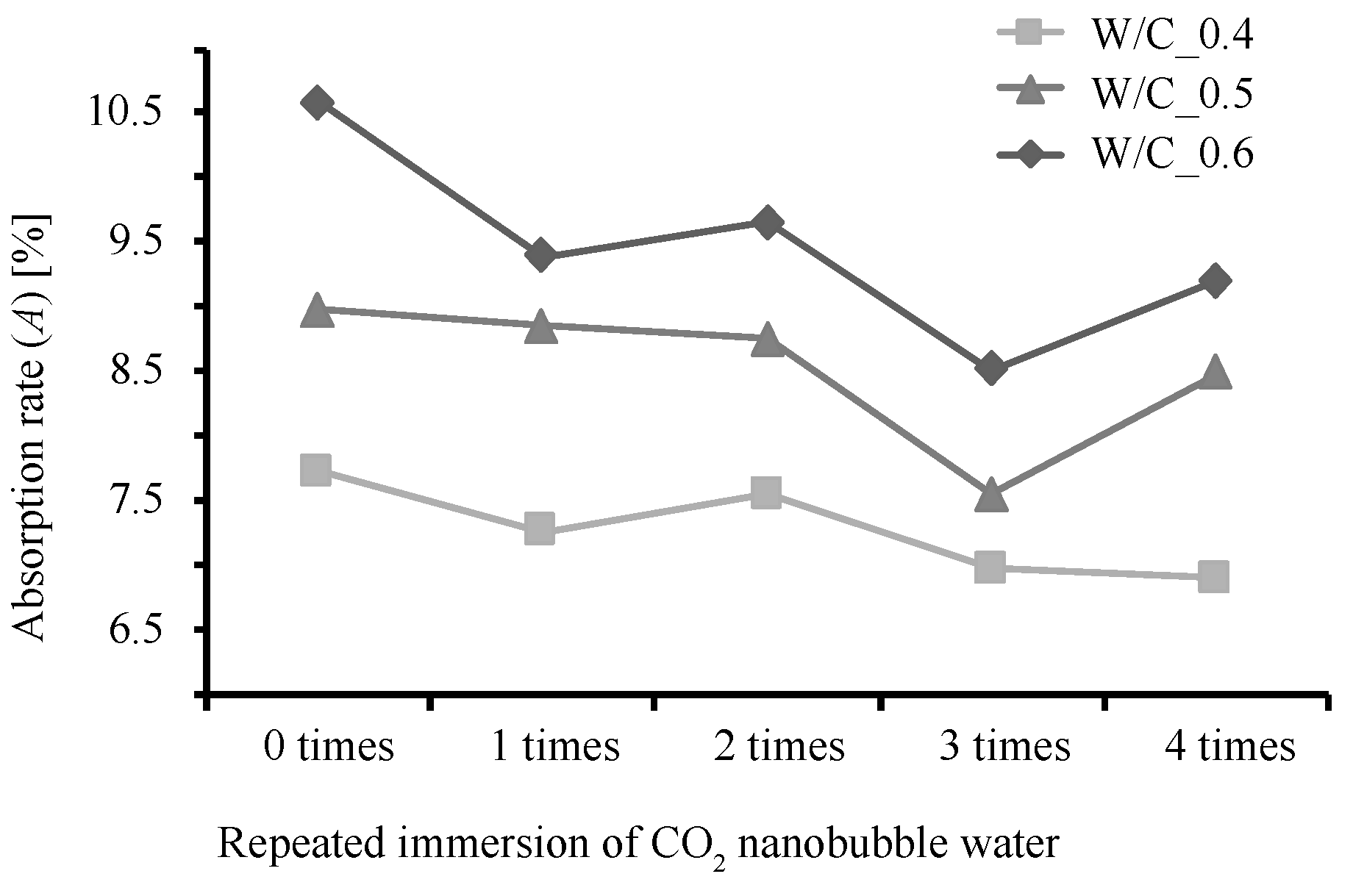
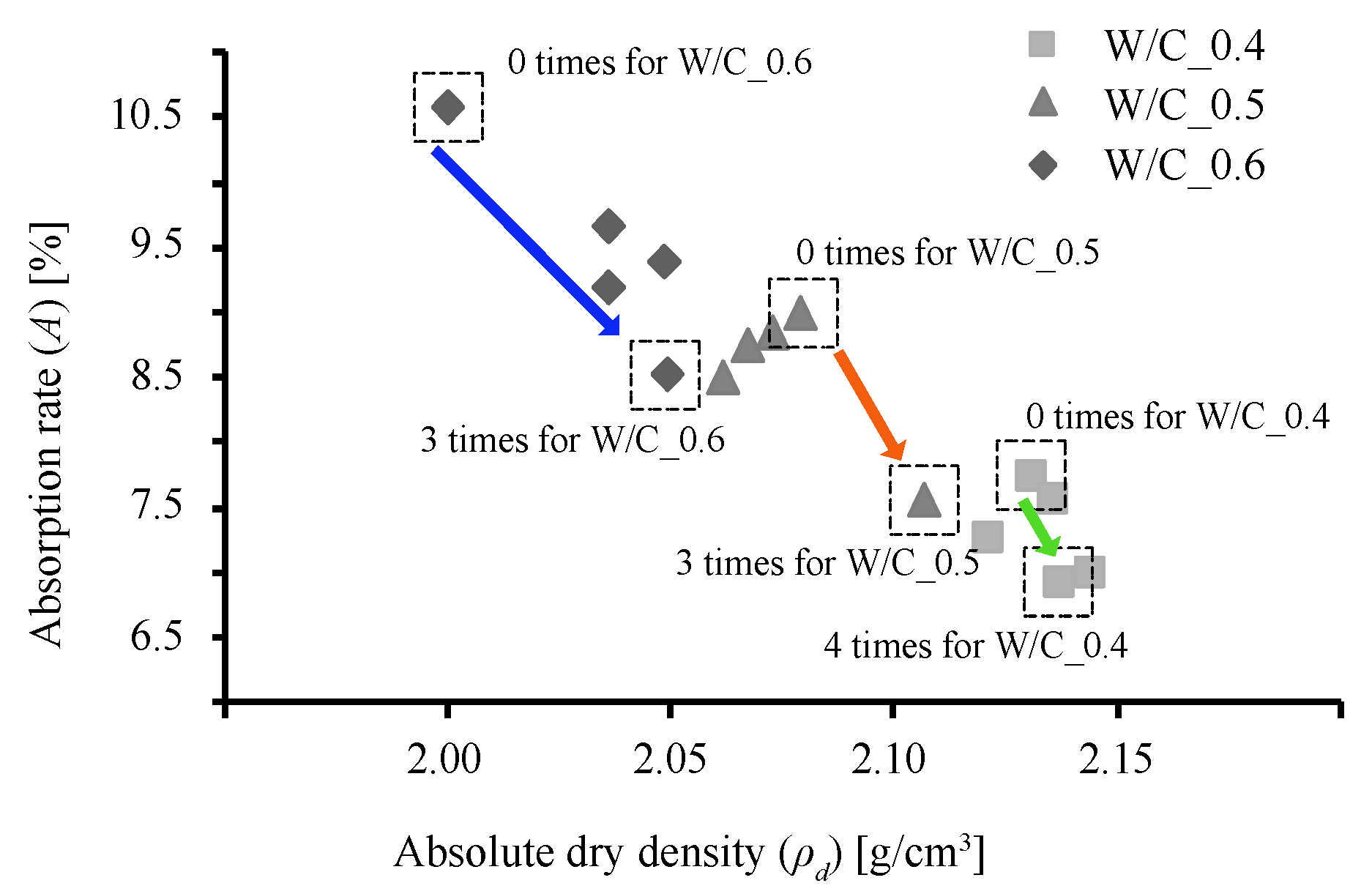
3.1. Rate of Weight Increase, Absolute Dry Density, and Rate of Water Absorption
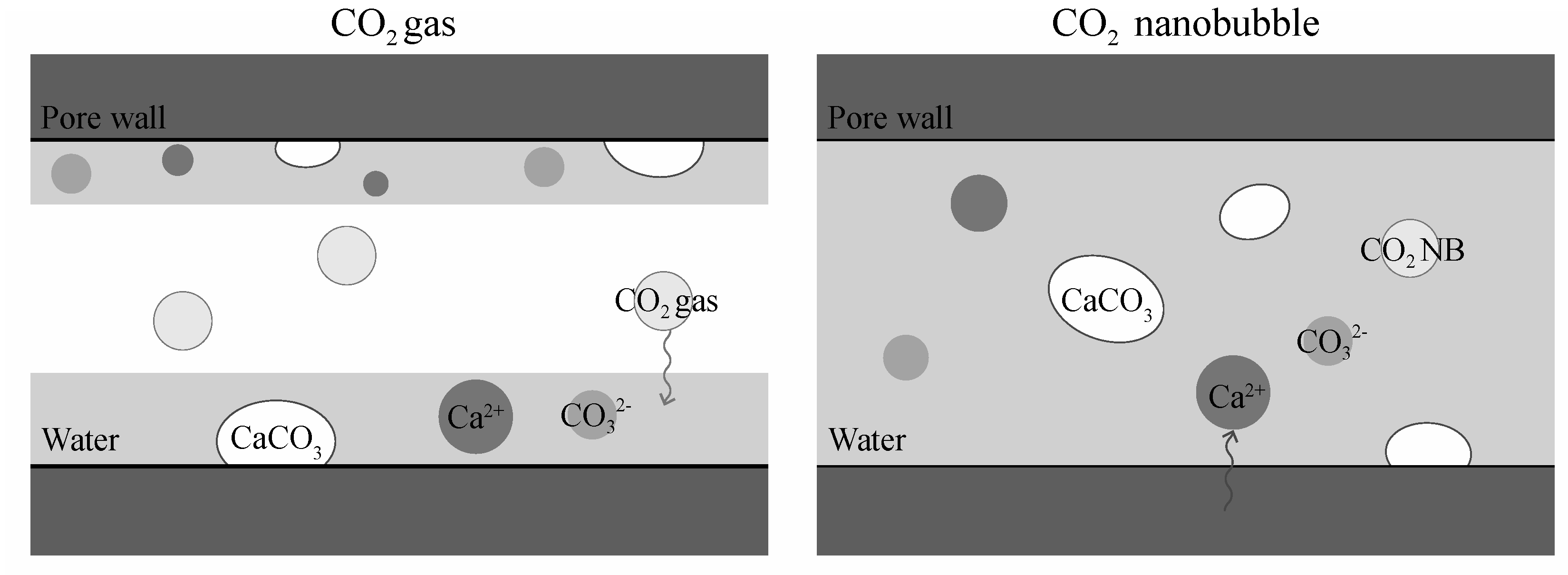
3.2. Pore Filling Effect of CO2 Nanobubble Water
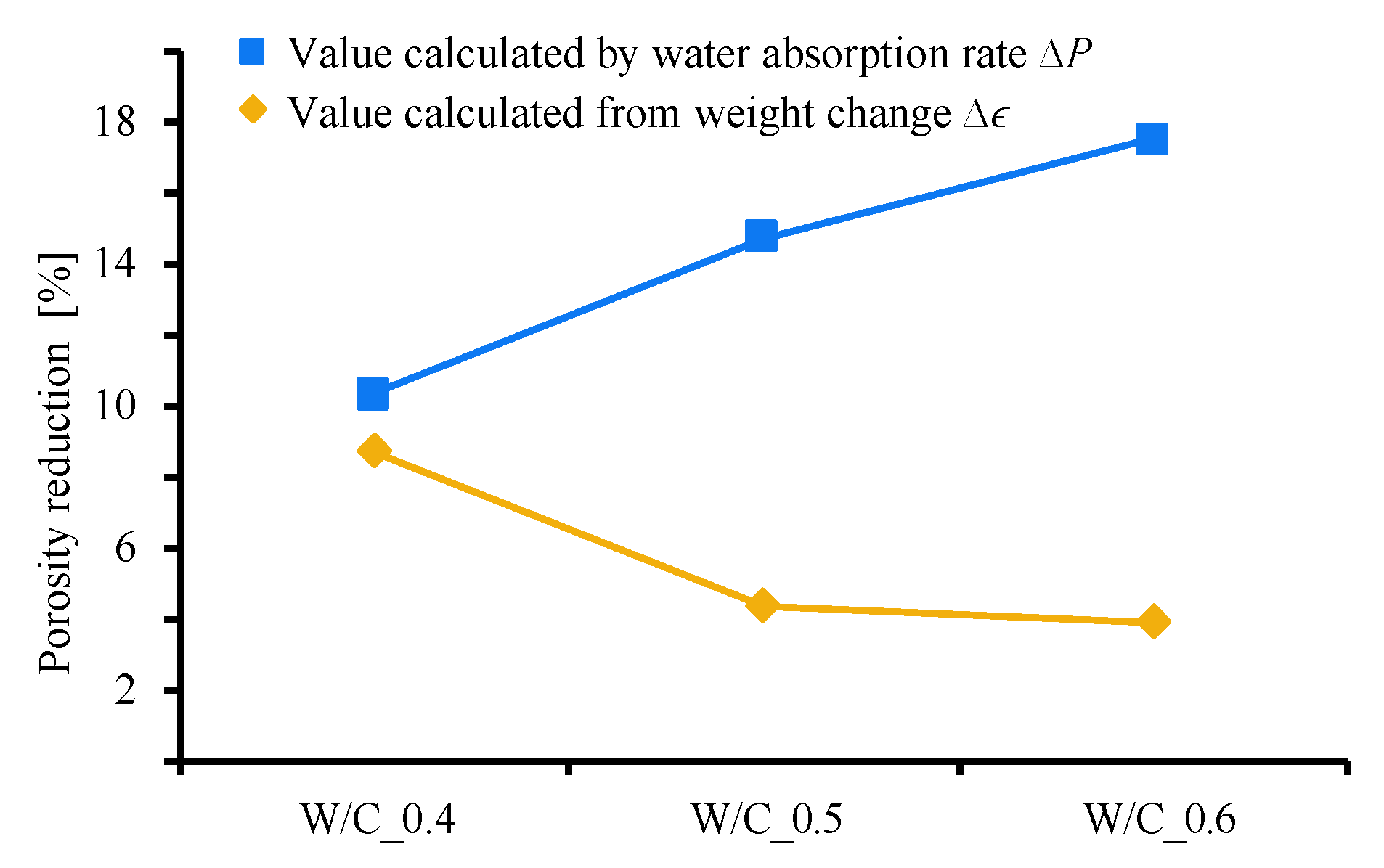
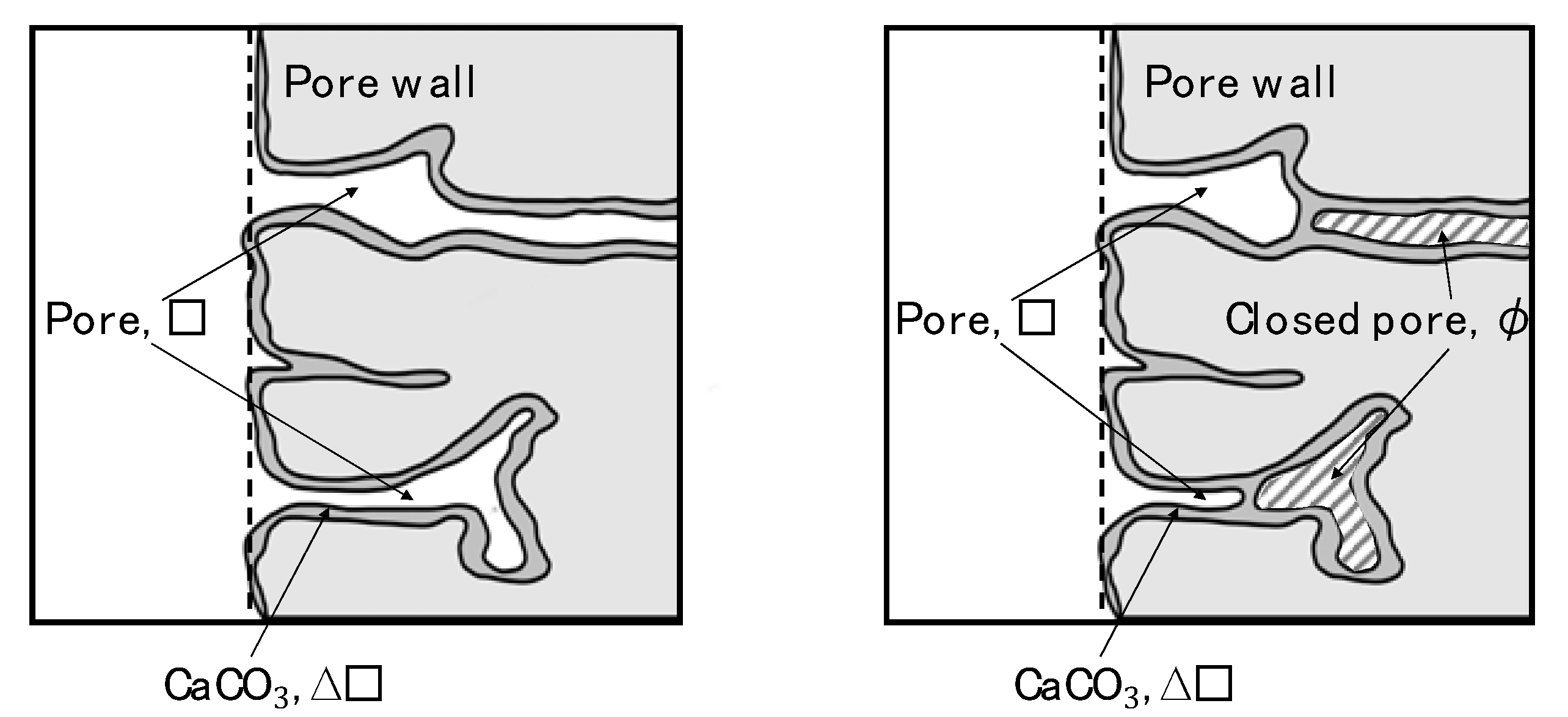
3.3. Quantification of Pore Closing Effect by CO2 Nanobubbles
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Yamabe, Y.; Tani, A.; Kawamura, H. Simulation of a recurrent architecture network based circulation society. J. Environ. Eng. 2008, 73, 253–260. (In Japanese) [Google Scholar] [CrossRef]
- Japan Concrete Institute. Practical Guideline for Investigation, Repair and Strengthening of Cracked Concrete Structure; Japan Concrete Institute (JCI): Tokyo, Japan, 2013. (In Japanese) [Google Scholar]
- CEB. Durable Concrete Structures; Thomas, T., Ed.; CEB Design Guide: London, UK, 1992. [Google Scholar]
- Branko, Š.; Mladena, L. Carbonation of cement paste: Understanding, challenges, and opportunities. Constr. Build. Mater. 2016, 117, 284–301. [Google Scholar]
- Vahid, R.; Yixin, S.; Andrew, J.B.; Zhen, H. Microstructure of cement paste subject to early carbonation curing. Cem. Concr. Res. 2012, 42, 186–193. [Google Scholar]
- Bao, J.Z.; Dong, X.X.; Chi, S.P.; Cai, J.S. Effect of curing parameters on CO2 curing of concrete blocks containing recycled aggregates. Cem. Concr. Compos. 2016, 71, 122–130. [Google Scholar]
- Shao, Y.; Mirza, M.S.; Wu, X. CO2 sequestration using calcium-silicate concrete. Can. J. Civ. Eng. 2006, 33, 776–784. [Google Scholar] [CrossRef]
- Kashef-Haghighi, S.; Ghoshal, S. CO2 sequestration in concrete through accelerated carbonation curing in a flow-through reactor. Ind. Eng. Chem. Res. 2009, 49, 1143–1149. [Google Scholar] [CrossRef]
- Shao, Y.; Monkman, S.; Boyd, A. Recycling carbon dioxide into concrete: A feasibility study. In Proceedings of the 2010 Concrete Sustainability Conference, Dubai, UAE, 13–14 December 2010. [Google Scholar]
- Rostami, V.; Shao, Y.; Boyd, A.J. Carbonation curing versus steam curing for precast concrete production. J. Mater. Civ. Eng. 2012, 24, 1221–1229. [Google Scholar] [CrossRef]
- Mo, L.; Panesar, D.K. Accelerated carbonation–a potential approach to sequester CO2 in cement paste containing slag and reactive MgO. Cem. Concr. Compos. 2013, 43, 69–77. [Google Scholar] [CrossRef]
- Chang, J.; Fang, Y.; Shang, X. The role of b-C2S and c-C2S in carbon capture and strength development. Mater. Struct. 2016, 49, 4417–4424. [Google Scholar] [CrossRef]
- Edvardsen, C. Water permeability and autogenous healing of cracks in concrete. ACI Mater. J. 1999, 96, 448–454. [Google Scholar]
- Jacobsen, S. SEM Observations of the microstructure of frost deteriorated and self-healed concrete. Cem. Concr. Res. 1995, 25, 1781–1790. [Google Scholar] [CrossRef]
- Sanjun, M.A. Effectiveness of crack control at early age on the corrosion of steel bars in low modulus sisal and coconut fiber-reinforced mortars. Cem. Concr. Res. 1998, 28, 555–565. [Google Scholar] [CrossRef]
- Shao, Y.; Rostami, V.; He, Z.; Boyd, A.J. Accelerated carbonation of Portland limestone cement. J. Mater. Civ. Eng. 2013, 26, 117–124. [Google Scholar] [CrossRef]
- Tsuge, H. Fundamentals of Microbubbles and Nanobubbles. Bull. Soc. Sea Water Sci. 2010, 64, 4–10. (In Japanese) [Google Scholar]
- Kitagaki, R. Rapid Carbonation Method to Concrete Surface Using Micro/Nano Bubble. J. Jap. Soc. Pre. Eng. 2017, 83, 647–650. [Google Scholar] [CrossRef]
- Xyla, A.G.; Koutsoukos, P.G. Quantitative Analysis of Calcium Carbonate Polymorphs by Infrared Spectroscopy. J. Chem. Soc. Faraday Trans. 1989, 85, 3165–3172. [Google Scholar] [CrossRef]
- Kojima, K.; Sadatomo, A.; Yasue, T.; Arai, Y. Control of Crystal Shape and Modification of Calcium Carbonate Prepared by Precipitation from Calcium Hydrogen Carbonate Solution. J. Ceram. Soc. 1992, 100, 1145–1153. [Google Scholar] [CrossRef] [Green Version]
- Chakraborty, D.; Agarwal, V.K.; Bhatia, S.K.; Bellare, J. Steady-State Transitions and Polymorph Transformations in Continuous Precipitation of Calcium Carbonate. Ind. Eng. Chem. Res. 1994, 33, 2187–2197. [Google Scholar] [CrossRef]
- Kitamura, M.; Konno, H.; Yasui, A.; Masuoka, H. Controlling Factors and Mechanism of Reactive Crystallization of Calcium Carbonate Polymorphs from Calcium Hydroxide Suspensions. J. Cryst. Growth 2002, 236, 323–332. [Google Scholar] [CrossRef]
- Matsumoto, M.; Fukunaga, T.; Suzuki, M.; Onoe, K. Reactive Crystallization of Calcium Carbonate Using CO2 Micro-Bubbles. Bull. Soc. Sea Water Sci. 2002, 56, 357–361. (In Japanese) [Google Scholar]
- Japanese Industrial Standards. Methods of Test for Density and Water Absorption of Fine Aggregates; JIS A 1109; Japanese Standards Association: Tokyo, Japan, 2020. [Google Scholar]
- Roncari, E.; Galassi, C.; Craciun, F.; Capiani, C.; Piancastelli, A. A microstructural study of porous piezoelectric ceramics obtained by different methods. J. Eur. Cer. Soc. 2001, 21, 409–417. [Google Scholar] [CrossRef]
- Japan Cement Association. Data Collection with Commentary on Cement and Concrete Chemistry; Japan Cement Association: Tokyo, Japan, 2016. [Google Scholar]
- Papadakis, V.G.; Vayenas, C.G.; Fardis, M.N. Physical and Chemical Characteristics Affecting the Durability of Concrete. ACI Mat. J. 1991, 8, 186–196. [Google Scholar]
- Brunauer, S.; Copeland, L.E. Chemistry of Concrete. Sci. Am. 1964, 210, 80–92. [Google Scholar] [CrossRef]
- Taylor, H.F.W. Chemistry of Cement Hydration. In Proceedings of the 8th International Congress on the Chemistry of Cement, Rio de Janeiro, Brazil, 22–27 September 1986; Volume 1, pp. 82–110. [Google Scholar]
- Bauer, T.; Lunkenheimer, P.; Loidl, A. Cooperativity and the freezing of molecular motion at the glass transition. Phys. Rev. Lett. 2013, 111, 225072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neves, J.A.; Toledo, F.R.D.; Fairbairn, E.D.M.R.; Dweck, J. A study of the carbonation profile of cement pastes by thermogravimetry and its effect on the compressive strength. J. Therm. Anal. Calorim. 2014, 116, 69–76. [Google Scholar] [CrossRef]
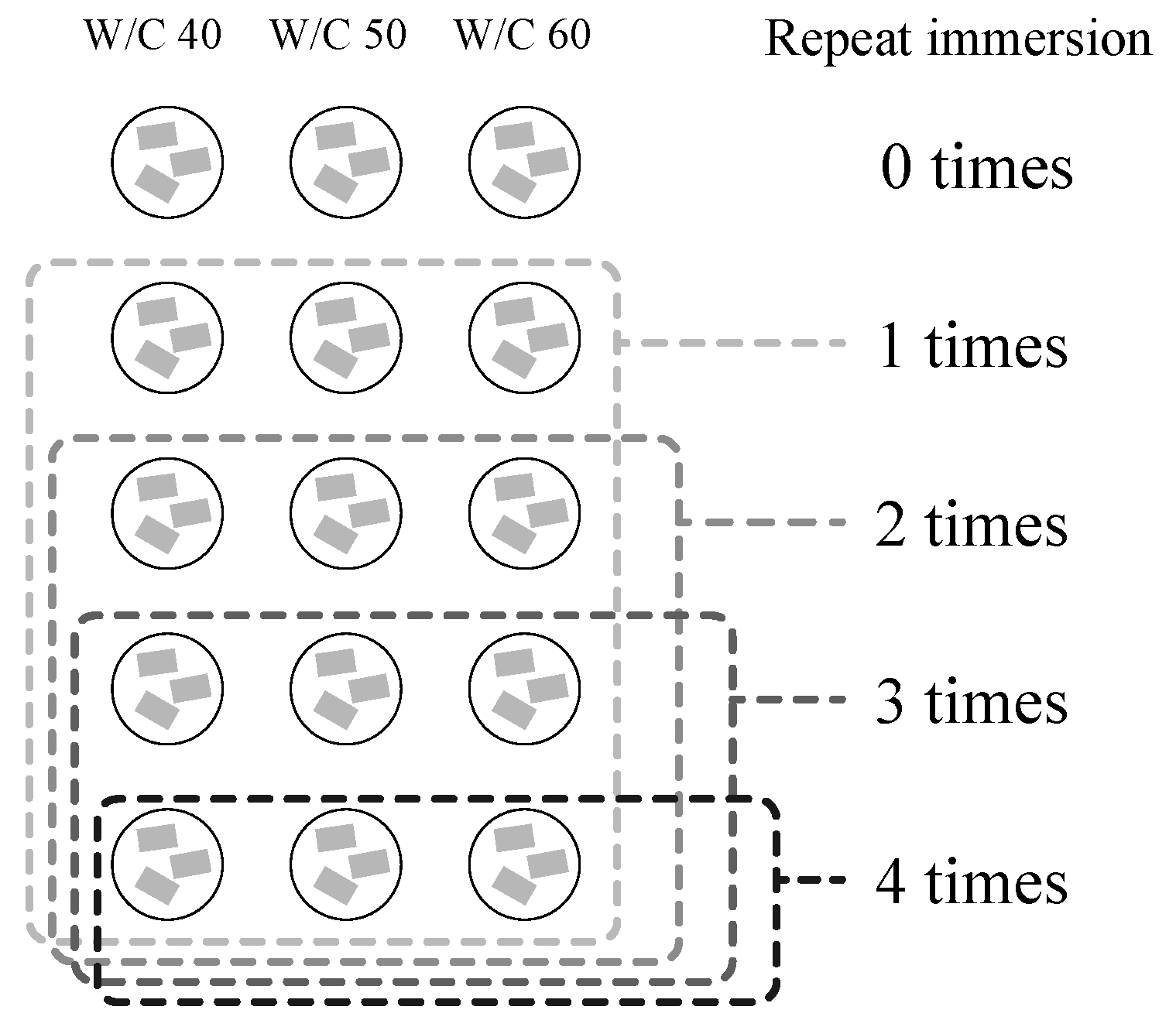

| Type | Water/Cement Ratio | Cement (g) | Water (g) | Fine-Aggregate (g) |
|---|---|---|---|---|
| W/C_0.4 | 0.4 | 114.0 | 45.6 | 225.0 |
| W/C_0.5 | 0.5 | 100.0 | 50.0 | 225.0 |
| W/C_0.6 | 0.6 | 89.0 | 53.4 | 225.0 |
| OPC | CaO | SiO2 | Al2O3 | MgO | SO3 | Fe2O3 | K2O | TiO2 | LOI |
|---|---|---|---|---|---|---|---|---|---|
| (wt%) | 68.11 | 18.43 | 2.85 | 2.37 | 3.03 | 2.17 | 1.10 | 0.12 | 1.82 |
| Turn Number | Process of Immersion | Method | Time |
|---|---|---|---|
| 1 | CO2 nanobubble water | Natural immersion | 30 min |
| 2 | Acetone | Natural immersion | 30 min |
| 3 | Dry | Vacuum desiccator | 30 min |
| 4 | Acetone | Ultrasonic immersion | 2 min |
| 5 | Dry | Vacuum desiccator | 30 min |
| 6 | Ca(OH)2 saturated aqueous solution | Ultrasonic immersion | 2 min |
| 7 | Acetone | Natural immersion | 30 min |
| 8 | Acetone | Ultrasonic immersion | 2 min |
| 9 | Dry | Vacuum desiccator | ~Until next day |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.; Kitagaki, R.; Choi, H. Pore Filling Effect of Forced Carbonation Reactions Using Carbon Dioxide Nanobubbles. Materials 2020, 13, 4343. https://doi.org/10.3390/ma13194343
Kim J, Kitagaki R, Choi H. Pore Filling Effect of Forced Carbonation Reactions Using Carbon Dioxide Nanobubbles. Materials. 2020; 13(19):4343. https://doi.org/10.3390/ma13194343
Chicago/Turabian StyleKim, Jihoon, Ryoma Kitagaki, and Heesup Choi. 2020. "Pore Filling Effect of Forced Carbonation Reactions Using Carbon Dioxide Nanobubbles" Materials 13, no. 19: 4343. https://doi.org/10.3390/ma13194343
APA StyleKim, J., Kitagaki, R., & Choi, H. (2020). Pore Filling Effect of Forced Carbonation Reactions Using Carbon Dioxide Nanobubbles. Materials, 13(19), 4343. https://doi.org/10.3390/ma13194343





