Incorporation of Waste Glass as an Activator in Class-C Fly Ash/GGBS Based Alkali Activated Material
Abstract
1. Introduction
2. Materials and Methods
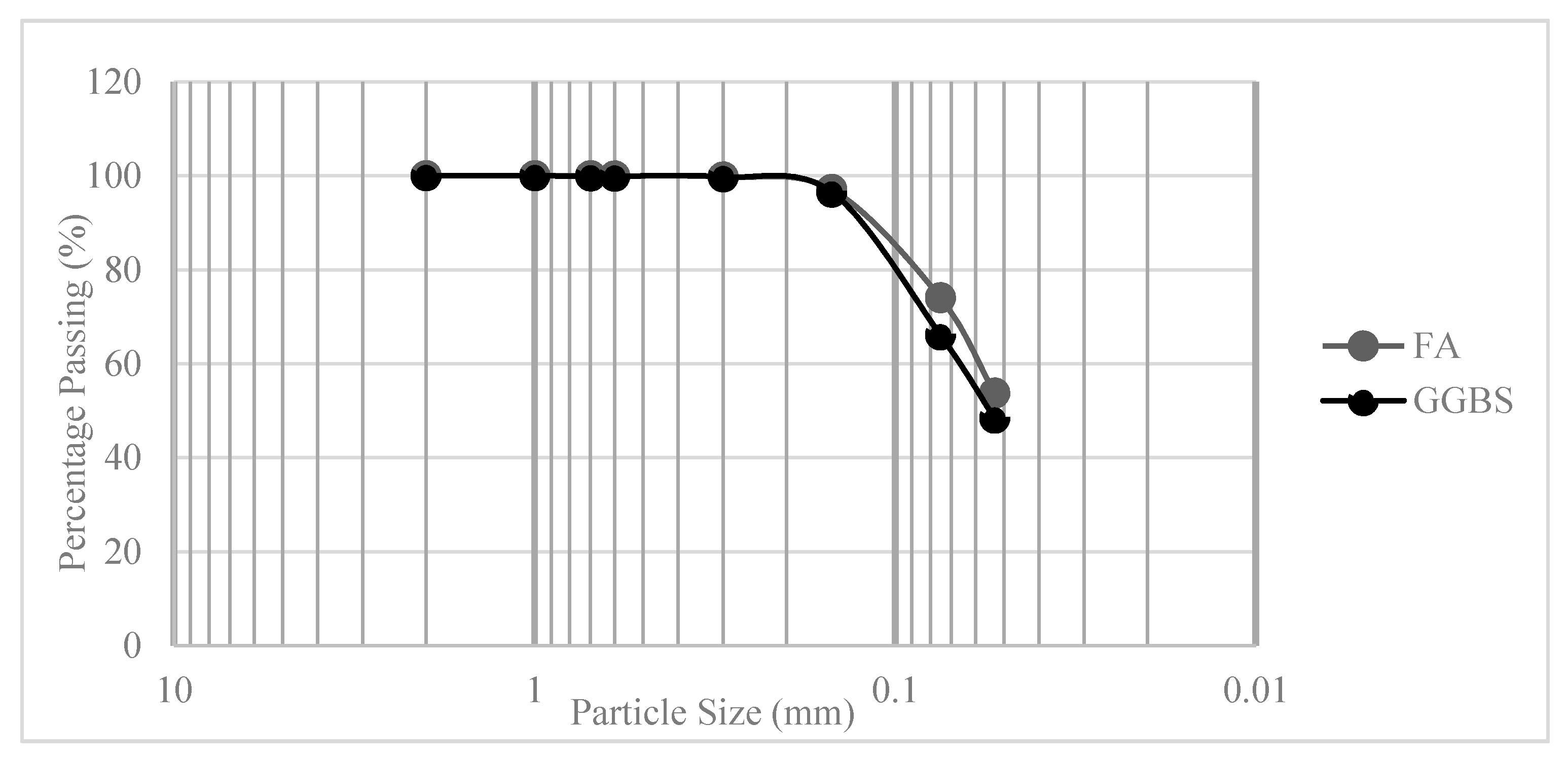
2.1. Materials, Mixture Proportions and Samples Synthesis Method
2.2. Experimental Plan
3. Experimental Results and Discussion
3.1. Chemical Composition of WGA-10, WGA-20 and WGA-30 Solution
3.2. Addition of Water to FA/GGBS Blends
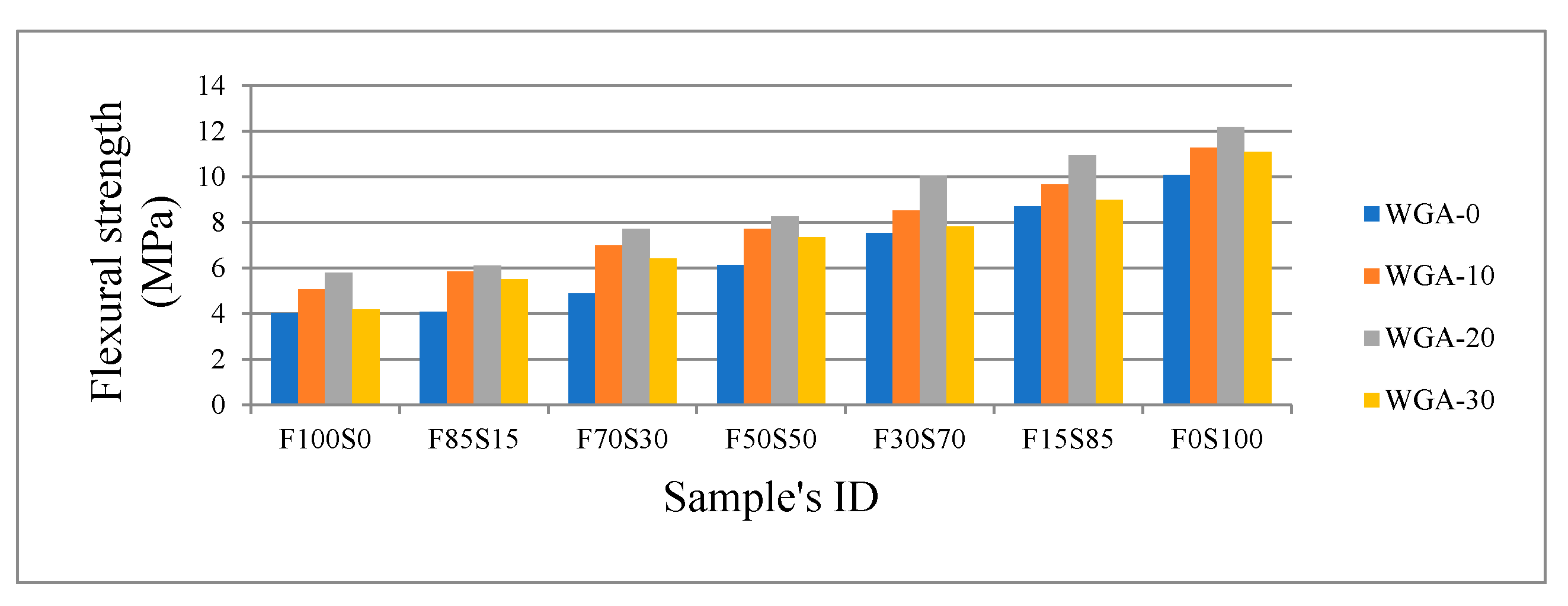
3.3. Mechanical Performance: Compressive Strength and Flexural Strength
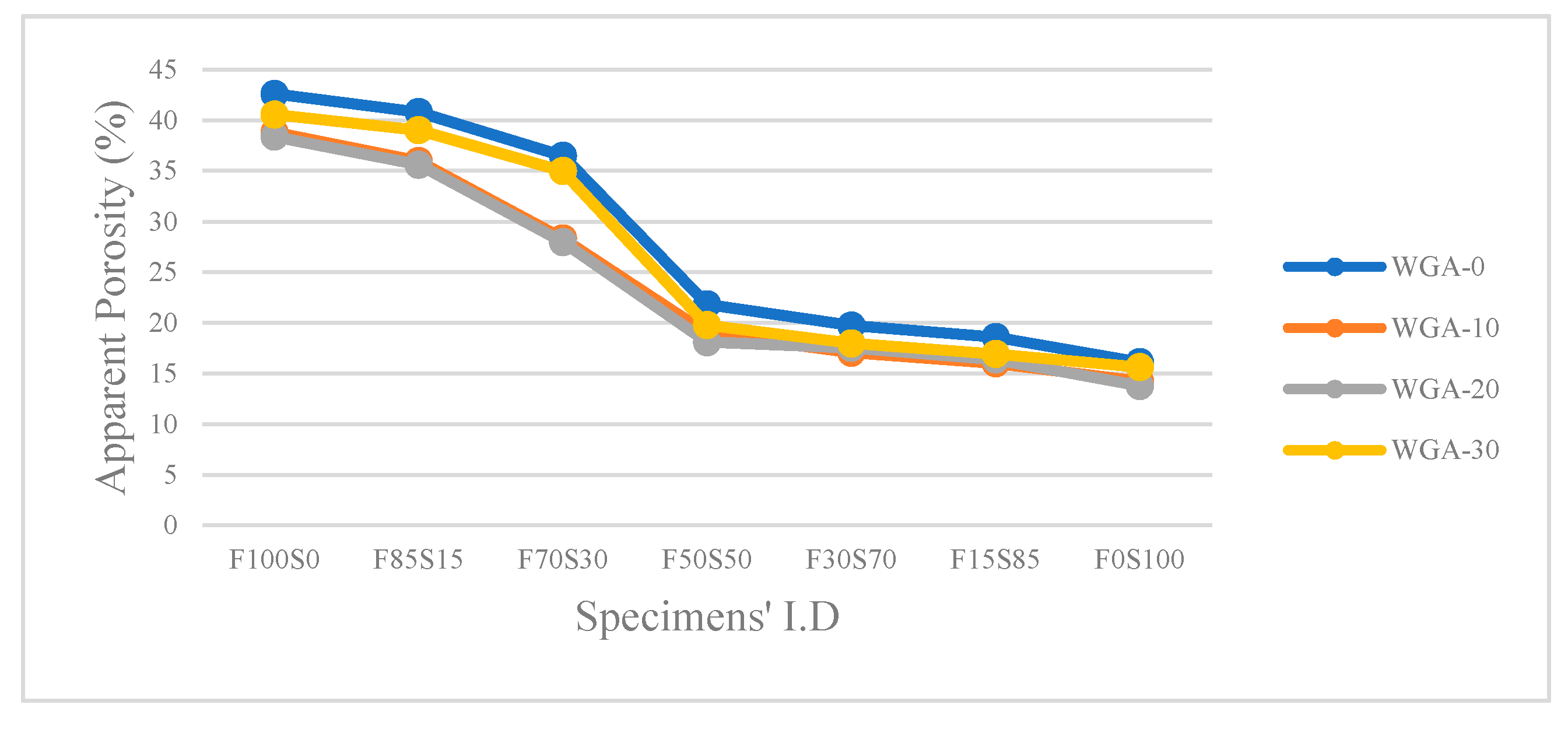
3.4. Apparent Porosity and Water Absorption
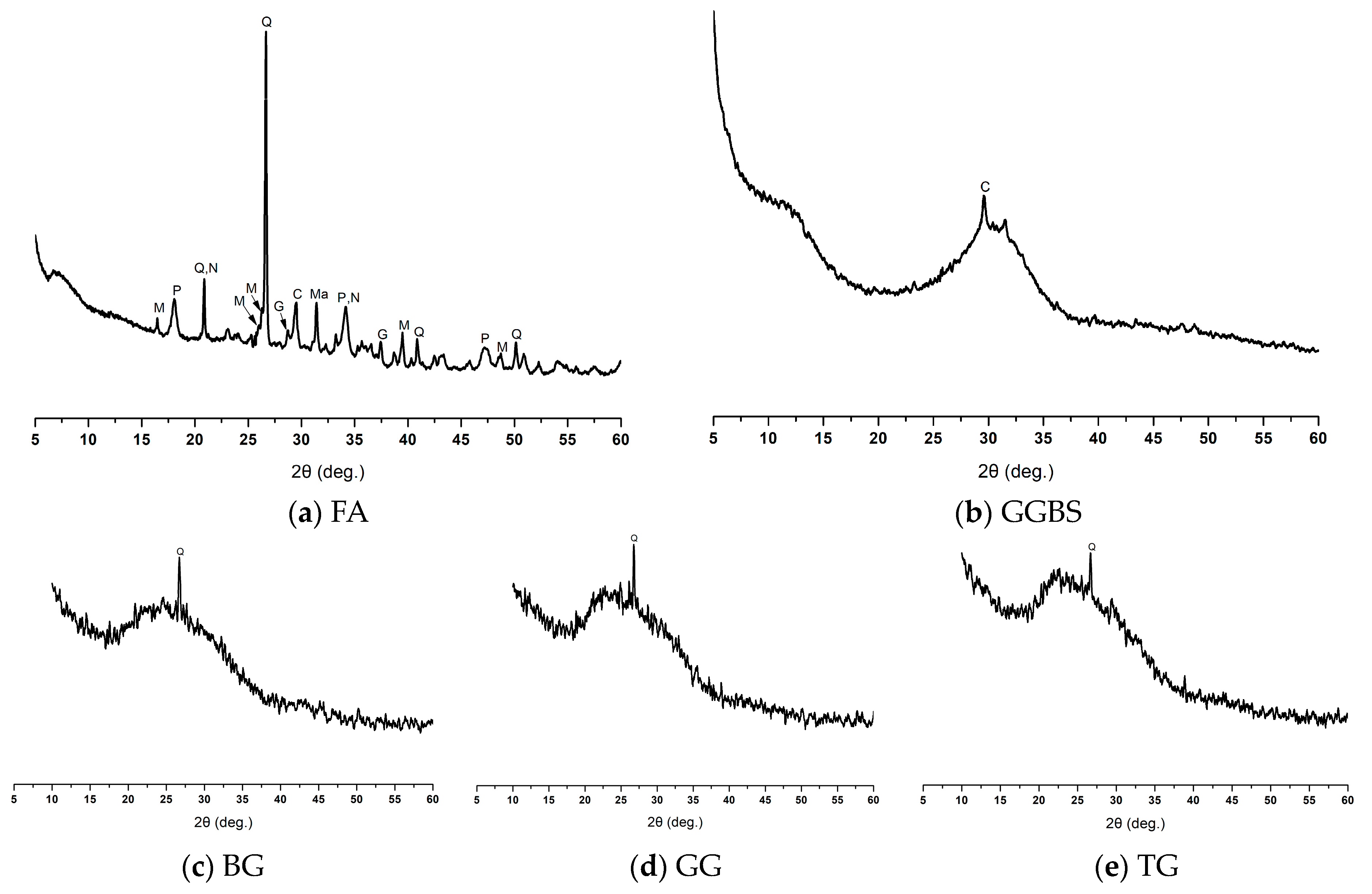
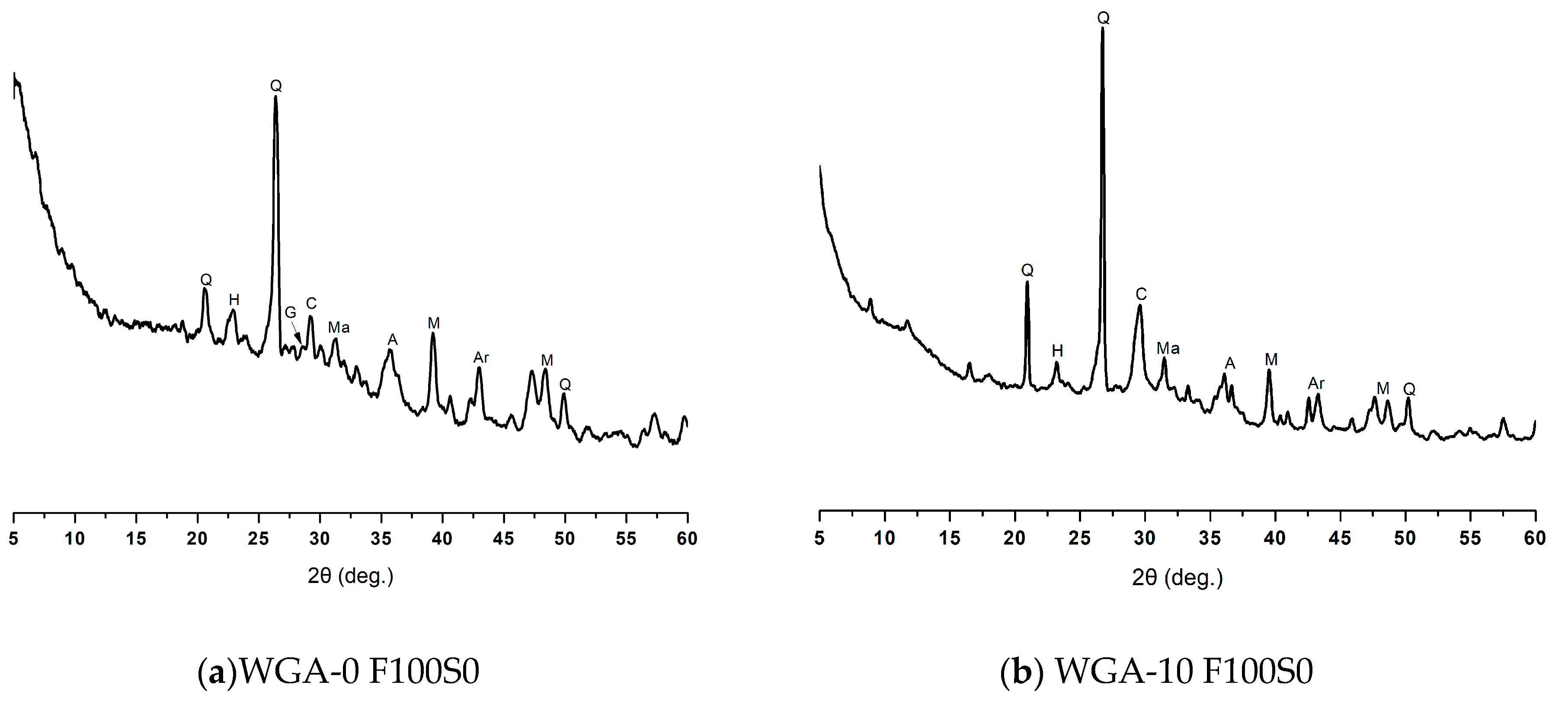
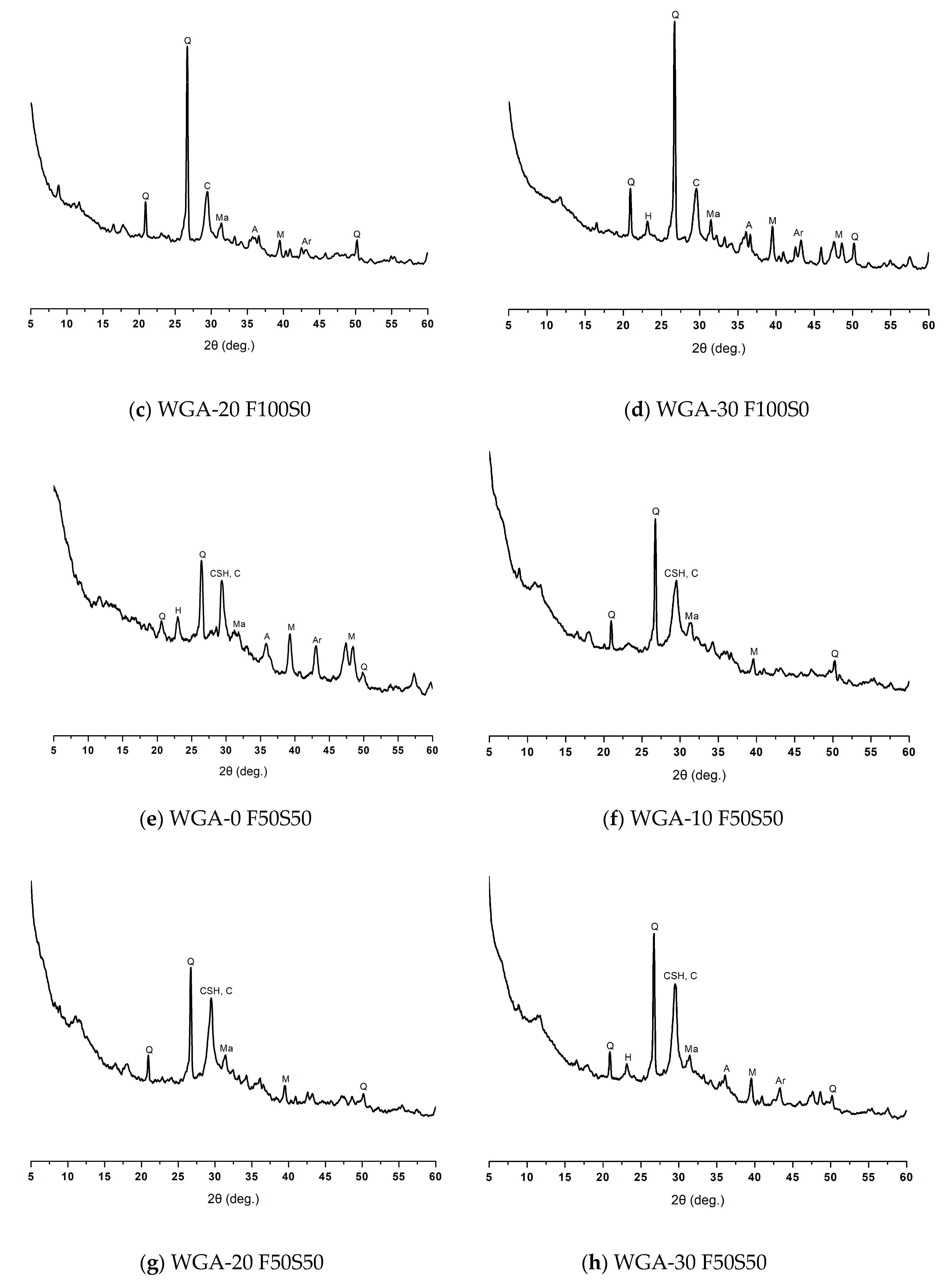
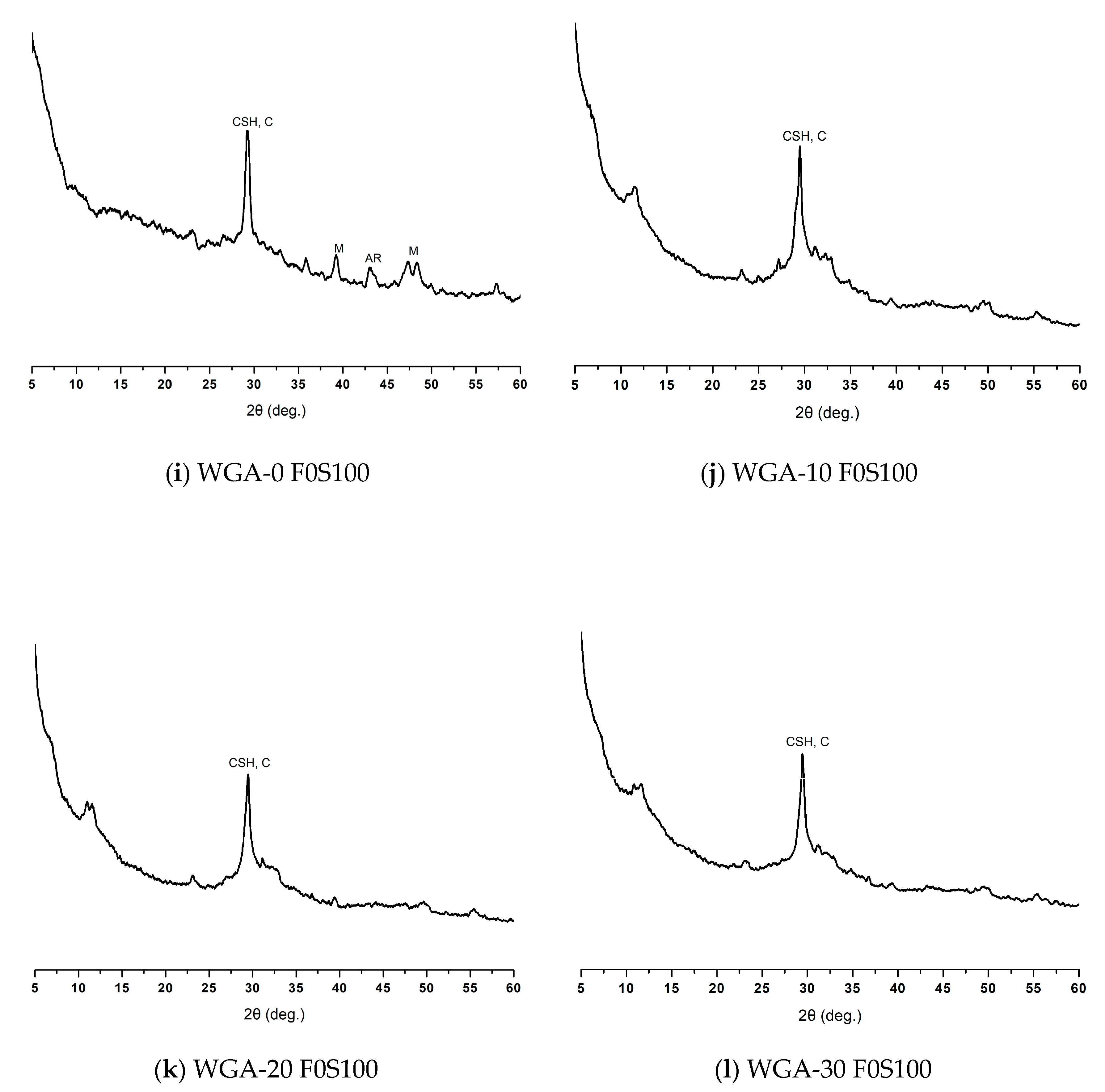
3.5. XRD Diffraction
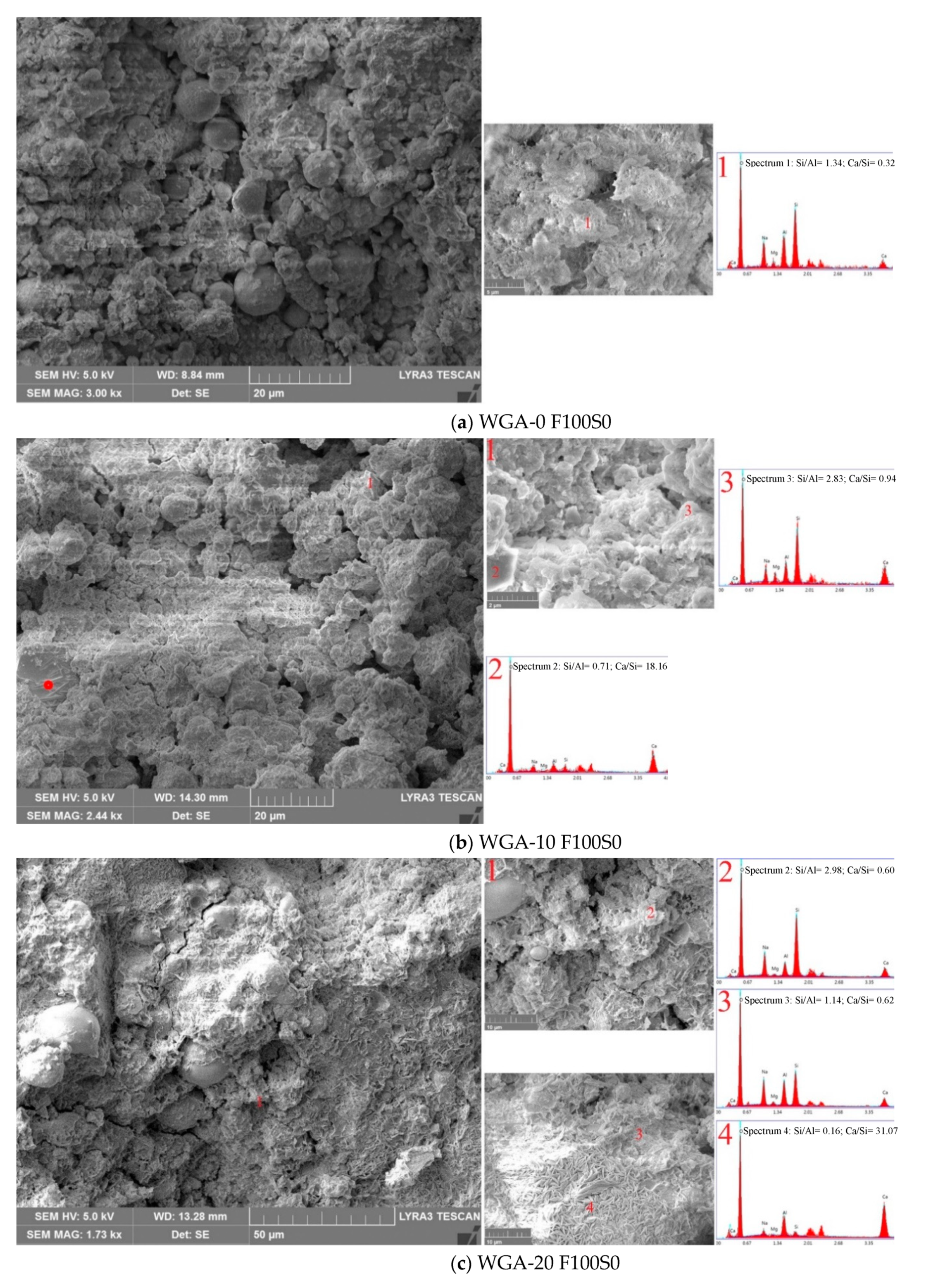
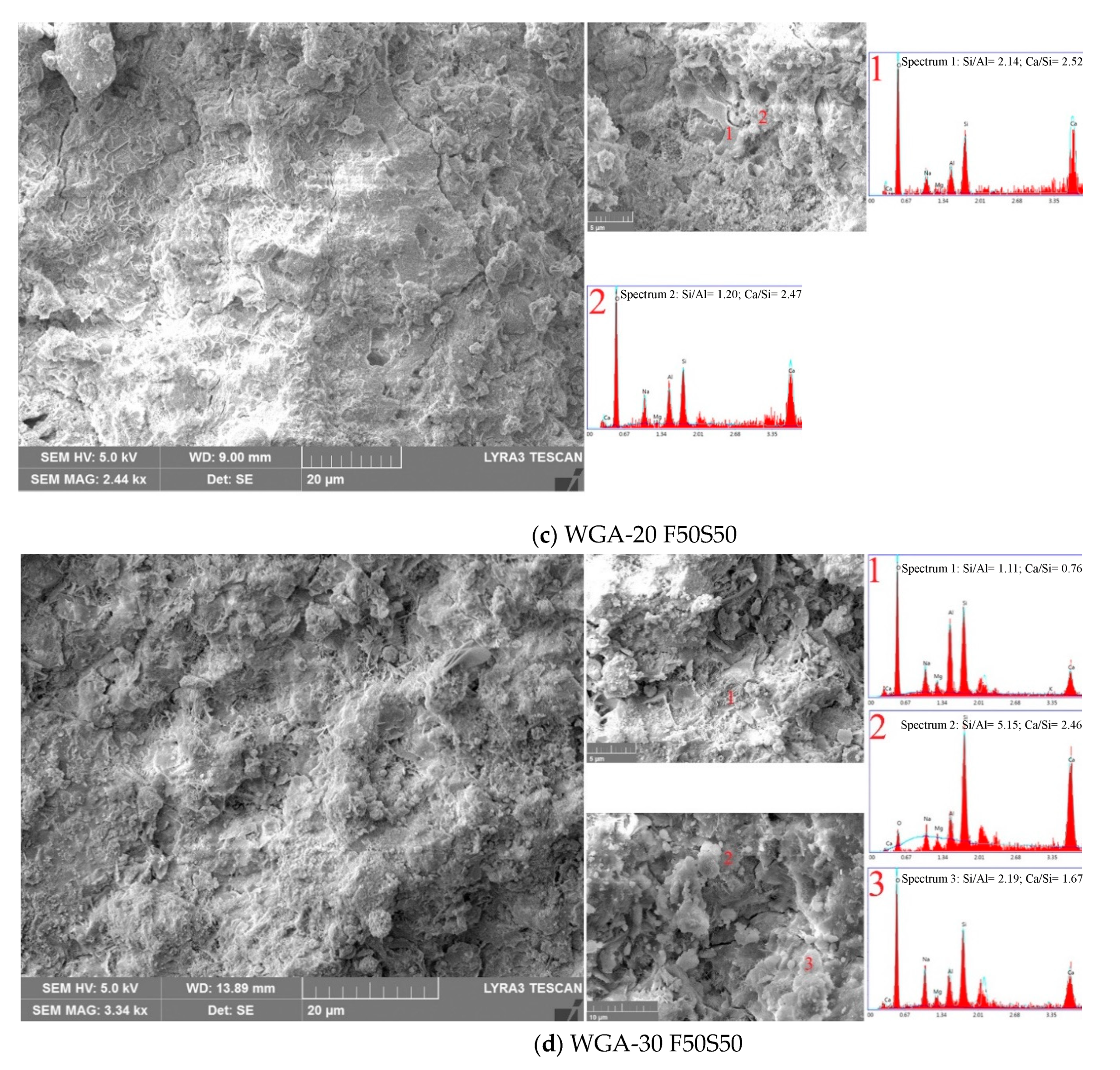
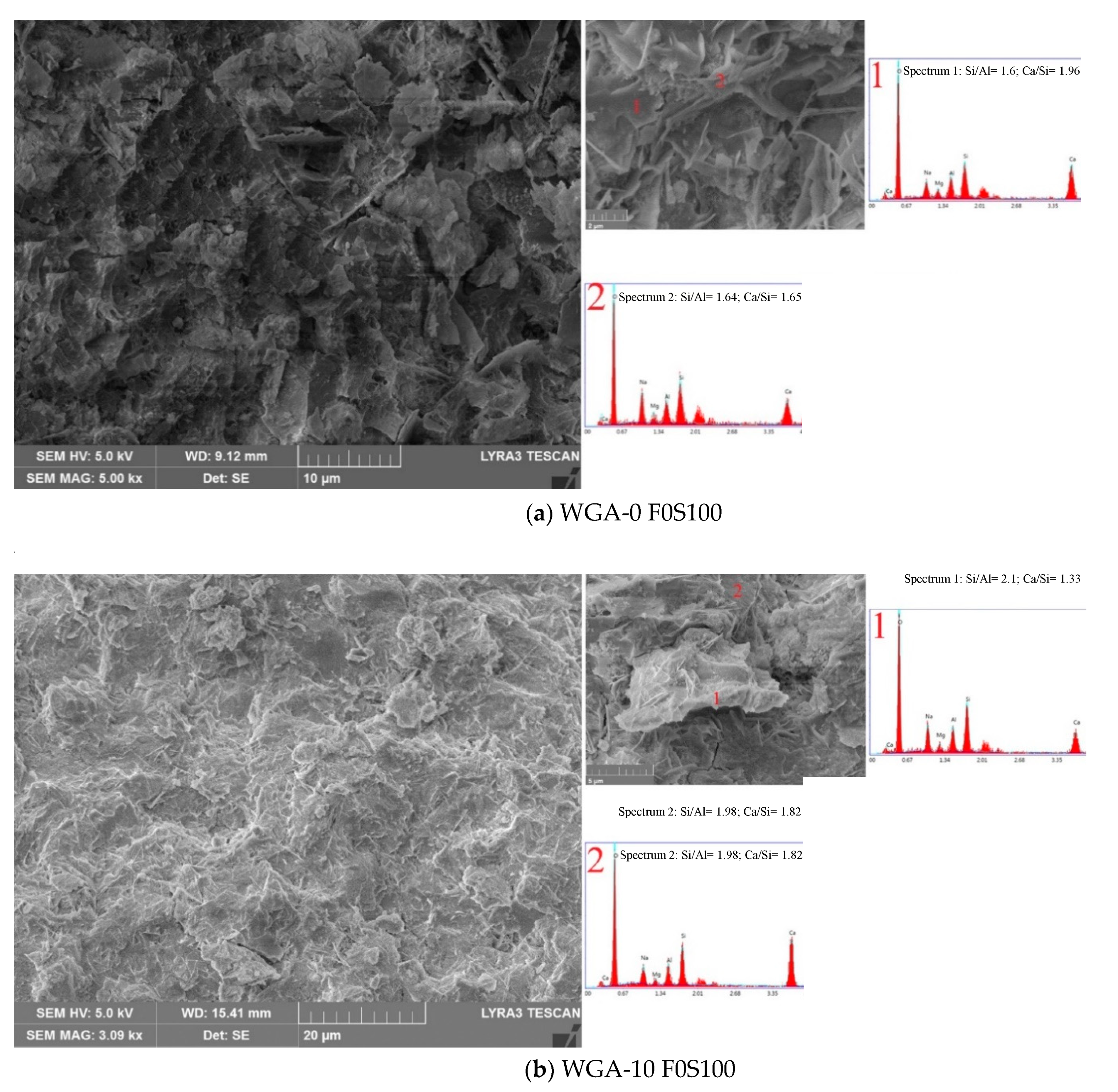
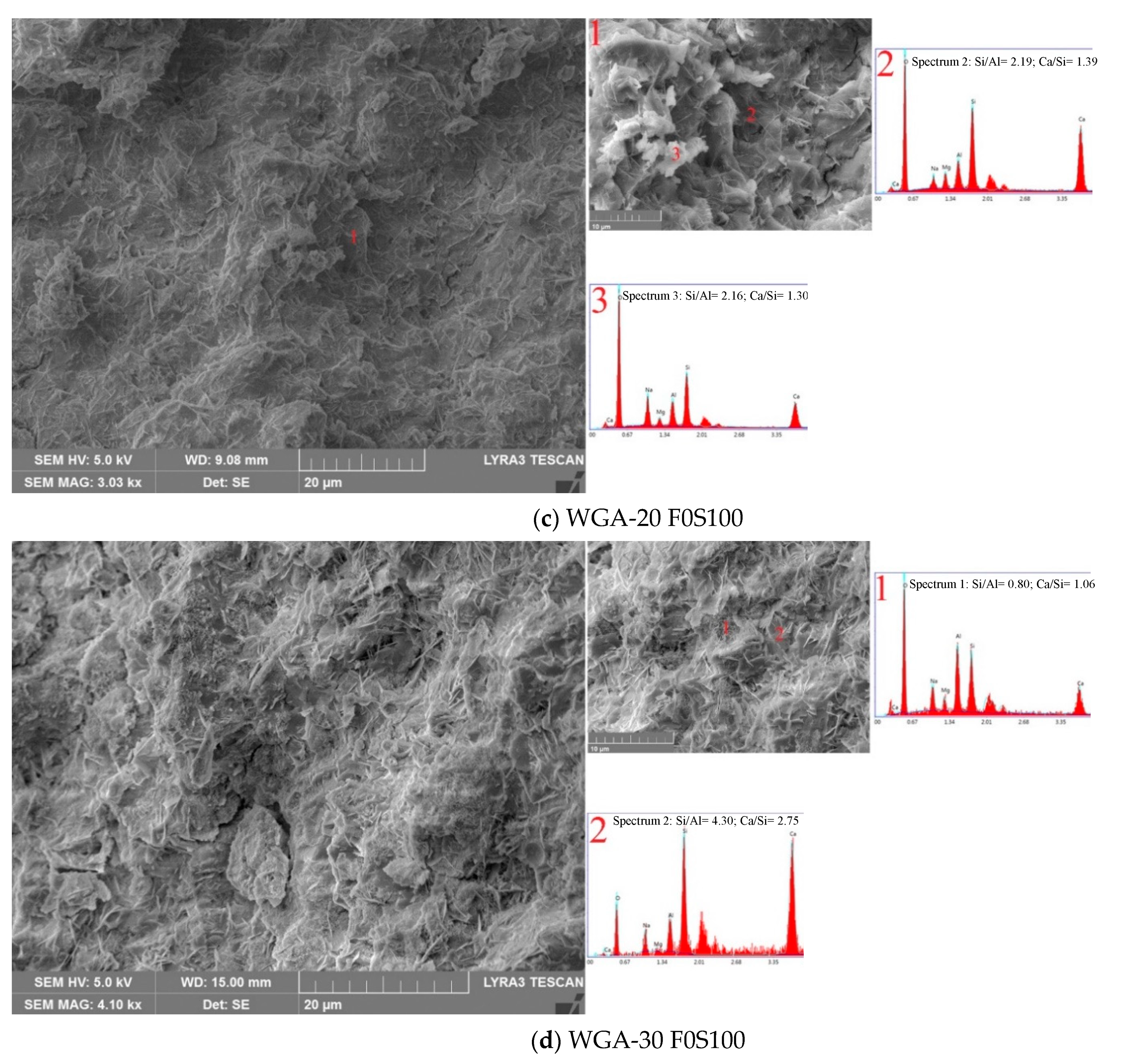
3.6. SEM/EDX Micrographs
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mohajerani, A.; Syter, D.; Jeffrey-Bailey, T.; Song, T.; Arulrajah, A.; Horpibulsuk, S.; Law, D. Recycling waste materials in geopolymer concrete. Clean Technol. Environ. 2019, 21, 439–515. [Google Scholar] [CrossRef]
- Davidovits, J.; Izquierdo, M.; Querol, X.; Antennuci, D.; Nugteren, H.; Butselaar-Orthlieb, V.; Fernández-Pereira, C.; Luna, Y. The European Research Project GEOASH: Geopolymer Cement Based on European Coal Fly Ashes; Technical Paper; Geopolymer Institute: Saint-Quentin, France, 2014. [Google Scholar]
- Phoo-ngernkham, T.; Maegawa, A.; Mishima, N.; Hatanaka, S.; Chindaprasirt, P. Effects of sodium hydroxide and sodium silicate solutions on compressive and shear bond strengths of FA–GBFS geopolymer. Constr. Build. Mater. 2015, 91, 1–8. [Google Scholar] [CrossRef]
- Arulrajah, A.; Kua, T.A.; Horpibulsuk, S.; Phetchuay, C.; Suksiripattanapong, C.; Du, Y.J. Strength and microstructure evaluation of recycled glass-fly ash geopolymer as low-carbon masonry units. Constr. Build. Mater. 2016, 114, 400–406. [Google Scholar] [CrossRef]
- Toniolo, N.; Rincón, A.; Roether, J.A.; Ercole, P.; Bernardo, E.; Boccaccini, A.R. Extensive reuse of soda-lime waste glass in fly ash-based geopolymers. Constr. Build. Mater. 2018, 188, 1077–1084. [Google Scholar] [CrossRef]
- Sasui, S.; Kim, G.; Nam, J.; Koyama, T.; Chansomsak, S. Strength and microstructure of class-C fly ash and GGBS blend geopolymer activated in NaOH & NaOH + Na2SiO3. Materials 2020, 13, 59. [Google Scholar] [CrossRef]
- Fernández-Jiménez, A.; Palomo, A.; Sobrados, I.; Sanz, J. The role played by the reactive alumina content in the alkaline activation of fly ashes. Micropor. Mesopor. Mater. 2006, 91, 111–119. [Google Scholar] [CrossRef]
- Gunasekara, C.; Disgantara, R.; Law, D.W.; Setunge, S. Effects of curing consitions on microstructure and pore-structure of brown fly ash geopolymer. Appl. Sci. 2019, 9, 3138. [Google Scholar] [CrossRef]
- Turner, L.K.; Collins, F.G. Carbon dioxide equivalent (CO2-e) emissions: A comparison between geopolymer and OPC cement concrete. Constr. Build. Mater. 2013, 43, 125–130. [Google Scholar] [CrossRef]
- Commonwealth of Australia. National Greenhouse Accounts (NGA) Factors; Department of Climate Change and Energy Efficiency: Canberra, Australia, 2012; ISBN 978-1-922003-56-0.
- Fawer, M.; Concannon, M.; Rieber, W. Life cycle inventories for the production of sodium silicates. Int. J. Life Cycle Assess. 1999, 4, 207. [Google Scholar] [CrossRef]
- Mathew, M.B.J.; Sudhakar, M.M.; Natarajan, D.C. Strength, economic and sustainability characteristics of coal ash-GGBS based geopolymer concrete. IJCER 2013, 3, 207–212. [Google Scholar]
- Redden, R.; Neithalath, N. Microstructure, strength, and moisture stability of alkali activated glass powder-based binders. Cem. Concr. Compos. 2014, 45, 46–56. [Google Scholar] [CrossRef]
- Cyr, M.; Idir, R.; Poinot, T. Properties of inorganic polymer (geopolymer) mortars made of glass cullet. J. Mater. Sci. 2012, 47, 2782–2797. [Google Scholar] [CrossRef]
- Rao, M.N.; Sultana, R.; Kota, S.H. (Eds.) Municipal solid waste. In Solid and Hazardous Waste Management, 1st ed.; Butterworth-Heinemann: Oxford, UK, 2017; pp. 3–120. [Google Scholar]
- Goto, K. States of silica in aqueous solution. II. Solubility of amorphous silica. Nippon Kagaku Zassi 1955, 76, 1364–1366. [Google Scholar] [CrossRef][Green Version]
- Paul, A. Chemical durability of glasses; a thermodynamic approach. J. Mater. Sci. 1977, 12, 2246–2268. [Google Scholar] [CrossRef]
- Torres-Carrasco, M.; Puertas, F. Waste glass in the geopolymer preparation, Mechanical and microstructural characterisation. J. Clean. Prod. 2015, 90, 397–408. [Google Scholar] [CrossRef]
- Carrasco, M.; Puertas, F.; Torroja, E. Re-Use of Waste Glass as Alkaline Activator in the Preparation of Alkali-Activated Materials. In Proceedings of the 34th Annual Cement and Concrete Science Conference, University of Sheffield, Sheffield, UK, 14–16 September 2014; pp. 207–210. [Google Scholar]
- Puertas, F.; Torres-Carrasco, M. Use of glass waste as an activator in the preparation of alkali-activated slag, Mechanical strength and paste characterisation. Cem. Concr. Res. 2014, 57, 95–104. [Google Scholar] [CrossRef]
- Tchakouté, H.K.; Rüscher, C.H.; Kong, S.; Kamseu, E.; Leonelli, C. Geopolymer binders from metakaolin using sodium waterglass from waste glass and rice husk ash as alternative activators: A comparative study. Constr. Build. Mater. 2016, 114, 276–289. [Google Scholar] [CrossRef]
- ASTM C618-19. Standard Specification for Coal Fly Ash and Raw or Calcined Natural Pozzolan for Use in Concrete; ASTM Internation: West Conshohocken, PA, USA, 2019. [Google Scholar]
- Fernández-Jiménez, A.; Puertas, F. Effect of activator mix on the hydration and strength behaviour of alkali-activated slag cements. Adv. Cem. Res. 2003, 15, 129–136. [Google Scholar] [CrossRef]
- Song, S.; Sohn, D.; Jennings, H.; Mason, T.O. Hydration of alkali-activated ground granulated blast furnace slag. J. Mater. Sci. 2000, 35, 249–257. [Google Scholar] [CrossRef]
- Zhang, S.; Keulen, A.; Arbi, K.; Ye, G. Waste glass as partial mineral precursor in alkali-activated slag/fly ash system. Cem. Concr. Res. 2017, 102, 29–40. [Google Scholar] [CrossRef]
- Tan, Z.; Bernal, S.A.; Provis, J.L. Reproducible mini-slump test procedure for measuring the yield stress of cementitious pastes. Matter. Struct. 2017, 50, 235. [Google Scholar] [CrossRef]
- Chindaprasirt, P.; Chareerat, T.; Sirivivatnanon, V. Workability and strength of coarse high calcium fly ash geopolymer. Cem. Concr. Compos. 2017, 29, 224–229. [Google Scholar] [CrossRef]
- ASTM C109/C109M-16a. Standard Test Method for Compressive Strength of Hydraulic Cement Mortars (Using 2-in. or [50-mm] Cube Specimens); ASTM Internation: West Conshohocken, PA, USA, 2018. [Google Scholar]
- ASTM C348-18. Standard Test Method for Flexural Strength of Hydraulic Cement Mortars; ASTM Internation: West Conshohocken, PA, USA, 2016. [Google Scholar]
- ASTM C20-00. Standard Test Method for Apparent Porosity, Water absorption, Apparent Specific Gravity and Bulk Density of Burned Refractory Brick and Shapes by Boiling Water; ASTM Internation: West Conshohocken, PA, USA, 2015. [Google Scholar]
- Nedeljković, M.; Li, Z.; Ye, G. Setting, strength, and autogenous shrinkage of alkali-activated fly ash and slag pastes: Effect of slag content. Materials 2018, 11, 2121. [Google Scholar] [CrossRef] [PubMed]
- Duxson, P.; Provis, J.L.; Lukey, G.C.; Mallicoat, S.W.; Kriven, W.M.; Van Deventer, J.S. Understanding the relationship between geopolymer composition, microstructure and mechanical properties. Colloids Surf. A Physicochem. Eng. Asp. 2005, 269, 47–58. [Google Scholar] [CrossRef]
- Duxson, P.; Fernández-Jiménez, A.; Provis, J.L.; Lukey, G.C.; Palomo, A.; Van Deventer, J.S. Geopolymer technology: The current state of the art. J. Mater. Sci. 2007, 42, 2917–2933. [Google Scholar] [CrossRef]
- Zhang, Z.; Xiao, Y.; Zhu, H.; Chen, Y. Role of water in the synthesis of calcined kaolin-based geopolymer. Appl. Clay Sci. 2009, 43, 218–223. [Google Scholar] [CrossRef]
- Ruiz-Santaquiteria, C.; Skibsted, J.; Fernández-Jiménez, A.; Palomo, A. Alkaline solution/binder ratio as a determining factor in the alkaline activation of aluminosilicates. Cem. Concr. Res. 2012, 42, 1242–1251. [Google Scholar] [CrossRef]
- Kamarudin, H.; Mustafa, A.; Abdullah, M.M.A.B.; Binhussain, M.; Ruzaidi, C.M.; Luqman, M.; Heah, C.Y.; Liew, Y.M. Preliminary Study on Effect of NaOH Concentration on Early Age Compressive Strength of Kaolin-Based Green Cement. In Proceedings of the International Conference on Chemistry and Chemical Process IPCBEE, Bangkok, Thailand, 7–9 May 2011. [Google Scholar]
- Aziz, A.; EI Hassani, I.E.; Khadiri, A.; Sadik, C.; El Bouari, A.; Ballil, A.; EI Haddar, A. Effect of slaked lime on the geopolymers synthesis of natural pozzolan from Moroccan Middle Atlas. J. Aust. Ceram. Soc. 2020, 56, 67–78. [Google Scholar] [CrossRef]
- Ghosh, K.; Ghosh, P. Effect of % Na2O and % SiO2 on apperent porosity and sorptivity of fly ash based geopolymer. IOSR J. Eng. 2012, 2, 96–101. [Google Scholar] [CrossRef]
- Song, M.; Qian, J.; Zhong, L.J.; Liang, S. From a view of alkali solution: Alkali concentration to determine hydration process of alkali activating metakaolin. In Calcined Clays for Sustainable Concrete; Scrivener, K., Aurélie, F., Eds.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 305–313. [Google Scholar] [CrossRef]
- Ismail, I.; Bernal, S.A.; Provis, J.L.; San Nicolas, R.; Hamdan, S.; Van Deventer, J.S. Modification of phase evolution in alkali-activated blast furnace slag by the incorporation of fly ash. Cem. Concr. Comp. 2014, 45, 125–135. [Google Scholar] [CrossRef]
- Yousefi, E.; Majidi, B. Effects of free quartz on mechanical behaviour of kaolinite based geopolymers. Mater. Technol. 2011, 26, 96–99. [Google Scholar] [CrossRef]
- Shaikh, F.A. Effects of slag content on the residual mechanical properties of ambient air-cure geopolymers exposed to elevated temperature. J. Asian Ceram. Soc. 2018, 6, 342–358. [Google Scholar] [CrossRef]
- Nath, P.; Sarker, P.K. Effect of GGBFS on setting, workability and early strength properties of fly ash geopolymer concrete cured in ambient condition. Constr. Build. Mater. 2014, 66, 163–171. [Google Scholar] [CrossRef]
- Astutiningsih, S.; Liu, Y. Geopolymerisation of Australian Slag with Effective Dissolution by the Alkali. In Proceedings of the World Congress Geopolymer, Saint Quentin, France, 29 June–1 July 2005. [Google Scholar]
- Belkowitz, J.; Armentrout, D. An Investigation of Nano Silica in the Cement Hydration Process. In Masters Abstracts International, Proceedings of Concrete Sustainability Conference; 10 January 2009; p. 48. Available online: https://www.concrete.org/publications/internationalconcreteabstractsportal/m/details/id/51663285 (accessed on 3 September 2020).
- Bobirică, C.; Shim, J.H.; Pyeon, J.H.; Park, J.Y. Influence of waste glass on the microstructure and strength of inorganic polymers. Ceram. Int. 2015, 41 Pt 10, 13638–13649. [Google Scholar] [CrossRef]
- Garcia-Lodeiro, I.; Palomo, A.; Fernández-Jiménez, A.; Macphee, D. Compatibility studies between NASH and CASH gels. Study in the ternary diagram Na2O–CaO–Al2O3–SiO2–H2O. Cem. Concr. Res. 2011, 41, 923–931. [Google Scholar] [CrossRef]
- Vafaei, M.; Allahverdi, A.; Dong, P.; Bassim, N. Durability performance of geopolymer cement based on fly ash and calcium aluminate cement in mild concentration acid solutions. JSCM 2019, 8, 290–308. [Google Scholar] [CrossRef]
- Xie, J.; Wang, J.; Rao, R.; Wang, C.; Fang, C. Effects of combined usage of GGBS and fly ash on workability and mechanical properties of alkali activated geopolymer concrete with recycled aggregate. Compos. Part B-Eng. 2019, 164, 179–190. [Google Scholar] [CrossRef]
- Pardal, X.; Pochard, I.; Nonat, A. Experimental study of Si–Al substitution in calcium-silicate-hydrate (C-S-H) prepared under equilibrium conditions. Cem. Concr. Res. 2009, 39, 637–643. [Google Scholar] [CrossRef]
- Ramakrishna, C.; Thenepalli, T.; Ahn, J.W. A brief review of aragonite precipitated calcium carbonate (PCC) synthesis methods and its applications. Korean Chem. Eng. Res. 2017, 55, 443–455. [Google Scholar]
- García, R.; Frías, M.; Vigil de la Villa Mencía, R.; Martínez-Ramírez, S. Ca/Si and Si/Al Ratios of Metakaolinite-Based Wastes: Their Influence on Mineralogy and Mechanical Strengths. Appl. Sci. 2018, 8, 480. [Google Scholar] [CrossRef]
- Kunther, W.; Ferreiro, S.; Skibsted, J. Influence of the Ca/Si ratio on the compressive strength of cementitious Calcium-silicate-hydrate binders. J. Mater. Chem. A 2017, 5, 17401–17412. [Google Scholar] [CrossRef]







| Precursor Material | ||||||||
| Elements (wt.%) | Si | Al | Na | Ca | Mg | Fe | O | K |
| FA | 18.16 | 10.33 | 0.65 | 18.72 | 0.26 | 2.01 | 43.44 | 1.53 |
| GGBS | 14.57 | 7.37 | 0.47 | 39.92 | 0.41 | 0.51 | 35.43 | 0.35 |
| Oxides (wt.%) | SiO2 | Al2O3 | Na2O | CaO | Fe2O3 | K2O | MgO | |
| FA | 38.84 | 19.52 | 0.87 | 26.19 | 2.87 | 1.84 | 0.43 | |
| GGBS | 31.17 | 13.92 | 0.63 | 55.85 | 0.729 | 0.421 | 0.67 | |
| Soda-Lime Waste Glass | ||||||||
| Elements (wt.%) | Si | Al | Na | Ca | Mg | Fe | O | K |
| BG | 34.05 | 2.61 | 6.92 | 8.46 | 1.49 | 0.47 | 35.62 | 0.72 |
| GG | 27.07 | 0.08 | 8.30 | 0.28 | 0.03 | 0.35 | 53.31 | 0.08 |
| TG | 39.57 | 2.99 | 9.39 | 5.87 | 0.69 | 0.21 | 37.27 | 0.54 |
| Oxides (wt.%) | SiO2 | Al2O3 | Na2O | CaO | Fe2O3 | K2O | MgO | |
| BG | 72.84 | 4.93 | 9.32 | 11.84 | 0.67 | 0.86 | 2.47 | |
| GG | 57.90 | 0.15 | 11.18 | 0.39 | 0.60 | 0.09 | 0.05 | |
| TG | 84.64 | 5.64 | 12.65 | 8.21 | 0.30 | 0.65 | 1.14 | |
| Batch 1: WGA-0 | Alkaline Solution: NaOH-4M | ||||||
| pH of WGA-0 | 12.5 | ||||||
| Specimens ID | F100S0 | F85S15 | F70S30 | F50S50 | F30S70 | F15S85 | F0S100 |
| FA (wt.%) | 100 | 85 | 70 | 50 | 30 | 15 | 0 |
| GGBS (wt.%) | 0 | 15 | 30 | 50 | 70 | 85 | 100 |
| Liquid/solid | 0.4 | ||||||
| Batch 2: WGA-10 | Alkaline Solution: NaOH-4M + 10 g (10 g/100 mL) | ||||||
| pH of WGA-10 | 12.55 | ||||||
| Specimens ID | F100S0 | F85S15 | F70S30 | F50S50 | F30S70 | F15S85 | F0S100 |
| FA (wt.%) | 100 | 85 | 70 | 50 | 30 | 15 | 0 |
| GGBS (wt.%) | 0 | 15 | 30 | 50 | 70 | 85 | 100 |
| Liquid/solid | 0.4 | ||||||
| Batch 3: WGA-20 | Alkaline Solution: NaOH-4M + 20 g (20 g/100 mL) | ||||||
| pH of WGA-20 | 12.51 | ||||||
| Specimens ID | F100S0 | F85S15 | F70S30 | F50S50 | F30S70 | F15S85 | F0S100 |
| FA (wt.%) | 100 | 85 | 70 | 50 | 30 | 15 | 0 |
| GGBS (wt.%) | 0 | 15 | 30 | 50 | 70 | 85 | 100 |
| Liquid/solid | 0.4 | ||||||
| Batch 4: WGA-30 | Alkaline Solution: NaOH-4M + 30 g (30 g/100 mL) | ||||||
| pH of WGA-30 | 12.47 | ||||||
| Specimens ID | F100S0 | F85S15 | F70S30 | F50S50 | F30S70 | F15S85 | F0S100 |
| FA (wt.%) | 100 | 85 | 70 | 50 | 30 | 15 | 0 |
| GGBS (wt.%) | 0 | 15 | 30 | 50 | 70 | 85 | 100 |
| Liquid/solid | 0.4 | ||||||
| Elements (ppm) | Si | Na | Al | Ca | Mg |
|---|---|---|---|---|---|
| WGA-10 | 3508 | 84,400 | 56.9 | 0.20 | <0.1 |
| WGA-20 | 6244 | 94,880 | 94.1 | 0.18 | 0.22 |
| WGA-30 | 8752 | 97,840 | 106.3 | 0.41 | 5.82 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sasui, S.; Kim, G.; Nam, J.; van Riessen, A.; Eu, H.; Chansomsak, S.; Alam, S.F.; Cho, C.H. Incorporation of Waste Glass as an Activator in Class-C Fly Ash/GGBS Based Alkali Activated Material. Materials 2020, 13, 3906. https://doi.org/10.3390/ma13173906
Sasui S, Kim G, Nam J, van Riessen A, Eu H, Chansomsak S, Alam SF, Cho CH. Incorporation of Waste Glass as an Activator in Class-C Fly Ash/GGBS Based Alkali Activated Material. Materials. 2020; 13(17):3906. https://doi.org/10.3390/ma13173906
Chicago/Turabian StyleSasui, Sasui, Gyuyong Kim, Jeongsoo Nam, Arie van Riessen, Hamin Eu, Sant Chansomsak, Syed Fakhar Alam, and Churl Hee Cho. 2020. "Incorporation of Waste Glass as an Activator in Class-C Fly Ash/GGBS Based Alkali Activated Material" Materials 13, no. 17: 3906. https://doi.org/10.3390/ma13173906
APA StyleSasui, S., Kim, G., Nam, J., van Riessen, A., Eu, H., Chansomsak, S., Alam, S. F., & Cho, C. H. (2020). Incorporation of Waste Glass as an Activator in Class-C Fly Ash/GGBS Based Alkali Activated Material. Materials, 13(17), 3906. https://doi.org/10.3390/ma13173906








