1. Introduction
Dispersive and soft clayey soils are some of the most problematic soils due to their poor and vulnerable engineering properties [
1,
2], such as their expansive nature, excessive cracking, low compressive and shear strengths, low modulus, large settlement under loading, high volumetric shrinkage, and poor durability against wetting/drying and freezing/thawing cycles [
3], which imposes severe damage to and/or failure of geotechnical structures [
4,
5,
6]. For decades, various industrial-based chemical stabilizers, such as Portland cement, lime, asphalt, and polymers, have been proven to improve the quality of clayey soils [
7,
8,
9], but can be considered to demand high economic and/or environmental costs. The increased cost associated with traditional chemical stabilizers has led researchers to develop alternative soil modifiers from industrial by-products [
10,
11,
12,
13,
14], such as ground granulated blast furnace slag, fly ash, and cement kiln dust, which provide both economic and environmental solutions to resource conservation in soil engineering.
Steel furnace slag (SFS) from the steelmaking and steel refining processes is a common by-product produced around the world [
15,
16,
17,
18,
19]. An estimated 169–254 million tons of SFS were produced worldwide in 2017 [
20]. Aside from its abundance, SFS has been regarded as a potential material for use in civil infrastructure applications [
16], such as Portland cement concrete, asphalt concrete, road base, ballast, embankment, and soil stabilization. From recent studies, there is limited usage of SFS, particularly in bound applications (e.g., aggregate in Portland cement concrete or in cement-treated bases) due to the deleterious expansion that it undergoes when the free calcium oxide (CaO) and magnesium oxide (MgO) present in the SFS react with water [
16,
21,
22,
23,
24]. Free CaO and free MgO expand by 92% and 120%, respectively, when reacting with water to form hydroxides [
25]. The free CaO content ranges for two types of SFS, which include basic oxygen furnace (BOF) slag and electric arc furnace (EAF) slag, are 1–10% and 0–4%, respectively [
19]. This expansion can be extensive and result in structural failures (e.g., failure due to swelling, expansive cracking, loss in strength, etc.) [
26,
27,
28].
However, the free CaO and free MgO in SFS can be effectively used in geotechnical engineering applications, such as stabilization of clayey soils and improvement of their engineering properties. Similar to the performance of lime in stabilizing dispersive soils [
29,
30,
31], the cation exchange, flocculation and agglomeration, and pozzolanic reaction can occur as a clayey soil reacts with certain free oxides in SFS. Various research studies have been conducted to employ SFS to enhance the properties of problematic soils, while primarily demonstrating that SFS can be an effective stabilization agent [
32,
33,
34,
35,
36,
37,
38,
39,
40,
41,
42,
43,
44,
45,
46,
47,
48,
49]. From the early 1990s, Akinmusuru [
43] pioneered the attempt to use SFS in soil stabilization for rural roads with low traffic volume, which suggests that SFS possesses the potential to improve the soil properties by increasing the strength, California bearing ratio (CBR), and dry density. Subsequent results by Poh et al. [
45] showed that using BOF slag for soil improvement was not encouraging. Despite the employment of chemical activators, the engineering properties (dry density, strength, and durability) of BOF-stabilized samples were lower than those properties improved by cement stabilization. A recent study of Akinwumi [
42] concluded that the addition of SFS to lateritic soil increased the dry density, decreased the optimum moisture content, and, as the percent SFS increased, the soaked and un-soaked CBR and the unconfined compressive strength increased. Zumrawi and Babikir [
47] studied the effectiveness of adding 5%, 10%, 15%, 20%, and 30% of SFS to an expansive soil and reported that the addition of SFS improved soil properties. Abdi [
49] investigated stabilizing a kaolinite soil with a combination of BOF slag and hydrated lime, which demonstrated that higher BOF slag with lime contents yielded higher compressive strengths. This finding also agreed with the work of Yildirim et al. [
44], which evaluated soil stabilization with the blend of SFS and either Class C fly ash or ground granulated blast furnace slag. Manso et al. [
36] found that clay soils stabilized with ladle furnace slag could have bearing capacities similar to lime-stabilized clay soils.
One major concern in using SFS, relative to more traditional stabilizing materials, is the slow rate of hydration at early stages due to the low activity of calcium silicates in SFS [
50]. Some studies have attempted to accelerate the hydration of SFS by adding a chemical activator such as quicklime, sodium hydroxide (NaOH), calcium chloride (CaCl
2), sodium chloride (NaCl), or sodium metasilicate pentahydrate (Na
2SiO
3-5H
2O) [
50]. Although studies have shown that quicklime and Na
2SiO
3-5H
2O were effective in accelerating the hydration of the SFS for soil stabilization [
45], CaCl
2 was found to be more effective to increase the SFS hydration rate both not only for soil improvement but also in SFS paste and as a replacement in concrete [
51].
Therefore, this study focuses on evaluating the use of SFS for improving the engineering properties of a clayey soil. The clayey soil selected has a low bearing capacity, which makes it unsuitable for any road base, foundation, and other construction projects. The soil stabilization was carried out at 10% and 15% additions of SFS by weight. Ladle metallurgy furnace (LMF) slag, also known as secondary refining slag, was employed in this study, which is a promising SFS type and shares common chemical features with other steel slags [
18,
21,
36,
52,
53,
54,
55]. In addition, CaCl
2 was selected as a potential chemical admixture to accelerate the hydration of the dicalcium silicate phase in the SFS. The primary contribution of the research is the use of LMF slag for clayey soil stabilization, which has seen little focus in the literature (e.g., References [
36,
38] used LMF slag powder), and particularly LMF slag fines, which have not been used in the literature as a soil stabilizer and which offer potential for both chemical and mechanical stabilization mechanisms.
Overview of SFS Selection and Properties
SFS is a by-product generated during the steelmaking process. Most SFS are generally classified as either by the conversion of iron to steel in a basic oxygen furnace (BOF) or the melting of scrap to make steel in an electric arc furnace (EAF). In fact, 99.6% of the steel produced worldwide is produced by either the BOF or the EAF process [
56], with around 71% of the worldwide production using the BOF process [
56]. Further refinement of the steel during secondary steelmaking can occur after the BOF or EAF processes from which ladle metallurgy furnace slag (LMF) is produced. For BOF production, liquid blast furnace metal, scrap, and various fluxes, consisting of lime or dolomitic lime, are charged to the furnace, and high-pressure oxygen is injected through a lance. The impurities, which include carbon monoxide, silicon, manganese, phosphorus, and liquid oxides, combine with the lime or dolomitic lime to form the slag. The EAF process is different in that it electrically charges cold steel scraps such as iron scrap, pig iron, and direct reduced iron. The steel scrap is melted with the charge and brought up to the required chemical composition by adding other metals. Oxygen is then blown into the EAF to purify the steel, which creates a slag layer that will float on the top and can be poured off.
The chemical composition of SFS may vary by plant and even by batch. As a byproduct of steel production, it is dependent on the raw materials, types of steel produced, furnace conditions, cooling processes, etc. [
15,
16]. The primary components in most SFS are oxides of calcium, magnesium, aluminum, silicon, and iron [
15,
16]. Mineralogically, BOF slags consist by weight mainly of 30–60% dicalcium silicate (2CaO-SiO
2), 0–30% tricalcium silicate (3CaO-SiO
2), 0–10% free CaO, 10–40% wüstite (FeO), and 5–20% dicalcium ferrite (2CaO-Fe
2O
3) [
19]. Comparatively, Portland cement is composed of about 55% tricalcium silicate (3CaO-SiO
2), 18% dicalcium silicate (2CaO-SiO
2), 10% tricalcium aluminate (3CaO-Al
2O
3), 8% tetracalcium aluminoferrite (4CaO-Al
2O
3-Fe
2O
3), and 6% gypsum (CaSO
4-2H
2O) [
57]. Therefore, there is potential for SFS to behave as a slow-reacting cementitious material [
58,
59,
60,
61,
62].
2. Materials and Methods
2.1. Material Selection
A clayey soil with a low bearing capacity was selected for this experiment. The soil and the LMF slag were characterized using several methods. The gradations of both the LMF and the clayey soil were evaluated by ASTM C136 while the LMF slag specific gravity and absorption were characterized by ASTM C128. ASTM C29 was used to evaluate the LMF unit weight (rodding method). Additionally, the soil for this stabilization project was a refractory clay, which is commonly utilized to make ceramics but is useful in soil studies for its plastic properties. The plastic limit (PL), liquid limit (LL), and plasticity index (PI) were determined according to ASTM D4318.
For this experimental study, the SFS provided for stabilization was a ladle metallurgy furnace (LMF) slag, which was produced by a modified EAF process. The LMF process can introduce more free lime (CaO) in the slag than the typical EAF process. The LMF slag was provided by the Edw. C. Levy Co. from a plant in Crawfordsville, IN, USA.
2.2. Chemical and Mineralogical Characterization
Mineralogical characterization of the LMF slag and the clayey soil was conducted using powder X-ray diffraction (XRD). The LMF slag was crushed to a particle size passing the No. 100 sieve (≤150 μm). The material passing the No. 200 sieve (≤75 μm) was used to determine the mineralogy of the clayey soil. A Siemens-Bruker D5000 XRD (Bruker, Billerica, MA, USA) was used, which utilizes copper (Cu) Kα radiation and has a graphite monochromator and a scintillation detector. The machine was operated at 40 kV and 30 mA. The sample size was 0.5 cm3 (0.03 in3). The 2θ scan range was from 10° to 80° with an increment of 0.02° and a scan speed of 0.5°/min.
Likewise, additional quantification of the LMF slag composition was conducted using thermogravimetric analysis (TGA), which has been commonly utilized to better assess the total calcium oxide (CaO) and calcium hydroxide (Ca(OH)
2) contents present in SFS, based on the method proposed by Brand and Roesler [
21]. In this study, a TA Instruments Q50 TGA (TA Instruments, New Castle, DE, USA) was utilized, which heated the sample to 1000 °C at a heating rate of 10 °C per minute to derive the weight loss. Nitrogen was used as the purge gas at flow rates of 60 mL/min for the sample purge and 40 mL/min for the balance purge.
Complexometric titration was utilized to determine the free lime content. In this technique, a sample of SFS is mixed with hot ethylene glycol, filtered, and then titrated with acid after an indicator has been added. Ethylene glycol extraction methods were originally developed to rapidly determine the free lime content of Portland cement and clinker [
63], but have since been adopted for SFS (e.g., References [
64,
65,
66,
67]). Specifically, the method from Brand and Roesler [
21,
68] was followed: about 1 g of SFS passing the No. 100 sieve (≤150 μm) was weighed and continuously stirred with 50 mL of ethylene glycol in a water bath at 95 ± 5 °C for 30 min. After filtering, 10 drops of a phenolphthalein indicator were added and then titrated with 0.1 N hydrochloric acid (HCl). The “ethylene glycol number” (EGN) is calculated as follows based on the initial mass of the SFS sample (m), the normality of the HCl (
NHCl), the volume of HCl titrated (
VHCl), a correction for the volume of HCl titrated in a blank ethylene glycol sample (
Vblank), and an equivalency factor (
F).
The correction factor
F for this method is 28 [
21,
69,
70]. The correction
Vblank is specified in other standards [
71] to account for the amount of HCl needed to titrate a plain solvent sample (i.e., plain ethylene glycol). It was found that
Vblank = 0 mL, which is a reasonable result since the pH of ethylene glycol is close to neutral. The EGN value accounts for the available Ca
2+ ions from the free CaO and Ca(OH)
2, so the free lime content needs to be adjusted based on the Ca(OH)
2 determined by TGA [
21].
Furthermore, the experimental design consisted of five total mixtures to be compacted at optimum moisture content, including the unmodified and SFS-stabilized clay. Moisture-density relationships were conducted to determine the optimum moisture content for the unmodified clay and the two SFS content mixtures (10% and 15% by weight). Based on the literature review, it was deemed that calcium chloride (CaCl2) may act as a suitable accelerator for the dicalcium silicate phase in the SFS. Therefore, additional mixtures at 10% and 15% SFS were made with 2% CaCl2 (by weight of total water). All five mixtures were then tested for unconfined compressive strength and dynamic modulus.
2.3. Moisture-Density Relationships
The standard Proctor test according to ASTM D698 was employed to determine the moisture-density relationship for unmodified clay and the clay samples modified with 10% and 15% SFS by weight. The materials were compacted at different moisture contents until the maximum dry density was achieved. The mixing was carried out using a mechanical mixer to accomplish uniform mixing. The compacted samples were weighed and recovered for determining actual moisture content. The optimum moisture content corresponding to maximum dry density was then estimated using the moisture density curves.
2.4. Unconfined Compressive Strength (UCS)
To evaluate the suitability of using the SFS modified mixtures in subgrade stabilization, it is important to quantify the effects of these stabilizers on the sample strength gain characteristics in comparison to unmodified clay strength properties. For this purpose, an unconfined compressive test for the unmodified clay was carried out using ASTM D2166, which was followed by testing the SFS-modified mixtures using ASTM D5102. This is for determining the UCS of compacted soil-lime mixtures.
The mold used for preparing the samples was 2.8 inches (7 cm) in diameter with a height of 5.6 inches (14 cm) with a diameter to height ratio of 1:2. The samples were compacted in the mold at the optimum moisture content to achieve the maximum dry density. The maximum dry density and optimum moisture content information was also employed to determine the exact weight of clay, SFS, and water to result in a specimen of required dimensions as mentioned above. The specimen was compacted into three equal layers.
The unmodified sample was tested immediately after compaction, whereas the modified specimens were wrapped in plastic to avoid moisture loss and then subjected to accelerated curing at 49 °C for 48 h. The accelerated curing used for this research has been tested as equivalent to the 28-day strength of soil-lime mixtures at 23 °C [
72,
73]. The cured samples were tested for unconfined compressive strength at a displacement rate of 1.0 mm/min. The test performed was stress controlled. The peak load measured was recorded as the unconfined compressive strength.
2.5. Vibration Resonance
In addition to the UCS testing, the improvement to the properties of the soil with the addition of SFS was also investigated by studying the dynamic modulus of the compacted soil. One such method of measuring the dynamic modulus is by vibration resonance. An impact event, when incident on a specimen of finite size, will generate various waves in the specimen, namely primary, secondary, and surface waves. The multiple reflections of the primary and secondary waves will eventually set up a vibration resonance in the sample, which is a function of the dynamic modulus and density of that material [
74]. Since the vibration resonance acts to “homogenize” the specimen, the test method can be applied to heterogeneous materials to determine the dynamic modulus, provided that the size of the specimen is larger than the constituents. The dynamic modulus (
Ed) can be computed based on the density (
ρ), length (
L), and fundamental longitudinal frequency (
fl) of the specimen as follows.
Vibration resonance testing, while more commonly applied to concrete materials according to ASTM C215, has been applied to both stabilized and un-stabilized soils [
75,
76,
77,
78,
79,
80]. Guimond-Barrett et al. [
75] found repeatable resonance tests between multiple specimens of soil-cement mixtures, which indicates that it is useful for such heterogeneous materials as stabilized soils. A good agreement was reached between the resonant frequency dynamic modulus measured in the laboratory and the modulus measured in the field [
76]. In addition, Hilbrich and Scullion [
77] determined that a reasonable agreement existed between the resonant frequency dynamic modulus and the resilient modulus of stabilized soils.
In this laboratory experiment, a compacted cylindrical specimen for the unstabilized and stabilized clay mixtures were tested for longitudinal resonance using three impactor sizes (8, 14, and 18 mm). The experiment configuration followed ASTM C215 for the support arrangement, impact location, and accelerometer location (
Figure 1). The accelerometer voltage and time were recorded and post-processed by a fast Fourier transform (FFT) algorithm and plotted in the frequency domain to determine the resonance frequency. For each signal, 50,000 data points were collected with a sample interval of 2 μs for a spectral line spacing of 10 Hz.
4. Conclusions
The study was undertaken to investigate the potential for stabilizing a clayey soil with steel furnace slag (SFS). The SFS employed was specifically a finely graded ladle metallurgy furnace (LMF) slag. The LMF slag was found to be composed of wüstite, larnite (β-dicalcium silicate), mayenite, and periclase with approximately 2.5% free lime, 1.3% calcium hydroxide, and 3.2% magnesium hydroxide. The soil, selected for its plastic properties, was a refractory clay composed of quartz and kaolinite and was classified as an AASHTO A-2-6 soil with a plasticity index of 14.
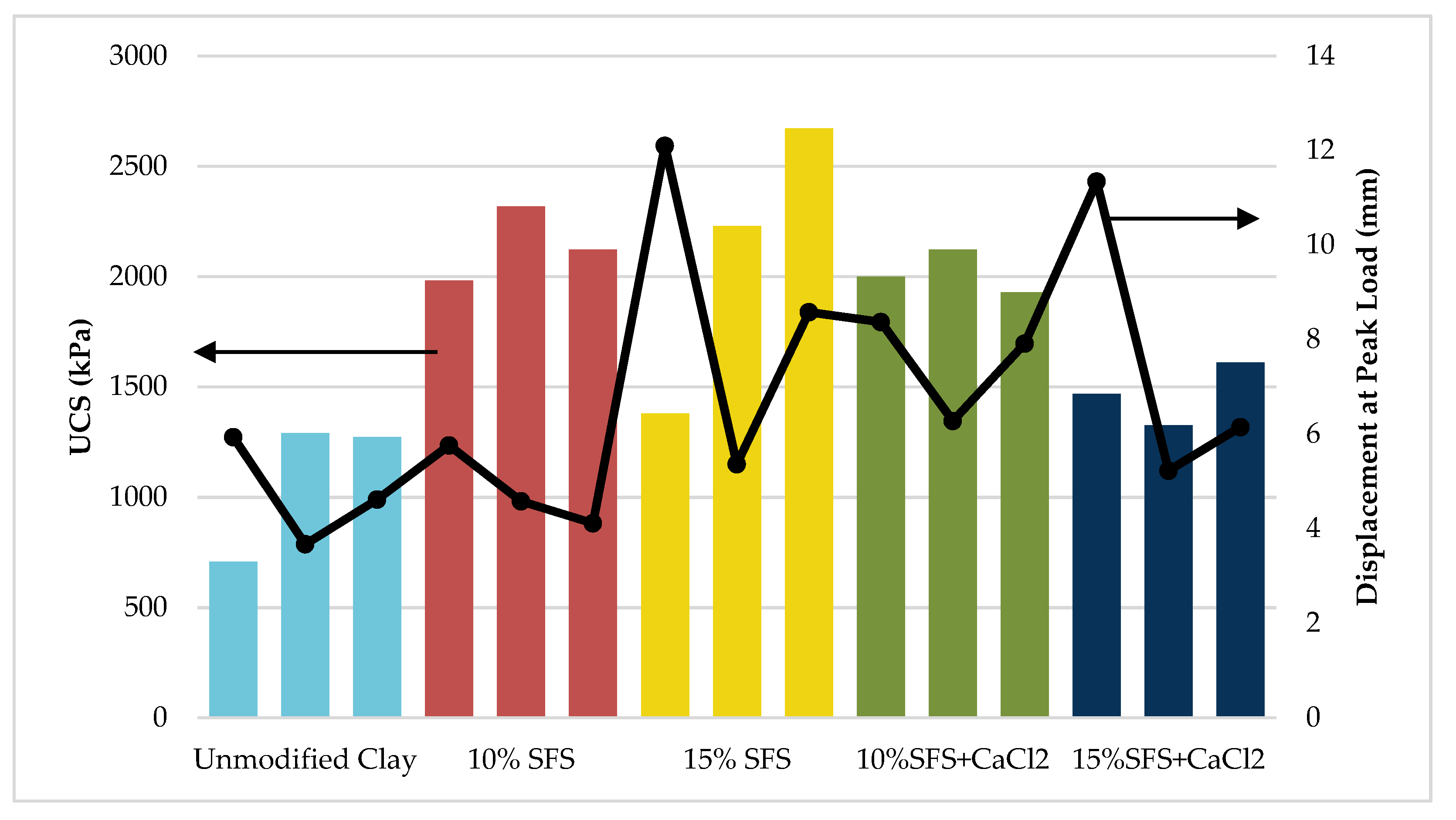
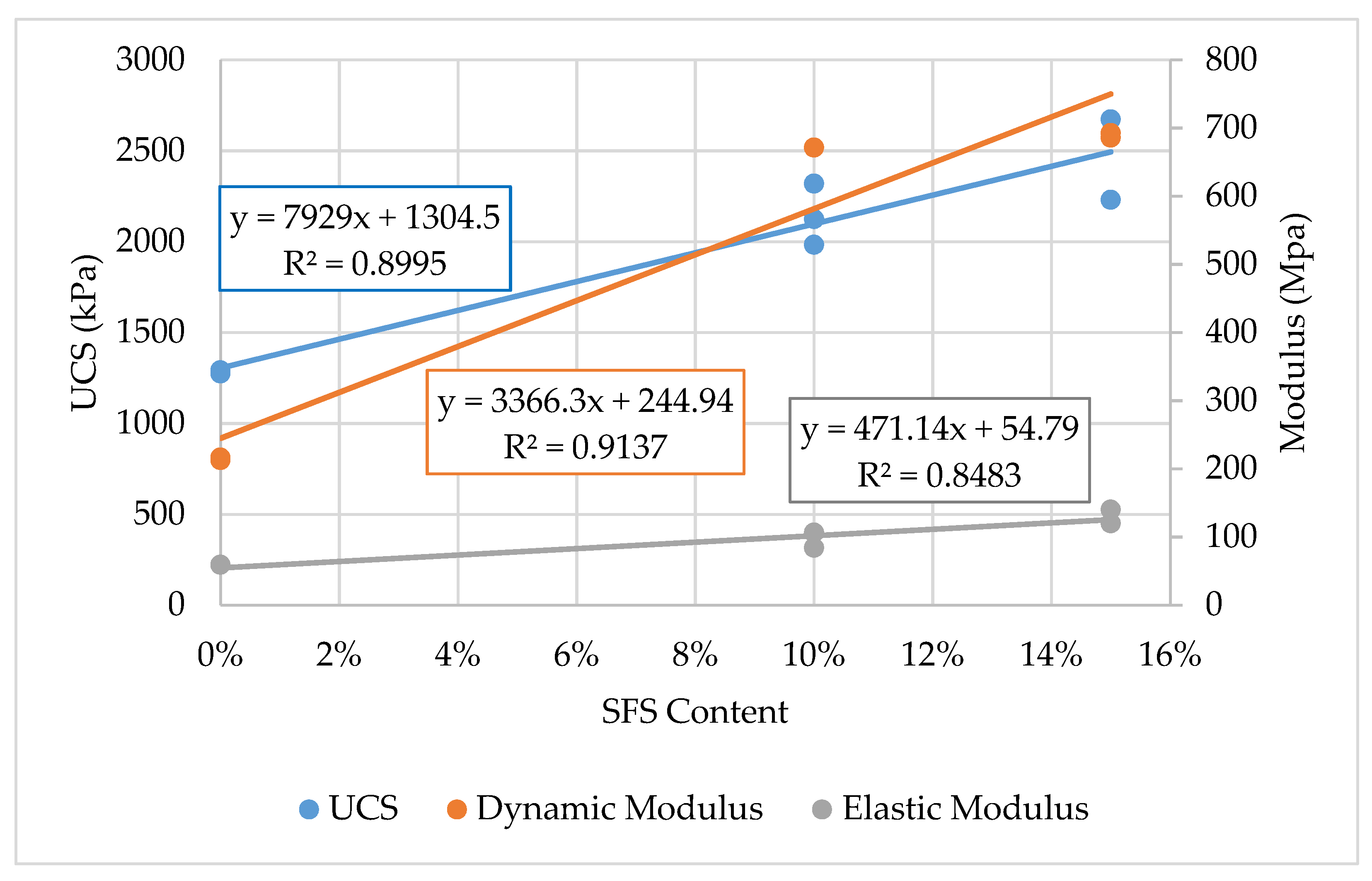
Two SFS contents were tested, which included 10% and 15% LMF slag by weight. The moisture-density relationships revealed that the maximum dry density and optimum moisture content increased with increasing SFS content. The results indicated a linear trend for increasing unconfined compressive strength (UCS) and dynamic modulus with increasing SFS content. Relative to the unmodified clay, the UCS increased by 67% and 91% when 10% and 15% SFS were utilized, respectively. The elastic modulus increased by 60% and 75% when 10% and 15% SFS were used, respectively, and the dynamic modulus increased by 212% and 221% when 10% and 15% SFS were added, respectively. Based on the literature, additional SFS modified samples were created with calcium chloride added at 2% by weight of the total water in an attempt to accelerate the hydration of the dicalcium silicate in the LMF slag, but the results suggested that calcium chloride was not effective.
These findings suggest that LMF slag fines are suitable for stabilizing clayey soils. While there was insufficient evidence of a chemical stabilization mechanism, it is likely that the SFS at least contributed to a mechanical stabilization mechanism. The results also indicate that 15% SFS provides the most improvement to the UCS and dynamic modulus of the stabilized soil, even though further testing is required to validate and improve upon these findings.