Characteristics of CO2 and Energy-Saving Concrete with Porous Feldspar
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Conditions
2.2.1. Characteristics of Strength
2.2.2. Method of Activation and Experimental
2.2.3. Strength Test of Feldspar and Mortar
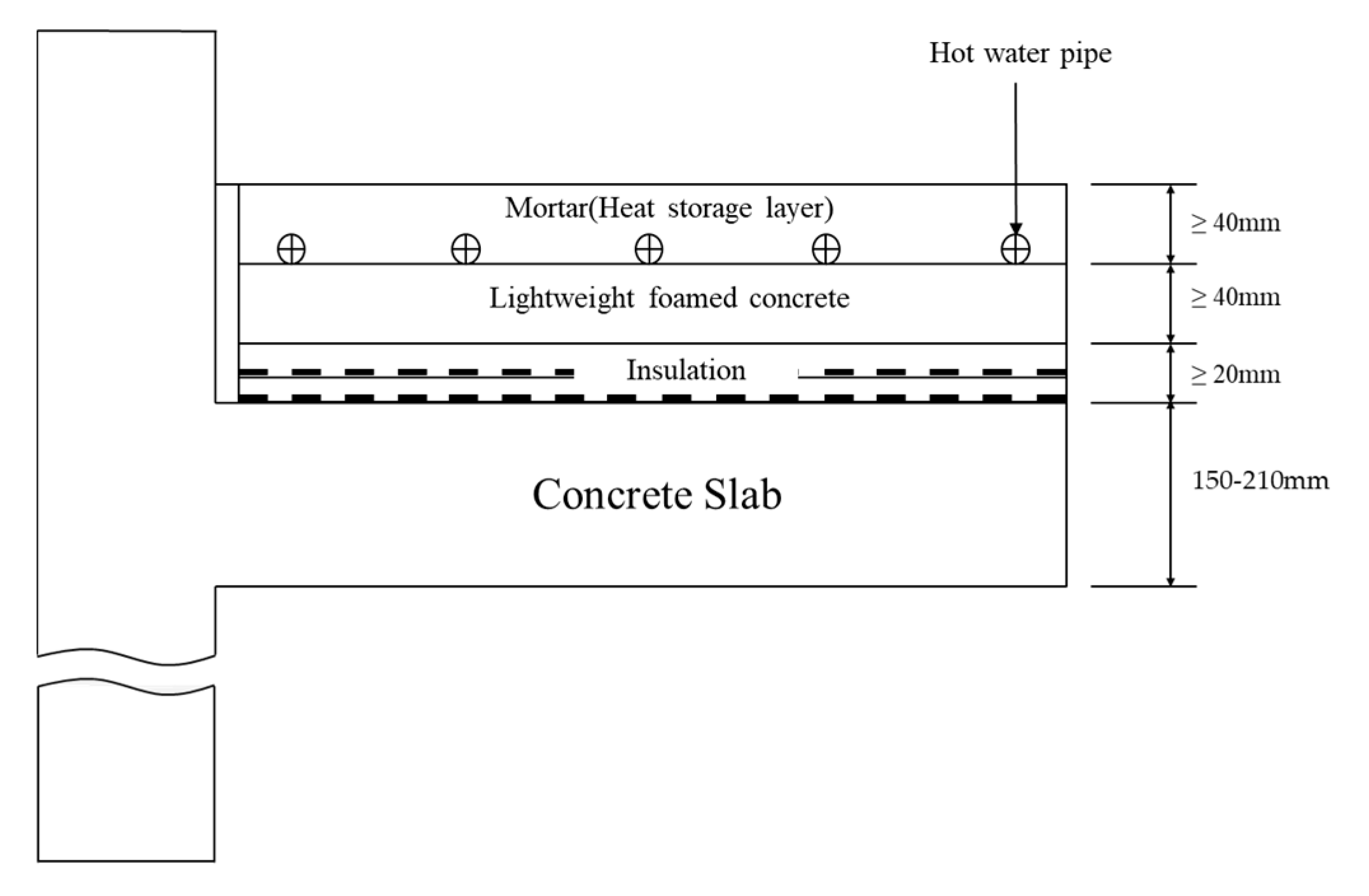
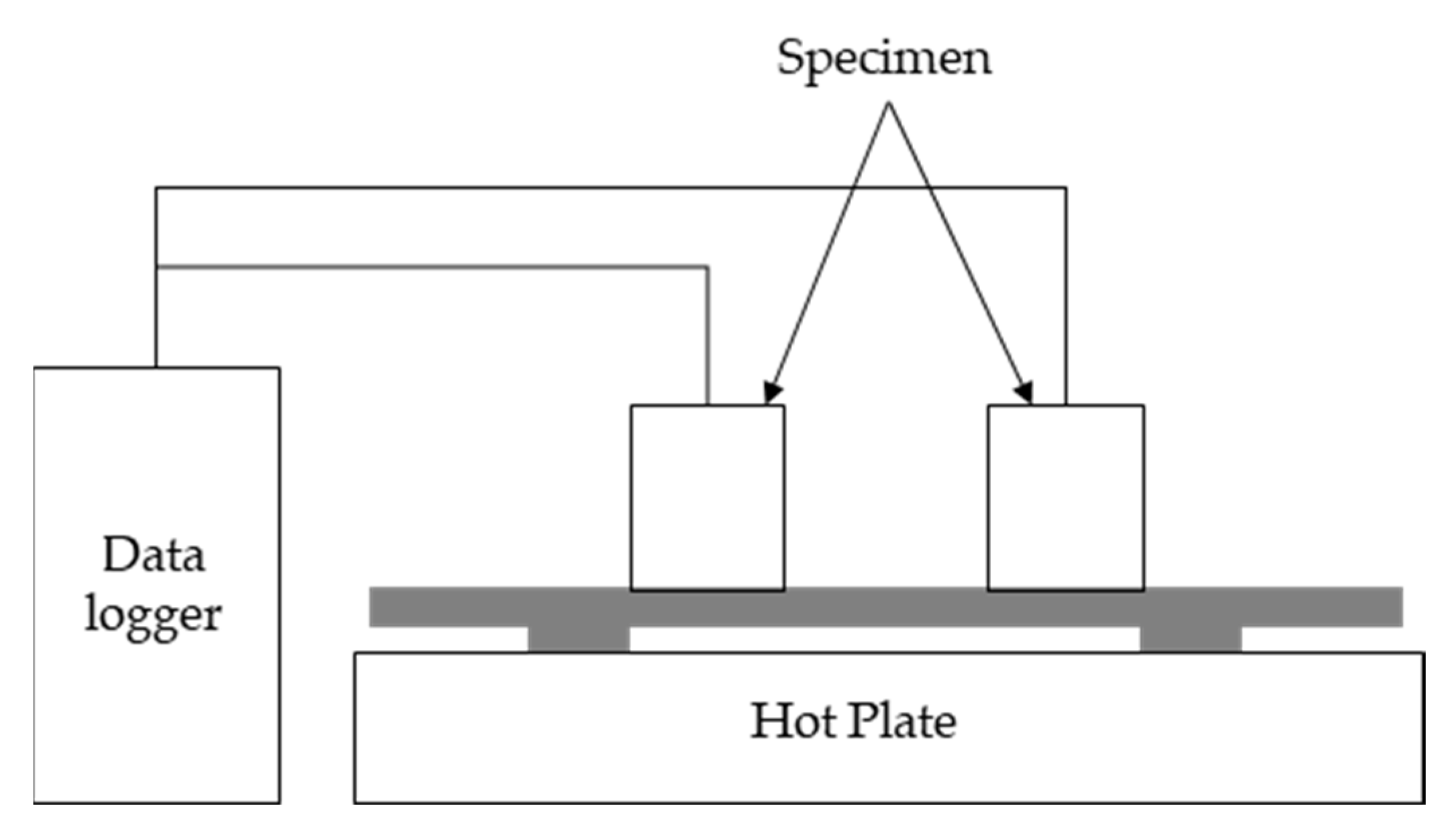
2.2.4. Thermal Diffusion and Heat Storage Test
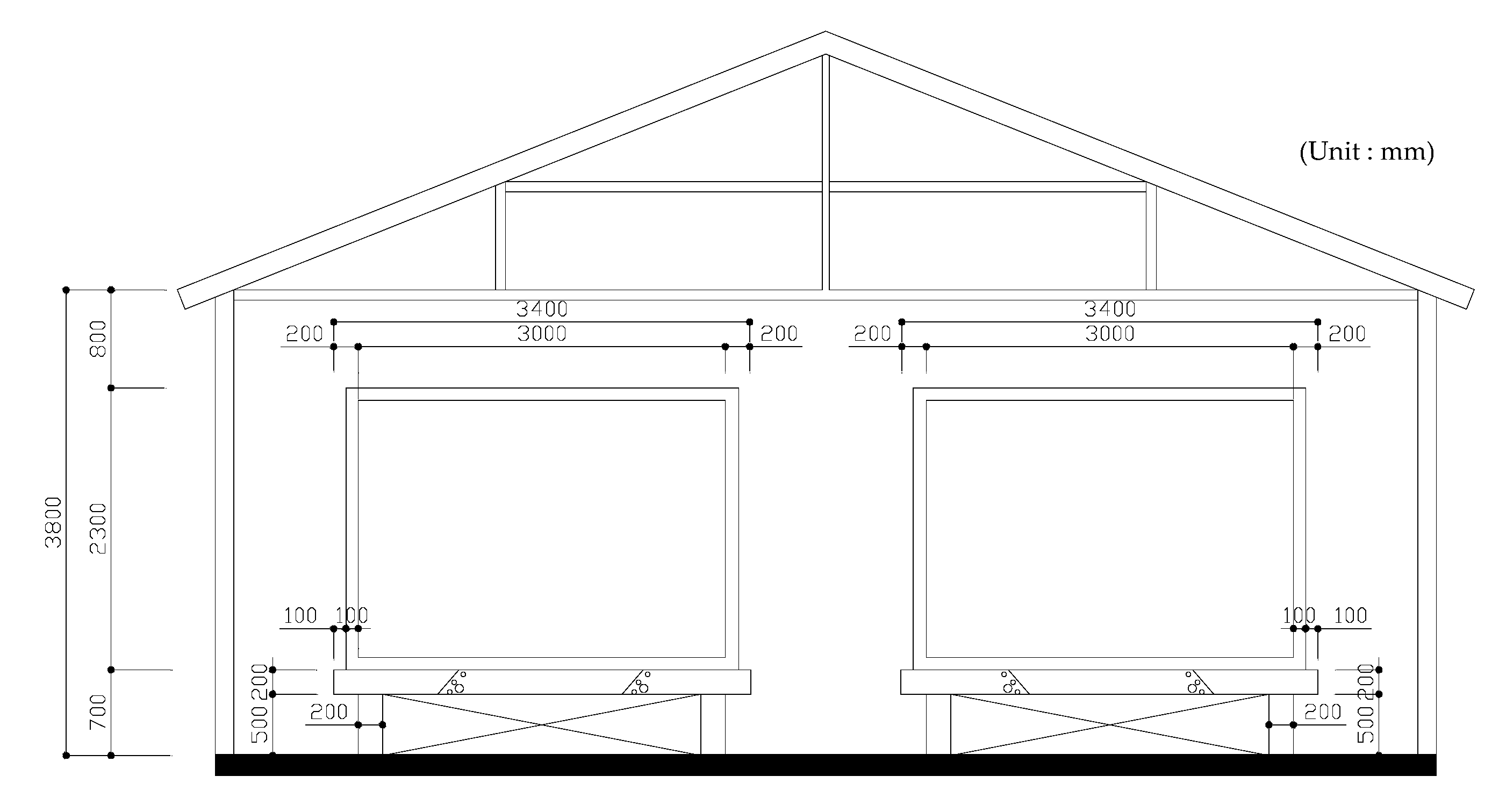
2.3. Pilot Test
3. Results and Discussion
3.1. Response Characteristics
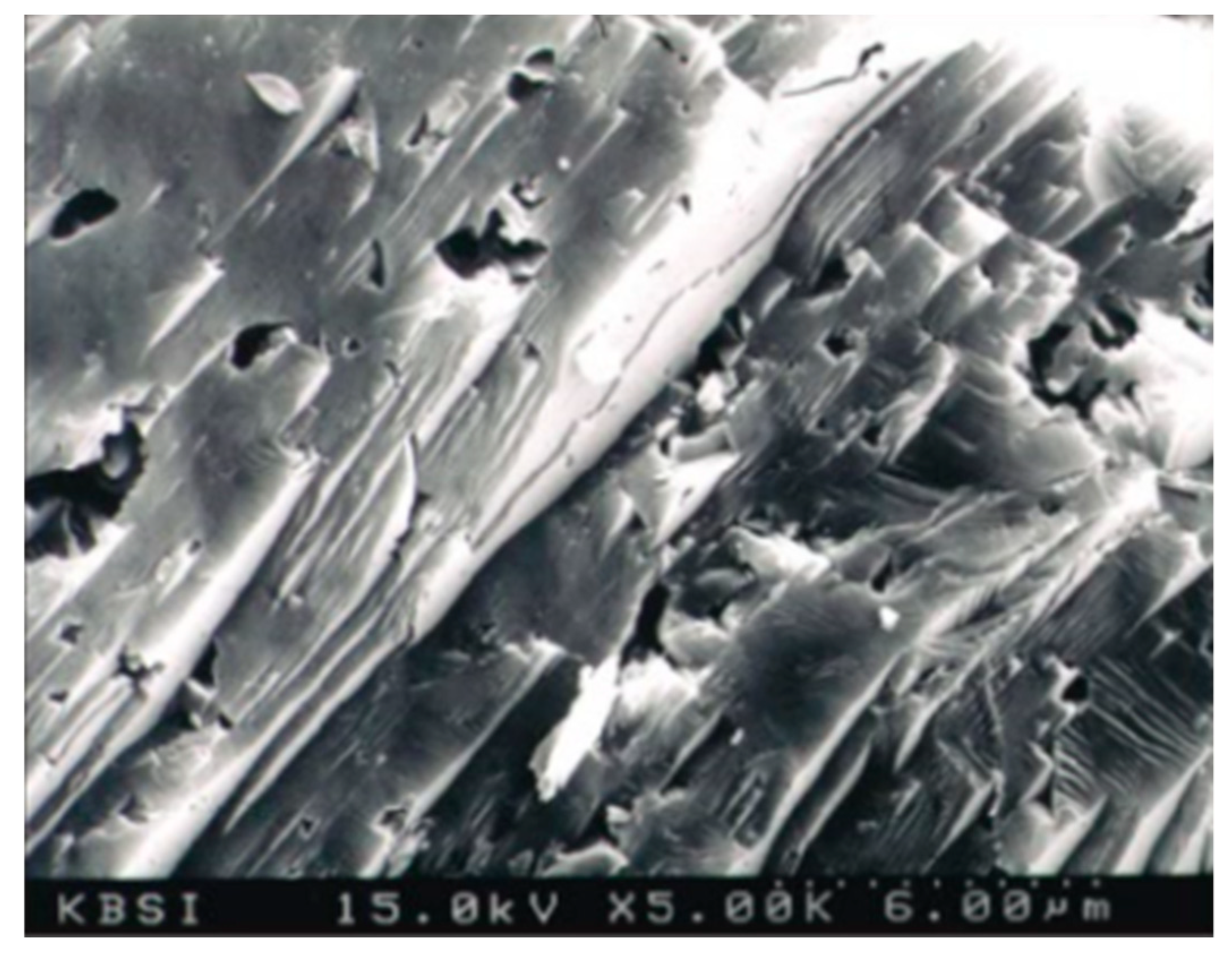
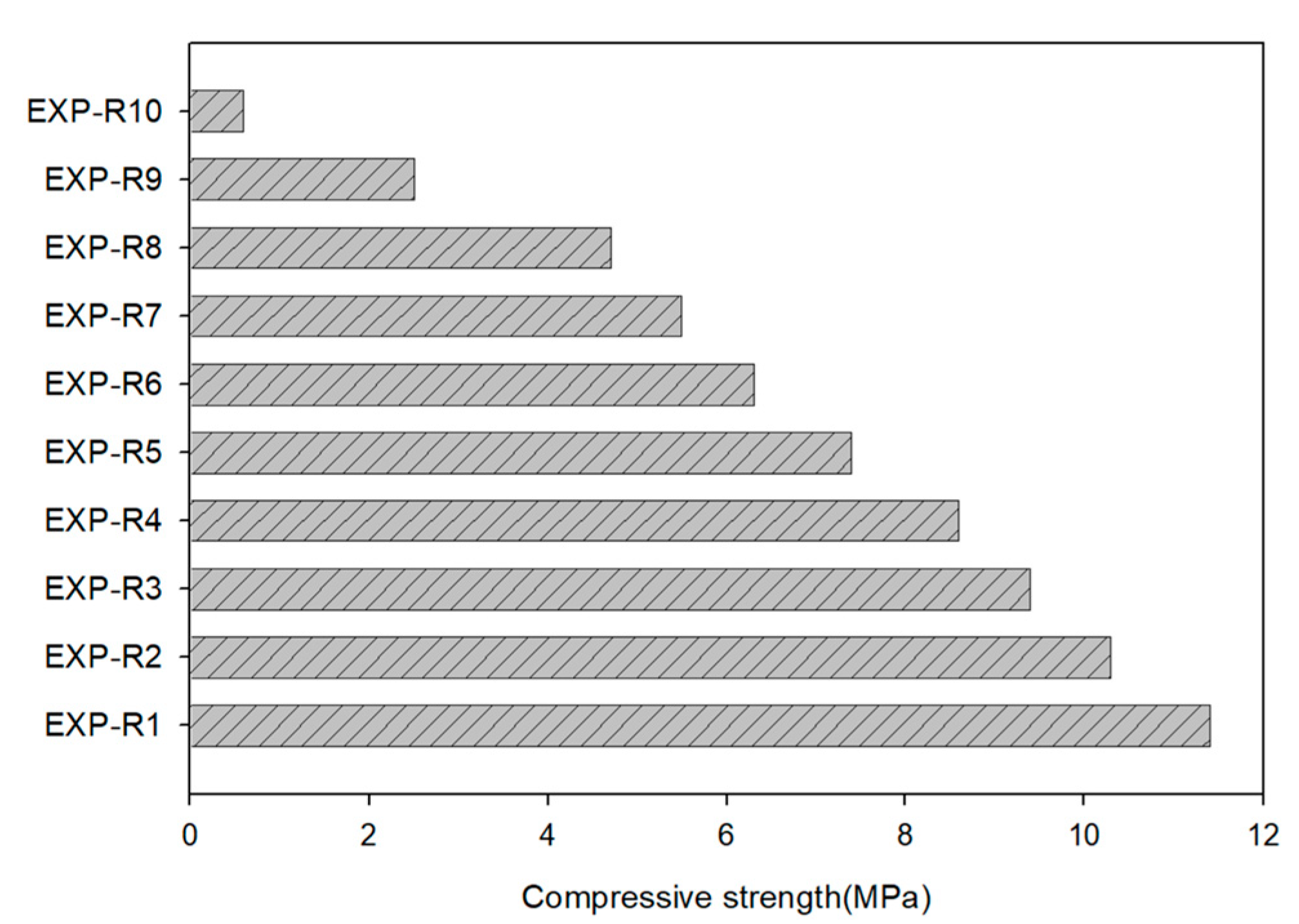
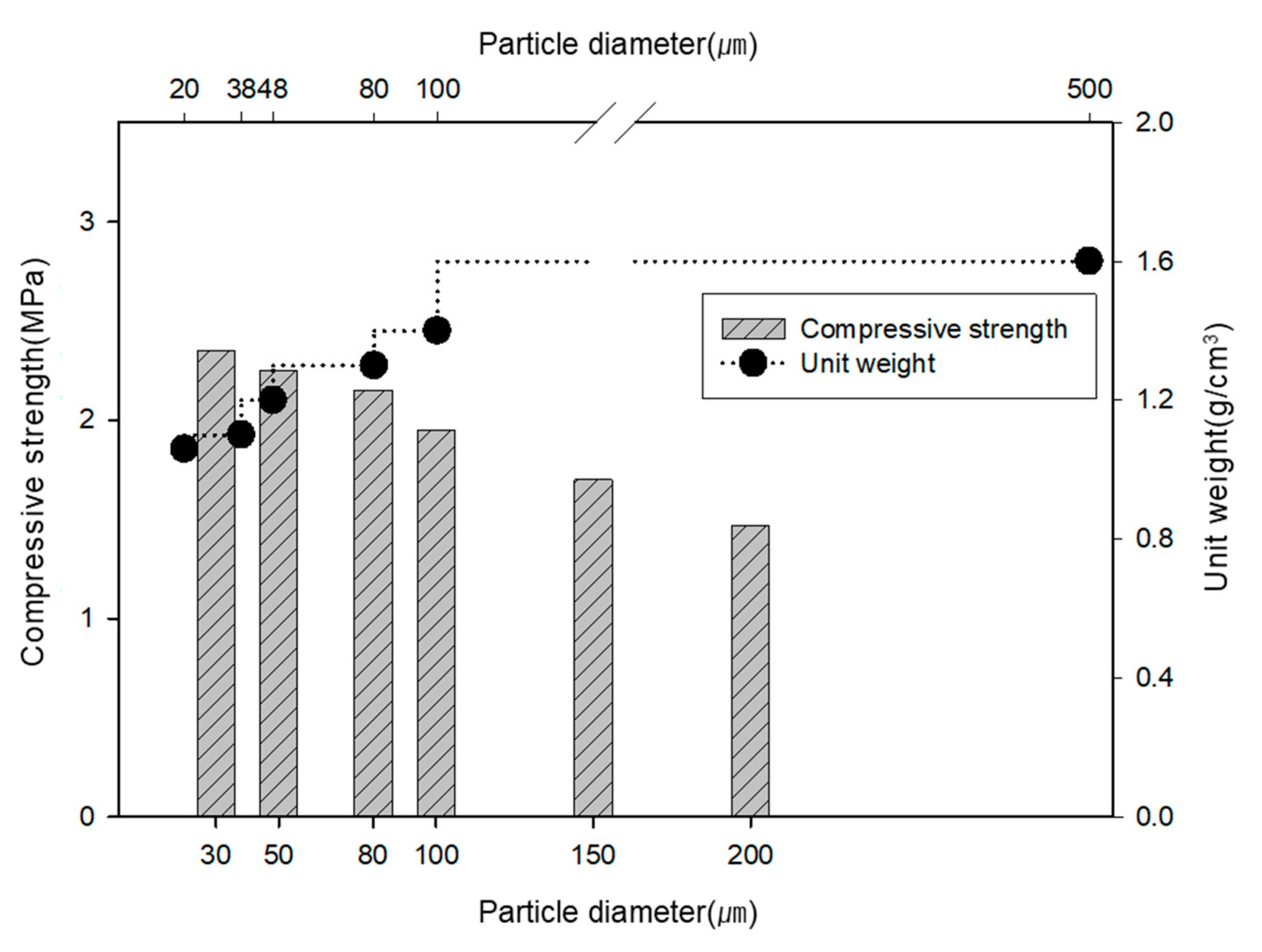
3.2. Mechanical Activation
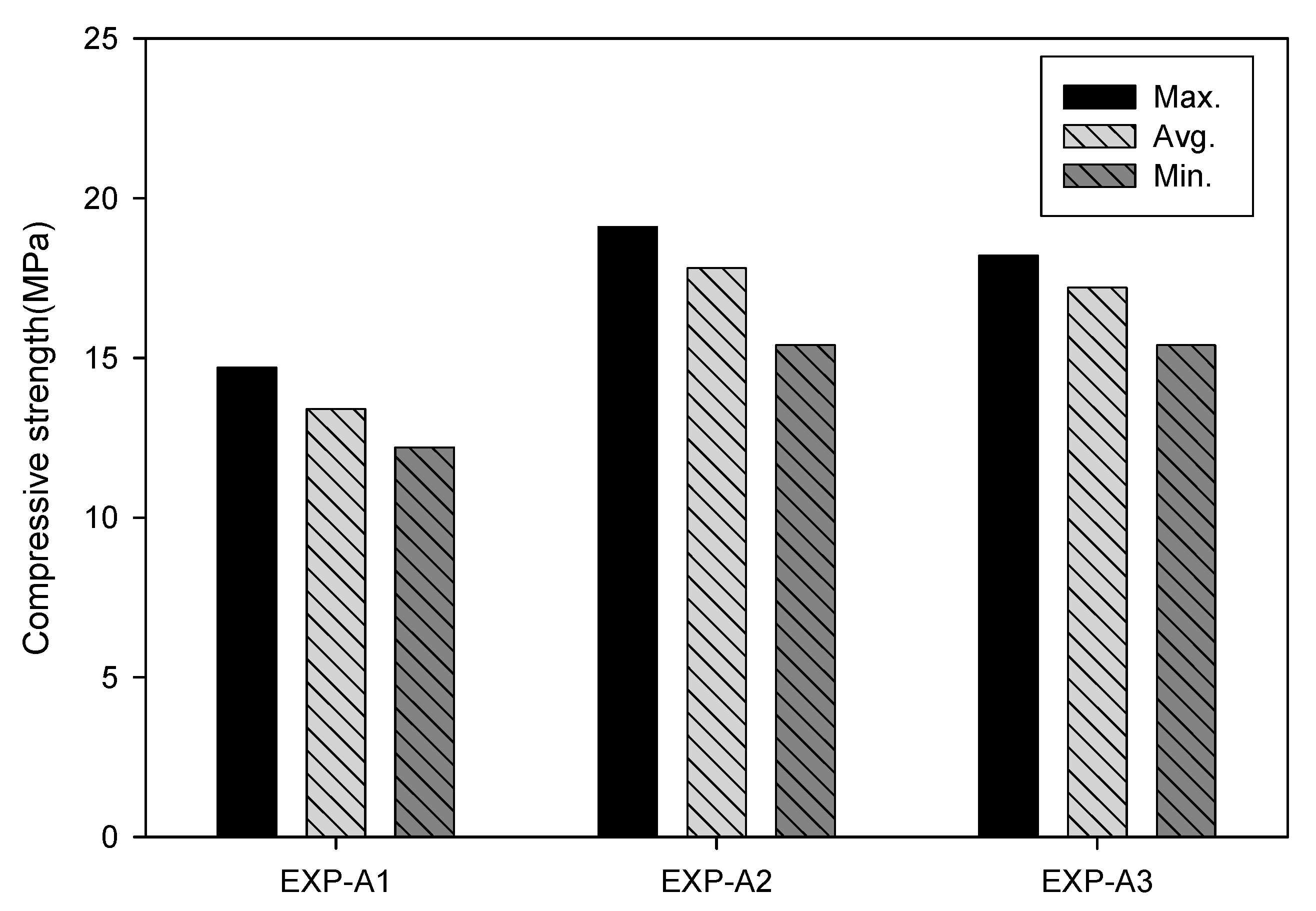
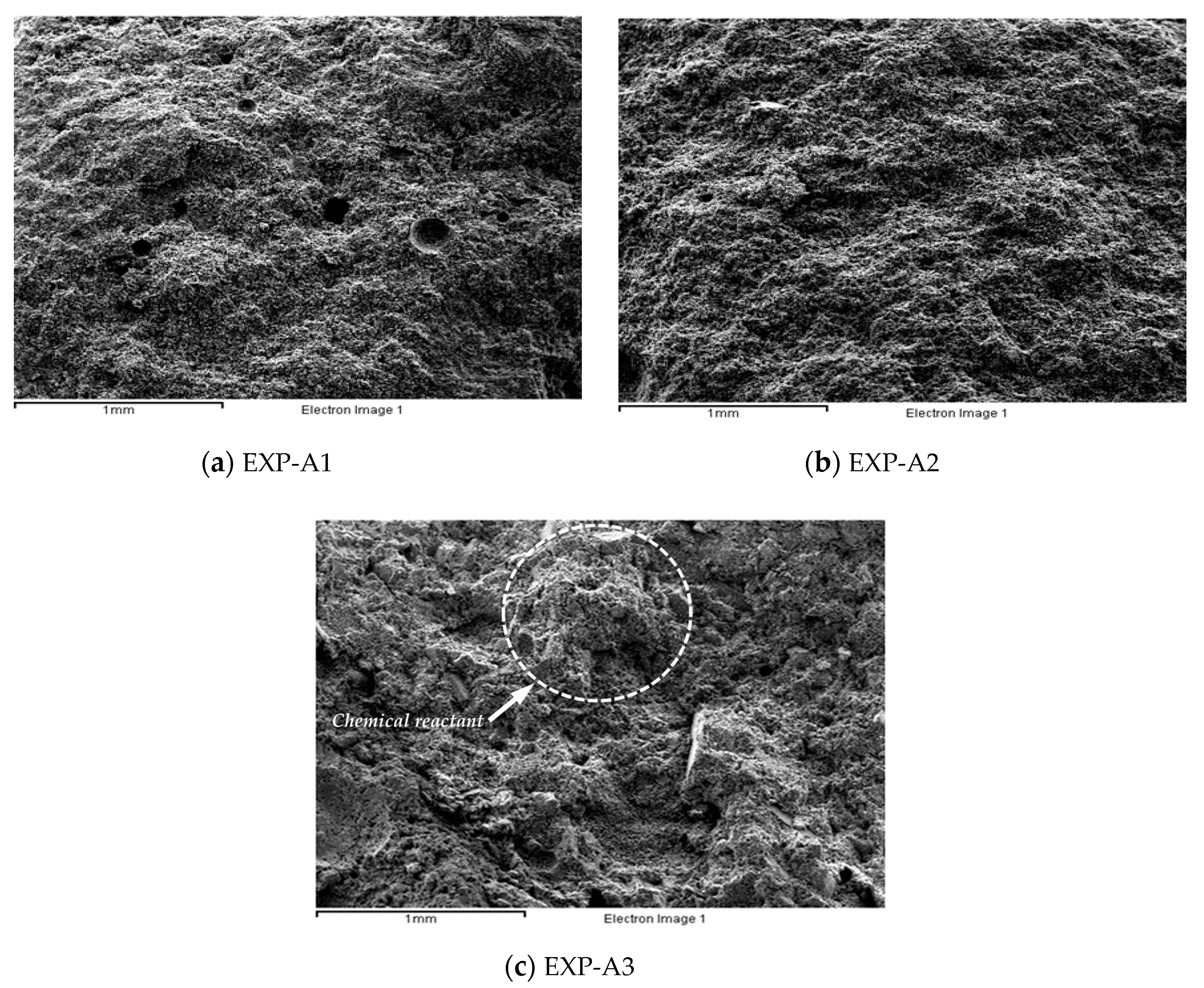
3.3. Chemical Activation
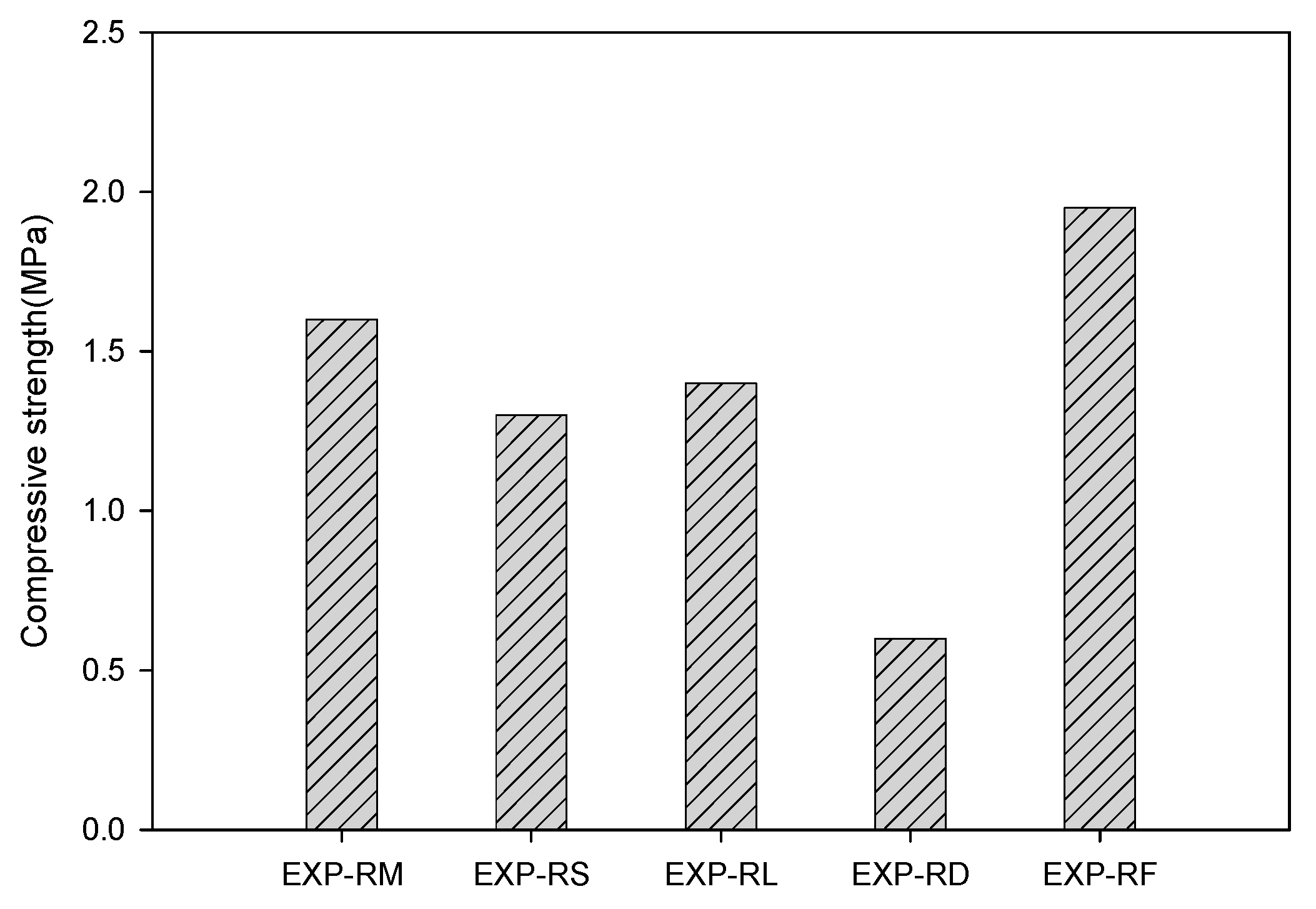
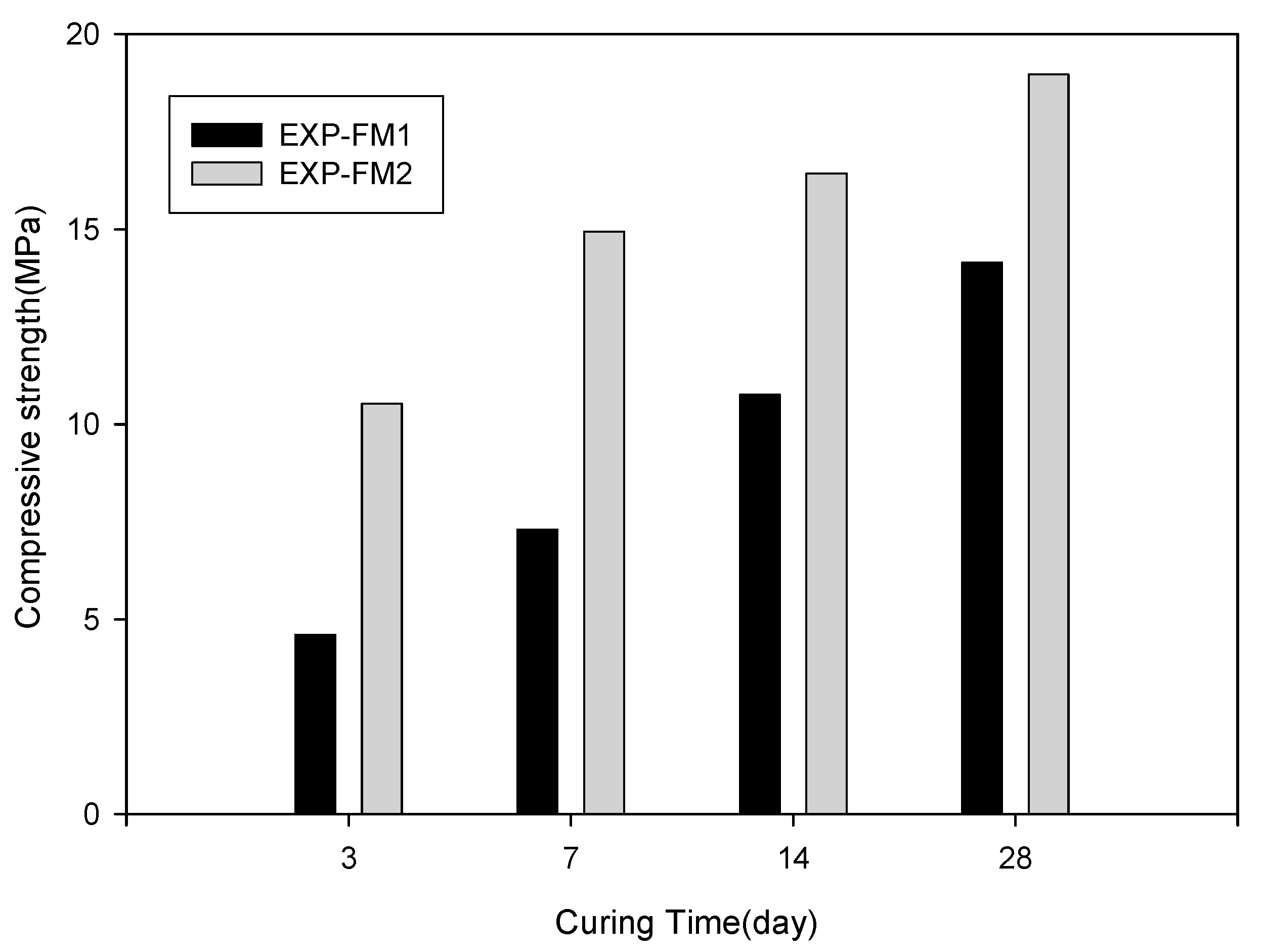
3.4. Evaluation of Substitute Materials
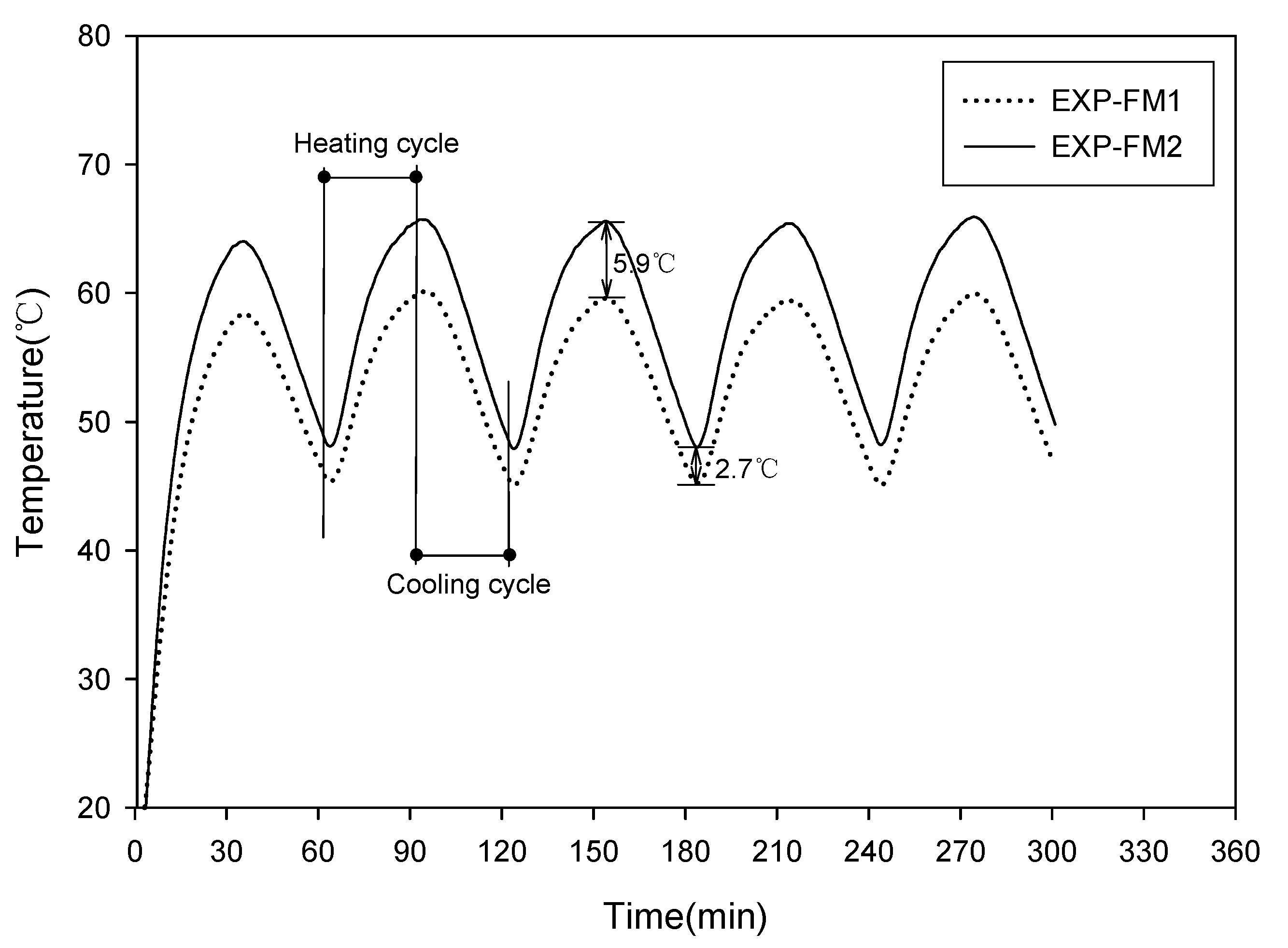
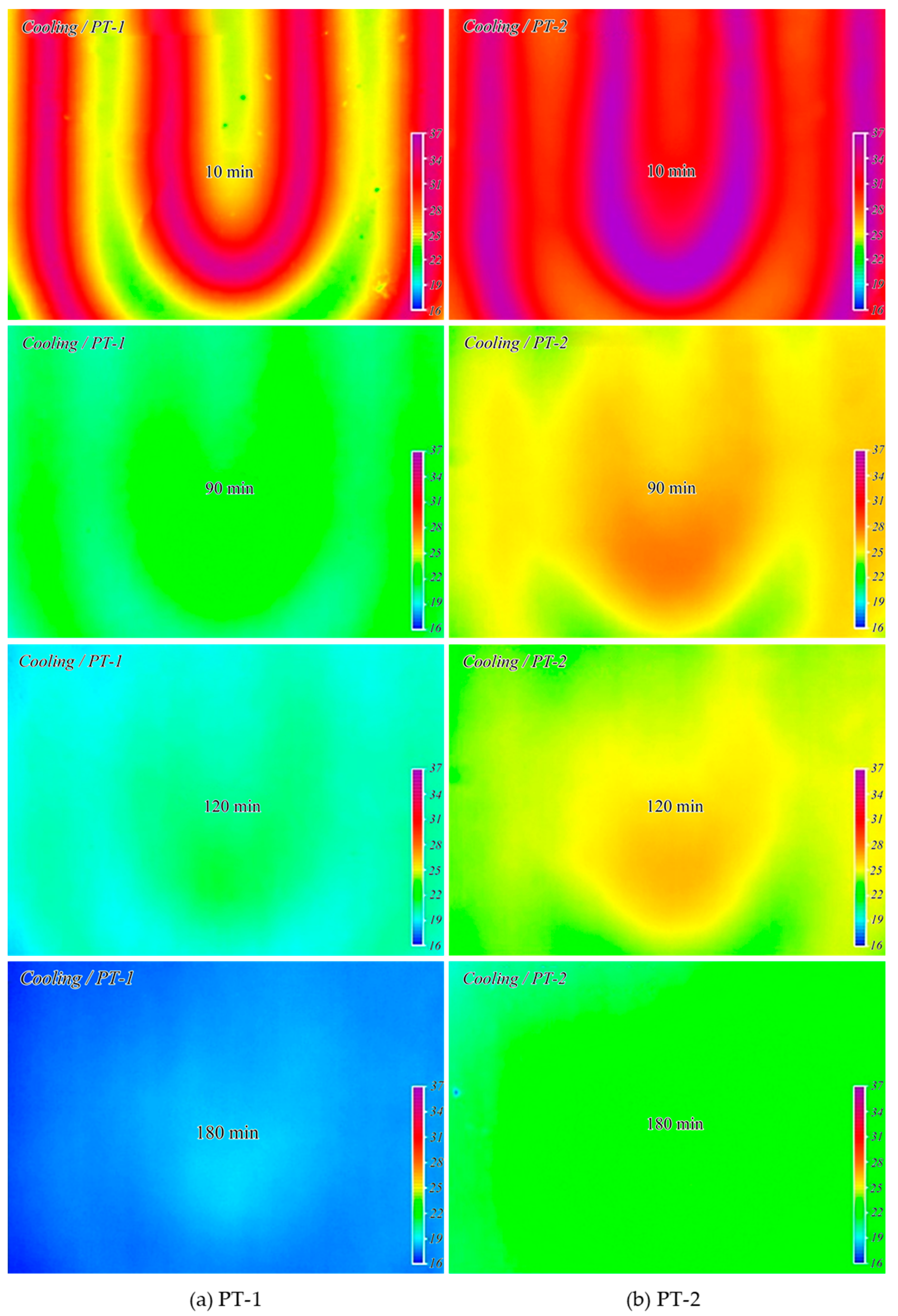
3.5. Characteristics of Thermal Diffusion and Heat Storage
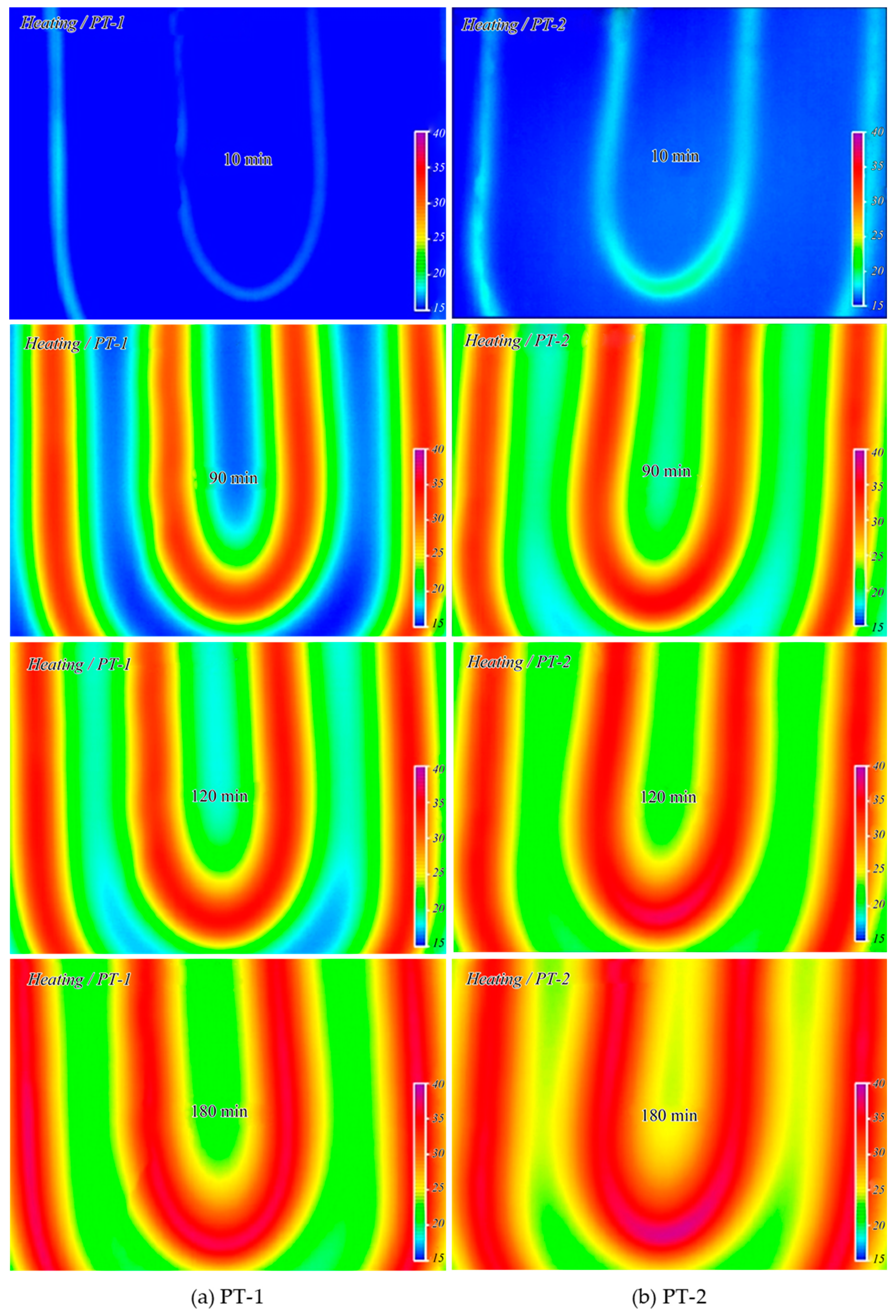
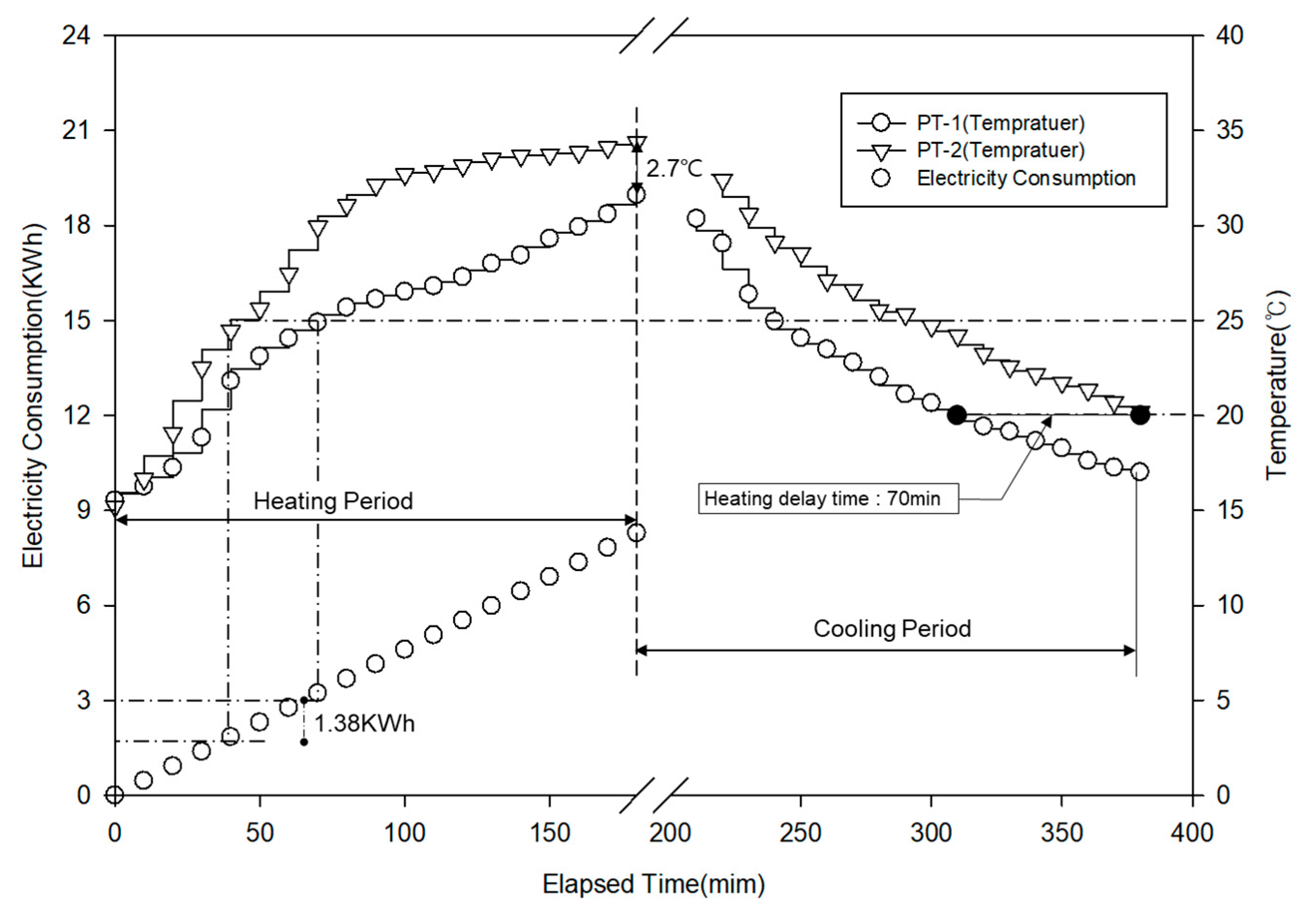
3.6. Pilot Test
3.6.1. Characteristics of Thermal Conductivity and Heat Storage
3.6.2. Energy Efficiency
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mehraj, S.S.; Bhat, G.A.; Balkhi, H.M. Cement factories and human health. Int. J. Cur. Res. Rev. 2013, 5, 47–54. [Google Scholar]
- Greenhouse gas inventory and research center of Korea. 2018 Korean Emissions Trading System (K-ETS) Summary Report; Greenhouse gas inventory and research center of Korea: Seoul, Korea, 2020. [Google Scholar]
- Kewalramani, M.A.; Syed, Z.I. Application of nanomaterials to enhance microstructure and mechanical properties of concrete. Int. J. Civ. Eng. Technol. 2018, 9, 115–129. [Google Scholar] [CrossRef]
- Islam, A.; Alengaram, U.J.; Jumaat, M.Z.; Bashar, I.I. The development of compressive strength of ground granulated blast furnace slag-palm oil fuel ash-fly ash based geopolymer mortar. Mater. Des. 2004, 56, 833–841. [Google Scholar] [CrossRef]
- Bernal, S.A.; Rodríguez, E.D.; Mejía de Gutiérrez, R.; Gordillo, M.; Provis, J.L. Mechanical and thermal characterisation of geopolymers based on silicate-activated metakaolin/slag blends. J. Mater. Sci. 2001, 42, 5477–5486. [Google Scholar] [CrossRef]
- Deb, P.S.; Nath, P.; Sarker, P.K. Strength and Permeation Properties of Slag Blended Fly Ash Based Geopolymer Concrete. Adv. Mater. Res. 2013, 651, 168–173. [Google Scholar]
- Malhotra, S.K.; Dave, N.G. Investigation into the effect of addition of fly-ash and burnt clay pozzolana on certain engineering properties of cement composites. Cem. Concr. Comp. 1999, 21, 285–291. [Google Scholar] [CrossRef]
- Indraratna, B.; Nutalaya, P.; Koo, K.S.; Kuganenthira, N. Engineering behaviour of a low carbon, pozzolanic fly ash and its potential as a construction fill. J. Can. Geotech. 1991, 28, 542–555. [Google Scholar] [CrossRef]
- Idawati, I.; Susan, A.B.; John, L.P.; Rackel, S.N.; Sinin, H.; Jannie, S.J. Modification of phase evolution in alkali-activated blast furnace slag by the incorporation of fly ash. Cem. Concr. Comp. 2014, 45, 125–135. [Google Scholar]
- Thanongsak, N.; Watcharapong, W.; Arnon, C. Utilization of fly ash with silica fume and properties of Portland cement–fly ash–silica fume concrete. Fuel 2010, 89, 768–774. [Google Scholar]
- Xinghua, F.; Wenping, H.; Chunxia, Y.; Dongxue, L.; Xuequan, W. Study on high-strength slag and fly ash compound cement. Cem. Concr. Res. 2000, 30, 1239–1243. [Google Scholar]
- Li, D.; Shen, J.; Chen, Y.; Cheng, L.; Xuequan, W. Study of properties on fly ash-slag complex cement. Cem. Concr. Res. 2000, 30, 1381–1387. [Google Scholar] [CrossRef]
- Roy, D.M.; Silsbee, M.R. Alkali activated cementilious materials, an overview. Mater. Res. Soc. 1992, 245, 153–164. [Google Scholar] [CrossRef]
- Arkaiusz, D.; Wojciech, F.; Halina, W.N.; Adriana, C.Z. Textural properties vs. CEC and EGME retention of Na–X zeolite prepared from fly ash at room temperature. Int. J. Miner. Process. 2007, 82, 57–68. [Google Scholar]
- Malik, R.; Ramteke, D.S.; Wate, S.R. Adsorption of malachite green on groundnut shell waste based powdered activated carbon. Waste Manag. 2007, 27, 1129–1138. [Google Scholar] [CrossRef] [PubMed]
- Jha, B.; Singh, D.N. A review on synthesis, characterization and industrial application of fly ash zeolites. J. Mater. Educ. 2011, 33, 65–132. [Google Scholar]
- Cho, K.W. Engineering properties of weathered alumino-silicate minerals as an eco-friendly construction materials. Ph.D. Thesis, Chung-Ang University, Seoul, Korea, 2018. [Google Scholar]
- Kauffman, R.A.; Van Dyk, D. Feldspars: In Industrial Minerals and Rocks, 6th ed.; Carr, D.D., Ed.; Society for Mining, Metallurgy, and Exploration Inc.: Littleton, CO, USA, 1994; pp. 473–481. [Google Scholar]
- Potter, M.J. Feldspar and nepheline syenite. In Minerals Yearbook; US Dept. of Interior, US Geological Survey: Reston, VA, USA, 1996. [Google Scholar]
- Cecen, F. Activated Carbon, Kirk-Othmer Encyclopedia of Chemical Technology; John Wiley and Sons: New York, NY, USA, 2014. [Google Scholar]
- Ministry of Land, Infrastructure and Transport. Standard Floor Finishing and Structures for Interlayer Noise Prevention; Ministry of Land, Infrastructure and Transport: Governing City, Korea, 2015.
- KS L ISO 679. Methods of Testing Cement-Determination of Strength; Korea standard association: Seoul, Korea, 2016.
- Brooks, J.J.; Megat Johari, M.A. Effect of metakaolin on creep and shrinkage of concrete. Cem. Concr. Comp. 2001, 23, 495–502. [Google Scholar] [CrossRef]
- ASTM C128. Standard Test. Method for Relative Density (Specific Gravity) and Absorption of Fine Aggregate; ASTM International: West Conshohocken, PA, USA, 2015. [Google Scholar]
- KS F 2504. Standard Test. Method for Density and Absorption of Fine Aggregates; Korea standard association: Seoul, Korea, 2019.
- ASTM C109/C109M-16a. Standard Test. Method for Compressive Strength of Hydraulic Cement Mortars (Using 2-in. or [50-mm] Cube Specimens]; ASTM International: West Conshohocken, PA, USA, 2016. [Google Scholar]
- KS L 5105. Testing Method for Compressive Strength of Hydraulic Cement Mortar; Korea standard Association: Seoul, Korea, 2017.
- Park, Y.S. Experimental Study on the Mortar using Alumino-Silicate Minerals as Aggregate. Master’s Thesis, Chung-Ang University, Seoul, Korea, 2020. [Google Scholar]
- Ferdoush, S.; Li, X. Wireless Sensor Network System Design Using Raspberry Pi and Arduino for Environmental Monitoring Applications. Procedia Computer Sci. 2014, 34, 103–110. [Google Scholar] [CrossRef]
- Choi, E.J.; Lee, W.J.; Lee, K.H.; Kim, J.K.; Kim, J.H. Real-time pedestrian recognition at night based on far infrared image sensor. In Proceedings of the 2nd International Conference on Communication and Information Processing, Singapore, 26–29 November 2016; pp. 115–119. [Google Scholar]
- KS L 5220. Dry Ready Mixed Cement Mortar; Korea standard Association: Seoul, Korea, 2018.

| I. Mineral Composition of Feldspar Porphyry | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Albite (NaAlSi3O8) | Quartz (SiO2) | Orthoclase (KAlSi3O8) | Chlorite | |||||||
| (%) | ||||||||||
| 38.8 | 26.5 | 22.7 | 8.3 | |||||||
| II. Chemical Composition of Feldspar | ||||||||||
| Location | SiO2 | Al2O3 | K2O | Na2O | CaO | Fe2O | MgO | TiO2 | LOI | Other |
| (%) | ||||||||||
| Chung-ju | 69.59 | 13.07 | 2.7 | 4.53 | 2.56 | 2.49 | 1.61 | 0.49 | 2.46 | 0.50 |
| Muan | 67.10 | 15.26 | 5.02 | 3.84 | 1.88 | 3.34 | 0.73 | 0.40 | 1.59 | 0.84 |
| Namwon | 68.95 | 14.59 | 4.63 | 3.39 | 1.29 | 3.03 | 0.67 | 0.37 | 2.71 | 0.37 |
| Mixed Ratio (Cement: Feldspar) | Feldspar Size | Curing Time | W/C Ratio | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EXP-R1 | EXP-R2 | EXP-R3 | EXP-R4 | EXP-R5 | EXP-R6 | EXP-R7 | EXP-R8 | EXP-R9 | EXP-R10 | 20–500 µm | 7 day | 0.5 |
| 10:0 | 9:1 | 8:2 | 7:3 | 6:4 | 5:5 | 4:6 | 3:7 | 2:8 | 1:9 | |||
| Mixed Ratio (Cement: Clay Mineral) | Metakaolin | Silica Fume | Ilite | Dolomite | Feldspar | Curing Time (Day) | W/C Ratio |
|---|---|---|---|---|---|---|---|
| 7:3 | EXP-RM | EXP-RS | EXP-RL | EXP-RD | EXP-RF | 3 | 0.5 |
| I. Mechanical Activation | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Test Methods | Feldspar Size (µm) | Mixed Ratio (PC:FS) * | Curing Time (day) | W/C Ratio | |||||
| Unit weight test | 20 | 38 | 48 | 80 | 100 | 500 | 0:10 | - | - |
| Compressive strength test | 30 | 50 | 80 | 100 | 150 | 200 | 70:30 | 3 | 0.5 |
| II. Chemical Activation | Curing time (day) | W/C Ratio | |||||||
| Test No. | EXP-A1 | EXP-A2 | EXP-A3 | Feldspar size (µm) | 28 | 0.5 | |||
| Mixed ratio (PC:FS:S) * | 100:0:0 | 100:0:0.1 | 30:70:0.1 | 80 | |||||
| Test Method | Mixed Ratio | Curing Time (Day) | W/C Ratio | |
| EXP-FM1 (PC:AG) * | EXP-FM2 (PC:AGF:PS:S) * | 3, 7, 14, 28 | 0.5 | |
| Compressive strength test | 25:75 | 20:40:4%:0.1 | ||
| Component | Si | Al | Ca | Na | Cl | Mg | K | S | Fe | |
|---|---|---|---|---|---|---|---|---|---|---|
| Sample No. | (%) | |||||||||
| EXP-A1 | 14.1 | 2.9 | 73.8 | N.D * | N.D * | 1.9 | 1.2 | 3.5 | 2.6 | |
| EXP-A2 | 11.2 | 3.1 | 73.6 | 0.8 | 0.9 | 1.7 | 3.2 | 2.7 | 2.8 | |
| EXP-A3 | 29.6 | 7.2 | 43.9 | 1.6 | 1.3 | 3.1 | 4.7 | 2.5 | 6.1 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, J.-G.; Cho, J.-W.; Kim, S.-W.; Park, Y.-S.; Lee, J.-Y. Characteristics of CO2 and Energy-Saving Concrete with Porous Feldspar. Materials 2020, 13, 4204. https://doi.org/10.3390/ma13184204
Han J-G, Cho J-W, Kim S-W, Park Y-S, Lee J-Y. Characteristics of CO2 and Energy-Saving Concrete with Porous Feldspar. Materials. 2020; 13(18):4204. https://doi.org/10.3390/ma13184204
Chicago/Turabian StyleHan, Jung-Geun, Jin-Woo Cho, Sung-Wook Kim, Yun-Suk Park, and Jong-Young Lee. 2020. "Characteristics of CO2 and Energy-Saving Concrete with Porous Feldspar" Materials 13, no. 18: 4204. https://doi.org/10.3390/ma13184204
APA StyleHan, J.-G., Cho, J.-W., Kim, S.-W., Park, Y.-S., & Lee, J.-Y. (2020). Characteristics of CO2 and Energy-Saving Concrete with Porous Feldspar. Materials, 13(18), 4204. https://doi.org/10.3390/ma13184204






