Effect of Annealing on the Microstructure and SERS Performance of Mo-48.2% Ag Films
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
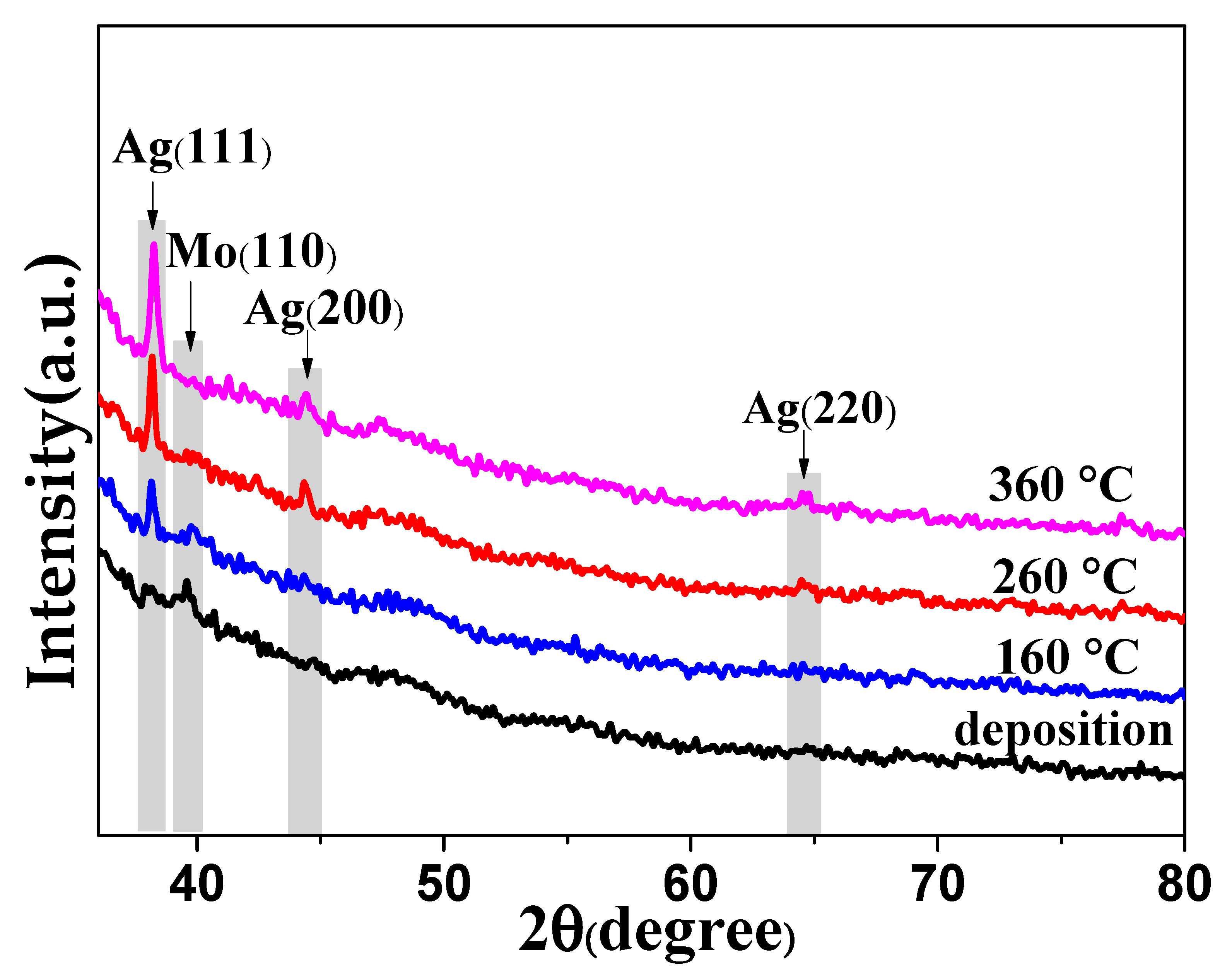
3.1. X-ray Diffraction (XRD) Patterns of the Mo-Ag Films with Different Annealing Temperature
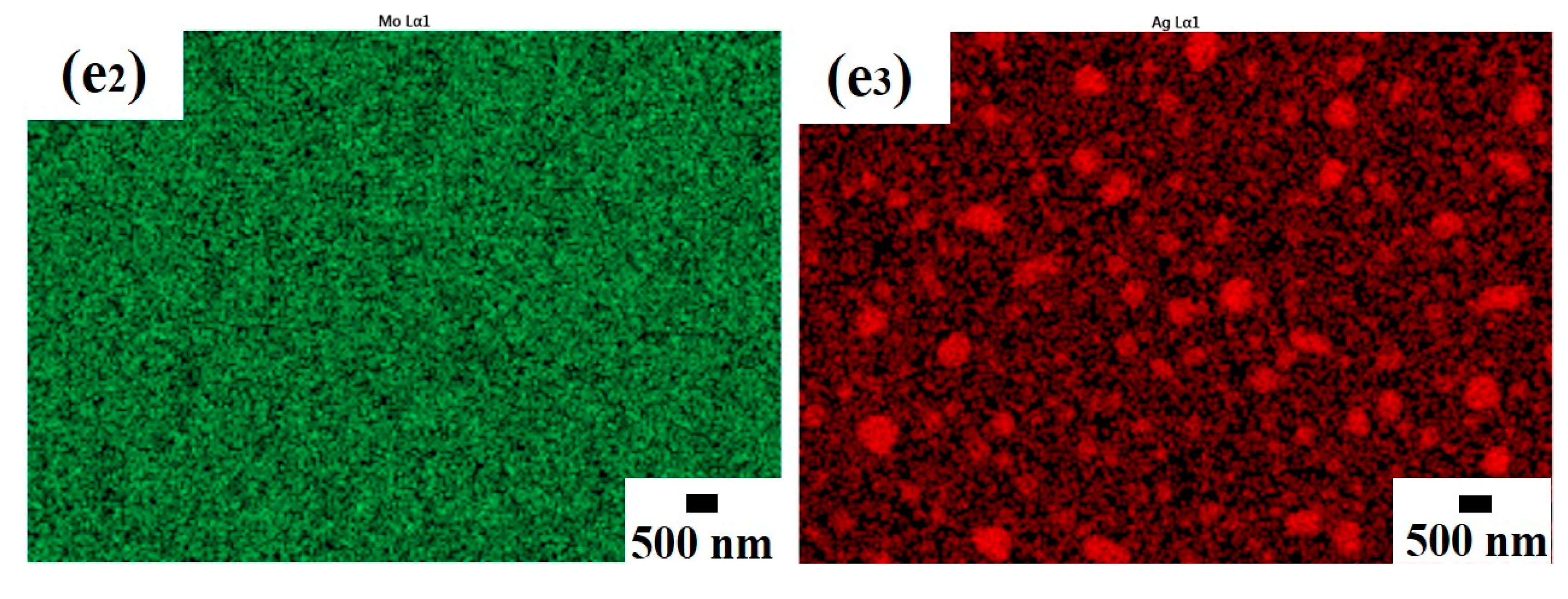
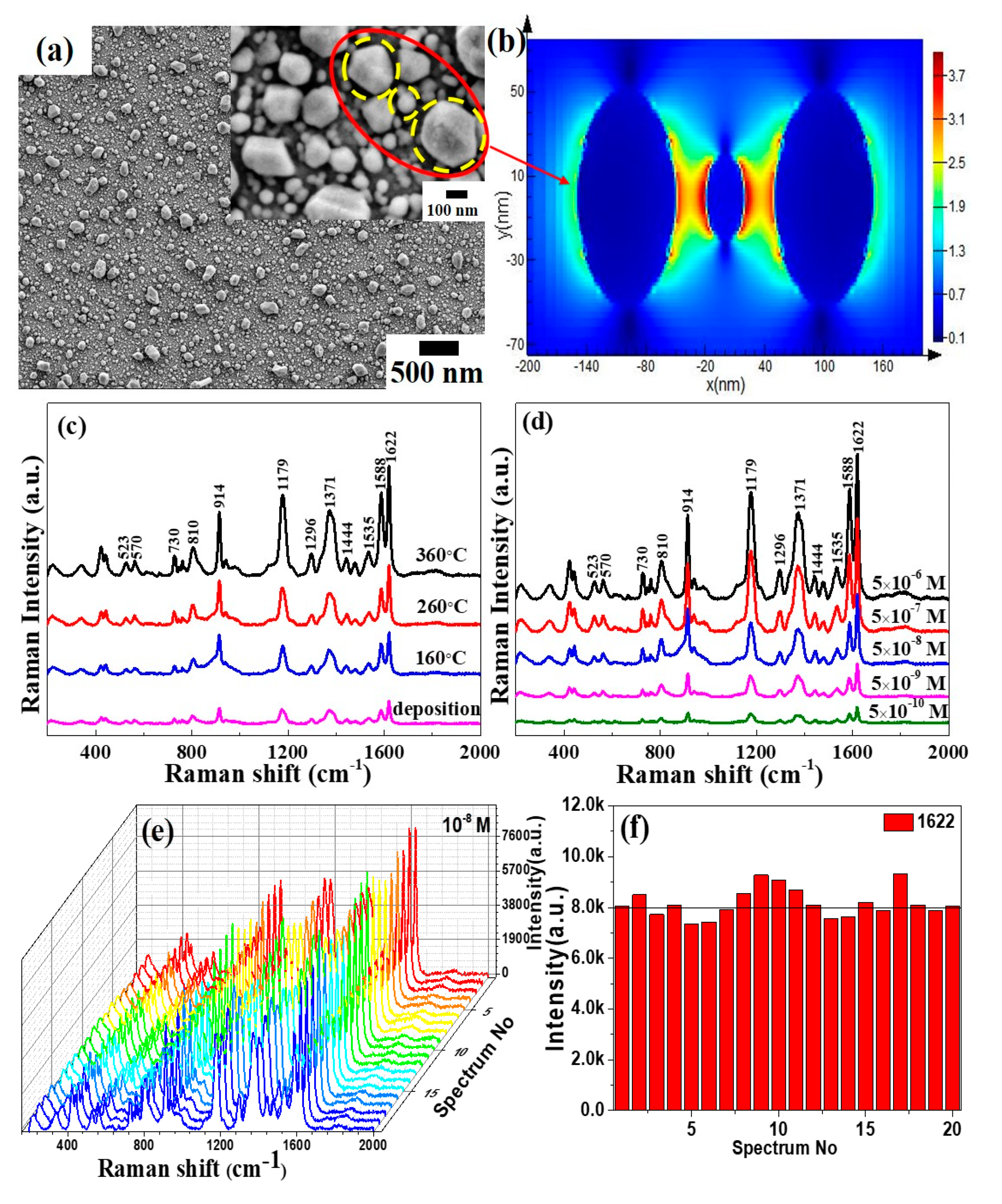
3.2. Morphology Characterization of Annealed Mo-Ag Films
3.3. Preparation and Raman Measurement of the Ag Layer/Annealed Mo-48.2% Ag Film
3.4. Electrical Performance of the Annealed Mo-48.2% Ag Film
3.5. Surface-Enhanced Raman Scattering (SERS) Activity of the Ag Layer/Annealed Mo-48.2% Ag Films
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lee, C.H.; Hankus, M.E.; Tian, L.; Pellegrino, P.M.; Singamaneni, S. Highly sensitive surface enhanced Raman scattering substrates based on filter paper loaded with plasmonic nanostructures. Anal. Chem. 2011, 83, 8953–8958. [Google Scholar] [CrossRef] [PubMed]
- Langer, J.; Aberasturi, D.J.D.; Aizpurua, J.; Alvarez-Puebla, R.A.; Auguié, B.; Baumberg, J.J.; Bazan, G.C.; Bell, S.E.J.; Boisen, A.; Brolo, A.G.; et al. Present and Future of Surface Enhanced Raman Scattering. ACS Nano 2020, 14, 28–117. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Qi, G.; Xu, S.; Xu, W. Construction of high sensitive surface-enhanced Raman scattering (SERS) nanosensor aimed for the test of glucose in urine. RSC Adv. 2016. [CrossRef]
- Wang, T.; Zhou, J.; Wang, Y. Simple, Low-Cost Fabrication of Highly Uniform and Reproducible SERS Substrates Composed of Ag–Pt Nanoparticles. Nanomaterials 2018, 8, 331. [Google Scholar] [CrossRef] [PubMed]
- Polavarapu, L.; Liz-Marzán, L.M. Towards low-cost flexible substrates for nanoplasmonic sensing. Phys. Chem. Chem. Phys. 2013, 15, 5288. [Google Scholar] [CrossRef]
- Fei, X.; Liu, Z.; Hou, Y.; Li, Y.; Yang, G.; Su, C.; Wang, Z.; Zhong, H.; Zhuang, Z.; Guo, Z. Synthesis of Au NP@MoS2 Quantum Dots Core@Shell Nanocomposites for SERS Bio-Analysis and Label-Free Bio-Imaging. Materials 2017, 10, 650. [Google Scholar] [CrossRef]
- Tseng, S.C.; Yu, C.C.; Wan, D.; Chen, H.L.; Wang, L.A.; Wu, M.C.; Su, W.F.; Han, H.C.; Chen, L.C. Eco-Friendly Plasmonic Sensors: Using the Photothermal Effect to Prepare Metal Nanoparticle-Containing Test Papers for Highly Sensitive Colorimetric Detection. Anal. Chem. 2012, 84, 5140. [Google Scholar] [CrossRef]
- Zhang, C.; Huang, Q.; Cui, Q.; Ji, C.; Zhang, Z.; Chen, X.; George, T.; Zhao, S.; Guo, L.J. High-Performance Large-Scale Flexible Optoelectronics Using Ultrathin Silver Films with Tunable Properties. ACS Appl. Mater. Interfaces 2019, 11. [Google Scholar] [CrossRef]
- Leopold, N.; Lendl, B. A New Method for Fast Preparation of Highly Surface-Enhanced Raman Scattering (SERS) Active Silver Colloids at Room Temperature by Reduction of Silver Nitrate with Hydroxylamine Hydrochloride. J. Phys. Chem. B 2003, 107, 5723–5727. [Google Scholar] [CrossRef]
- Ambroziak, R.; Hodyński, M.; Pociński, T.; Pisarek, M.; Kudelski, A. Cubic Silver Nanoparticles Fixed on TiO2 Nanotubes as Simple and Efficient Substrates for Surface Enhanced Raman Scattering. Materials 2019, 12, 3373. [Google Scholar] [CrossRef]
- Yang, C.; Xiang, X.; Zhang, Y.; Peng, Z.; Cao, Z.; Wang, J.; Xuan, L. Large-scale controlled fabrication of highly roughened flower-like silver nanostructures in liquid crystalline phase. Sci. Rep. 2015, 5, 12355. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.W.; Chang, S.H.; Lai, Y.S.; Lin, C.C.; Tsai, C.M.; Lee, Y.C.; Chen, J.C.; Huang, C.L. Effect of Temperature on the Growth of Silver Nanoparticles Using Plasmon-Mediated Method under the Irradiation of Green LEDs. Materials 2014, 7, 7781–7798. [Google Scholar] [CrossRef]
- Tao, A.; Kim, F.; Hess, C.; Goldberger, J.; He, R.; Sun, Y.; Xia, Y.; Yang, P. Langmuir−Blodgett Silver Nanowire Monolayers for Molecular Sensing Using Surface-Enhanced Raman Spectroscopy. Nano Lett. 2003, 3, 1229–1233. [Google Scholar] [CrossRef]
- Yang, Y.; Li, Z.Y.; Yamaguchi, K.; Tanemura, M.; Huang, Z.; Jiang, D.; Chen, Y.; Zhou, F.; Nogami, M. Controlled fabrication of silver nanoneedles array for SERS and their application in rapid detection of narcotics. Nanoscale 2012, 4, 2663–2669. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.P.; Chu, H.Y.; Abell, J.; Tripp, R.A.; Zhao, Y. Flexible and mechanical strain resistant large area SERS active substrates. Nanoscale 2012, 4, 3410–3414. [Google Scholar] [CrossRef]
- Wang, K.; Sun, D.W.; Pu, H.; Wei, Q.Y.; Huang, L. A Stable, Flexible and High-Performance SERS Chip Enabled by Ternary Films-Packaged Plasmonic Nanoparticles Array. ACS Appl. Mater. Interfaces 2019, 11. [Google Scholar] [CrossRef]
- Bhattacharyya, S.R.; Datta, D.; Shyjumon, I.; Smirnov, B.M.; Chini, T.K.; Ghose, D.; Hippler, R. Growth and melting of silicon supported silver nanocluster films. J. Phys. D Appl. Phys. 2009, 42, 035306. [Google Scholar] [CrossRef]
- Tao, A.; Sinsermsuksakul, P.; Yang, P. Polyhedral silver nanocrystals with distinct scattering signatures. Angew. Chem. 2010, 45, 4597–4601. [Google Scholar] [CrossRef]
- Zhang, R.; Xu, B.B.; Liu, X.Q.; Zhang, Y.L.; Xu, Y.; Chen, Q.D.; Sun, H.B. Highly efficient SERS test strips. Chem. Commun. 2012, 48, 5913–5915. [Google Scholar] [CrossRef]
- Xu, B.B.; Ma, Z.C.; Wang, H.; Liu, X.Q.; Zhang, Y.L.; Zhang, X.L.; Zhang, R.; Jiang, H.B.; Sun, H.B. A SERS-active microfluidic device with tunable surface plasmon resonances. Electrophoresis 2011, 32, 3378–3384. [Google Scholar] [CrossRef]
- Ma, Y.; Yang, L.; Yang, Y.; Peng, Y.; Wei, Y.; Huang, Z. Multifunctional Ag-decorated g-C3N4 nanosheets as recyclable SERS substrates for CV and RhB detection. RSC Adv. 2018, 8, 22095. [Google Scholar] [CrossRef]
- Zhu, S.Q.; Zhang, T.; Guo, X.L.; Wang, L.D. Self-Assembly of Two-Dimensional Hexagon Shaped Gold Nanoparticle Film Using Solvent Evaporation Method. Nanosci. Nanotechnol. Lett. 2013, 5, 253–256. [Google Scholar] [CrossRef]
- Bozzini, B.; Gaudenzi, G.P.D.; Mele, C. A SERS investigation of the electrodeposition of Ag–Au alloys from free-cyanide solutions. J. Electroanal. Chem. 2004, 570, 29–34. [Google Scholar] [CrossRef]
- Mahato, M.; Sarkar, R.; Pal, P.; Talapatra, G.B. Formation of silver nanoparticle at phospholipid template using Langmuir–Blodgett technique and its Surface-enhanced Raman Spectroscopy application. Indian J. Phys. 2015, 89, 997–1005. [Google Scholar] [CrossRef]
- Fasolato, C.; Domenici, F.; Brasili, F.; Mura, F.; Sennato, S.; Angelis, D.L.; Mazzi, E.; Bordi, F.; Postorino, P. Self-Assembled Nanoparticle Aggregates: Organizing Disorder for High Performance Surface-Enhanced Spectroscopy. AIP Conf. Proc. 2015. [CrossRef]
- Zhu, W.; Wu, Y.; Yan, C.; Wang, C.; Zhang, M.; Wu, Z. Facile Synthesis of Mono-Dispersed Polystyrene (PS)/Ag Composite Microspheres via Modified Chemical Reduction. Materials 2013, 6, 5625–5638. [Google Scholar] [CrossRef]
- Kim, S.M.; Lee, T.; Gil, Y.G.; Kim, G.H.; Park, C.; Jang, H.; Min, J. Fabrication of Bioprobe Self-Assembled on Au-Te Nanoworm Structure for SERS Biosensor. Materials 2020, 13, 3234. [Google Scholar] [CrossRef]
- Lian, X.X.; Sun, H.L.; Lv, Y.J.; Wang, G.X. Room temperature self-assembled Ag nanoparticles/Mo-37.5% Ag film as efficient flexible SERS substrate. Mater. Lett. 2020. [Google Scholar] [CrossRef]
- Huang, X.X.; Sun, H.L.; Wang, G.X.; Stock, H.R. Self-formation of Ag particles/Ag-Zr alloy films on flexible polyimide as SERS substrates. Appl. Surf. Sci. 2019, 487, 1341–1347. [Google Scholar] [CrossRef]
- Lian, X.X.; Lv, Y.J.; Sun, H.L.; Hui, D.; Wang, G.X. Effects of Ag contents on the microstructure and SERS performance of self-grown Ag nanoparticles/Mo-Ag alloy films. Nanotechnol. Rev. 2020, 9, 751–759. [Google Scholar] [CrossRef]
- Sarakinos, K.; Greczynski, G.; Elofsson, V.; Magnfält, D.; Högberg, H.; Alling, B. Theoretical and experimental study of metastable solid solutions and phase stability within the immiscible Ag-Mo binary system. J. Appl. Phys. 2016, 119, 095303. [Google Scholar] [CrossRef]
- Sun, H.L.; Huang, X.X.; He, M.J.; Lian, X.X.; Wang, G.X. Preparation and controllability of Cu particles on annealed Mo-Cu alloy films. Mater. Lett. 2019, 254, 175–177. [Google Scholar] [CrossRef]
- Sun, H.L.; Huang, X.X.; Lian, X.X.; Wang, G.X. Discrepancies in the Microstructures of Annealed Cu–Zr Bulk Alloy and Cu–Zr Alloy Films. Materials 2019, 12, 2467. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.L.; Huang, X.X.; Lian, X.X.; Wang, G.X. Adjustment Cu3Si growth in the interface of annealed Cu-Zr alloy films/Si substrate to form inverted pyramid structure. Mater. Lett. 2019. [Google Scholar] [CrossRef]
- Kweon, S.Y.; Choi, S.K.; Yeom, S.J.; Roh, J.S. Platinum Hillocks in Pt/Ti Film Stacks Deposited on Thermally Oxidized Si Substrate. Jpn. J. Appl. Phys. 2001, 40, 5850–5855. [Google Scholar] [CrossRef]
- Chen, C.; Suganuma, K. Low temperature SiC die-attach bonding technology by hillocks generation on Al sheet surface with stress self-generation and self-release. Sci. Rep. 2020, 10. [Google Scholar] [CrossRef]
- Huang, X.X.; Sun, H.L.; Shen, J.; Cui, K.; Wang, G.X. Different Effects of Annealing on Microstructure Evolution and SERS Performance for Cu-Cr Alloy Film and Bulk Alloy. Materials 2019, 12, 2990. [Google Scholar] [CrossRef]
- Ding, Q.; Zhang, L.; Yang, L. A simple approach for the synthesis of Ag-coated Ni@TiO2 nanocomposites as recyclable photocatalysts and SERS substrate to monitor catalytic degradation of dye molecules. Mater. Res. Bull. 2014, 53, 205–210. [Google Scholar] [CrossRef]
- Zhang, K.; Zeng, T.; Tan, X.; Wu, W.; Tang, Y.; Zhang, H. A facile surface-enhanced Raman scattering (SERS) detection of rhodamine 6G and crystal violet using Au nanoparticle substrates. Appl. Surf. Sci. 2015, 347, 569–573. [Google Scholar] [CrossRef]
- Stiles, P.L.; Dieringer, J.A.; Shah, N.C.; Van Duyne, R.P. Surface-enhanced Raman spectroscopy. Annu. Rev. Anal. Chem. 2008, 1, 601–626. [Google Scholar] [CrossRef]



| Annealing Temperature | 2θ (Degree) | d (Å) | β (Degree) | D (nm) |
|---|---|---|---|---|
| Room temperature | 39.573 | 2.275 | 0.319 | 26 |
| 160 | 38.250 | 2.351 | 0.268 | 31 |
| 260 | 38.148 | 2.357 | 0.258 | 32 |
| 360 | 38.271 | 2.350 | 0.249 | 33 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, H.; Lian, X.; Lv, Y.; Liu, Y.; Xu, C.; Dai, J.; Wu, Y.; Wang, G. Effect of Annealing on the Microstructure and SERS Performance of Mo-48.2% Ag Films. Materials 2020, 13, 4205. https://doi.org/10.3390/ma13184205
Sun H, Lian X, Lv Y, Liu Y, Xu C, Dai J, Wu Y, Wang G. Effect of Annealing on the Microstructure and SERS Performance of Mo-48.2% Ag Films. Materials. 2020; 13(18):4205. https://doi.org/10.3390/ma13184205
Chicago/Turabian StyleSun, Haoliang, Xinxin Lian, Yuanjiang Lv, Yuanhao Liu, Chao Xu, Jiwei Dai, Yilin Wu, and Guangxin Wang. 2020. "Effect of Annealing on the Microstructure and SERS Performance of Mo-48.2% Ag Films" Materials 13, no. 18: 4205. https://doi.org/10.3390/ma13184205
APA StyleSun, H., Lian, X., Lv, Y., Liu, Y., Xu, C., Dai, J., Wu, Y., & Wang, G. (2020). Effect of Annealing on the Microstructure and SERS Performance of Mo-48.2% Ag Films. Materials, 13(18), 4205. https://doi.org/10.3390/ma13184205




