Thermochemical Route for Extraction and Recycling of Critical, Strategic and High-Value Elements from By-Products and End-of-Life Materials, Part II: Processing in Presence of Halogenated Atmosphere
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Elemental and Mineralogical Analysis of Copper Anode Slime (CAS) and Polyvinyl Chloride (PVC) Samples
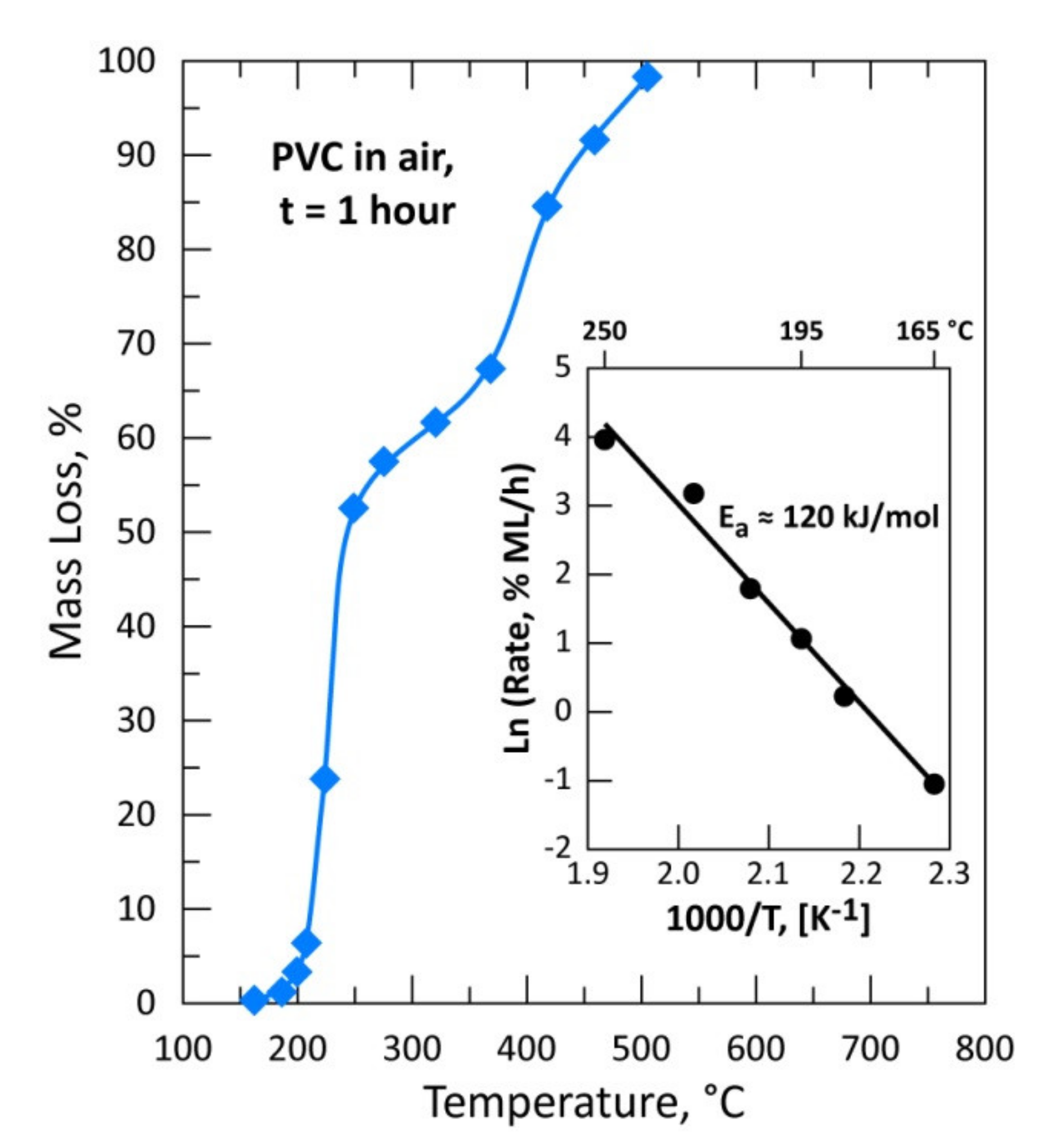
3.2. Thermal Treatment of a Mixture of (CAS + PVC) and PVC Sample in Air for 1 h
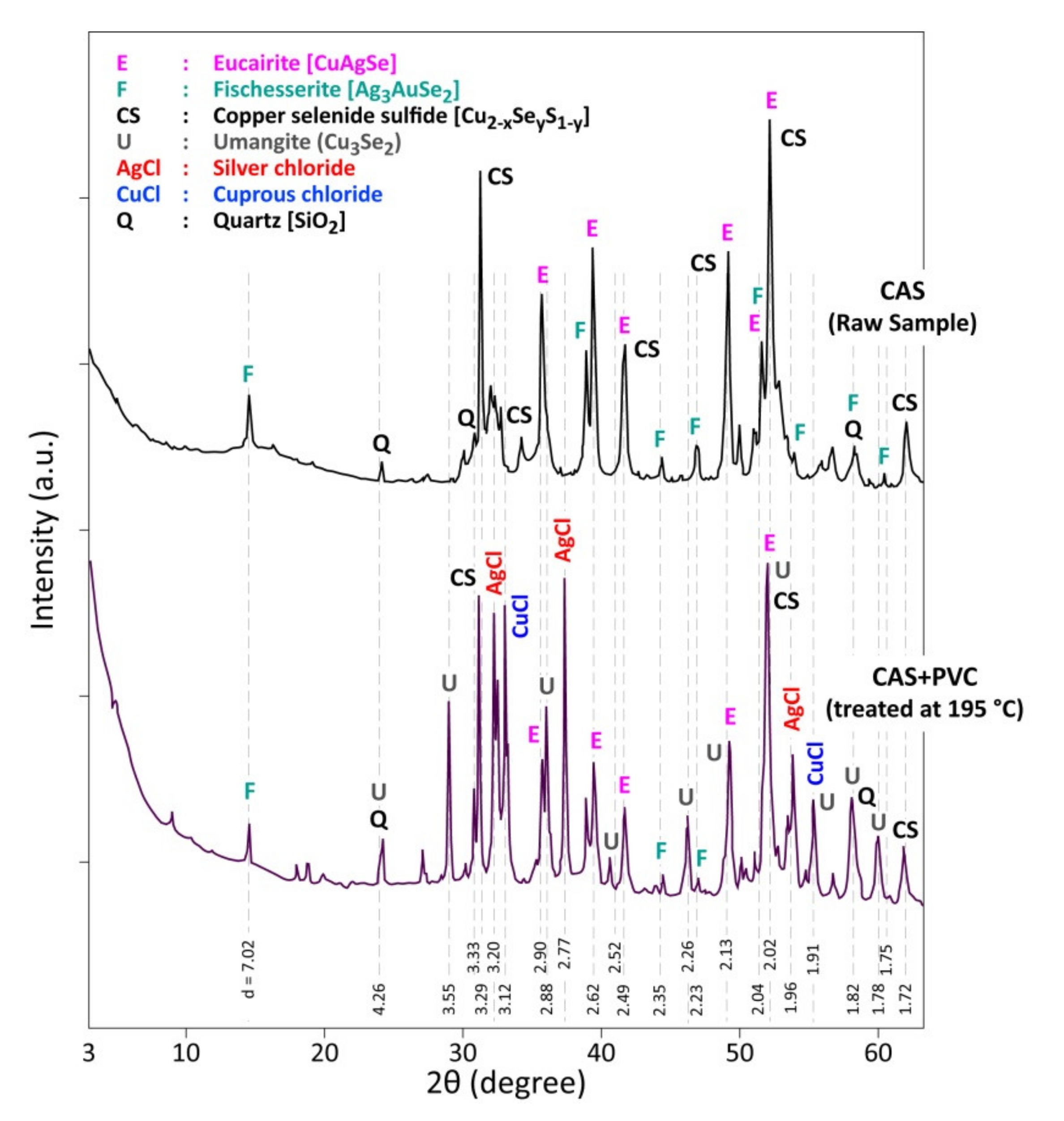
3.3. Analysis of the Reaction Products
3.4. Preliminary Results for Treatment of E-Waste in Air
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kanari, N.; Allain, E.; Shallari, S.; Diot, F.; Diliberto, S.; Patisson, F.; Yvon, J. Thermochemical route for extraction and recycling of critical, strategic and high value elements from by-products and end-of-life materials, Part I: Treatment of a copper by-product in air atmosphere. Materials 2019, 12, 1625. [Google Scholar] [CrossRef]
- Critical raw materials—European Commission—Europa EU. Available online: http://ec.europa.eu/growth/sectors/raw-materials/specific-interest/critical_en (accessed on 15 March 2019).
- Allain, E.; Kanari, N.; Diot, F.; Yvon, J. Development of a process for the concentration of the strategic tantalum and niobium oxides from tin slags. Miner. Eng. 2019, 134, 97–103. [Google Scholar] [CrossRef]
- Holgersson, S.; Steenari, B.-M.; Björkman, M.; Cullbrand, K. Analysis of the metal content of small-size Waste Electric and Electronic Equipment (WEEE) printed circuit boards-part 1: Internet routers, mobile phones and smartphones. Resour. Conserv. Recycl. 2018, 133, 300–308. [Google Scholar] [CrossRef]
- Kumar, S.; Rawat, S. Future e-waste: Standardisation for reliable assessment. Gov. Inform. Q. 2018, 35, S33–S42. [Google Scholar] [CrossRef]
- Otto, S.; Kibbe, A.; Henn, L.; Hentschke, L.; Kaiser, F.G. The economy of e-waste collection at the individual level: A practice oriented approach of categorizing determinants of e-waste collection into behavioral costs and motivation. J. Clean. Prod. 2018, 204, 33–40. [Google Scholar] [CrossRef]
- Jayaraman, K.; Vejayon, S.; Raman, S.; Mostafiz, I. The proposed e-waste management model from the conviction of individual laptop disposal practices-An empirical study in Malaysia. J. Clean. Prod. 2019, 208, 688–696. [Google Scholar] [CrossRef]
- Abbondanza, M.N.M.; Souza, R.G. Estimating the generation of household e-waste in municipalities using primary data from surveys: A case study of Sao Jose dos Campos, Brazil. Waste Manage. 2019, 85, 374–384. [Google Scholar] [CrossRef] [PubMed]
- Cho, B.-G.; Lee, J.-C.; Yoo, K. Valuable Metal Recycling. Metals 2018, 8, 345. [Google Scholar] [CrossRef]
- Ding, Y.; Zhang, S.; Liu, B.; Zheng, H.; Chang, C.-C.; Ekberg, C. Recovery of precious metals from electronic waste and spent catalysts: A review. Resour. Conserv. Recy. 2019, 141, 284–298. [Google Scholar] [CrossRef]
- Avarmaa, K.; Klemettinen, L.; O’Brien, H.; Taskinen, P. Urban mining of precious metals via oxidizing copper smelting. Miner. Eng. 2019, 133, 95–102. [Google Scholar] [CrossRef]
- Amer, A.M. Processing of copper anodic-slimes for extraction of valuable metals. Waste Manage. 2003, 23, 763–770. [Google Scholar] [CrossRef]
- Khaleghi, A.; Ghader, S.; Afzali, D. Ag recovery from copper anode slime by acid leaching at atmospheric pressure to synthesize silver nanoparticles. Int. J. Min. Sci. Technol. 2014, 24, 251–257. [Google Scholar] [CrossRef]
- Li, X.J.; Yang, H.Y.; Jin, Z.N.; Chen, G.B.; Tong, L.L. Transformation of selenium-containing phases in copper anode slimes during leaching. JOM 2017, 69, 1932–1938. [Google Scholar] [CrossRef]
- Xiao, L.; Wang, Y.L.; Yu, Y.; Fu, G.Y.; Han, P.W.; Sun, Z.H.I.; Ye, S.F. An environmentally friendly process to selectively recover silver from copper anode slime. J. Clean. Prod. 2018, 187, 708–716. [Google Scholar] [CrossRef]
- Kilic, Y.; Kartal, G.; Timur, S. An investigation of copper and selenium recovery from copper anode slimes. Int. J. Miner. Process. 2013, 124, 75–82. [Google Scholar] [CrossRef]
- Wang, Z.; Wei, R.; Wang, X.; He, J.; Wang, J. Pyrolysis and combustion of polyvinyl chloride (PVC) sheath for new and aged cables via thermogravimetric analysis-Fourier transform infrared (TG-FTIR) and calorimeter. Materials 2018, 11, 1997. [Google Scholar] [CrossRef]
- Niu, L.; Xu, J.; Yang, W.; Ma, J.; Zhao, J.; Kang, C.; Su, J. Study on the synergetic fire-retardant effect of nano-Sb2O3 in PBT Matrix. Materials 2018, 11, 1060. [Google Scholar] [CrossRef]
- Rani, M.; Marchesi, C.; Federici, S.; Rovelli, G.; Alessandri, I.; Vassalini, I.; Ducoli, S.; Borgese, L.; Zacco, A.; Bilo, F.; et al. Miniaturized near-infrared (MicroNIR) spectrometer in plastic waste sorting. Materials 2019, 12, 2740. [Google Scholar] [CrossRef]
- Hermosillo-Nevárez, J.J.; Bustos-Terrones, V.; Bustos-Terrones, Y.A.; Uriarte-Aceves, P.M.; Rangel-Peraza, J.G. Feasibility study on the use of recycled polymers for malathion adsorption: Isotherms and kinetic modeling. Materials 2020, 13, 1824. [Google Scholar] [CrossRef]
- Mun, S.-Y.; Hwang, C.-H. Experimental and numerical studies on major pyrolysis properties of flame retardant PVC cables composed of multiple materials. Materials 2020, 13, 1712. [Google Scholar] [CrossRef]
- Kaczorek-Chrobak, K.; Fangrat, J. PVC-based copper electric wires under various fire conditions: Toxicity of fire effluents. Materials 2020, 13, 1111. [Google Scholar] [CrossRef] [PubMed]
- Allain, E.; Gaballah, I.; Djona, M. Extraction of tantalum and niobium from tin slags by chlorination and carbochlorination. Metall. Mat. Trans. B 1997, 28B, 359–369. [Google Scholar] [CrossRef]
- Kanari, N.; Allain, E.; Gaballah, I. Reactions of wüstite and hematite with different chlorinating agents. Thermochim. Acta 1999, 335, 79–86. [Google Scholar] [CrossRef][Green Version]
- Kanari, N.; Gaballah, I.; Allain, E. Kinetics of oxychlorination of chromite Part I. Effect of temperature. Thermochim. Acta 2001, 371, 143–154. [Google Scholar] [CrossRef]
- Kanari, N.; Gaballah, I.; Allain, E. Kinetics of oxychlorination of chromite part II. Effect of reactive gases. Thermochim. Acta 2001, 371, 75–86. [Google Scholar] [CrossRef]
- Kanari, N.; Allain, E.; Joussemet, R.; Mochón, J.; Ruiz-Bustinza, I.; Gaballah, I. An overview study of chlorination reactions applied to the primary extraction and recycling of metals and to the synthesis of new reagents. Thermochim. Acta 2009, 495, 42–50. [Google Scholar] [CrossRef]
- Kanari, N.; Menad, N.; Diot, F.; Allain, E.; Yvon, J. Phosphate valorization by dry chlorination route. J. Min. Metall. Sect. B Metall. 2016, 52, 17–24. [Google Scholar] [CrossRef]
- Kanari, N.; Menad, N.-E.; Ostrosi, E.; Shallari, S.; Diot, F.; Allain, E.; Yvon, J. Thermal behavior of hydrated iron sulfate in various atmospheres. Metals 2018, 8, 1084. [Google Scholar] [CrossRef]
- Kanari, N.; Evrard, O.; Neveux, N.; Ninane, L. Recycling ferrous sulfate via super-oxidant synthesis. JOM 2001, 53, 32–33. [Google Scholar] [CrossRef]
- Kanari, N.; Ostrosi, E.; Ninane, L.; Neveux, N.; Evrard, O. Synthesizing alkali ferrates using a waste as a raw material. JOM 2005, 57, 39–42. [Google Scholar] [CrossRef]
- Kanari, N.; Filippov, L.; Diot, F.; Mochón, J.; Ruiz-Bustinza, I.; Allain, E.; Yvon, J. Synthesis of potassium ferrate using residual ferrous sulfate as iron bearing material. J. Phys. Conf. Ser. 2013, 416, art. no. 012013. [Google Scholar] [CrossRef]
- Kanari, N.; Filippova, I.; Diot, F.; Mochón, J.; Ruiz-Bustinza, I.; Allain, E.; Yvon, J. Utilization of a waste from titanium oxide industry for the synthesis of sodium ferrate by gas-solid reactions. Thermochim. Acta 2014, 575, 219–225. [Google Scholar] [CrossRef]
- Kanari, N.; Ostrosi, E.; Diliberto, C.; Filippova, I.; Shallari, S.; Allain, E.; Diot, F.; Patisson, F.; Yvon, J. Green process for industrial waste transformation into super-oxidizing materials named alkali metal ferrates (VI). Materials 2019, 12, 1977. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.T.; Dutrizac, J.E. A mineralogical study of the deportment and reaction of silver during copper electrorefining. Metall. Mat. Trans. B 1989, 20B, 345–361. [Google Scholar] [CrossRef]
- Petkova, E.N. Microscopic examination of copper electrorefining slimes. Hydrometallurgy 1990, 24, 351–359. [Google Scholar] [CrossRef]
- Petkova, E.N. Hypothesis about the origin of copper electrorefining slime. Hydrometallurgy 1994, 34, 343–358. [Google Scholar] [CrossRef]
- Chen, T.T.; Dutrizac, J.E. Mineralogical characterization of a copper anode and the anode slimes from the La Caridad copper refinery of Mexicana de Cobre. Metall. Mat. Trans. B 2005, 36B, 229–240. [Google Scholar] [CrossRef]
- Roine, A. Outokumpu HSC Chemistry for Windows, Version 3.0; Outokumpu Research: Pori, Finland, 1997. [Google Scholar]
- ASM Handbook—Volume 3, Alloy Phase Diagrams. Available online: http://s1.iran-mavad.com/ASM%20hanbooks/Vol_3_ASM%20handbooks_iran-mavad.com.pdf (accessed on 7 July 2020).
- Kanari, N.; Gaballah, I.; Allain, E.; Menad, N. Chlorination of chalcopyrite concentrates. Metall. Mat. Trans. B 1999, 30, 567–576. [Google Scholar] [CrossRef]
- Kanari, N.; Gaballah, I.; Allain, E. A low temperature chlorination–volatilization process for the treatment of chalcopyrite concentrates. Thermochim. Acta 2001, 373, 75–93. [Google Scholar] [CrossRef]
- Fokina, E.L.; Klimova, E.V.; Charykova, M.V.; Krivovichev, V.G.; Platonova, N.V.; Semenova, V.V.; Depmeier, W. The thermodynamics of arsenates, selenites, and sulfates in the oxidation zone of sulfide ores: VIII. Field of thermal stability of synthetic analog of chalcomenite, its dehydration and dissociation. Geol. Ore Deposit. 2014, 56, 538–545. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, Z. A critical review of material flow, recycling technologies, challenges and future strategy for scattered metals from minerals to wastes. J. Clean. Prod. 2018, 202, 1001–1025. [Google Scholar] [CrossRef]
- Davidsson, S.; Höök, M. Material requirements and availability for multi-terawatt deployment of photovoltaics. Energy Policy 2017, 108, 574–582. [Google Scholar] [CrossRef]
- Padoan, F.C.S.M.; Altimari, P.; Pagnanelli, F. Recycling of end of life photovoltaic panels: A chemical prospective on process development. Sol. Energy 2019, 177, 746–761. [Google Scholar] [CrossRef]
- Domínguez, A.; Geyer, R. Photovoltaic waste assessment of major photovoltaic installations in the United States of America. Renew. Energy 2019, 133, 1188–1200. [Google Scholar] [CrossRef]
- Hino, T.; Agawa, R.; Moriya, Y.; Nishida, M.; Tsugita, Y.; Araki, T. Techniques to separate metal from waste printed circuit boards from discarded personal computers. J. Matter Cycles Waste Manag. 2009, 11, 42–54. [Google Scholar] [CrossRef]
- Bizzo, W.A.; Figueiredo, R.A.; De Andrade, V.F. Characterization of printed circuit boards for metal and energy recovery after milling and mechanical separation. Materials 2014, 7, 4555–4566. [Google Scholar] [CrossRef]
- Chiang, H.-L.; Lin, K.-H. Exhaust constituent emission factors of printed circuit board pyrolysis processes and its exhaust control. J. Hazard. Mater. 2014, 264, 545–551. [Google Scholar] [CrossRef]
- Szałatkiewicz, J. Metals recovery from artificial ore in case of printed circuit boards, using plasmatron plasma reactor. Materials 2016, 9, 683. [Google Scholar] [CrossRef]
- Wan, X.; Fellman, J.; Jokilaakso, A.; Klemettinen, L.; Marjakoski, M. Behavior of waste printed circuit board (WPCB) materials in the copper matte smelting process. Metals 2018, 8, 887. [Google Scholar] [CrossRef]

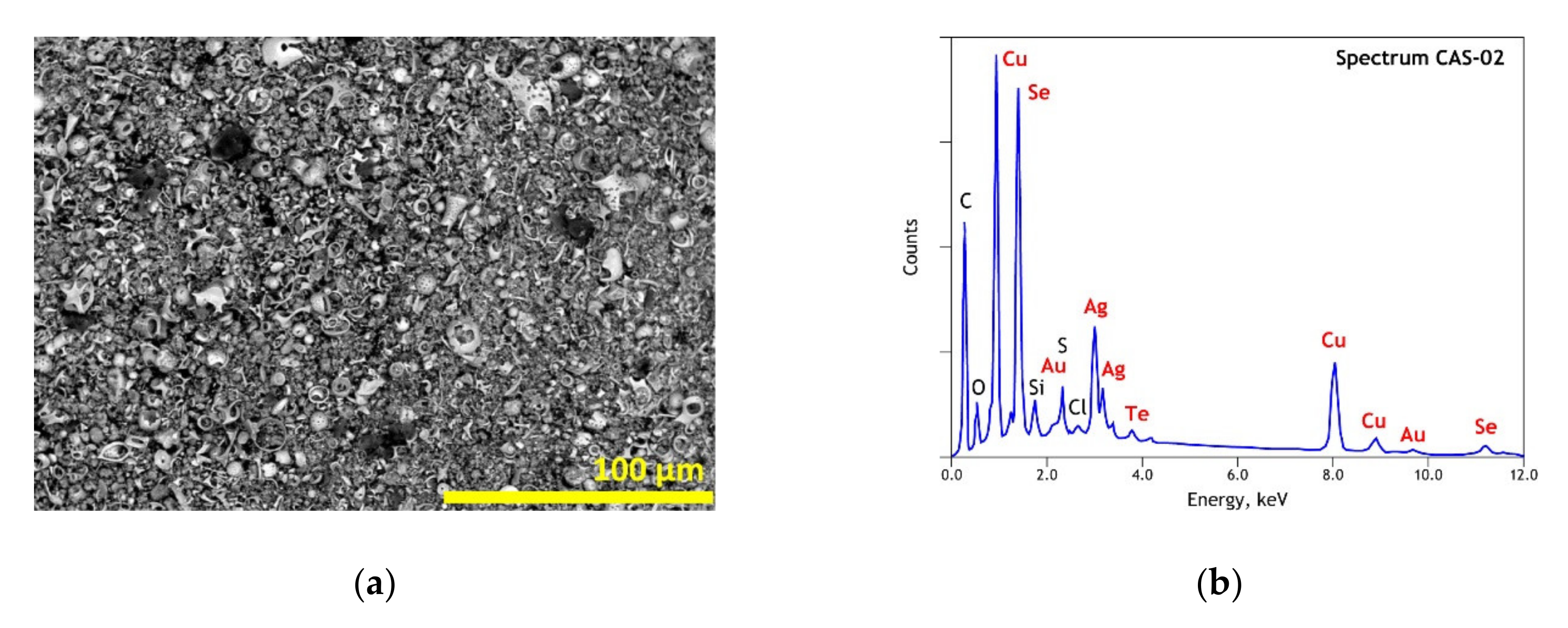


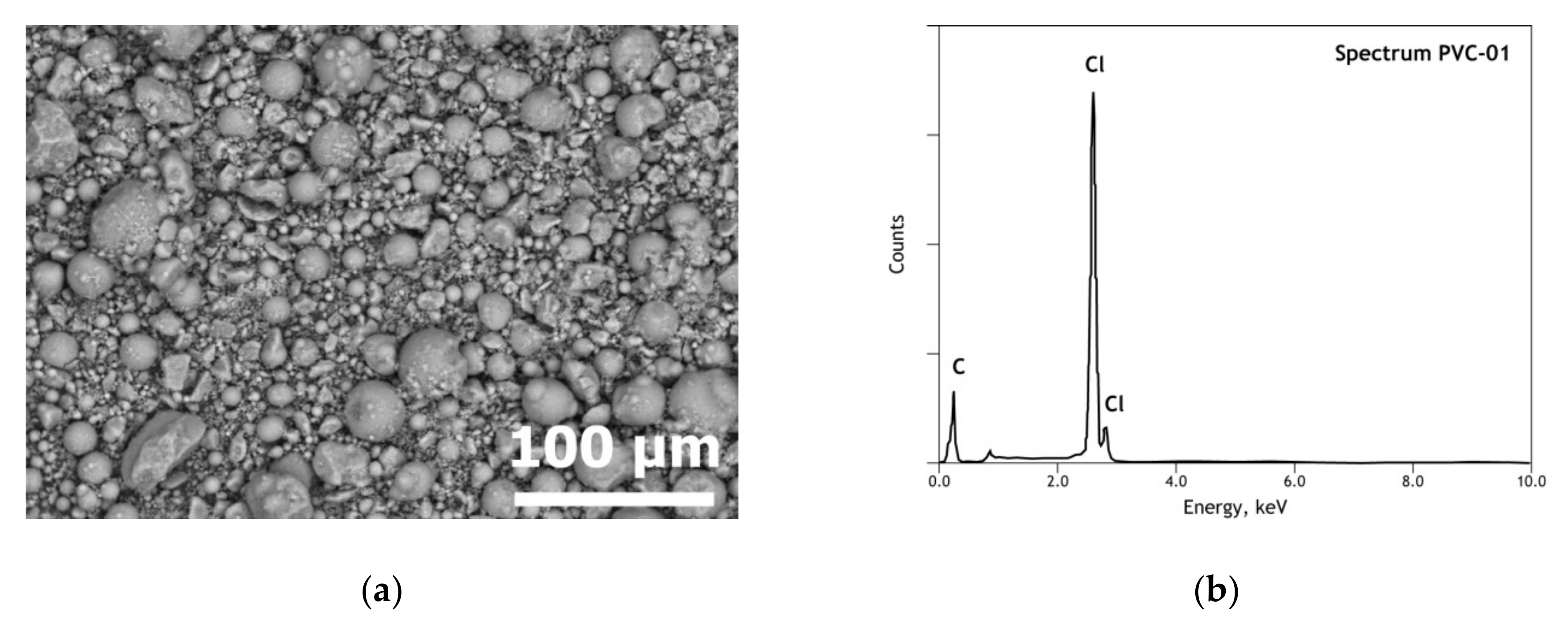


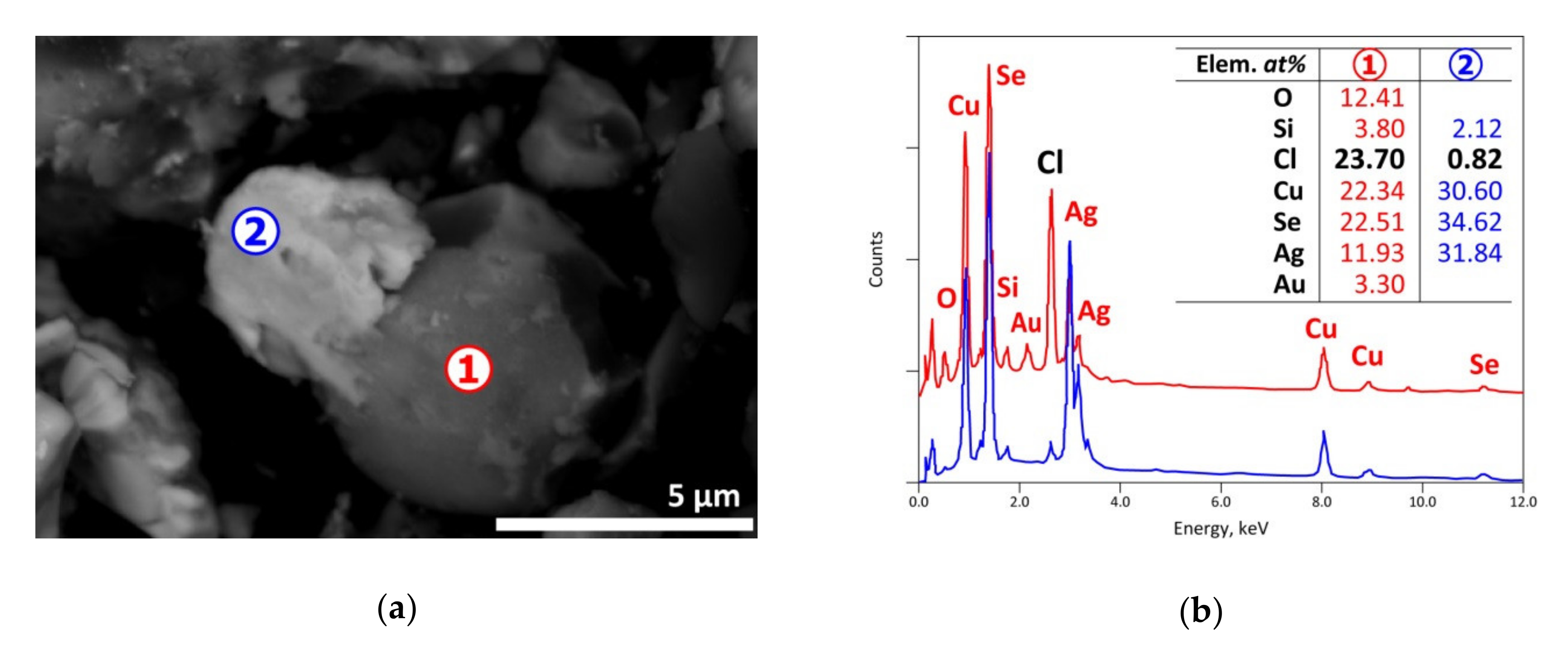
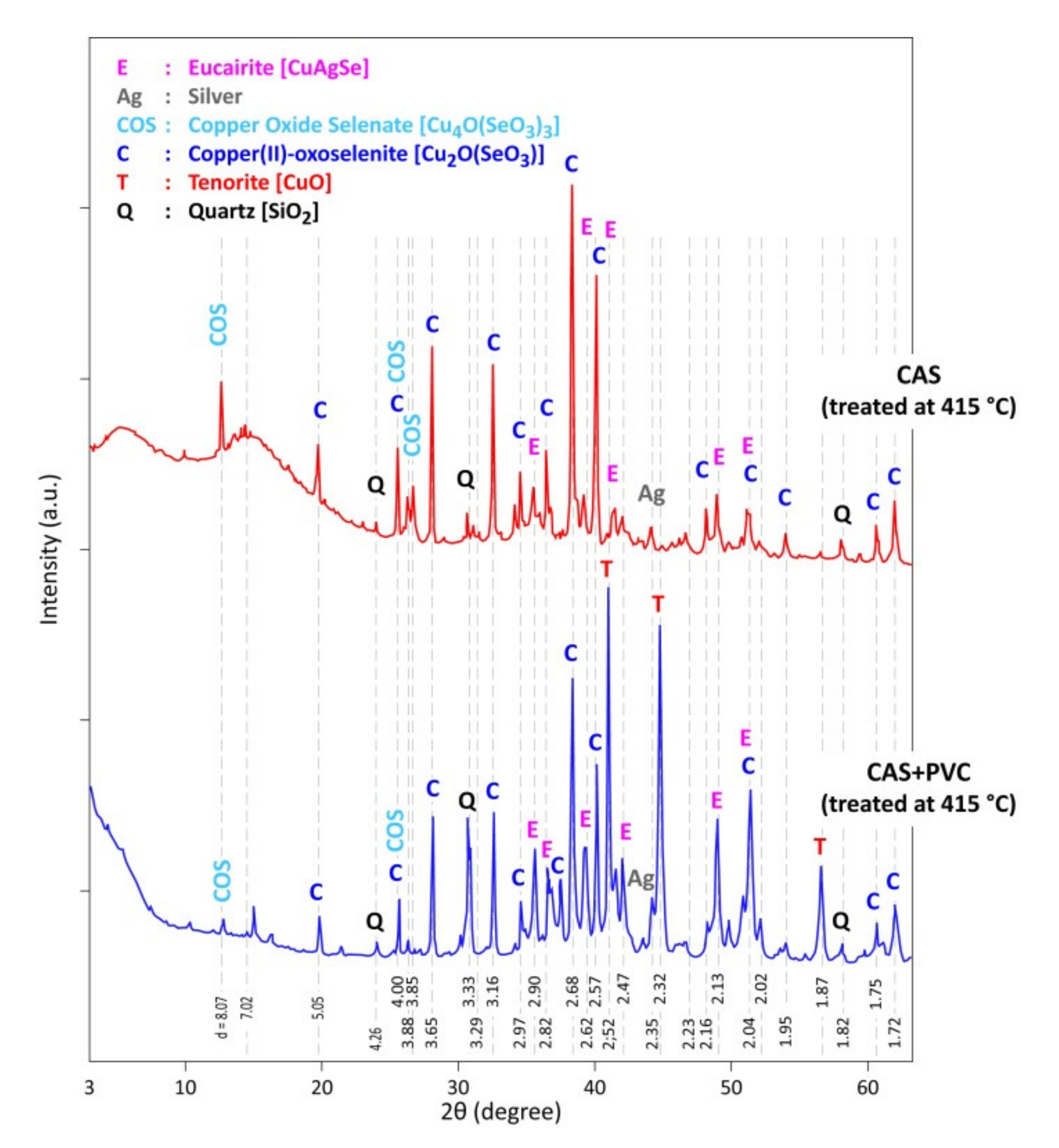


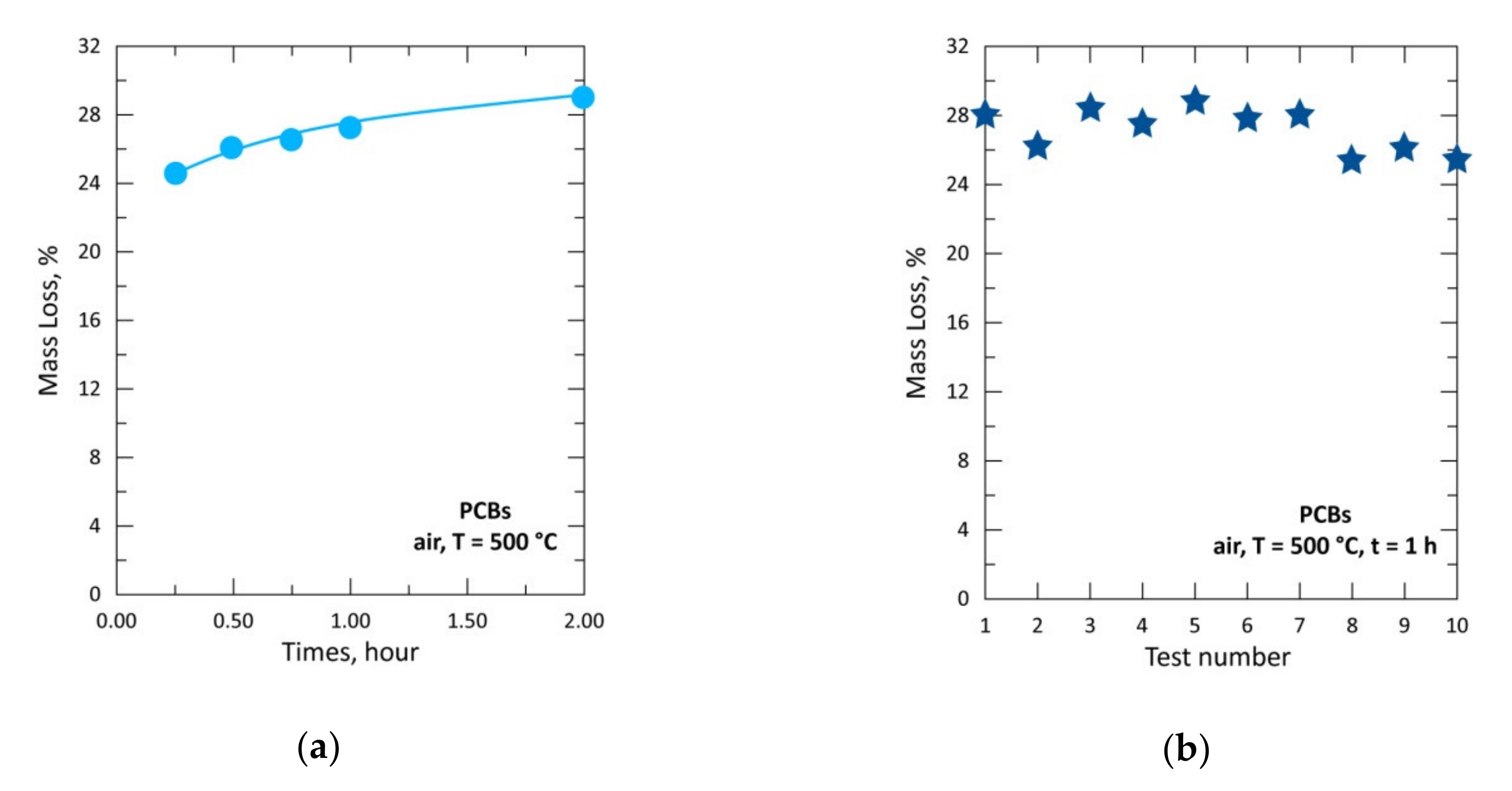






| Elements | Spot n° 1 | Spot n° 2 | Spot n° 3 | Spot n° 4 | Spot n° 5 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1wt% | 1at% | wt% | at% | wt% | at% | wt% | at% | wt% | at% | |
| O | - | - | - | - | - | - | 1.75 | 8.15 | 3.49 | 15.70 |
| Si | - | - | - | - | - | - | 0.31 | 0.82 | 0.49 | 1.25 |
| S | 1.17 | 2.87 | 3.65 | 7.84 | 2.05 | 4.44 | 3.30 | 7.66 | 2.06 | 4.62 |
| Cu | 32.40 | 40.13 | 47.16 | 51.11 | 48.48 | 53.10 | 32.60 | 38.22 | 28.05 | 31.73 |
| Se | 33.15 | 33.05 | 41.97 | 36.60 | 44.68 | 39.37 | 28.99 | 27.34 | 23.06 | 20.99 |
| Ag | 30.27 | 22.09 | 5.50 | 3.51 | 4.79 | 3.09 | 17.01 | 11.74 | 33.38 | 22.24 |
| Te | 3.01 | 1.86 | 1.71 | 0.92 | - | - | - | - | - | - |
| Au | - | - | - | - | 16.04 | 6.07 | 9.47 | 3.46 | ||
| Identified Phases | IS 1 | 225 °C | 320 °C | 415 °C | 505 °C | 685 °C | 770 °C |
|---|---|---|---|---|---|---|---|
| CuAgSe | |||||||
| Ag3AuSe2 | |||||||
| Cu2−xSeyS1−y | |||||||
| Silver (Ag) | |||||||
| Cu4O(SeO3)3 | |||||||
| Cu2O(SeO3) | |||||||
| CuO | |||||||
| SiO2 |
| Identified Phases | IS 1 | 195 °C | 225 °C | 320 °C | 415 °C | 505 °C | 685 °C | 770 °C |
|---|---|---|---|---|---|---|---|---|
| CuAgSe | ||||||||
| Ag3AuSe2 | ||||||||
| Cu2−xSeyS1−y | ||||||||
| Cu3Se2 | ||||||||
| CuCl | ||||||||
| Silver (Ag) | ||||||||
| AgCl | ||||||||
| Cu4O(SeO3)3 | ||||||||
| Cu2O(SeO3) | ||||||||
| CuO | ||||||||
| SiO2 |
| Elements | Spot n° 1 | Spot n° 2 | Spot n° 3 | |||
|---|---|---|---|---|---|---|
| 1wt% | 1at% | wt% | at% | wt% | at% | |
| O | - | - | 12.85 | 37.29 | 4.38 | 17.85 |
| Al | - | - | - | - | 0.14 | 0.35 |
| Si | - | - | - | - | 0.11 | 0.26 |
| Cu | 2.16 | 3.84 | 83.92 | 61.32 | 60.52 | 62.04 |
| Ag | 84.40 | 88.45 | 3.23 | 1.39 | 18.40 | 11.11 |
| Au | 13.44 | 7.71 | - | - | - | - |
| Te | - | - | - | - | 16.45 | 8.40 |
| Elements | Spot n° 1 | Spot n° 2 | Spot n° 3 | Spot n° 4 | ||||
|---|---|---|---|---|---|---|---|---|
| 1wt% | 1at% | wt% | at% | wt% | at% | wt% | at% | |
| O | 1.89 | 15.62 | 21.39 | 65.63 | 20.75 | 66.29 | 46.96 | 66.27 |
| Na | - | - | - | - | - | - | 3.21 | 3.16 |
| Al | - | - | 0.65 | 1.19 | 1.64 | 3.11 | 11.92 | 9.97 |
| Si | 0.30 | 1.40 | 0.70 | 1.22 | - | - | 17.41 | 14.00 |
| P | - | - | - | - | - | - | 2.12 | 1.54 |
| Ca | 1.81 | 5.99 | - | - | - | - | 3.37 | 1.90 |
| Ti | - | - | - | - | - | - | 2.09 | 0.98 |
| Mn | - | - | - | - | 12.83 | 11.94 | - | - |
| Cu | 5.79 | 12.06 | - | - | - | - | 1.47 | 0.52 |
| Br | 7.13 | 11.82 | - | - | - | - | - | - |
| Sn | - | - | 77.26 | 31.96 | 2.38 | 1.02 | 5.02 | 0.95 |
| Ta | - | - | - | - | 62.40 | 17.63 | - | - |
| Pb | 83.08 | 53.11 | - | - | - | - | 6.43 | 0.70 |
| Elements | Spot n° 1 | Spot n° 2 | Spot n° 3 | |||
|---|---|---|---|---|---|---|
| 1wt% | 1at% | wt% | at% | wt% | at% | |
| O | 17.75 | 60.63 | 14.56 | 54.58 | 16.91 | 58.83 |
| Cu | 4.42 | 3.80 | 4.95 | 4.67 | 8.53 | 7.47 |
| Br | 9.88 | 6.75 | 16.52 | 12.39 | 16.84 | 11.73 |
| Ag | 4.39 | 2.22 | 9.73 | 5.41 | 2.57 | 1.32 |
| Sn | 49.28 | 22.69 | 33.07 | 16.71 | 28.60 | 13.41 |
| W | 4.18 | 1.24 | 2.99 | 0.98 | 2.92 | 0.88 |
| Pb | 10.11 | 2.67 | 18.17 | 5.26 | 23.64 | 6.35 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kanari, N.; Allain, E.; Shallari, S.; Diot, F.; Diliberto, S.; Patisson, F.; Yvon, J. Thermochemical Route for Extraction and Recycling of Critical, Strategic and High-Value Elements from By-Products and End-of-Life Materials, Part II: Processing in Presence of Halogenated Atmosphere. Materials 2020, 13, 4203. https://doi.org/10.3390/ma13184203
Kanari N, Allain E, Shallari S, Diot F, Diliberto S, Patisson F, Yvon J. Thermochemical Route for Extraction and Recycling of Critical, Strategic and High-Value Elements from By-Products and End-of-Life Materials, Part II: Processing in Presence of Halogenated Atmosphere. Materials. 2020; 13(18):4203. https://doi.org/10.3390/ma13184203
Chicago/Turabian StyleKanari, Ndue, Eric Allain, Seit Shallari, Frédéric Diot, Sébastien Diliberto, Fabrice Patisson, and Jacques Yvon. 2020. "Thermochemical Route for Extraction and Recycling of Critical, Strategic and High-Value Elements from By-Products and End-of-Life Materials, Part II: Processing in Presence of Halogenated Atmosphere" Materials 13, no. 18: 4203. https://doi.org/10.3390/ma13184203
APA StyleKanari, N., Allain, E., Shallari, S., Diot, F., Diliberto, S., Patisson, F., & Yvon, J. (2020). Thermochemical Route for Extraction and Recycling of Critical, Strategic and High-Value Elements from By-Products and End-of-Life Materials, Part II: Processing in Presence of Halogenated Atmosphere. Materials, 13(18), 4203. https://doi.org/10.3390/ma13184203






