The Influence of the Third Element on Nano-Mechanical Properties of Iron Borides FeB and Fe2B Formed in Fe-B-X (X = C, Cr, Mn, V, W, Mn + V) Alloys
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Phase Composition and Microstructure
3.1.1. Fe-B-C Alloys
3.1.2. Fe-B-Cr Alloys
3.1.3. Fe-B-Mn Alloys
3.1.4. Fe-B-V Alloys
3.1.5. Fe-B-W Alloys
3.1.6. Fe-B-Mn-V Alloys
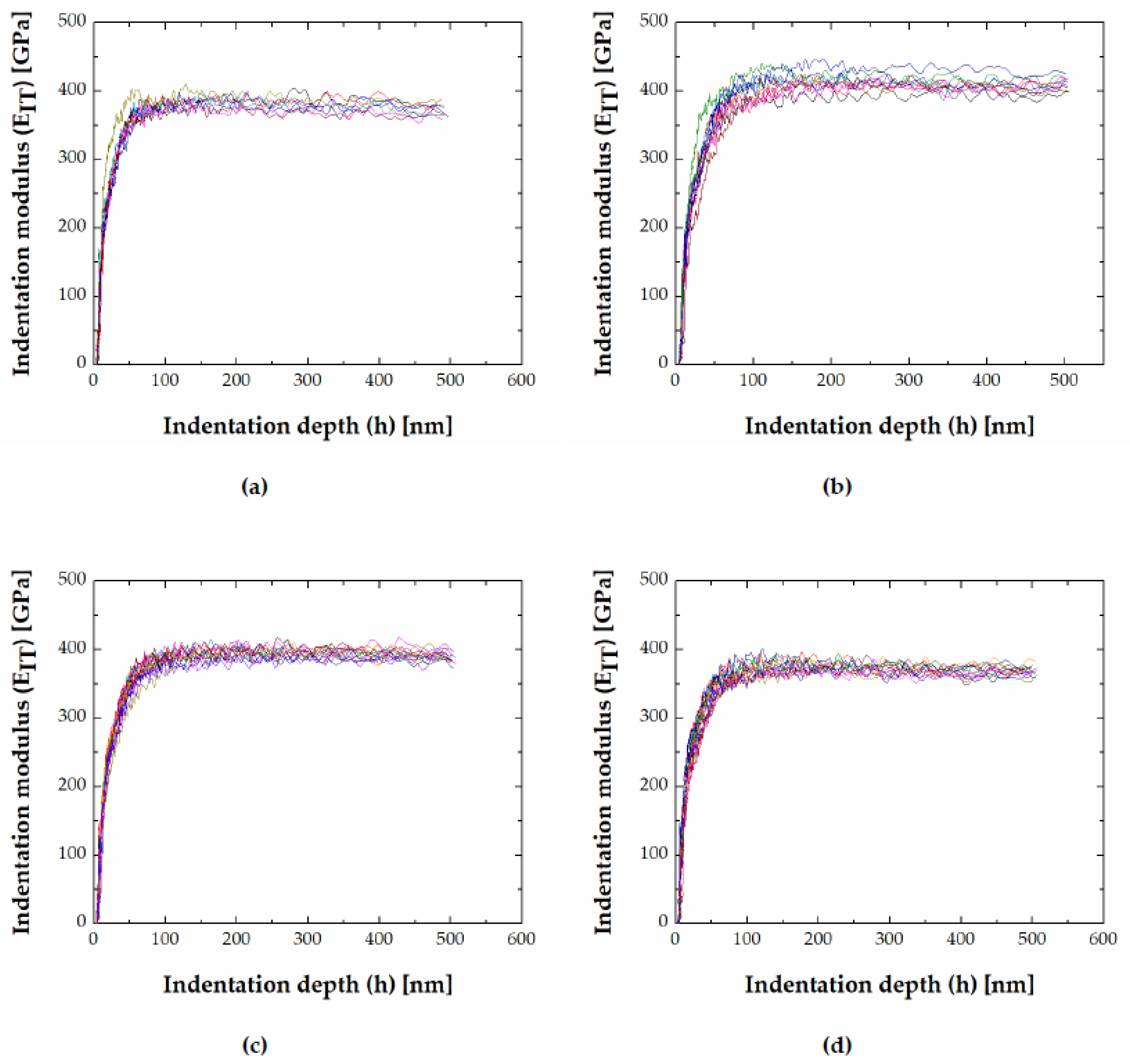
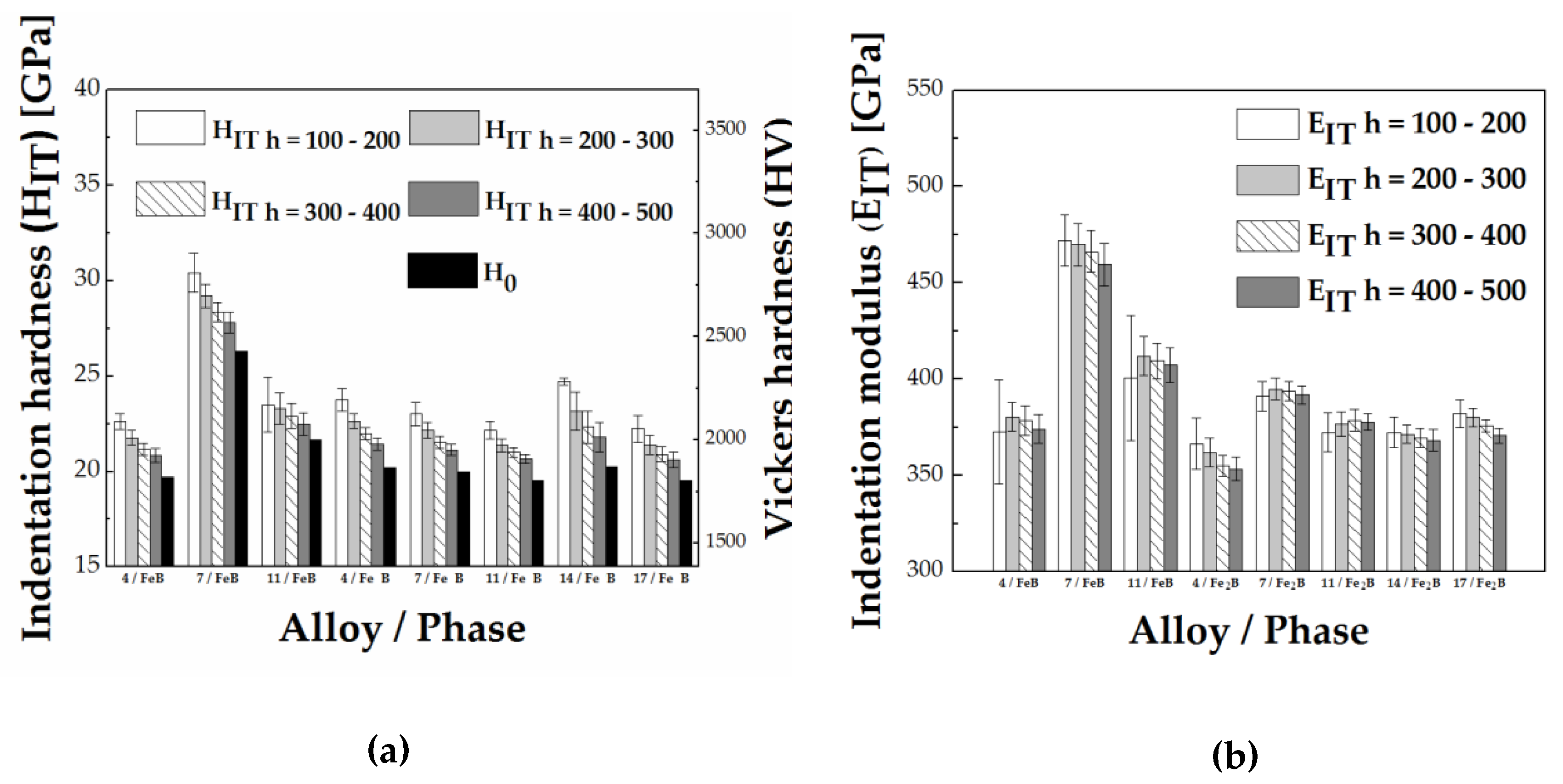
3.2. Hardness and Modulus of FeB and Fe2B Borides
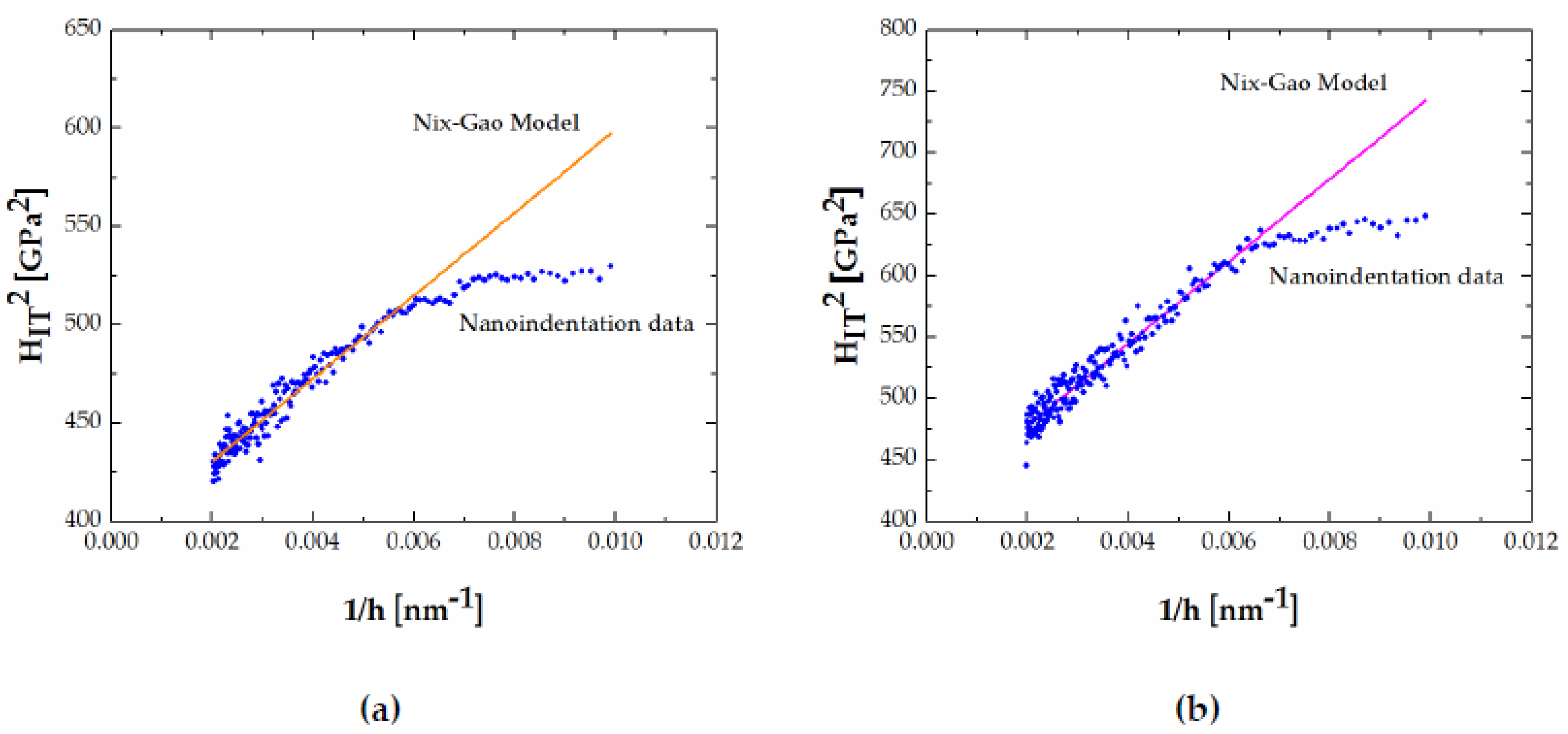
3.3. Indentation Size Effect
4. Conclusions
- (1)
- The determined indentation hardness under the influence of different amounts and type of alloying elements showed the highest hardness of FeB formed in Fe-B-X (X = C, Cr, Mn) systems in the presence of Cr as an alloying element with a hardness value of HIT = 26.9 ± 1.4 GPa. The highest hardness in the Fe2B was measured in the presence of W additions HIT = 20.8 ± 0.9 GPa. The lowest hardness in both alloys was determined in Mn alloyed FeB and Fe2B.
- (2)
- The highest hardness for FeB boride was measured at VEC = 5.28, and for the Fe2B boride, at VEC = 6.368. Comparison between FeB and Fe2B showed, overall, that the indentation hardness decreases with increasing VEC, which is associated with the increase of the ‘metallic’ character of the materials and easier slip on a given slip system or the activation of more systems.
- (3)
- The determined indentation modulus under the influence of different amounts and type of alloying elements showed that Cr alloyed FeB and Fe2B are stiffest, EIT = 485.5 ± 22.3 GPa and EIT = 391.7 ± 10.9 GPa, respectively. The lowest modulus in the case of FeB formed in Fe-B-X (X = C, Cr, Mn) was measured in the presence of C as an alloying element, while for Fe2B, in Mn alloyed Fe2B.
- (4)
- The indentation size effect was observed in both FeB and Fe2B phases and the hardness decreased with an increase in the indentation depth. The nanoindentation data has been successfully fitted to a dislocation-based model for determining the real hardness, H0.
Author Contributions
Funding
Conflicts of Interest
References
- Krukovich, M.G.; Prusakov, B.A.; Sizov, I.G. Plasticity of Boronized Layers; Springer International Publishing: Cham, Switzerland, 2016; pp. 301–310. [Google Scholar]
- Kulka, M. Current Trends in Boriding; Springer International Publishing: Cham, Switzerland, 2019; pp. 9–16. [Google Scholar]
- Winter, K.-M.; Kalucki, J.; Koshel, D. Process technologies for thermochemical surface engineering. In Thermochemical Surface Engineering of Steels; Mittemeijer, E., Somers, A.J.M., Eds.; Woodhead Publishing: Cambridge, UK, 2015; Volume 3, pp. 154–196. [Google Scholar]
- Behrens, B.; Bräuer, G.; Paschke, H.; Bistron, M. Reduction of wear at hot forging dies by using coating systems containing boron. J. Prod. Eng. 2015, 5, 497–506. [Google Scholar] [CrossRef]
- Medvedovski, E.; Chinski, F.; Stewart, J. Wear- and Corrosion-Resistant Boride-Based Coatings Obtained through Thermal Diffusion CVD Processing. Adv. Eng. Mater. 2014, 16, 713–728. [Google Scholar] [CrossRef]
- Röttger, A.; Lentz, J.; Theisen, W. Boron-alloyed Fe–Cr–C–B tool steels—Thermodynamic calculations and experimental validation. Mater. Des. 2015, 88, 420–429. [Google Scholar] [CrossRef]
- Lentz, J.; Röttger, A.; Theisen, W. Microstructures, Heat Treatment, and Properties of Boron-Alloyed Tool Steels. Steel Res. Int. 2019, 91, 1900416. [Google Scholar] [CrossRef]
- Liu, Z.; Li, Y.; Chen, X.; Hu, K. Microstructure and mechanical properties of high boron white cast iron. Mater. Sci. Eng. A 2008, 486, 112–116. [Google Scholar] [CrossRef]
- Chen, X.; Li, Y.; Zhang, H. Microstructure and mechanical properties of high boron white cast iron with about 4 wt% chromium. J. Mater. Sci. 2010, 46, 957–963. [Google Scholar] [CrossRef]
- Chen, X.; Li, Y. Effect of heat treatment on microstructure and mechanical properties of high boron white cast iron. Mater. Sci. Eng. A 2010, 528, 770–775. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, X.; Li, Y.; Hu, K. Effect of Chromium on Microstructure and Properties of High Boron White Cast Iron. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. A 2008, 39, 636–641. [Google Scholar] [CrossRef]
- Chen, Z.; Miao, S.; Kong, L.; Wei, X.; Zhang, F.; Yu, H. Effect of Mo Concentration on the Microstructure Evolution and Properties of High Boron Cast Steel. Materials 2020, 13, 975. [Google Scholar] [CrossRef]
- Xu, D.; Xu, X.; Su, Z.; An, J.; Lu, Y. Mechanical properties, wear, and corrosion of boronized N80 tube steel. J. Mater. Sci. 2012, 48, 106–112. [Google Scholar] [CrossRef]
- Su, Z.; Tian, X.; An, J.; Lu, Y.; Yang, Y.; Sun, S. Investigation on Boronizing of N80 Tube Steel. ISIJ Int. 2009, 49, 1776–1783. [Google Scholar] [CrossRef][Green Version]
- Selçuk, B.; Ipek, R.; Karamış, M. A study on friction and wear behaviour of carburized, carbonitrided and borided AISI 1020 and 5115 steels. J. Mater. Process. Technol. 2003, 141, 189–196. [Google Scholar] [CrossRef]
- Calik, A.; Duzgun, A.; Ekinci, A.; Karakas, S.; Ucar, N. Comparison of Hardness and Wear Behaviour οf Boronized and Carburized AISI 8620 Steels. Acta Phys. Pol. A. 2009, 116, 1029–1032. [Google Scholar] [CrossRef]
- Zong, X.; Jiang, W.; Fan, Z. Characteristics and wear performance of borided AISI 440C matensitic stainless steel. Mater. Express. 2018, 8, 500–510. [Google Scholar] [CrossRef]
- Kayali, Y.; Güneş, İ.; Ulu, S. Diffusion kinetics of borided AISI 52100 and AISI 440C steels. Vacuum 2012, 86, 1428–1434. [Google Scholar] [CrossRef]
- Ozbek, I. Mechanical Properties and Kinetics of Borided AISI M50 Bearing Steel. Arab. J. Sci. Eng. 2014, 39, 5185–5192. [Google Scholar] [CrossRef]
- Vera Cárdenas, E.; Lewis, R.; Martínez Pérez, A.; Bernal Ponce, J.; Pérez Pinal, F.; Domínguez, M.; Rivera Arreola, E. Characterization and wear performance of boride phases over tool steel substrates. Adv. Mater. Sci. Eng. 2016, 8, 168781401663025. [Google Scholar] [CrossRef]
- Campos-Silva, I.; Martínez-Trinidad, J.; Doñu-Ruíz, M.; Rodríguez-Castro, G.; Hernández-Sánchez, E.; Bravo-Bárcenas, O. Interfacial indentation test of FeB/Fe2B coatings. Surf. Coat. Technol. 2011, 206, 1809–1815. [Google Scholar] [CrossRef]
- Campos-Silva, I.; Bernabé-Molina, S.; Bravo-Bárcenas, D.; Martínez-Trinidad, J.; Rodríguez-Castro, G.; Meneses-Amador, A. Improving the Adhesion Resistance of the Boride Coatings to AISI 316L Steel Substrate by Diffusion Annealing. J. Mater. Eng. Perform. 2016, 25, 3852–3862. [Google Scholar] [CrossRef]
- Campos-Silva, I.; Palomar-Pardavé, M.; Pérez Pastén-Borja, R.; Kahvecioglu Feridun, O.; Bravo-Bárcenas, D.; López-García, C.; Reyes-Helguera, R. Tribocorrosion and cytotoxicity of FeB-Fe2B layers on AISI 316 L steel. Surf. Coat. Technol. 2018, 349, 986–997. [Google Scholar] [CrossRef]
- Rodríguez-Castro, G.; Campos-Silva, I.; Chávez-Gutiérrez, E.; Martínez-Trinidad, J.; Hernández-Sánchez, E.; Torres-Hernández, A. Mechanical properties of FeB and Fe2B layers estimated by Berkovich nanoindentation on tool borided steel. Surf. Coat. Technol. 2013, 215, 291–299. [Google Scholar] [CrossRef]
- Hernández-Sánchez, E.; Domínguez-Galicia, Y.; Orozco-Álvarez, C.; Carrera-Espinoza, R.; Herrera-Hernández, H.; Velázquez, J. A Study on the Effect of the Boron Potential on the Mechanical Properties of the Borided Layers Obtained by Boron Diffusion at the Surface of AISI 316L Steel. Adv. Mater. Sci. Eng. 2014, 2014, 1–9. [Google Scholar] [CrossRef]
- Campos-Silva, I.; Hernández-Sánchez, E.; Rodríguez-Castro, G.; Rodríguez-Pulido, A.; López-García, C.; Ortiz-Domínguez, M. Indentation size effect on the Fe2B/substrate interface. Surf. Coat. Technol. 2011, 206, 1816–1823. [Google Scholar] [CrossRef]
- Kulka, M.; Makuch, N.; Piasecki, A. Nanomechanical characterization and fracture toughness of FeB and Fe2B iron borides produced by gas boriding of Armco iron. Surf. Coat. Technol. 2017, 325, 515–532. [Google Scholar] [CrossRef]
- Campos-Silva, I.; Hernández-Sánchez, E.; Rodríguez-Castro, G.; Cimenoglu, H.; Nava-Sánchez, J.; Meneses-Amador, A.; Carrera-Espinoza, R. A study of indentation for mechanical characterization of the Fe2B layer. Surf. Coat. Technol. 2013, 232, 173–181. [Google Scholar] [CrossRef]
- Lentz, J.; Röttger, A.; Theisen, W. Hardness and modulus of Fe2B, Fe3(C, B), and Fe23(C, B)6 borides and carboborides in the Fe-C-B system. Mater. Charact. 2018, 135, 192–202. [Google Scholar] [CrossRef]
- Jian, Y.; Huang, Z.; Xing, J.; Zheng, B.; Sun, L.; Liu, Y.; Liu, Y. Effect of improving Fe2B toughness by chromium addition on the two-body abrasive wear behavior of Fe–3.0wt% B cast alloy. Tribol. Int. 2016, 101, 331–339. [Google Scholar] [CrossRef]
- Huang, Z.; Xing, J.; Guo, C. Improving fracture toughness and hardness of Fe2B in high boron white cast iron by chromium addition. Mater. Des. 2010, 31, 3084–3089. [Google Scholar] [CrossRef]
- Huang, Z.; Xing, J.; Lv, L. Effect of tungsten addition on the toughness and hardness of Fe2B in wear-resistant Fe–B–C cast alloy. Mater. Charact. 2013, 75, 63–68. [Google Scholar] [CrossRef]
- Huang, Z.; Xing, J.; Tao, X. Effect of molybdenum addition on fracture toughness and hardness of Fe2B in Fe–B–C cast alloy. Int. J. Mater. Res. 2012, 103, 1539–1543. [Google Scholar] [CrossRef]
- Jian, Y.; Huang, Z.; Xing, J.; Gao, Y. Effects of chromium on the morphology and mechanical properties of Fe2B intermetallic in Fe-3.0B alloy. J. Mater. Sci. 2018, 53, 5329–5338. [Google Scholar] [CrossRef]
- Wei, X.; Chen, Z.; Zhong, J.; Wang, L.; Yang, W.; Wang, Y. Effect of alloying elements on mechanical, electronic and magnetic properties of Fe2B by first-principles investigations. Comput. Mater. Sci. 2018, 147, 322–330. [Google Scholar] [CrossRef]
- Akopov, G.; Pangilinan, L.; Mohammadi, R.; Kaner, R. Perspective: Superhard metal borides: A look forward. APL Mater. 2018, 6, 070901. [Google Scholar] [CrossRef]
- Mohammadi, R.; Xie, M.; Lech, A.; Turner, C.; Kavner, A.; Tolbert, S.; Kaner, R. Toward Inexpensive Superhard Materials: Tungsten Tetraboride-Based Solid Solutions. J. Am. Chem. Soc. 2012, 134, 20660–20668. [Google Scholar] [CrossRef] [PubMed]
- Homolová, V.; Čiripová, L. Experimental Investigation of Isothermal Section of the B-Cr-Fe Phase Diagram at 1353 K. Adv. Mater. Sci. Eng. 2017, 2017, 1–7. [Google Scholar] [CrossRef]
- Repovský, P.; Homolová, V.; Čiripová, L.; Kroupa, A.; Zemanová, A. Experimental study and thermodynamic modelling of the B-Fe-Mn ternary system. Calphad 2016, 55, 252–259. [Google Scholar] [CrossRef]
- Homolová, V.; Kroupa, A.; Výrostková, A. Calculation of Fe–B–V ternary phase diagram. J. Alloys Compd. 2012, 520, 30–35. [Google Scholar] [CrossRef]
- Homolová, V.; Repovský, P.; Výrostková, A.; Kroupa, A. Experimental and Theoretical Determination of Phase Fraction in the Fe-B-V Alloys. J. Phase Equilib. Diffus. 2014, 35, 172–177. [Google Scholar] [CrossRef]
- Homolová, V.; Kepič, J.; Zemanová, A.; Zobač, O. Experimental Study of Phase Composition of B-Fe-Mn-V Alloys and Thermodynamic Calculations of Phase Equilibria in the B-Mn-V and B-Fe-Mn-V Systems. Adv. Mater. Sci. Eng. 2018, 2018, 1–10. [Google Scholar] [CrossRef]
- Homolová, V.; Čiripová, L.; Výrostková, A. Experimental Study of Phase Composition of Fe-(30-60) B-C Alloys and Boron-Rich Corner of Fe-B-C Phase Diagram. J. Phase. Equilibria. Diffus. 2015, 36, 599–605. [Google Scholar] [CrossRef]
- Homolová, V.; Čiripová, L.; Kepič, J. Isothermal Section of the B-Cr-Fe System at 873 K. J. Phase. Equilibria. Diffus. 2019, 40, 79–85. [Google Scholar] [CrossRef]
- Kirkovska, I.; Homolová, V. Experimental investigation of phase equilibrai in Fe-W-B alloys. In Proceedings of the Metalurgia Junior 2019, Herľany, Slovakia, 11–12 June 2019; Heželová, M., Pikna, L., Eds.; Technical University of Košice: Košice, Slovakia, 2019. [Google Scholar]
- Kirkovska, I.; Homolová, V.; Čiripová, L. Experimental study of the relationship between hardness and phase volume fraction in Fe–W–B alloys. In Proceedings of the Metalurgia Junior 2020, Košice, Slovakia, 25 May 2020; Heželová, M., Pikna, L., Eds.; Technical University of Košice: Košice, Slovakia, 2020. [Google Scholar]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Natu. Met. 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Rogl, P. Boron–Iron–Tungsten. In Ternary Alloy Systems Phase Diagrams, Crystallographic and Thermodynamic Data, Iron Systems, Part 1; Effenberg, G., Ilyenko, S., Eds.; Springer: Heidelberg, Germany, 2008; Volume 11D1, pp. 455–463. [Google Scholar]
- Kirkovska, I.; Homolová, V.; Čiripová, L.; Petryshynets, I. Nanomechanical Characterization of Iron Borides in Fe–Mn–B Ternary Alloys. Met. Fractography 2019. submitted. [Google Scholar]
- Wei, X.; Chen, Z.; Zhong, J.; Wang, L.; Wang, Y.; Shu, Z. First-principles investigation of Cr-doped Fe2B: Structural, mechanical, electronic and magnetic properties. J. Magn. Magn. Mater. 2018, 456, 150–159. [Google Scholar] [CrossRef]
- Jian, Y.; Huang, Z.; Xing, J.; Guo, X.; Wang, Y.; Lv, Z. Effects of Mn addition on the two-body abrasive wear behavior of Fe-3.0 wt% B alloy. Tribol. Int. 2016, 103, 243–251. [Google Scholar] [CrossRef]
- Lentz, J.; Röttger, A.; Großwendt, F.; Theisen, W. Enhancement of hardness, modulus and fracture toughness of the tetragonal (Fe, Cr)2B and orthorhombic (Cr, Fe)2B phases with addition of Cr. Mater. Des. 2018, 156, 113–124. [Google Scholar] [CrossRef]
- Li, K.; Huang, Z.; Zhang, L.; Wang, Y.; Shen, Y.; He, L.; Zheng, Q.; Li, H. Influence of Cr addition on tribological properties of bulk Fe2B under dry friction and water lubrication. Mater. Res. Express. 2018, 5, 076506. [Google Scholar] [CrossRef]
- Ma, R.; Fan, Y.; Cao, X.; Wen, M. Influence of Micro-Addition Chromium on the Valence Electron Structure and Toughness of Fe2B Phase. Appl. Mech. Mater. 2010, 34–35, 1135–1139. [Google Scholar] [CrossRef]
- Jhi, S.; Ihm, J.; Louie, S.; Cohen, M. Electronic mechanism of hardness enhancement in transition-metal carbonitrides. Nature. 1999, 399, 132–134. [Google Scholar] [CrossRef]
- Yang, Q.; Lengauer, W.; Koch, T.; Scheerer, M.; Smid, I. Hardness and elastic properties of Ti(CxN1-x), Zr(CxN1-x) and Hf(CxN1-x). J. Alloys Compd. 2000, 309, L5. [Google Scholar] [CrossRef]
- Balasubramanian, K.; Khare, S.; Gall, D. Valence electron concentration as an indicator for mechanical properties in rocksalt structure nitrides, carbides and carbonitrides. Acta Mater. 2018, 152, 175–185. [Google Scholar] [CrossRef]
- Ge, H.; Tian, F. A Review of Ab Initio Calculation on Lattice Distortion in High-Entropy Alloys. JOM 2019, 71, 4225–4237. [Google Scholar] [CrossRef]
- Gou, Y.; Fu, Z.; Liang, Y.; Zhong, Z.; Wang, S. Electronic structures and mechanical properties of iron borides from first principles. Solid State Commun. 2014, 187, 28–32. [Google Scholar] [CrossRef]
- Pharr, G.; Herbert, E.; Gao, Y. The Indentation Size Effect: A Critical Examination of Experimental Observations and Mechanistic Interpretations. Annu. Rev. Mater. Res. 2010, 40, 271–292. [Google Scholar] [CrossRef]
- Nix, W.; Gao, H. Indentation size effects in crystalline materials: A law for strain gradient plasticity. J. Mechan. Phys. Sol. 1998, 46, 411–425. [Google Scholar] [CrossRef]
- Elmustafa, A.; Stone, D. Indentation size effect in polycrystalline F.C.C. metals. Acta Mater. 2002, 50, 3641–3650. [Google Scholar] [CrossRef]
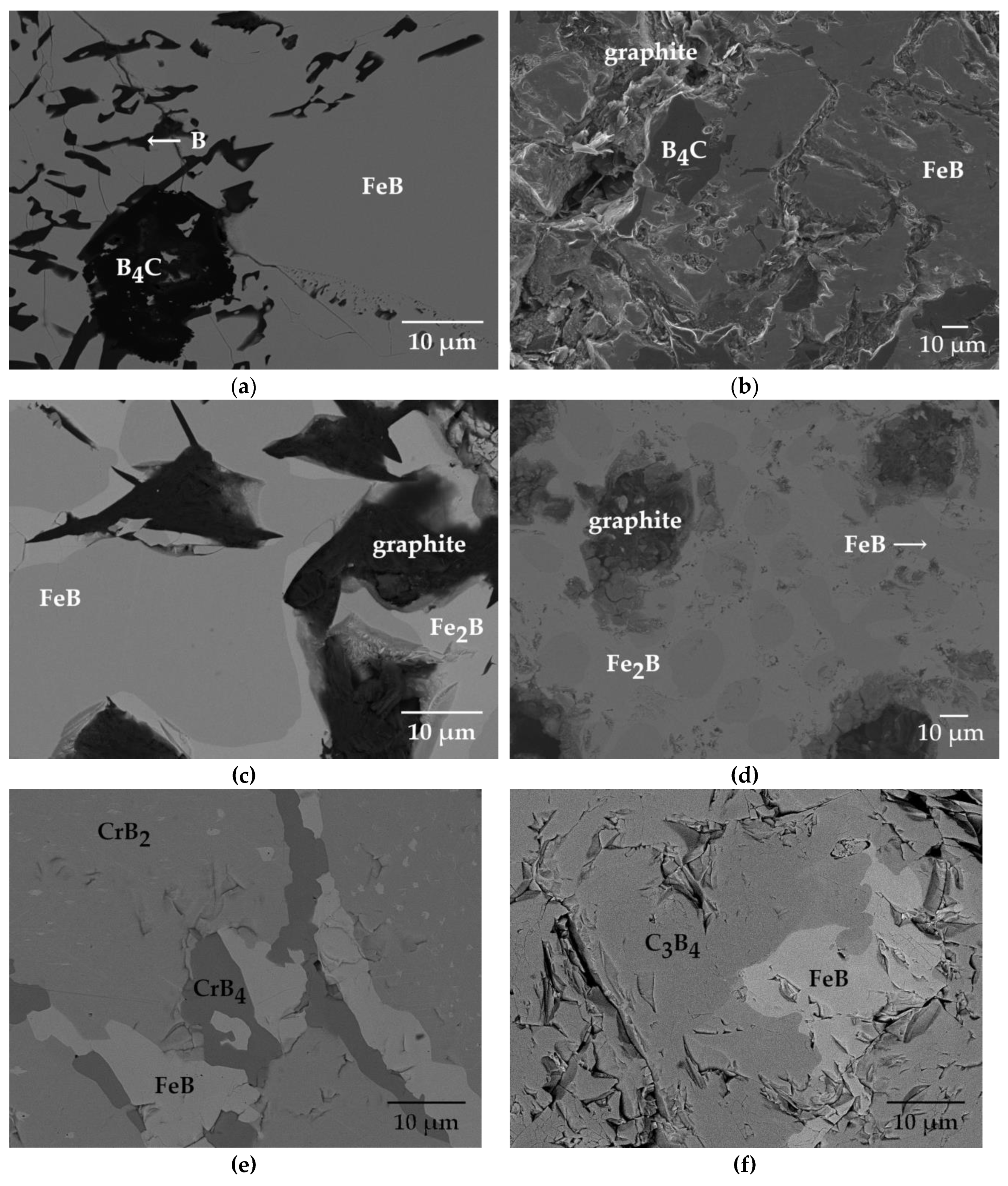
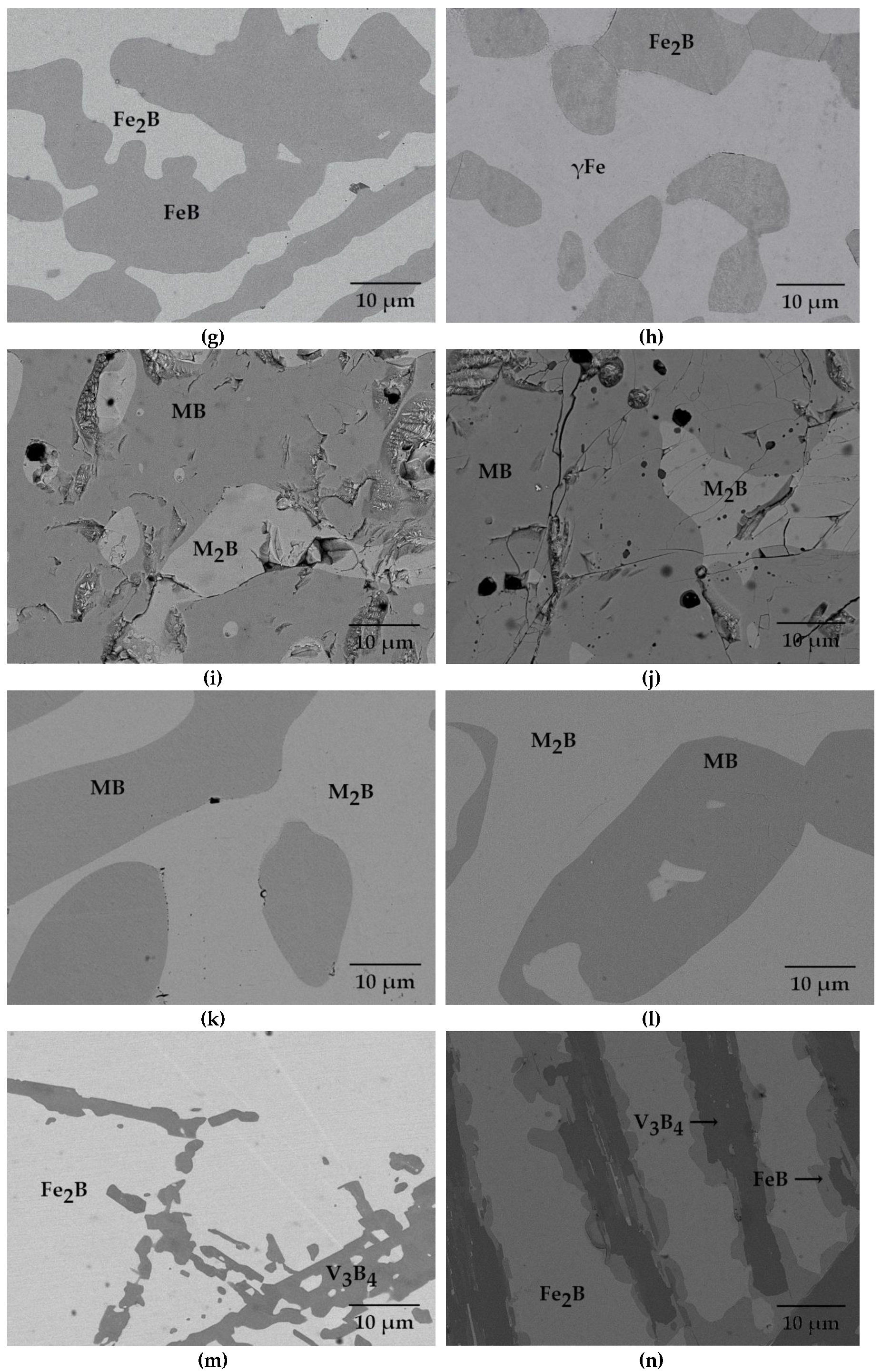
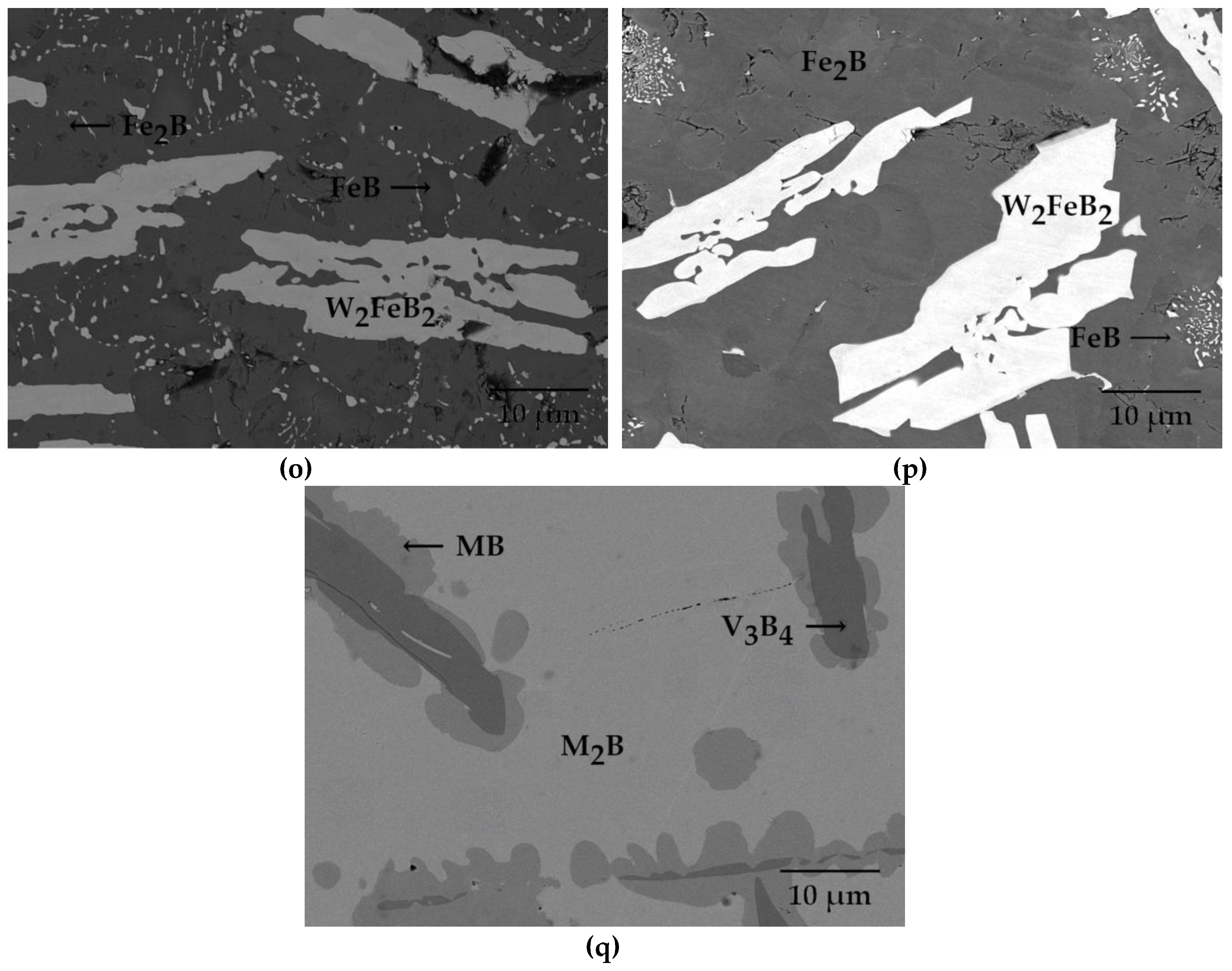
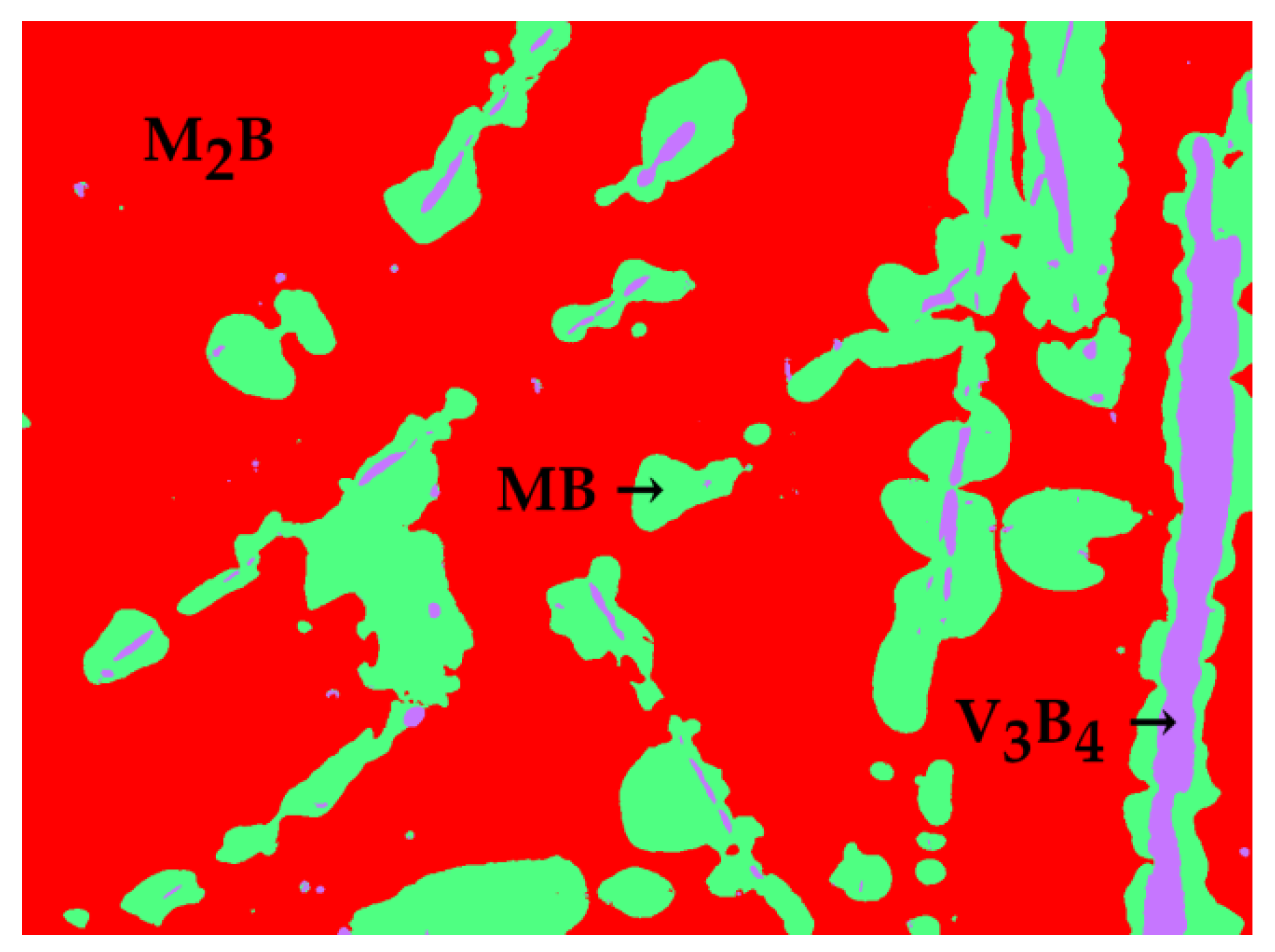



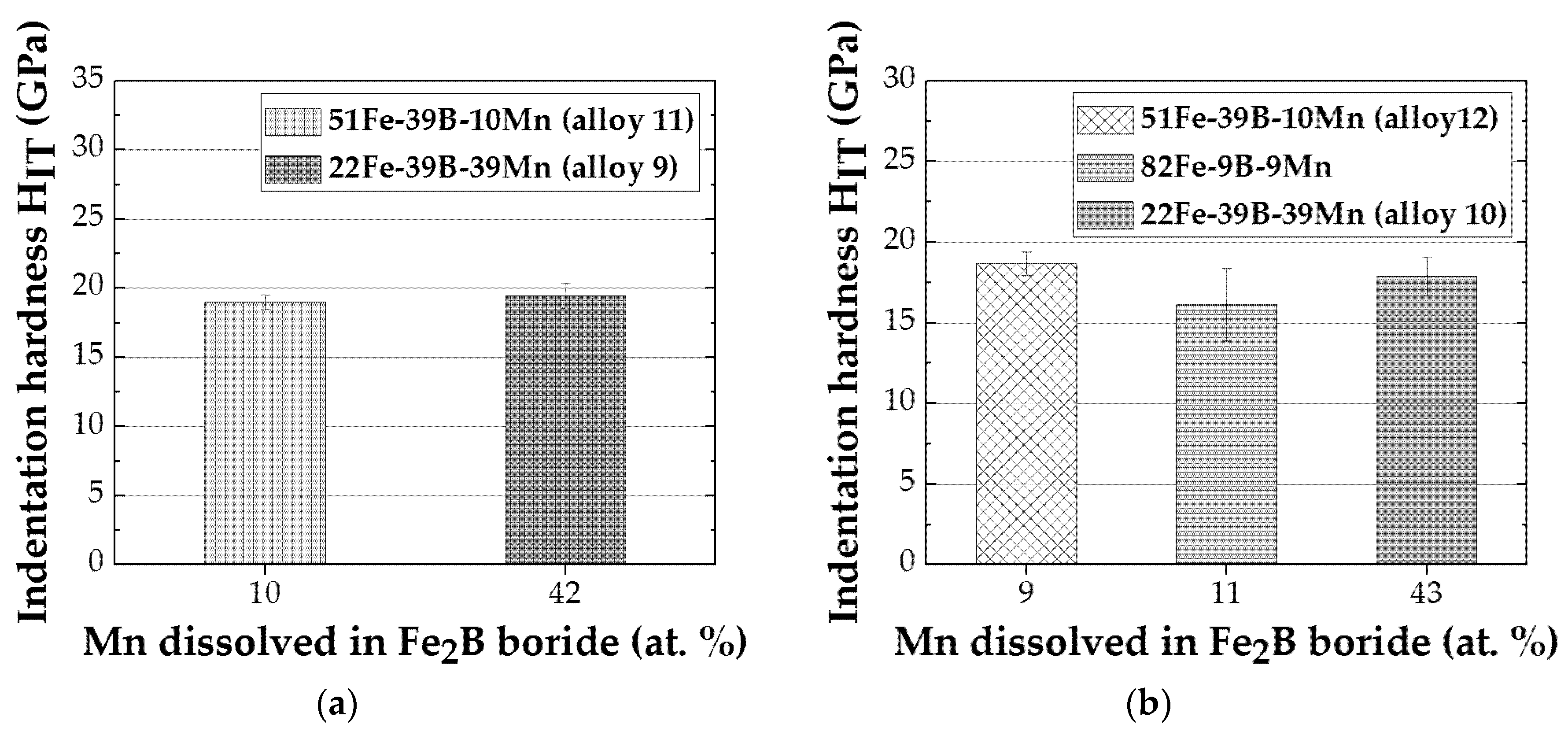
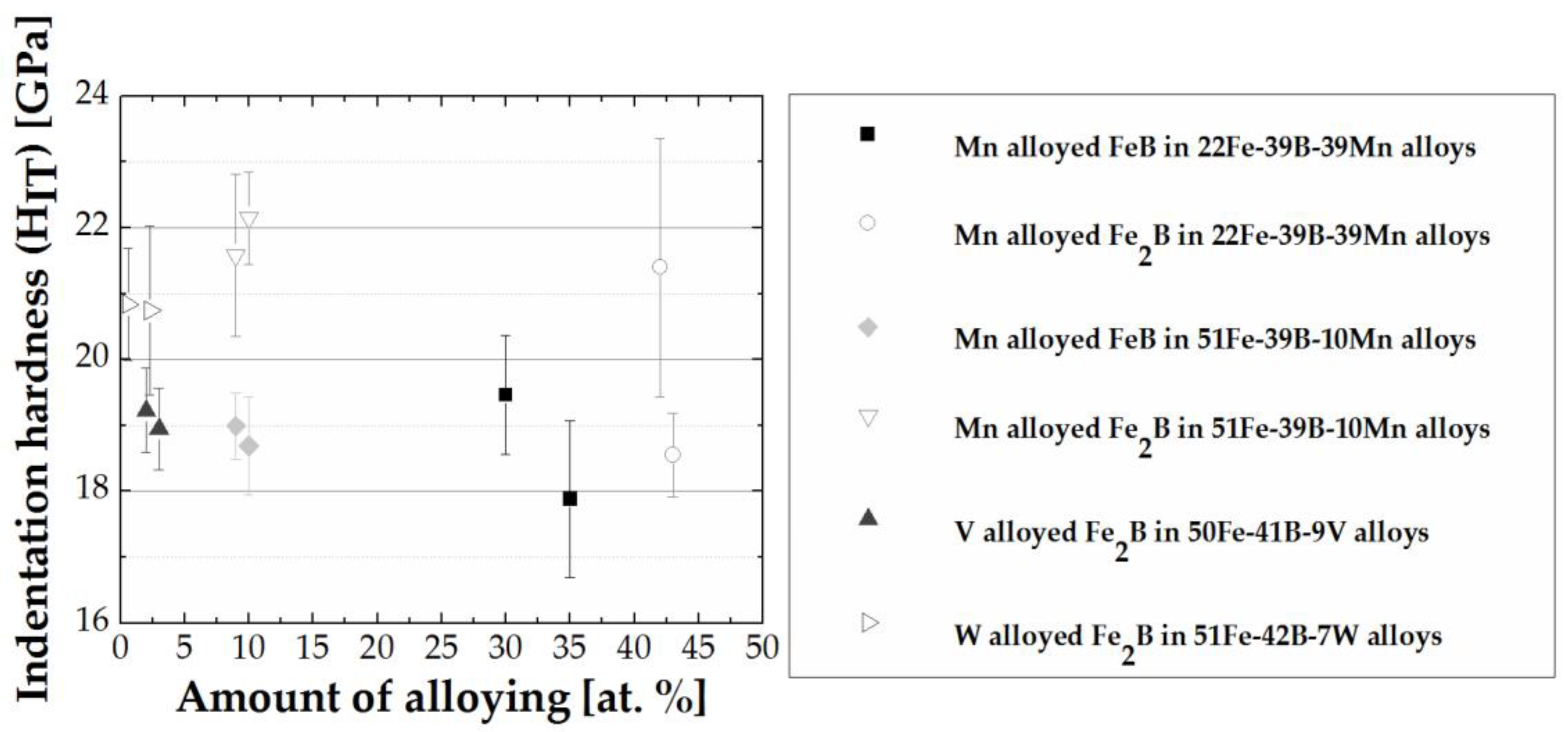

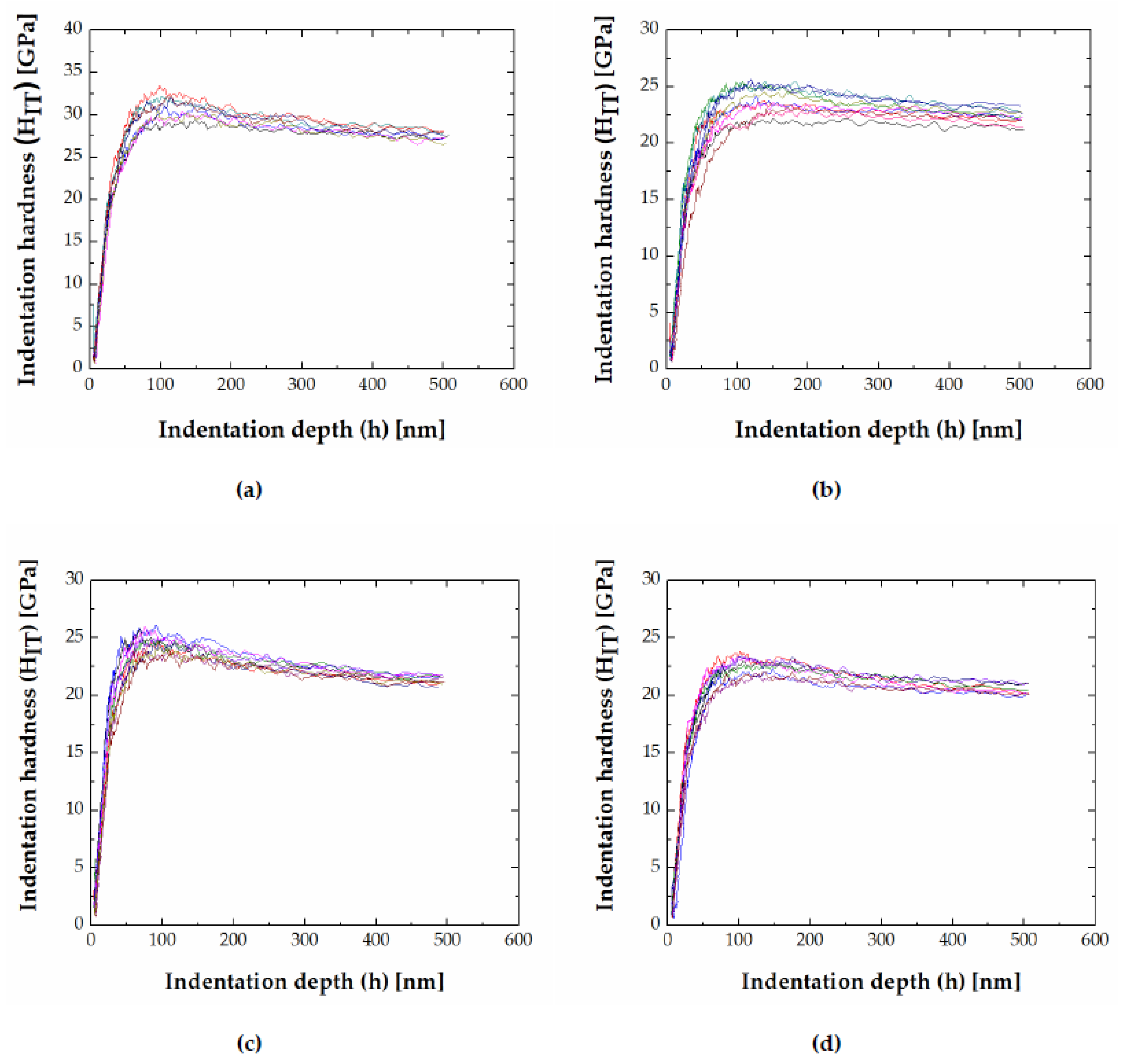

| Alloy | Alloy Composition (at.%) | Annealing Conditions T (K)/time (h) | Phase Composition | Fe2B (M2B) Chemical Composition (at.%) | FeB (MB) Chemical Composition (at.%) |
|---|---|---|---|---|---|
| 1 | 38.5 Fe–59.2 B–2.3 C | 1173/1000 | FeB + B4C + B | - | 48.96 Fe, 50 B, 1.04 C |
| 2 | 34.6 Fe–52 B–13.4 C | 1173/1000 | FeB + B4C + graphite | - | 47.3 Fe, 50 B, 2.7 C |
| 3 | 37 Fe–34 B–29 C | 873/1000 | Fe2B + FeB + graphite | 30.01 Fe, 66.67B, 3.32C | 47.92 Fe, 50 B, 2.08 C |
| 4 | 39.7 Fe–33 B–27.3 C | 1173/1000 | Fe2B + FeB + graphite | 31.06 Fe, 66.67B, 2.27C | 47.31 Fe, 50 B, 2.69 C |
| 5 | 17 Fe–65 B–18 Cr | 1353/1848 | FeB + CrB4 + CrB2 | - | 39 Fe, 50 B, 11 Cr |
| 6 | 8 Fe–56 B–36 Cr | 873/2300 | FeB + Cr3B4 | - | 37 Fe, 51 B, 12 Cr |
| 7 | 50 Fe–40 B–10 Cr | 1353/1848 | Fe2B + FeB | 59 Fe, 33 B, 8 Cr | 35 Fe, 49 B, 16 Cr |
| 8 | 82 Fe–9 B–9 Mn | 1223/1440 | Fe2B + γFe | 56 Fe, 33 B, 11 Mn | - |
| 9 | 22 Fe–39 B–39 Mn | 873/2160 | M2B + MB | 28 Fe, 30 B, 42 Mn | 19 Fe, 51 B, 30 Mn |
| 10 | 22 Fe–39 B–39 Mn | 1223/1440 | M2B + MB | 27 Fe, 30 B, 43 Mn | 15 Fe, 50 B, 35 Mn |
| 11 | 51 Fe–39 B–10 Mn | 873/2160 | M2B + MB | 57 Fe, 33 B, 10 Mn | 41 Fe, 50 B, 9 Mn |
| 12 | 51 Fe–39 B–10 Mn | 1223/1440 | M2B +MB | 58 Fe, 33 B, 9 Mn | 40 Fe, 50 B, 10 Mn |
| 13 | 50 Fe–41 B–9 V | 1353/1440 | Fe2B + V3B4 | 64 Fe, 34 B, 2 V | - |
| 14 | 50 Fe–41 B–9 V | 903/4560 | Fe2B + FeB + V3B4 | 66 Fe, 31 B, 3 V | 39.2 Fe, 51 B, 9.8 V |
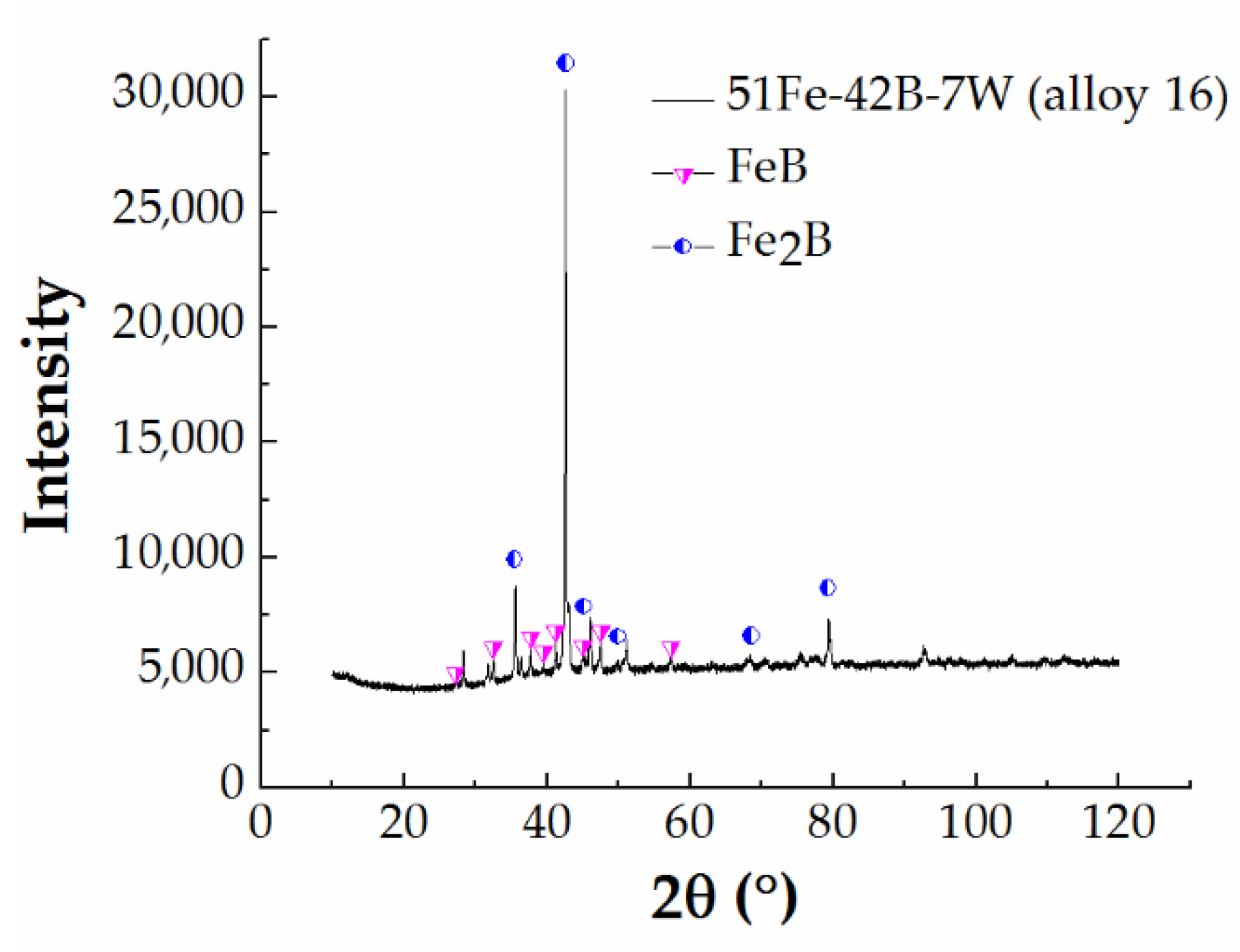
| 15 | 51 Fe–42 B–7 W | 1323/2000 | Fe2B + FeB + W2FeB2 | 67 Fe, 32.4 B, 0.6 W | 41 Fe, 50 B, 1 W |
| 16 | 51 Fe–42 B–7 W | 950/4224 | Fe2B + FeB + W2FeB2 | 68.89 Fe, 28.81 B, 2.3 W | 47.86 Fe, 49.54 B, 2.6 W |
| 17 | 45 Fe–40 B–5 Mn–10 V | 900/2040 | M2B + MB + V3B4 | 58 Fe, 34 B, 5 Mn, 3 V | 36 Fe, 50 B, 5 Mn, 9 V |
| Alloy | Phase Fraction (vol. %) |
|---|---|
| 1 | FeB: 77.28, B4C: 11.44, B: 11.28 ** |
| 2 | FeB: 54.25, B4C: 6.32, graphite: 39.43 ** |
| 3 | Fe2B: 20,02, FeB: 52,09, graphite: 27,89 ** |
| 4 | Fe2B: 48.64, FeB: 23.42, graphite: 27.94 ** |
| 5 | FeB: 58.20, CrB4: 21.56, CrB2: 20.24 ** |
| 6 | FeB: 2.87, Cr3B4: 97.13 ** |
| 7 | Fe2B: 71.35, FeB: 28.65 ** |
| 8 | Fe2B: 27, γ-Fe:73 * |
| 9 | M2B: 66, MB: 34 * |
| 10 | M2B: 67, MB: 33 * |
| 11 | M2B: 68.6, MB: 31.4 * |
| 12 | M2B: 68.3, MB: 31.7 * |
| 13 | Fe2B: 76.7, V3B4: 23.3 * |
| 14 | Fe2B: 63.8, FeB: 21.6, V3B4: 14.6 * |
| 15 | Fe2B: 25.65, FeB: 48.58, W2FeB2: 25.77 ** |
| 16 | Fe2B: 37.78, FeB: 44.85, W2FeB2: 17.37 ** |
| 17 | M2B: 60.02, MB: 21.64, V3B4: 18.34 ** |
| Alloy | Alloy’s Chemical Composition | HIT FeB | EIT FeB | HIT Fe2B | EIT Fe2B |
|---|---|---|---|---|---|
| 1 | 38.5 Fe–59.2 B–2.3 C | 20.5 ± 1.0 | 370.3 ± 20.6 | - | - |
| 2 | 34.6 Fe–52 B–13.4 C | 22.3 ± 1.3 | 358.5 ± 30.0 | - | - |
| 3 | 37 Fe–34 B–29 C | 20.4 ± 0.5 | 315.6 ± 32.8 | 19.4 ± 0.7 | 315.7 ± 21.7 |
| 4 | 39.7 Fe–33 B–27.3 C | 20.6 ± 0.8 | 375.8 ± 22.2 | 20.3 ± 0.6 | 350.4 ± 14.3 |
| 5 | 17 Fe–65 B–18 Cr | 26.9 ± 1.4 | 485.5 ± 22.3 | - | - |
| 6 | 8 Fe–56 B–36 Cr | 26.4 ± 1.9 | 410.1 ± 27.3 | - | - |
| 7 | 50 Fe–40 B–10 Cr | 24.5 ± 0.7 | 437.4 ± 17.1 | 18.3 ± 0.7 | 391.7 ± 10.9 |
| 8 | 82 Fe–9 B–9 Mn | - | - | 16.1 ± 2.2 | 297.5 ± 24.6 |
| 9 | 22 Fe–39 B–39 Mn | 21.4 ± 2.0 | 374.3 ± 36.6 | 19.5 ± 0.9 | 354.4 ± 11.2 |
| 10 | 22 Fe–39 B–39 Mn | 18.6 ± 0.6 | 375.0 ± 11.2 | 17.9 ± 1.2 | 315.1 ± 35.9 |
| 11 | 51 Fe–39 B–10 Mn | 22.2 ± 0.7 | 385.5 ± 25.4 | 19.0 ± 0.5 | 375.2 ± 8.8 |
| 12 | 51 Fe–39 B–10 Mn | 21.6 ± 1.2 | 400.5 ± 15.0 | 18.7 ± 0.7 | 367.9 ± 8.9 |
| 13 | 50 Fe–41 B–9 V | - | - | 19.2 ± 0.6 | 354.5 ± 9.5 |
| 14 | 50 Fe–41 B–9 V | - | - | 19.0 ± 0.6 | 364.1 ± 8.0 |
| 15 | 51 Fe–42 B–7 W | - | - | 20.8 ± 0.9 | 383.1 ± 8.7 |
| 16 | 51 Fe–42 B–7 W | - | - | 20.7 ± 1.3 | 377.8 ± 8.7 |
| 17 | 45 Fe–40 B–5 Mn–10 V | - | - | 18.1 ± 1.2 | 367.4 ± 18.7 |
| Alloy | Chemical Composition | H0-Fe2B (M2B) | H0-FeB (MB) |
|---|---|---|---|
| 4 | 39.7 Fe–33 B–27.3 C | 20.19 | 19.70 |
| 7 | 50 Fe–40 B–10 Cr | 19.98 | 26.31 |
| 11 | 51 Fe–39 B–10 Mn | 19.54 | 21.67 |
| 14 | 50 Fe–41 B–9 V | 20.26 | - |
| 17 | 45 Fe–40 B–5 Mn–10 V | 19.54 | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kirkovska, I.; Homolová, V.; Petryshynets, I.; Csanádi, T. The Influence of the Third Element on Nano-Mechanical Properties of Iron Borides FeB and Fe2B Formed in Fe-B-X (X = C, Cr, Mn, V, W, Mn + V) Alloys. Materials 2020, 13, 4155. https://doi.org/10.3390/ma13184155
Kirkovska I, Homolová V, Petryshynets I, Csanádi T. The Influence of the Third Element on Nano-Mechanical Properties of Iron Borides FeB and Fe2B Formed in Fe-B-X (X = C, Cr, Mn, V, W, Mn + V) Alloys. Materials. 2020; 13(18):4155. https://doi.org/10.3390/ma13184155
Chicago/Turabian StyleKirkovska, Ivana, Viera Homolová, Ivan Petryshynets, and Tamás Csanádi. 2020. "The Influence of the Third Element on Nano-Mechanical Properties of Iron Borides FeB and Fe2B Formed in Fe-B-X (X = C, Cr, Mn, V, W, Mn + V) Alloys" Materials 13, no. 18: 4155. https://doi.org/10.3390/ma13184155
APA StyleKirkovska, I., Homolová, V., Petryshynets, I., & Csanádi, T. (2020). The Influence of the Third Element on Nano-Mechanical Properties of Iron Borides FeB and Fe2B Formed in Fe-B-X (X = C, Cr, Mn, V, W, Mn + V) Alloys. Materials, 13(18), 4155. https://doi.org/10.3390/ma13184155





