Bioactive (Co)oligoesters as Potential Delivery Systems of p-Anisic Acid for Cosmetic Purposes
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Measurements
2.2.1. Performance of Electrospray Mass Spectrometry (ESI-MSn) Analyses
2.2.2. Performing of HPLC Analyses
2.3. Assessment of Cytocompatibility of (Homo) and (Co)oligoesters Containing the p-Anisic Acid Moiety
2.3.1. Statistical Analysis
2.3.2. SRB (Sulforhodamine B) Cell Proliferation Assay
2.4. Hydrolytic Degradation Tests of Bioactive (p-AA-CH2-PL)n Oligoester and [(p-AA-CH2-HP)x-co-(HB)y] (Co)oligoester With the p-AA Moiety
3. Results and Discussion
3.1. Characterization of (p-AA-CH2-HP)n Oligoester Degradation Products
3.2. Characterization of [(p-AA-CH2-HP)x-co-(HB)y] (Co)oligoester Degradation Products
3.3. Comparative Studies of the Release of p-Anisic Acid from (p-AA-CH2-HP)n Oligoester and [(p-AA-CH2-HP)x-co-(HB)y] (Co)oligoesters
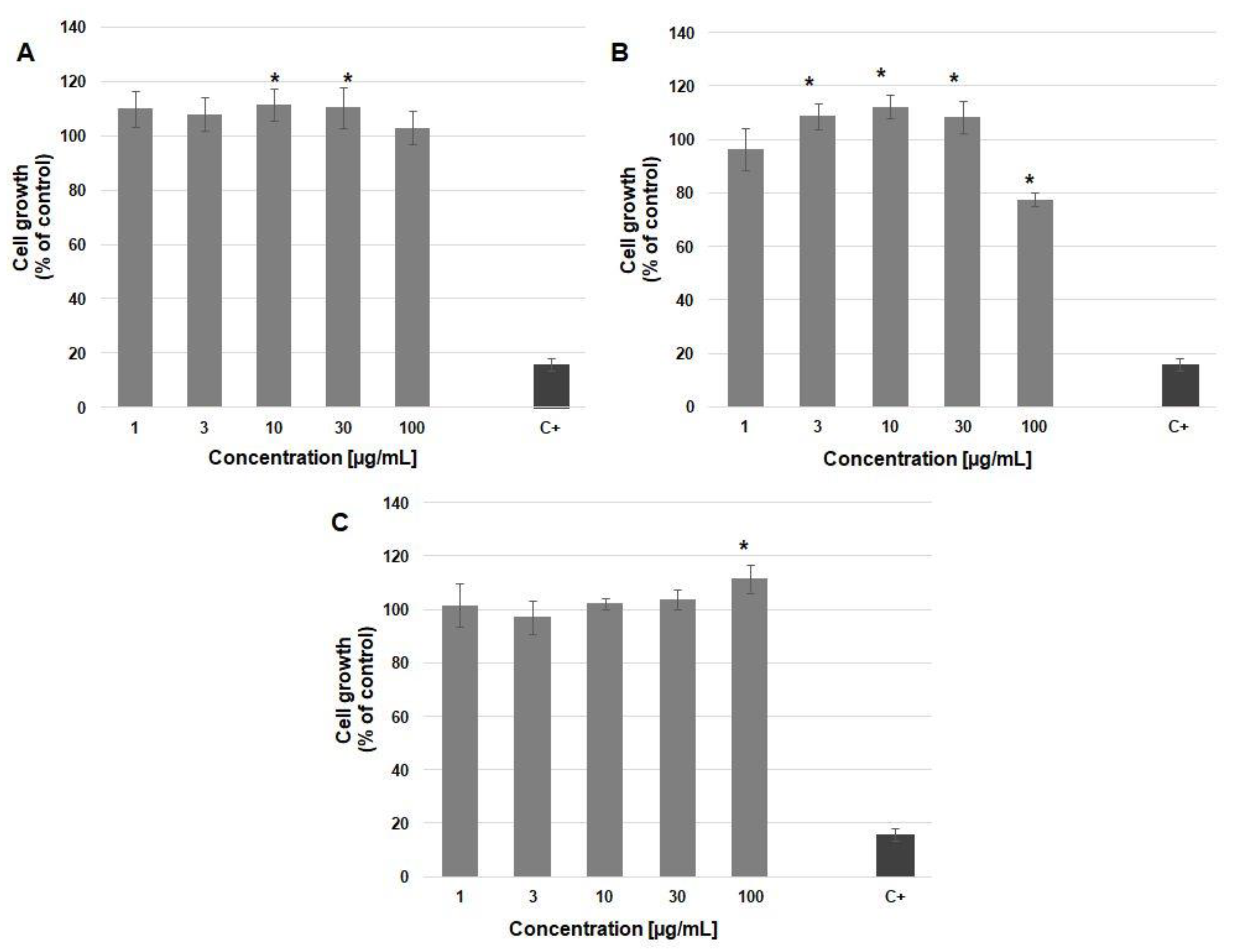
3.4. Cytocompatibility of (Homo)- and (Co)oligoesters Containing p-AA Moiety
SRB Assay for Cell Proliferation
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Parrado, C.; Mascaraque, M.; Gilaberte, Y.; Juarranz, A.; Gonzalez, S. Fernblock (Polypodium leucotomos Extract): Molecular Mechanisms and Pleiotropic Effects in Light-Related Skin Conditions, Photoaging and Skin Cancers, a Review. Int. J. Mol. Sci. 2016, 17, 1026. [Google Scholar] [CrossRef] [PubMed]
- Othman, A.; Norton, L.; Finny, S.; Andreescu, S. Easy-to-use and inexpensive sensors for assessing the quality and traceability of cosmetic antioxidants. Talanta 2020, 208, 120473. [Google Scholar] [CrossRef] [PubMed]
- Herman, A. Antimicrobial Ingredients as preservative booster and components of self-preserving cosmetic products. Curr. Microbiol. 2019, 76, 744–754. [Google Scholar] [CrossRef]
- Papageorgiou, S.; Varvaresou, A.; Tsirivas, E.; Demetzos, C. New alternatives to cosmetics preservation. J. Cosmet. Sci. 2010, 61, 107–123. [Google Scholar]
- Straetmans, U.; Janichen, J.; Petersen, W.; Kinder, M.; Johnson, C.; Reynolds, G. Concentrated, aqueous solutions of p-methoxybenzoic acid for use in cosmetic and dermatologic formulations. U.S. Patent 20060229291 A1, 12 October 2006. [Google Scholar]
- Sautour, M.; Mitaine-Offer, A.-C.; Lacaille-Dubois, M.-A. The Dioscorea genus: A review of bioactive steroid saponins. J. Nat. Med. 2007, 61, 91–101. [Google Scholar] [CrossRef]
- Varvaresou, A.; Papageorgiou, S.; Tsirivas, E.; Protopapa, E.; Kintziou, H.; Kefala, V.; Demetzos, C. Self-preserving cosmetics. Int. J. Cosm. Sci. 2009, 31, 163–175. [Google Scholar] [CrossRef]
- Lupo, M.P. Antioxidants and vitamins in cosmetics. Clin. Dermatol. 2001, 19, 467–473. [Google Scholar] [CrossRef]
- Costa, R.; Santos, L. Delivery systems for cosmetics—From manufacturing tothe skin of natural antioxidants. Powder Technol. 2017, 322, 402–416. [Google Scholar] [CrossRef]
- Rancan, F.; Blume-Peytavi, U.; Vogt, A. Utilization of biodegradable polymeric materials as delivery agents in dermatology. Clin. Cosmet. Investig. Dermatol. 2014, 7, 23–34. [Google Scholar] [CrossRef]
- Albertsson, A.-C.; Varma, I.K. Recent developments in ring opening polymerization of lactones for biomedical applications. Biomacromolecules 2003, 4, 1466–1486. [Google Scholar] [CrossRef]
- Michalak, M.; Jurczyk, S.; Jelonek, K.; Hakkarainen, M.; Kurcok, P. Polyhydroxyalkanoates as promising materials in biomedical systems. In Frontiers in Drug Design and Discovery; Rahman, A., Choudhary, I., Eds.; Bentham Science Publishers: Sharjah, UAE, 2017; Volume 8, Chapter 5; pp. 242–288. [Google Scholar]
- Pommier, A.; Pons, J.M. Recent Advances in β-Lactone Chemistry. Synthesis 1993, 5, 441–459. [Google Scholar] [CrossRef]
- Rieth, L.R.; Moore, D.R.; Lobkovsky, E.B.; Coates, G.W. Single-Site ß-Diiminate Zinc Catalysts for the Ring-Opening Polymerization of ß-Butyrolactone and ß-Valerolactone to Poly(3-hydroxyalkanoates). J. Am. Chem. Soc. 2002, 124, 15239–15248. [Google Scholar] [CrossRef] [PubMed]
- Adamus, G.; Kowalczuk, M. Synthesis of Poly(2-methyl-3-hydroxyoctanoate) via Anionic Polymerization of α-Methyl-β-pentyl-β-propiolactone. Biomacromolecules 2008, 9, 696–703. [Google Scholar] [CrossRef] [PubMed]
- Getzler, Y.D.Y.L.; Mahadevan, V.; Lobkovsky, E.B.; Coates, G.W. Synthesis of ß-Lactones: A highly active and selective catalyst for epoxide carbonylation. J. Am. Chem. Soc. 2002, 124, 1174–1175. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, J.A.R.; Mahadevan, V.; Getzler, Y.D.Y.L.; Coates, G.W. A readily synthesized and highly active epoxide carbonylation catalyst based on a chromium porphyrin framework. Expanding the range of available ß-Lactones. Org. Lett. 2004, 6, 373–376. [Google Scholar] [CrossRef]
- Kramer, J.W.; Lobkovsky, E.B.; Coates, G.W. Practical ß-Lactone Synthesis: Epoxide Carbonylation at 1 atm. Org. Lett. 2006, 8, 3709–3712. [Google Scholar] [CrossRef]
- Mundlia, J.; Ahuja, M.; Kumar, P. Enhanced biological activity of polyphenols on conjugation with gellan gum. Int. J. Polym. Mater. 2020. [CrossRef]
- Maksymiak, M.; Dębowska, R.; Jelonek, K.; Kowalczuk, M.; Adamus, G. Structural characterization of biocompatible lipoic acid–oligo-(3-hydroxybutyrate) conjugates by electrospray ionization mass spectrometry. Rapid Commun. Mass Spectrom. 2013, 27, 773–783. [Google Scholar] [CrossRef]
- Maksymiak, M.; Kowalczuk, M.; Adamus, G. Electrospray tandem mass spectrometry for the structural characterization of p-coumaric acid–oligo(3-hydroxybutyrate) conjugates. Int. J. Mass Spectrom. 2014, 359, 6–11. [Google Scholar] [CrossRef]
- Maksymiak, M.; Debowska, R.; Bazela, K.; Dzwigalowska, A.; Orchel, A.; Jelonek, K.; Dolegowska, B.; Kowalczuk, M.; Adamus, G. Designing of Biodegradable and Biocompatible Release and Delivery Systems of Selected Antioxidants Used in Cosmetology. Biomacromolecules 2015, 16, 3603–3612. [Google Scholar] [CrossRef] [PubMed]
- Adamus, G.; Kwiecień, I.; Maksymiak, M.; Bałakier, T.; Jurczak, J.; Kowalczuk, M. Molecular level structure of novel synthetic analogues of aliphatic biopolyesters as revealed by multistage mass spectrometry. Anal. Chim. Acta. 2014, 808, 104–114. [Google Scholar] [CrossRef]
- Maksymiak, M.; Bałakier, T.; Jurczak, J.; Kowalczuk, M.; Adamus, G. Bioactive (co)oligoesters with antioxidant properties—Synthesis and structural characterization at the molecular level. RSC Adv. 2016, 6, 57751–57761. [Google Scholar] [CrossRef]
- Bałakier, T.; Chaładaj, W.; Jurczak, J.; Adamus, G.; Kowalczuk, M. An effective protocol for the synthesis enantiomerically pure 4-substituted oxetane-2-ones. Tetrahedron 2013, 69, 4990–4993. [Google Scholar] [CrossRef]
- Adamus, G. Molecular Level Structure of (R,S)-3-hydroxybutyrate/(R,S)-3-hydroxy-4-ethoxybutyrate Copolyesters with Dissimilar Architecture. Macromolecules 2009, 42, 4547–4557. [Google Scholar] [CrossRef]
- Kowalczuk, M.; Adamus, G. Mass spectrometry for the elucidation of the subtle molecular structure of biodegradable polymers and their degradation products. Mass Spectrom. Rev. 2016, 35, 188–198. [Google Scholar] [CrossRef]
- Adamus, G.; Hakkarainen, M.; Höglund, A.; Kowalczuk, M.; Albertsson, A.C. MALDI-TOF-MS reveals the molecular level structures of different hydrophilic-hydrophobic polyether-esters. Biomacromolecules 2009, 10, 1540–1546. [Google Scholar] [CrossRef]
- Boukamp, P.; Petrussevska, R.T.; Breitkreutz, D.; Hornung, J.; Markham, A.; Fusenig, N.E. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J. Cell Biol. 1988, 106, 761–771. [Google Scholar] [CrossRef]
- Schoop, V.M.; Mirancea, N.; Fusenig, N.E. Epidermal organization and differentiation of HaCaT keratinocytes in organotypic coculture with human dermal fibroblasts. J. Investig. Dermatol. 1999, 112, 343–353. [Google Scholar] [CrossRef]
- Georgousaki, K.; Tsafantakis, N.; Gumeni, S.; Lambrinidis, G.; González-Menéndez, V.; Tormo, J.R.; Genilloud, O.; Trougakos, I.P.; Fokialakis, N. Biological evaluation and in silico study of benzoic acid derivatives from Bjerkandera adusta targeting proteostasis network modules. Molecules 2020, 25, 666. [Google Scholar] [CrossRef]
- Kubo, I.; Chen, Q.X.; Nihei, K.; Calderón, J.S.; Céspedes, C.L. Tyrosinase inhibition kinetics of anisic acid. Z. Naturforsch. C J. Biosci. 2003, 58, 713–718. [Google Scholar] [CrossRef] [PubMed]
- El-Hawary, S.S.; Sayed, A.M.; Mohammed, R.; Hassan, H.M.; Zaki, M.A.; Rateb, M.E.; Mohammed, T.A.; Amin, E.; Abdelmohsen, U.R. Epigenetic modifiers induce bioactive phenolic metabolites in the marine-derived fungus Penicillium brevicompactum. Mar. Drugs 2018, 16, 253. [Google Scholar] [CrossRef] [PubMed]
- Zawidlak-Wegrzyńska, B.; Kawalec, M.; Bosek, I.; Łuczyk-Juzwa, M.; Adamus, G.; Rusin, A.; Filipczak, P.; Głowala-Kosińska, M.; Wolańska, K.; Krawczyk, Z.; et al. Synthesis and antiproliferative properties of ibuprofen-oligo(3-hydroxybutyrate) conjugates. Eur. J. Med. Chem. 2010, 45, 1833–1842. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Dai, Z.; Zhao, Y.; Chen, G.Q. In vitro effect of oligo-hydroxyalkanoates on the growth of mouse fibroblast cell line L929. Biomaterials 2007, 28, 3896–3903. [Google Scholar] [CrossRef] [PubMed]
- Guh, J.Y.; Chuang, T.D.; Chen, H.C.; Hung, W.C.; Lai, Y.H.; Shin, S.J.; Chuang, L.Y. Beta-hydroxybutyrate-induced growth inhibition and collagen production in HK-2 cells are dependent on TGF-beta and Smad3. Kidney Int. 2003, 64, 2041–2051. [Google Scholar] [CrossRef]
- Tasaki, O.; Hiraide, A.; Shiozaki, T.; Yamamura, H.; Ninomiya, N.; Sugimoto, H. The dimer and trimer of 3-hydroxybutyrate oligomer as a precursor of ketone bodies for nutritional care. JPEN J. Parenter. Enteral. Nutr. 1999, 23, 321–325. [Google Scholar] [CrossRef]






| Time (min) | Mobile Phase (%) | |
|---|---|---|
| A | B | |
| 0 | 75 | 25 |
| 5 | 75 | 25 |
| 20 | 60 | 40 |
| 25 | 55 | 45 |
| 45 | 45 | 55 |
| 50 | 75 | 25 |
| Series | Chemical Structure of the Ions | Composition of the Comonomers | m/z |
|---|---|---|---|
| A |  | (p-AA-CH2-HP)x = Ax | |
| p-AA-A1/HB3 | 645 | ||
| p-AA-A1/HB4 | 731 | ||
| p-AA-A1/HB5 | 817 | ||
| p-AA-A1/HB6 | 903 | ||
| p-AA-A1/HB7 | 989 | ||
| p-AA-A1/HB8 | 1075 | ||
| B |  | p-AA-A2/HB1 | 709 |
| p-AA-A2/HB2 | 795 | ||
| p-AA-A2/HB3 | 881 | ||
| p-AA-A2/HB4 | 967 | ||
| p-AA-A2/HB5 | 1053 | ||
| C |  | p-AA-HB6 | 667 |
| p-AA-HB7 | 753 | ||
| p-AA-HB8 | 839 | ||
| p-AA-HB9 | 925 | ||
| p-AA-HB10 | 1011 | ||
| p-AA-HB11 | 1097 | ||
| D |  | A1/HB5OH | 683 |
| A1/HB6OH | 769 | ||
| A1/HB7OH | 855 | ||
| A1/HB8OH | 941 | ||
| A1/HB9OH | 1027 | ||
| E |  | A2/HB2OH | 661 |
| A2/HB3OH | 747 | ||
| A2/HB4OH | 833 | ||
| A2/HB5OH | 919 | ||
| A2/HB6OH | 1005 | ||
| A2/HB7OH | 1091 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martinka Maksymiak, M.; Zięba, M.; Orchel, A.; Musiał-Kulik, M.; Kowalczuk, M.; Adamus, G. Bioactive (Co)oligoesters as Potential Delivery Systems of p-Anisic Acid for Cosmetic Purposes. Materials 2020, 13, 4153. https://doi.org/10.3390/ma13184153
Martinka Maksymiak M, Zięba M, Orchel A, Musiał-Kulik M, Kowalczuk M, Adamus G. Bioactive (Co)oligoesters as Potential Delivery Systems of p-Anisic Acid for Cosmetic Purposes. Materials. 2020; 13(18):4153. https://doi.org/10.3390/ma13184153
Chicago/Turabian StyleMartinka Maksymiak, Magdalena, Magdalena Zięba, Arkadiusz Orchel, Monika Musiał-Kulik, Marek Kowalczuk, and Grazyna Adamus. 2020. "Bioactive (Co)oligoesters as Potential Delivery Systems of p-Anisic Acid for Cosmetic Purposes" Materials 13, no. 18: 4153. https://doi.org/10.3390/ma13184153
APA StyleMartinka Maksymiak, M., Zięba, M., Orchel, A., Musiał-Kulik, M., Kowalczuk, M., & Adamus, G. (2020). Bioactive (Co)oligoesters as Potential Delivery Systems of p-Anisic Acid for Cosmetic Purposes. Materials, 13(18), 4153. https://doi.org/10.3390/ma13184153








