Synthesis, Absolute Configuration, Antibacterial, and Antifungal Activities of Novel Benzofuryl β-Amino Alcohols
Abstract
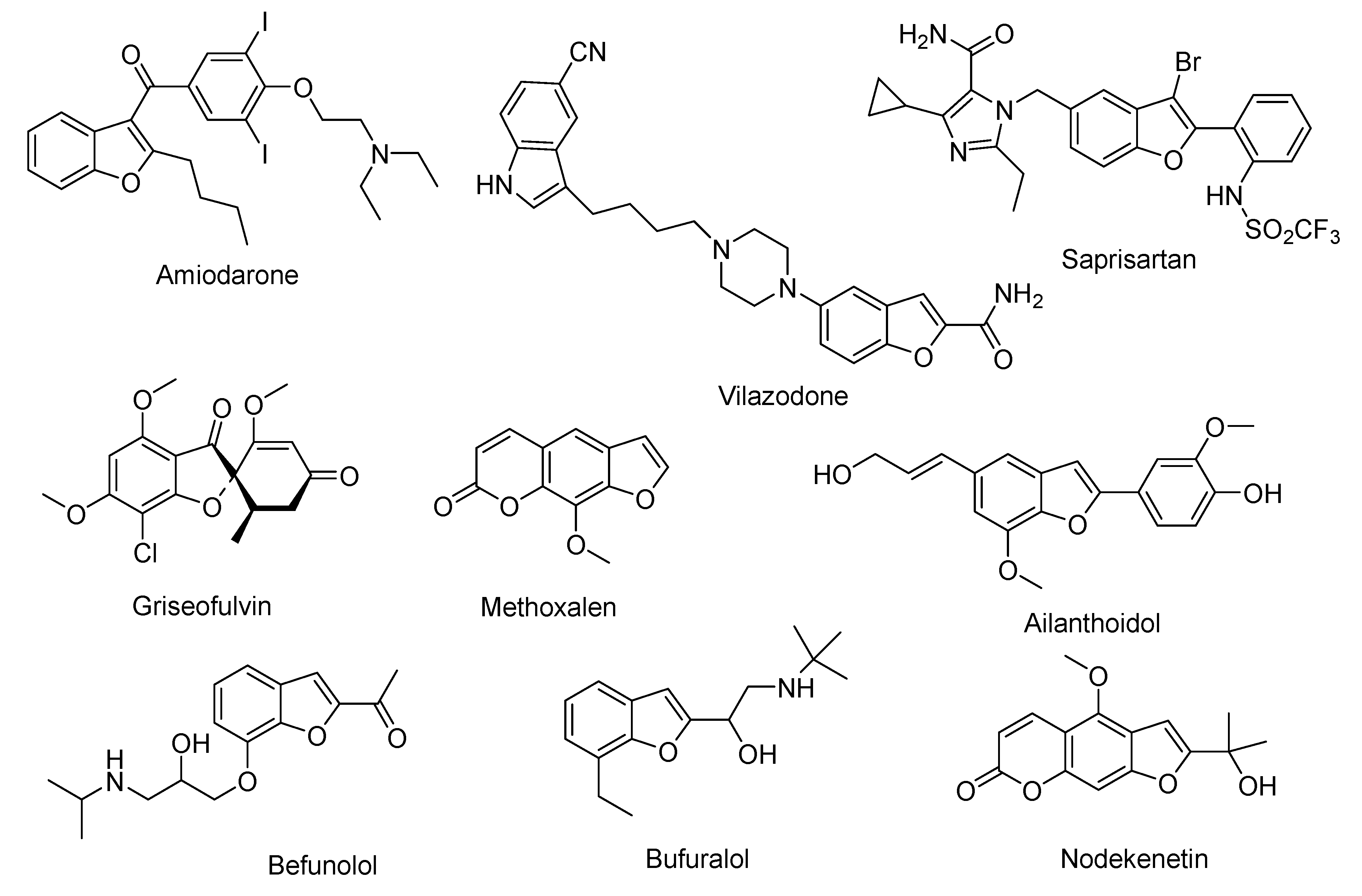
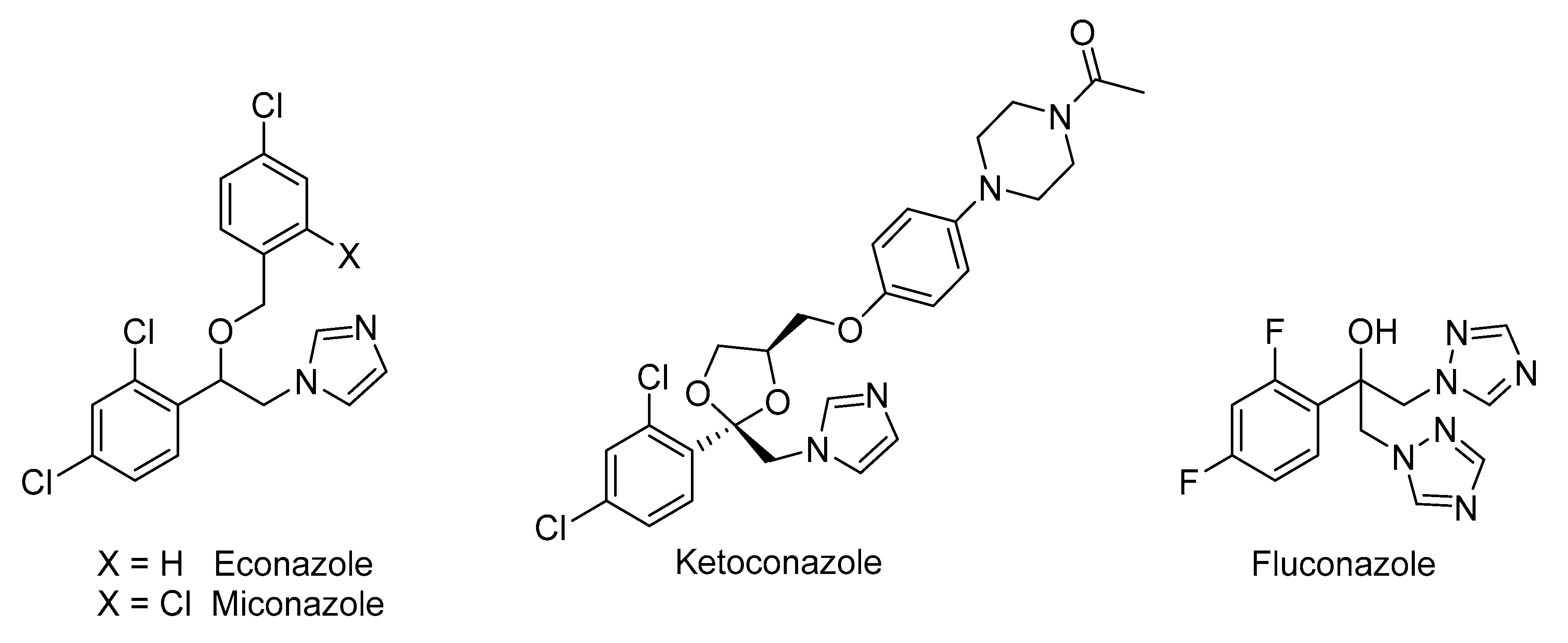
1. Introduction
2. Materials and Methods
2.1. General Information
- Nuclear magnetic resonance (NMR) spectra were recorded on Bruker AMX 300 MHz, Bruker Avance III 400 MHz and Brucker Avance III 700 MHz spectrometers (Ettlingen, Germany). Chemical shifts were reported in δ ppm from tetramethylsilane (TMS) as the internal standard.
- High-performance liquid chromatography (HPLC) analyses were performed on a Shimadzu LC-10AT chromatograph (Shimadzu, Kyoto, Japan).
- Optical rotations were measured on an automatic polarimeter PolAAr 3000 (Optical Activity Ltd., Ramsey, Cambridgeshire, UK) and recorded at 20 °C on a Jasco P-2000 polarimeter (Easton, MD, USA).
- The ECD and UV (ultraviolet) spectra were measured using a Jasco J-810 spectropolarimeter (Easton, MD, USA) at room temperature, in acetonitrile solution, and with the use of a quartz cell of 0.1 cm optical length. The analytes concentration ranged from 1.0 to 2.0 × 10−4 mol L−1.
- IR (infrared) analyses were recorded on an Alpha FT-IR spectrometer from Bruker (Ettlingen, Germany).
- Melting points were determined in open glass capillaries and are uncorrected.
- Elemental analyses were performer on a Vario MACRO CHN, ELEMENTAR Anaylsensysteme GmbH instrument (Langenselbold, Germany).
2.2. Materials
2.3. General Procedures
2.3.1. Method of α-Amino Ketones 5–12 Preparation
2.3.2. General Procedure for the Asymmetric Transfer Hydrogenation of α-Amino Ketones 5–12
2.3.3. General Procedure for α-Amino Ketones 5–12 Reduction by NaBH4
2.3.4. General Procedure for the Asymmetric Reduction of α-Amino Ketones 6, 9 and 12 by Saccharomyces Cerevisiae
2.3.5. General Procedure for the Asymmetric Reduction of α-Amino Ketones 6, 9 and 12 by Aureobasidium Pullulans without Additives
2.3.6. General Procedure for the Asymmetric Reduction of α-Amino Ketones 6, 9 and 12 by Aureobasidium Pullulans with Additives
2.4. Antibacterial and Antifungal Studies of Racemic and Chiral Benzofuryl Derivatives
3. Results and Discussion
3.1. Synthesis and Reduction of Novel Benzofuryl α-Azole Ketones
3.2. Determination of the Absolute Configuration of Chiral Benzofuryl β-Amino Alcohols
3.3. Antibacterial and Antifungal Activities of Racemic and Chiral Benzofuryl Derivatives
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shamsuzzaman, H.K. Bioactive benzofuran derivatives: A review. Eur. J. Med. Chem. 2015, 97, 483–504. [Google Scholar] [CrossRef]
- Bourgery, G.; Dostert, L.; Lacour, A.; Langlois, M.; Pourrias, B.; Tisne-Versailles, J. Synthesis and antiarrhythmic activity of new benzofuran derivatives. J. Med. Chem. 1981, 24, 159–167. [Google Scholar] [CrossRef]
- Nevagi, R.J.; Dighe, S.N.; Dighe, S.N. Biological and medicinal significance of benzofuran. Eur. J. Med. Chem. 2015, 97, 561–581. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.; Zmarlicka, M.; Ehret, M.J. Vilazodone: A novel antidepressant. Am. J. Health-Syst. Pharm. 2012, 69, 1551–1557. [Google Scholar] [CrossRef] [PubMed]
- Petersen, A.B.; Ronnest, M.H.; Larsen, T.O.; Clausen, M.H. The chemistry of griseofulvin. Chem. Rev. 2014, 114, 12088–12107. [Google Scholar] [CrossRef]
- Nore, P.; Honkanen, E. A new synthesis of methoxalen. J. Heterocycl. Chem. 1980, 17, 985–987. [Google Scholar] [CrossRef]
- Kao, C.L.; Chern, J.W. A convenient synthesis of naturally occuring benzofuran ailanthoidol. Tetrahedron Lett. 2001, 42, 1111–1113. [Google Scholar] [CrossRef]
- Miao, Y.; Hu, Y.; Yang, J.; Liu, T.; Sun, J.; Wang, X. Natural source, bioactivity and synthesis of benzofuran derivatives. RSC Adv. 2019, 9, 27510–27540. [Google Scholar] [CrossRef]
- Takayanagi, I.; Koike, K. A beta-adrenoceptor blocking agent, befunolol as a partial agonist in isolated organs. Gen. Pharmacol. 1985, 16, 265–267. [Google Scholar] [CrossRef]
- Narimatsu, S.; Takemi, C.; Kuramoto, S.; Tsuzuki, D.; Hichiya, H.; Tamagake, K.; Yamamoto, S. Stereoselectivity in the oxidation of bufuralol, a chiral substrate, by human cytochrome P450s. Chirality 2003, 15, 333–339. [Google Scholar] [CrossRef]
- Maschmeyer, G.; Haas, A. Voriconazole: A broad spectrum triazole for the treatment of serious and invasive fungal infections. Future Microbiol. 2006, 1, 365–385. [Google Scholar] [CrossRef] [PubMed]
- Sari, S.; Avci, A.; Koçak, E.; Kart, D.; Sabuncuoğlu, S.; Doğan, Ì.S.; Özdemir, Z.; Bozbey, Ì.; Karakurt, A.; Saraç, S.; et al. Antibacterial azole derivatives: Antibacterial activity, cytotoxicity, and in silico mechanistic studies. Drug Dev. Res. 2020, 1–11. [Google Scholar] [CrossRef]
- Alsterholm, M.; Karami, N.; Faergemann, J. Antimicrobial activity of topical skin pharmaceuticals—An in vitro study. Acta Derm. Venereol. 2010, 90, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Nenoff, P.; Koch, D.; Krüger, C.; Drechsel, C.; Mayser, P. New insights on the antibacterial efficacy of miconazole in vitro. Mycoses 2017, 60, 1–6. [Google Scholar] [CrossRef]
- Chatterjee, A.; Rai, S.; Guddattu, V.; Mukhopadhyay, C.; Saravu, K. Is methicillin-resistant Staphylococcus aureus infection associated with higher mortality and morbidity in hospitalized patients? A cohort study of 551 patients from South Western India. Risk Manag. Healthc. Policy 2018, 11, 243–250. [Google Scholar] [CrossRef]
- Khabnadideh, S.; Rezaei, Z.; Younes, G.; Montazeri-Najafabady, N. Antibacterial activity of some new azole compounds. Anti-Infect. Agents 2012, 10, 26–33. [Google Scholar] [CrossRef]
- Karakurt, A.; Dalkara, S.; Ozalp, M.; Ozbey, S.; Kendi, E.; Stables, J.P. Synthesis of some 1-(2-naphthyl)-2-(imidazole-1-yl)ethanone oxime and oxime ether derivatives and their anticonvulsant and antimicrobial activities. Eur. J. Med. Chem. 2001, 36, 421–433. [Google Scholar] [CrossRef]
- Doğan, Ì.S.; Saraç, S.; Sari, S.; Kart, D.; Gökhan, Ş.E.; Vural, Ì.; Dalkara, S. New azole derivatives showing antimicrobial effects and their mechanism of antifungal activity by molecular modeling studies. Eur. J. Med. Chem. 2017, 130, 124–138. [Google Scholar] [CrossRef]
- Chen, S.C.; Playford, E.G.; Sorrell, T.C. Antifungal therapy in invasive fungal infections. Curr. Opin. Pharmacol. 2010, 10, 522–530. [Google Scholar] [CrossRef]
- Regina, G.L.; D’Auria, F.D.; Tafi, A.; Piscitelli, F.; Olla, S.; Caporuscio, F.; Nencioni, L.; Cirilli, R.; La Torre, F.; De Molo, N.R.; et al. 1-[(3-Aryloxy-3-aryl)propyl]-1H-imidazoles, new imidazoles with potent activity against Candida albicans and Dermatophytes. Synthesis, structure-activity relationship, and molecular modeling studies. J. Med. Chem. 2008, 51, 3841–3855. [Google Scholar] [CrossRef]
- De Vita, D.; Scipione, L.; Tortorella, S.; Mellini, P.; Di Rienzo, B.; Simonetti, G.; D‘Auria, F.D.; Panella, S.; Cirilli, R.; Di Santo, R.; et al. Synthesis and antifungal activity of a new series of 2-(1H-imidazol-1-yl)-1-phenylethanol derivatives. Eur. J. Med. Chem. 2012, 49, 334–342. [Google Scholar] [CrossRef] [PubMed]
- De Camp, W.H. The FDA perspective on the development of the stereoisomers. Chirality 1989, 1, 2–6. [Google Scholar] [CrossRef] [PubMed]
- Machin, P.J.; Hurst, D.N.; Osbond, J.M. β-Adrenoceptor activity of the stereoisomers of the bufuralol alcohol and ketone metabolites. J. Med. Chem. 1985, 28, 1648–1651. [Google Scholar] [CrossRef] [PubMed]
- Kawamoto, A.; Wills, M. Enantioselective synthesis of β-hydroxy amines and aziridines using asymmetric transfer hydrogenation of α-amido ketones. Tetrahedron Asymmetry 2000, 11, 3257–3261. [Google Scholar] [CrossRef]
- Kawamoto, A.M.; Wills, M. Enantioselective synthesis of β-hydroxy amines and aziridines using asymmetric transfer hydrogenation of α-amino ketones. J. Chem. Soc. Perkin Trans. 2001, 1, 1916–1928. [Google Scholar] [CrossRef]
- Lennon, I.C.; Ramsden, J.A. An efficient catalytic asymmetric route to 1-aryl-2-imidazol-1-yl-ethanols. Org. Process Res. Dev. 2005, 9, 110–112. [Google Scholar] [CrossRef]
- Morris, D.J.; Hayes, A.M.; Wills, M. The „reverse-tethered“ ruthenium(II) catalyst for asymmetric transfer hydrogenation: Further applications. J. Org. Chem. 2006, 71, 7035–7044. [Google Scholar] [CrossRef]
- Friggeri, L.; Hargrove, T.Y.; Rachakonds, G.; Williams, A.D.; Wawrzak, Z.; Di Santo, R.; De Vita, D.; Waterman, M.R.; Tortorella, S.; Villaltra, F.; et al. Structural basis for rational design of inhibitors targeting Trypanosoma cruzi sterol 14α-demethylasa: Two regions of the enzyme molecule potentiate ist inhibition. J. Med. Chem. 2014, 57, 6704–6717. [Google Scholar] [CrossRef]
- Xu, Z.; Li, Y.; Liu, J.; Wu, N.; Li, K.; Zhu, S.; Zhang, R.; Liu, Y. Highy enantioselective asymmetric transfer hydrogenation (ATH) of α-phthalimide ketones. Org. Biomol. Chem. 2015, 13, 7513–7516. [Google Scholar] [CrossRef]
- Farina, V.; Reeves, J.T.; Senanayake, C.H.; Song, J.J. Asymmetric synthesis of active pharmaceutical ingredients. Chem. Rev. 2006, 106, 2734–2793. [Google Scholar] [CrossRef]
- Ohkuma, T.; Noyori, R. Hydrogenation of carbonyl groups. In Comprehensive Asymmetric Catalysis; Supplement 1; Jacobsen, E.N., Pflatz, A., Yamamoto, H., Eds.; Springer: Berlin/Heidelberg, Germany, 2004; pp. 1–41. [Google Scholar]
- Wang, F.; Liu, H.; Cun, L.; Zhu, J.; Deng, J.; Jiang, Y. Asymmetric transfer hydrogenation of ketones catalyzed by hydrophobic metal-amido complexes in aqueous micelles and vesicles. J. Org. Chem. 2005, 70, 9424–9429. [Google Scholar] [CrossRef] [PubMed]
- Berova, N.; Polavarapu, P.L.; Nakanishi, K.; Woody, R.W. Comprehensive Chiroptical Spectroscopy: Applications in Stereochemical Analysis of Synthetic Compounds, Natural Products, and Biomolecules; Wiley: Hoboken, NY, USA, 2012; Volume 2. [Google Scholar]
- Rodger, A.; Nordén, B. Circular Dichroism & Linear Dichroism; Oxford University Press Inc.: New York, NY, USA, 1997. [Google Scholar]
- Flack, H.D.; Bernardinelli, G. The use of X-ray crystallography to determine absolute configuration. Chirality 2008, 20, 681–690. [Google Scholar] [CrossRef]
- Pescitelli, G.; Di Bari, L.; Berova, N. Conformational aspects in the studies of organic compounds by electronic circular dichroism. Chem. Soc. Rev. 2011, 40, 4603–4625. [Google Scholar] [CrossRef] [PubMed]
- Lightner, D.A.; Gurst, J.E. Organic Conformational Analysis and Stereochemistry from Circular Dichroism Spectroscopy; Wiley-VCH: New York, NY, USA, 2000. [Google Scholar]
- Seco, J.M.; Riguera, R. Absolute Stereochemistry by NMR Spectroscopy. In Encyclopedia of Spectroscopy and Spectrometr, 3rd ed.; Lindon, J.C., Koppenaal, D.W., Tranter, G.E., Eds.; Elsevier: Kidlington, UK, 2017. [Google Scholar]
- Wenzel, T.J. Strategies for using NMR spectroscopy to determine absolute configuration. Tetrahedron Asymmetry 2017, 28, 1212–1219. [Google Scholar] [CrossRef]
- Harada, N. Chiral Molecular Science: How were the absolute configurations of chiral molecules determined? Experimental results and theories. Chirality 2017, 29, 774–797. [Google Scholar] [CrossRef]
- Seco, J.M.; Quiñoaá, E.; Riguera, R. Assignment of the Absolute Configuration of Polyfunctional Compounds by NMR Using Chiral Derivatizing Agents. Chem. Rev. 2012, 112, 4603–4641. [Google Scholar] [CrossRef] [PubMed]
- Tafelska-Kaczmarek, A.; Krzemiński, M.P.; Ćwiklińska, M. Asymmetric synthesis of benzofuryl β-amino alcohols by the transfer hydrogenation of α-functionalized ketones. Tetrahedron 2017, 73, 3883–3897. [Google Scholar] [CrossRef]
- Tafelska-Kaczmarek, A.; Prewysz-Kwinto, A.; Skowerski, K.; Pietrasiak, K.; Kozakiewicz, A.; Zaidlewicz, M. Asymmetric synthesis of β-amino alcohols by the transfer hydrogenation of α-keto imines. Tetrahedron Asymmetry 2010, 21, 2244–2248. [Google Scholar] [CrossRef]
- Zaidlewicz, M.; Tafelska-Kaczmarek, A.; Prewysz-Kwinto, A. Enantioselective reduction of benzofuryl halomethyl ketones: Asymmetric synthesis of (R)-bufuralol. Tetrahedron Asymmetry 2005, 16, 3205–3210. [Google Scholar] [CrossRef]
- Shriner, R.L.; Anderson, J. Derivatives of coumaran. VI. Reduction of 2-acetobenzofuran and its derivatives. J. Am. Chem. Soc. 1939, 61, 2705–2708. [Google Scholar] [CrossRef]
- Zaidlewicz, M.; Tafelska-Kaczmarek, A.; Prewysz-Kwinto, A.; Chechłowska, A. Asymmetric synthesis of (S)-bufuralol and a propafenone analogue. Tetrahedron Asymmetry 2003, 14, 1659–1664. [Google Scholar] [CrossRef]
- Nielek, B.; Lesiak, T. Chemistry of thiazole, I. Synthesis and properties of 2,3,5,6-tetrahydro-6-(3-methylbenzofuran-2-yl)imidazo[2,1-b]thiazole. Chem. Ber. 1982, 115, 1247–1251. [Google Scholar] [CrossRef]
- Mashima, K.; Abe, T.; Tani, K. Asymmetric transfer hydrogenation of ketonic substrates catalyzed by (η5-C5Me5)MCl complexes (M = Rh and Ir) of (1S,2S)-N-(p-toluenesulfonyl)-1,2-diphenylethylenediamine. Chem. Lett. 1998, 27, 1199–1200. [Google Scholar] [CrossRef]
- Wahbi, Y.; Caujolle, R.; Tournaire, C.; Payard, M.; Linas, M.D.; Seguela, J.P. Aromatic ethers of 1-aryl 2-(1H-azolyl)ethanol: Study of antifungal activity. Eur. J. Med. Chem. 1995, 30, 955–962. [Google Scholar] [CrossRef]
- Scherm, A.; Peteri, D.; Deuschle, E.; Schatton, W. Benzofuran-2-yl-ethyl Imidazole Derivatives, Process for their Preparation, and Pharmaceuticals. DE Patent 3413363A1, 17 October 1985. [Google Scholar]
- Clinical and Laboratory Standards Institute (CLSI). Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically, 9th ed.; CLSI document M07-A9; Clinical and Laboratory Standards Institute: Wayne, PE, USA, 2012. [Google Scholar]
- De Vita, D.; Pandolfi, F.; Cirilli, R.; Scipione, L.; Di Santo, R.; Friggeri, L.; Mori, M.; Fiorucci, D.; Maccari, G.; Christopher, R.S.A.; et al. Discovery of in vitro antitubercular agents through in silico ligand-based approaches. Eur. J. Med. Chem. 2016, 121, 169–180. [Google Scholar] [CrossRef]
- Kołodziejska, R.; Studzińska, R.; Tafelska-Kaczmarek, A.; Pawluk, H.; Stasiak, B.; Kwit, M.; Woźniak, A. Effect of chemical structure of benzofuran derivatives and reaction conditions on enantioselective properties of Aureobasidium pullulans microorganism contained in Boni Protect antifungal agent. Chirality 2020, 32, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Kołodziejska, R.; Studzińska, R.; Tafelska-Kaczmarek, A.; Pawluk, H.; Kwit, M.; Stasiak, B.; Woźniak, A. The application of safe for human and the environment polyversum antifungal agent containing living cells of Pythium oligandrum for biotransformation of prochiral ketones. Bioorg. Chem. 2019, 92, 103204. [Google Scholar] [CrossRef]
- Kołodziejska, R.; Studzińska, R.; Kwit, M.; Jelecki, M.; Tafelska-Kaczmarek, A. Microbiological bio-reduction of prochiral carbonyl compounds by antimycotic agent Boni Protect. Catal. Commun. 2017, 101, 81–84. [Google Scholar] [CrossRef]
- MacLeod, R.; Prosser, H.; Fikentscher, L.; Lanyi, J.; Mosher, H.S. Asymmetric reduction. XII. Stereoselective ketone reductions by fermenting yeast. Biochemistry 1964, 3, 838–846. [Google Scholar] [CrossRef]
- Gawroński, J.; Kwit, M.; Boyd, D.R.; Sharma, N.D.; Malone, J.F.; Drake, A.F. Absolute configuration, conformation, and circular dichroism of monocyclic arene dihydrodiol metabolites: It is all due to the heteroatom substituents. J. Am. Chem. Soc. 2005, 127, 4308–4319. [Google Scholar] [CrossRef]
- Kwit, M.; Gawroński, J.; Boyd, D.R.; Sharma, N.D.; Kaik, M.; More O’Ferrall, R.; Kudavalli, J.S. Toluene dioxygenase-catalyzed synthesis of cis-dihydrodiol metabolites from 2-substituted naphthalene substrates: Assignments of absolute configurations and conformations from circular dichroism and optical rotation measurements. Chem. Eur. J. 2008, 14, 11500–11511. [Google Scholar] [CrossRef] [PubMed]
- Kwit, M.; Gawroński, J.; Boyd, D.R.; Sharma, N.D.; Kaik, M. Circular dichroism, optical rotation and absolute configuration of 2-cyclohexenone-cis-diol type phenol metabolites: Redefining the role of substituents and 2-cyclohexenone conformation in electronic circular dichroism spectra. Org. Biomol. Chem. 2010, 8, 5635–5645. [Google Scholar] [CrossRef] [PubMed]
- Caldwell, D.J.; Eyring, H. The Theory of Optical Activity. Phys. Today 1972, 11, 53. [Google Scholar] [CrossRef]
- Grauso, L.; Teta, R.; Esposito, G.; Menna, M.; Mangoni, A. Computational prediction of chiroptical properties in structure elucidation of natural products. Nat. Prod. Rep. 2019, 36, 1005–1030. [Google Scholar] [CrossRef]
- Pescitelli, G.; Bruhn, T. Good computational practice in the assignment of absolute configurations by TDDFT calculations of ECD spectra. Chirality 2016, 28, 466–474. [Google Scholar] [CrossRef]
- Autschbach, J. Computing chiroptical properties with first-principles theoretical methods: Background and illustrative examples. Chirality 2009, 21, E116–E152. [Google Scholar] [CrossRef] [PubMed]
- Crawford, T.D.; Tam, M.C.; Abrams, M.L. The current state of ab initio calculations of optical rotation and electronic circular dichroism spectra. J. Phys. Chem. A 2007, 111, 12057–12068. [Google Scholar] [CrossRef]
- Crawford, T.D. Ab initio calculation of molecular chiroptical properties. Theor. Chem. Acc. 2005, 115, 227–245. [Google Scholar] [CrossRef]
- Kwit, M.; Rozwadowska, M.D.; Gawroński, J.; Grajewska, A. Density functional theory calculations of the optical rotation and electronic circular dichroism: The absolute configuration of the highly flexible trans-isocytoxazone revised. J. Org. Chem. 2009, 74, 8051–8063. [Google Scholar] [CrossRef]
- Scigress. Fujitsu, Ltd. Available online: https://www.fujitsu.com/global/solutions/business-technology/tc/sol/scigress/ (accessed on 11 September 2020).
- Gaussian 09. Available online: https://gaussian.com/glossary/g09/ (accessed on 11 September 2020).
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef] [PubMed]
- Tomasi, J.; Mennucci, B.; Cammi, R. Quantum mechanical continuum solvation models. Chem. Rev. 2005, 105, 2999–3093. [Google Scholar] [CrossRef] [PubMed]
- Yanai, T.; Tew, D.; Handy, N. A new hybrid exchange-correlation functional using the coulomb-attenuating method (CAM-B3LYP). Chem. Phys. Lett. 2004, 393, 51–57. [Google Scholar] [CrossRef]
- Zhao, Y.; Truhlar, D.G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc. 2008, 120, 215–241. [Google Scholar] [CrossRef]
- Chai, J.D.; Head-Gordon, M. Long-range corrected hybrid density functionals with damped atom-atom dispersion corrections. Phys. Chem. Chem. Phys. 2008, 10, 6615–6620. [Google Scholar] [CrossRef]
- Laniado-Laborín, R.; Cabrales-Vargas, M.N. Amphotericin B: Side effects and toxicity. Rev. Iberoam. Micol. 2009, 26, 223–227. [Google Scholar] [CrossRef]
- Kester, M.; Karpa, K.D.; Vrana, K.E. Treatment of infectious diseases. In Elsevier’s Integrated Review. Pharmacology, 2nd ed.; Kester, M., Karpa, K.D., Vrana, K.E., Eds.; Elsevier Saunders WB: Philadelphia, PE USA, 2012; pp. 41–78. [Google Scholar]
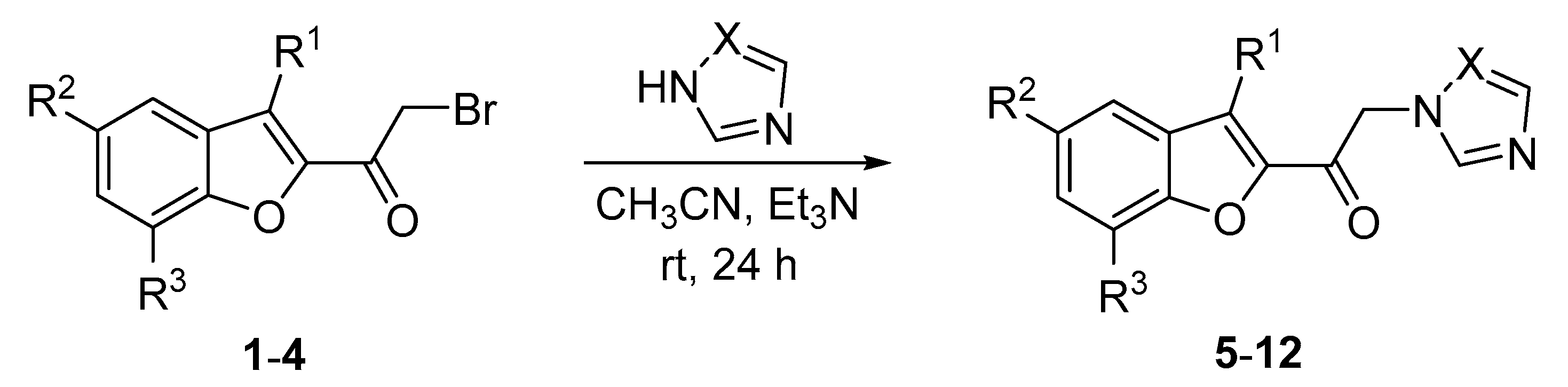
| No. | R1 | R2 | R3 | No. | R1 | R2 | R3 | X | Yield (%) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | H | H | H | 5 | H | H | H | CH | 53 |
| 2 | H | H | Et | 6 | H | H | Et | CH | 55 |
| 3 | Me | H | H | 7 | Me | H | H | CH | 49 |
| 4 | Me | Me | H | 8 | Me | Me | H | CH | 58 |
| 9 | H | H | H | N | 42 | ||||
| 10 | H | H | Et | N | 47 | ||||
| 11 | Me | H | H | N | 53 | ||||
| 12 | Me | Me | H | N | 83 |
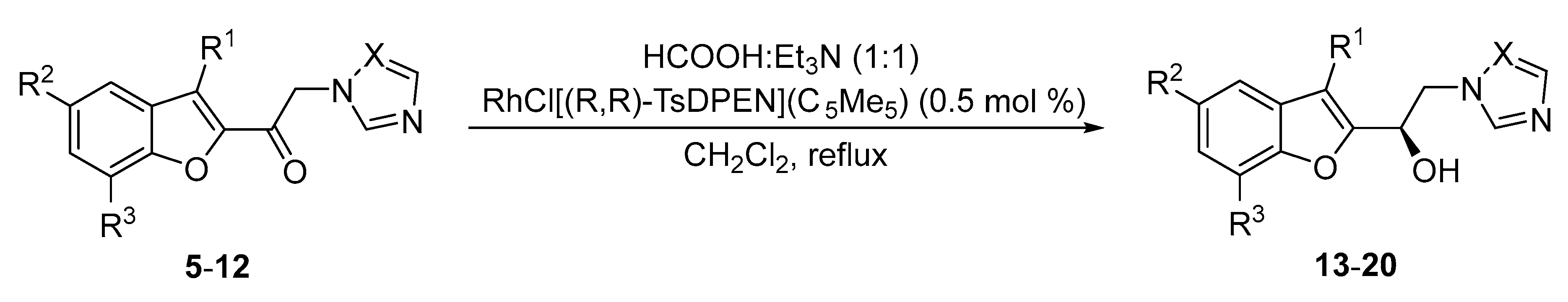
| No. | R1 | R2 | R3 | X | Time (h) | No. | Yield (%) | ee (%) |
|---|---|---|---|---|---|---|---|---|
| 5 | H | H | H | CH | 24 | 13 | 79 | 99 |
| 6 | H | H | Et | CH | 24 | 14 | 75 | 96 |
| 7 | Me | H | H | CH | 24 | 15 | 66 | 98 |
| 8 | Me | Me | H | CH | 24 | 16 | 76 | 98 |
| 9 | H | H | H | N | 48 | 17 | 71 | 96 |
| 10 | H | H | Et | N | 48 | 18 | 77 | 98 |
| 11 | Me | H | H | N | 48 | 19 | 57 | 97 |
| 12 | Me | Me | H | N | 48 | 20 | 48 | 97 |
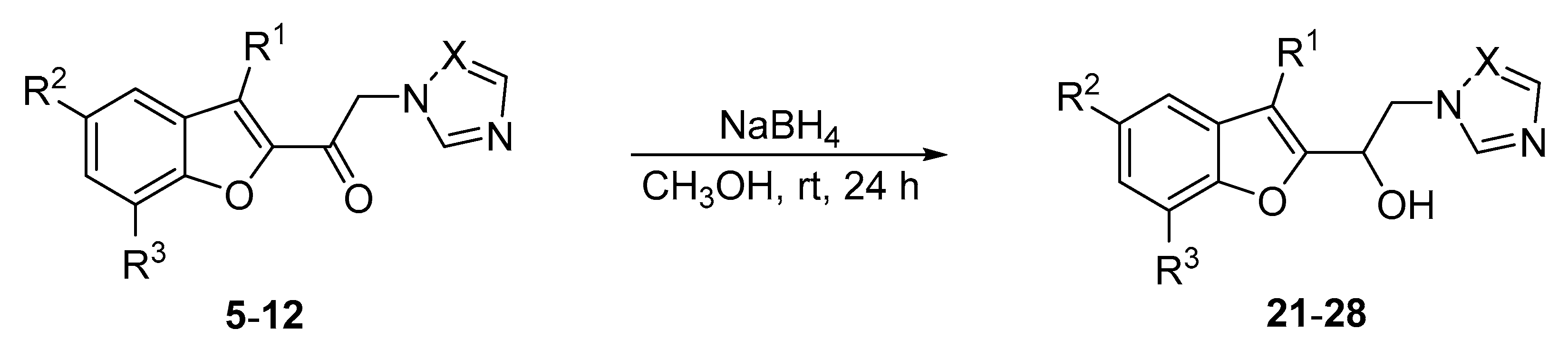
| No. | R1 | R2 | R3 | X | No. | Yield (%) |
|---|---|---|---|---|---|---|
| 5 | H | H | H | CH | 21 | 95 |
| 6 | H | H | Et | CH | 22 | 93 |
| 7 | Me | H | H | CH | 23 | 85 |
| 8 | Me | Me | H | CH | 24 | 88 |
| 9 | H | H | H | N | 25 | 88 |
| 10 | H | H | Et | N | 26 | 91 |
| 11 | Me | H | H | N | 27 | 87 |
| 12 | Me | Me | H | N | 28 | 64 |
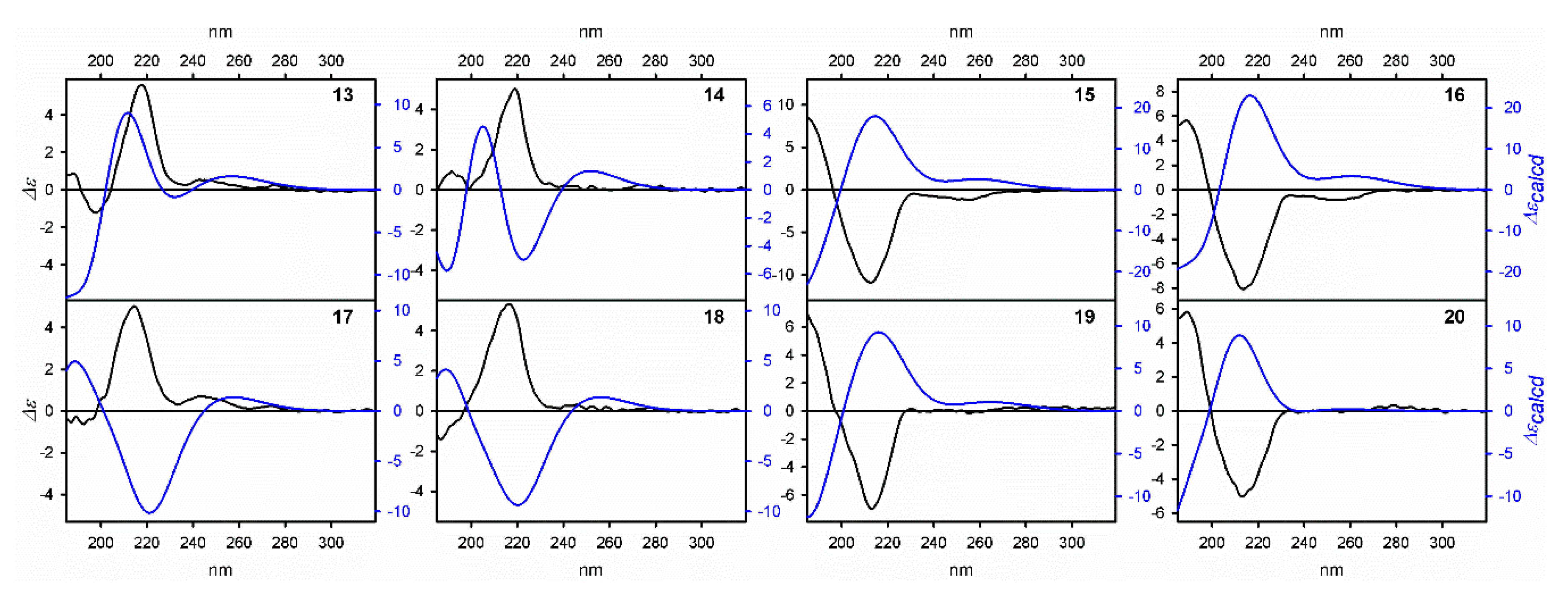
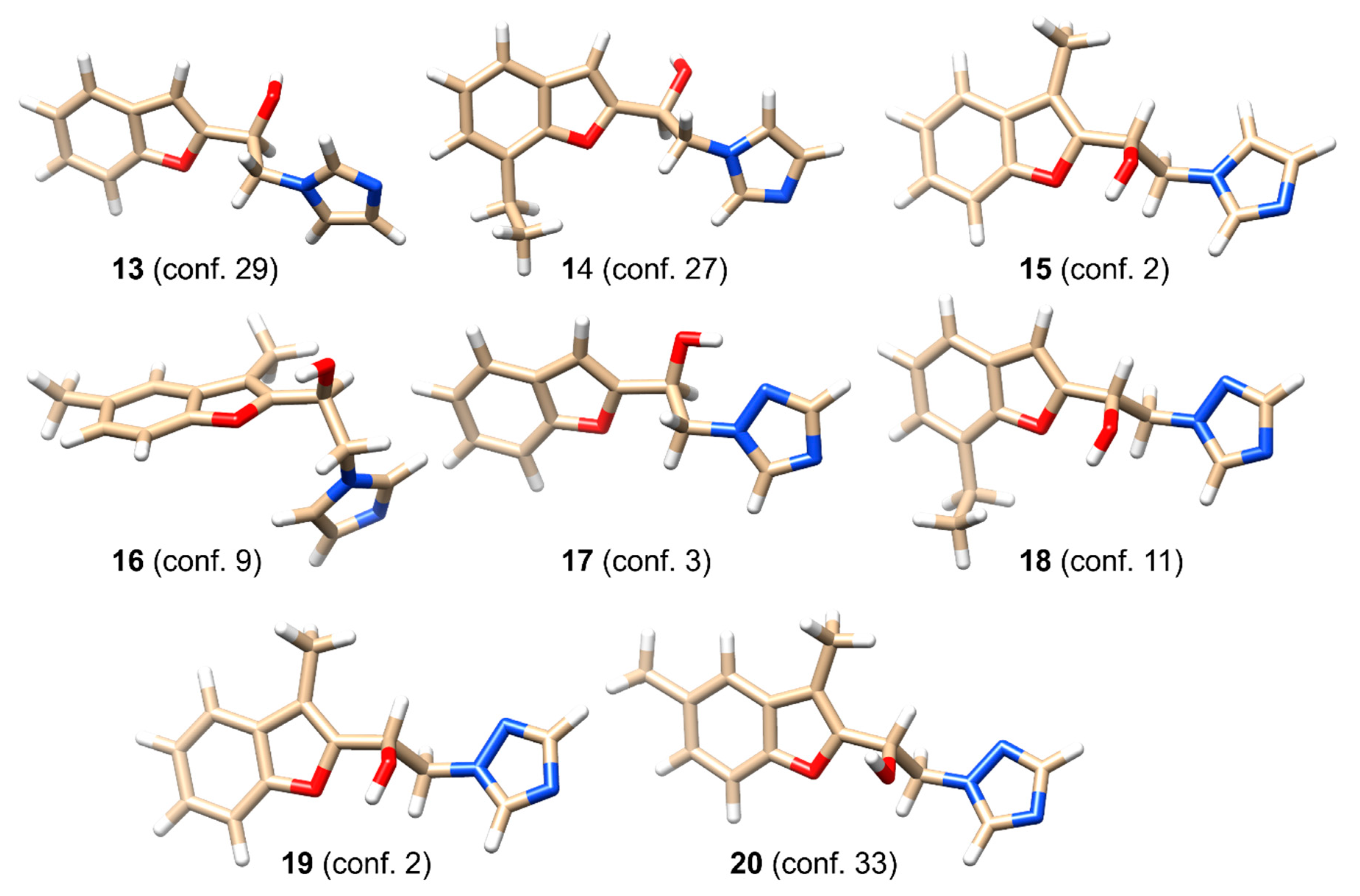
| No. | Conditions | Inhibitor 1.25 × 10−5 mol | Yield (%) | ee (%)/Conf. |
|---|---|---|---|---|
| 14 | Aureobasidium Pullulans Phosphate Buffer pH 7, 30 °C, 1 h | - | 76 | 59 (S) |
| Aureobasidium Pullulans Phosphate Buffer pH 7, 30 °C, 2 h | AMA-1 | 78 | 73 (S) | |
| Aureobasidium Pullulans Phosphate Buffer pH 7, 24 °C, 1 h | allyl alcohol | 79 | 82 (S) | |
| Aureobasidium Pullulans Phosphate Buffer pH 7, 24 °C, 1 h | cysteine | 79 | 99 (S) | |
| Saccharomyces Cerevisiae Phosphate Buffer pH 7, 30 °C, 24 h | - | 89 | 86 (R) | |
| 17 | Aureobasidium Pullulans Phosphate Buffer pH 7, 24 °C, 2 h | - | 88 | 10 (S) |
| Aureobasidium Pullulans Phosphate Buffer pH 7, 24 °C, 2 h | AMA-1 | 80 | 16 (S) | |
| Aureobasidium Pullulans Phosphate Buffer pH 7, 24 °C, 2 h | allyl alcohol | 80 | 28 (S) | |
| Aureobasidium Pullulans Phosphate Buffer pH 7, 24 °C, 2 h | cysteine | 80 | 50 (S) | |
| Saccharomyces Cerevisiae Phosphate Buffer pH 7, 30 °C, 24 h | - | 90 | 89 (R) | |
| 20 | Aureobasidium Pullulans Phosphate Buffer pH 7, 24 °C, 98 h | - | 65 | 51 (R) |
| Aureobasidium Pullulans Phosphate Buffer pH 7, 30 °C, 2 h | AMA-1 | 14 | 38 (R) | |
| Aureobasidium Pullulans Phosphate Buffer pH 7, 30 °C, 2 h | ethyl chloroacetate | 13 | 38 (R) | |
| Aureobasidium Pullulans Phosphate Buffer pH 7, 27 °C, 24 h | cysteine | 33 | 32 (R) | |
| Saccharomyces Cerevisiae Phosphate Buffer pH 7, 30 °C, 24 h | - | 95 | 99 (R) |
| No. | Optical Rotations (λ) a | ||||
|---|---|---|---|---|---|
| 589 nm | 578 nm | 546 nm | 436 nm | ||
| 13 | exp. | +79 | +84 | +95 | +159 |
| calcd. | +18 | +20 | +23 | +54 | |
| 14 | exp. | +55 | +71 | +72 | n.a. |
| calcd. | −21 | −22 | −24 | −35 | |
| 15 | exp. | −19 | −18 | −26 | −64 |
| calcd. | +108 | +114 | +134 | +268 | |
| 16 | exp. | −20 | −22 | −26 | −65 |
| calcd. | +76 | +79 | +93 | +185 | |
| 17 | exp. | +68 | +73 | +88 | +148 |
| calcd. | −91 | −95 | −109 | −197 | |
| 18 | exp. | +74 | +78 | +94 | +153 |
| calcd. | −41 | −43 | −49 | −84 | |
| 19 | exp. | +0.2 | +4 | −2 | −12 |
| calcd. | +28 | +29 | +35 | +80 | |
| 20 | exp. | −3 | +1 | +0.5 | −14 |
| calcd. | +9 | +10 | +22 | +33 | |
| No. | Escherichia Coli ATCC 25922 | Escherichia Coli ATCC 8739 | Staphylococcus Aureus ATCC 25923 | Staphylococcus Aureus ATCC 6538 | Candida Albicans ATCC 10231 | Malassezia Furfur DSM 6170 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | |
| 13 | 512 | 512 | 512 | >512 | >512 | >512 | >512 | >512 | 512 | >512 | 256 | 256 |
| 14 | >512 | >512 | >512 | >512 | >512 | >512 | >512 | >512 | >512 | >512 | >512 | >512 |
| 15 | 512 | 512 | 512 | >512 | 512 | >512 | >512 | >512 | 512 | 512 | 256 | 256 |
| 16 | 512 | 512 | 512 | 512 | 64 | 96 | >512 | >512 | 512 | 512 | 256 | 256 |
| 17 | 512 | >512 | 512 | >512 | >512 | >512 | 512 | >512 | 512 | >512 | 256 | 256 |
| 18 | 512 | >512 | 512 | >512 | >512 | >512 | >512 | >512 | 512 | >512 | 256 | 256 |
| 19 | 512 | >512 | 512 | >512 | >512 | >512 | >512 | >512 | 512 | 512 | 256 | 256 |
| 20 | 512 | >512 | 512 | >512 | >512 | >512 | >512 | >512 | 512 | 512 | 64 | 64 |
| 21 | 512 | >512 | 512 | 512 | >512 | >512 | >512 | >512 | 512 | >512 | 256 | 256 |
| 22 | 512 | 512 | 512 | >512 | 512 | 512 | 512 | 512 | 512 | 512 | 256 | 256 |
| 23a | - | - | - | - | - | - | - | - | - | - | - | - |
| 24 | 512 | 512 | 512 | >512 | 512 | >512 | >512 | >512 | 512 | 512 | 256 | 256 |
| 25 | 512 | >512 | 512 | >512 | >512 | >512 | >512 | >512 | 512 | 512 | 256 | 256 |
| 26 | 512 | >512 | 512 | >512 | >512 | >512 | >512 | >512 | 512 | >512 | 128 | 128 |
| 27 | 512 | >512 | 512 | >512 | >512 | >512 | >512 | >512 | 512 | 512 | 256 | 256 |
| 28 | 512 | >512 | 512 | >512 | >512 | >512 | >512 | >512 | 512 | 512 | 256 | 256 |
| No. | Escherichia Coli ATCC 25922 | Escherichia Coli ATCC 8739 | Staphylococcus Aureus ATCC 25923 | Staphylococcus Aureus ATCC 6538 | Candida Albicans ATCC 10231 | Malassezia Furfur DSM 6170 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | |
| 1 | 64 | 64 | 64 | 64 | 32 | 256 | 64 | 64 | 512 | 512 | 1.5 | 1.5 |
| 2 | 128 | 256 | 256 | 256 | 256 | 256 | 48 | 48 | 256 | 256 | 1.5 | 1.5 |
| 3 | 256 | 256 | 512 | 512 | 48 | 64 | 64 | 96 | >512 | >512 | 1.5 | 1.5 |
| 4 | >512 | >512 | >512 | >512 | 16 | 16 | 16 | 64 | >512 | >512 | 1.5 | 1.5 |
| 5 | 512 | >512 | >512 | >512 | >512 | >512 | >512 | >512 | 512 | >512 | 512 | >512 |
| 6 | >512 | >512 | >512 | >512 | 512 | >512 | 512 | 512 | 512 | 512 | 512 | 512 |
| 7 | >512 | >512 | >512 | >512 | >512 | >512 | >512 | >512 | 512 | >512 | 512 | 512 |
| 8 | >512 | >512 | >512 | >512 | >512 | >512 | >512 | >512 | >512 | >512 | 128 | 256 |
| 9 | >512 | >512 | >512 | >512 | >512 | >512 | >512 | >512 | >512 | >512 | 512 | >512 |
| 10 | >512 | >512 | >512 | >512 | >512 | >512 | >512 | >512 | >512 | >512 | 512 | >512 |
| 11 | >512 | >512 | >512 | >512 | >512 | >512 | >512 | >512 | >512 | >512 | >512 | >512 |
| 12 | 512 | 512 | 256 | 512 | 256 | 512 | 128 | 256 | 512 | 512 | 256 | 256 |
| Tested Microorganism | AMP | K | TE | |||
| MIC | MBC | MIC | MBC | MIC | MBC | |
| Escherichia Coli ATCC 8739 | >512 | >512 | 512 | >512 | 64 | 256 |
| Escherichia Coli ATCC 25922 | 4 | 4 | 32 | 32 | 1 | 256 |
| Staphylococcus Aureus ATCC 6538 | 1 | 64 | 8 | 16 | 4 | 256 |
| Staphylococcus Aureus ATCC 25923 | 0.5 | 32 | 64 | 128 | 0.5 | 512 |
| AMB | FLU | KCA | ||||
| MIC | MBC | MIC | MBC | MIC | MBC | |
| Candida Albicans ATCC 10231 | 0.25 | 0.5 | >512 | >512 | >512 | >512 |
| Malassezia Furfur DSM 6170 | >512 | >512 | 4 | 96 | >512 | >512 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tafelska-Kaczmarek, A.; Kołodziejska, R.; Kwit, M.; Stasiak, B.; Wypij, M.; Golińska, P. Synthesis, Absolute Configuration, Antibacterial, and Antifungal Activities of Novel Benzofuryl β-Amino Alcohols. Materials 2020, 13, 4080. https://doi.org/10.3390/ma13184080
Tafelska-Kaczmarek A, Kołodziejska R, Kwit M, Stasiak B, Wypij M, Golińska P. Synthesis, Absolute Configuration, Antibacterial, and Antifungal Activities of Novel Benzofuryl β-Amino Alcohols. Materials. 2020; 13(18):4080. https://doi.org/10.3390/ma13184080
Chicago/Turabian StyleTafelska-Kaczmarek, Agnieszka, Renata Kołodziejska, Marcin Kwit, Bartosz Stasiak, Magdalena Wypij, and Patrycja Golińska. 2020. "Synthesis, Absolute Configuration, Antibacterial, and Antifungal Activities of Novel Benzofuryl β-Amino Alcohols" Materials 13, no. 18: 4080. https://doi.org/10.3390/ma13184080
APA StyleTafelska-Kaczmarek, A., Kołodziejska, R., Kwit, M., Stasiak, B., Wypij, M., & Golińska, P. (2020). Synthesis, Absolute Configuration, Antibacterial, and Antifungal Activities of Novel Benzofuryl β-Amino Alcohols. Materials, 13(18), 4080. https://doi.org/10.3390/ma13184080





