Impact of Calcium Oxide on Hygienization and Self-Heating Prevention of Biologically Contaminated Polymer Materials
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
- UFMSW: 35, 50 and 60% (control sample: 35%);
- RDF: 20 and 30% (control sample: 20%).
2.2. Sampling and Experiment
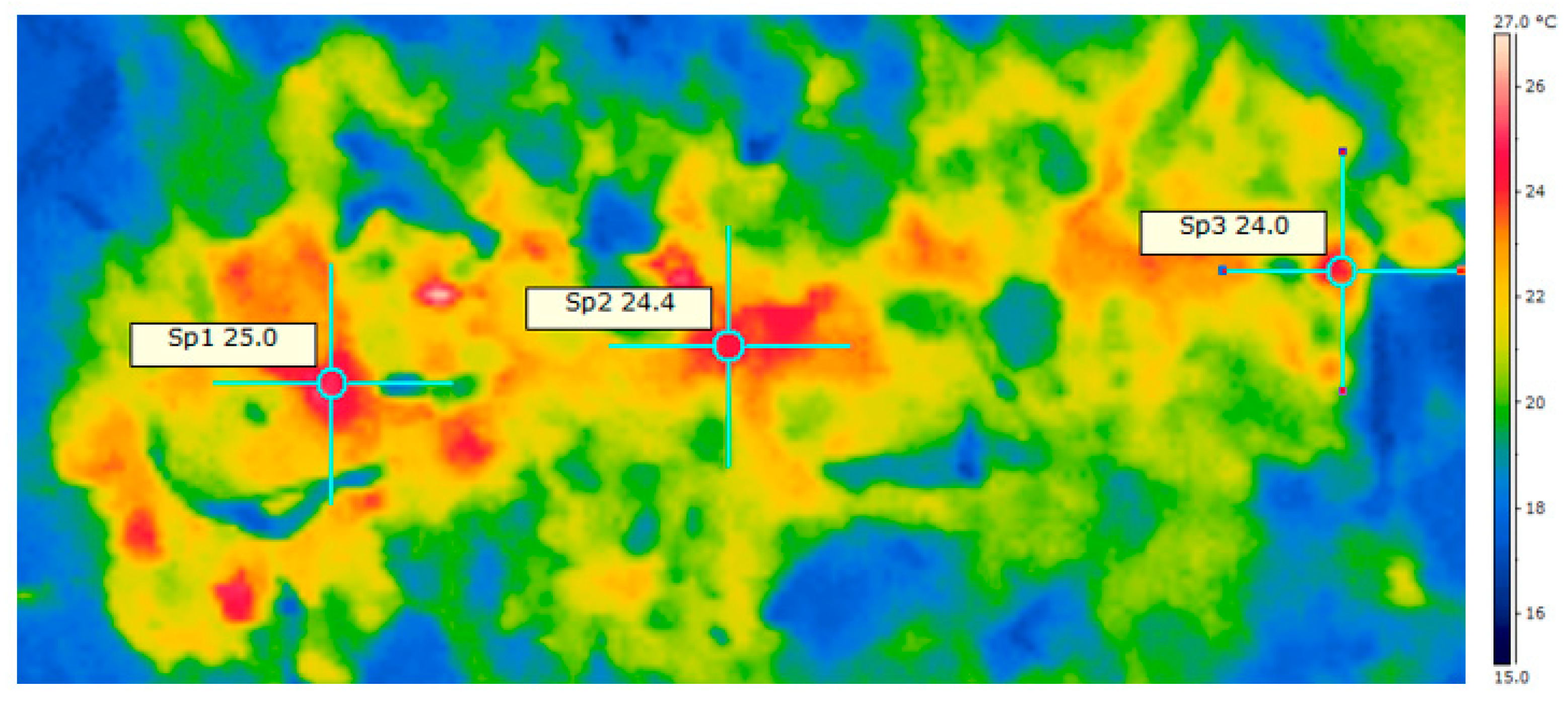
2.3. Thermographic Analysis
2.4. Microbiological, Physical, and Chemical Analyses
3. Results
3.1. Characteristics of RDF and UFMSW
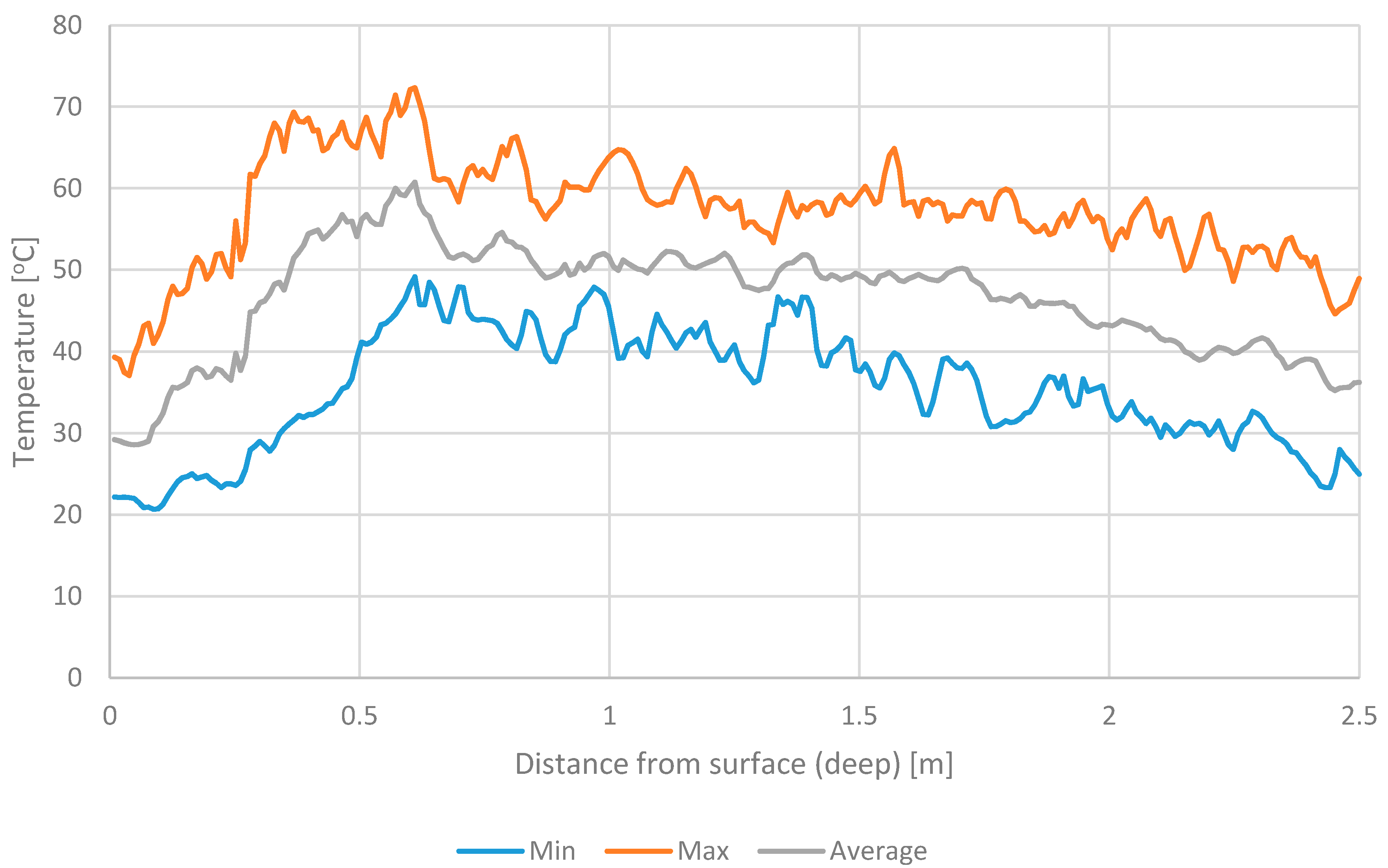
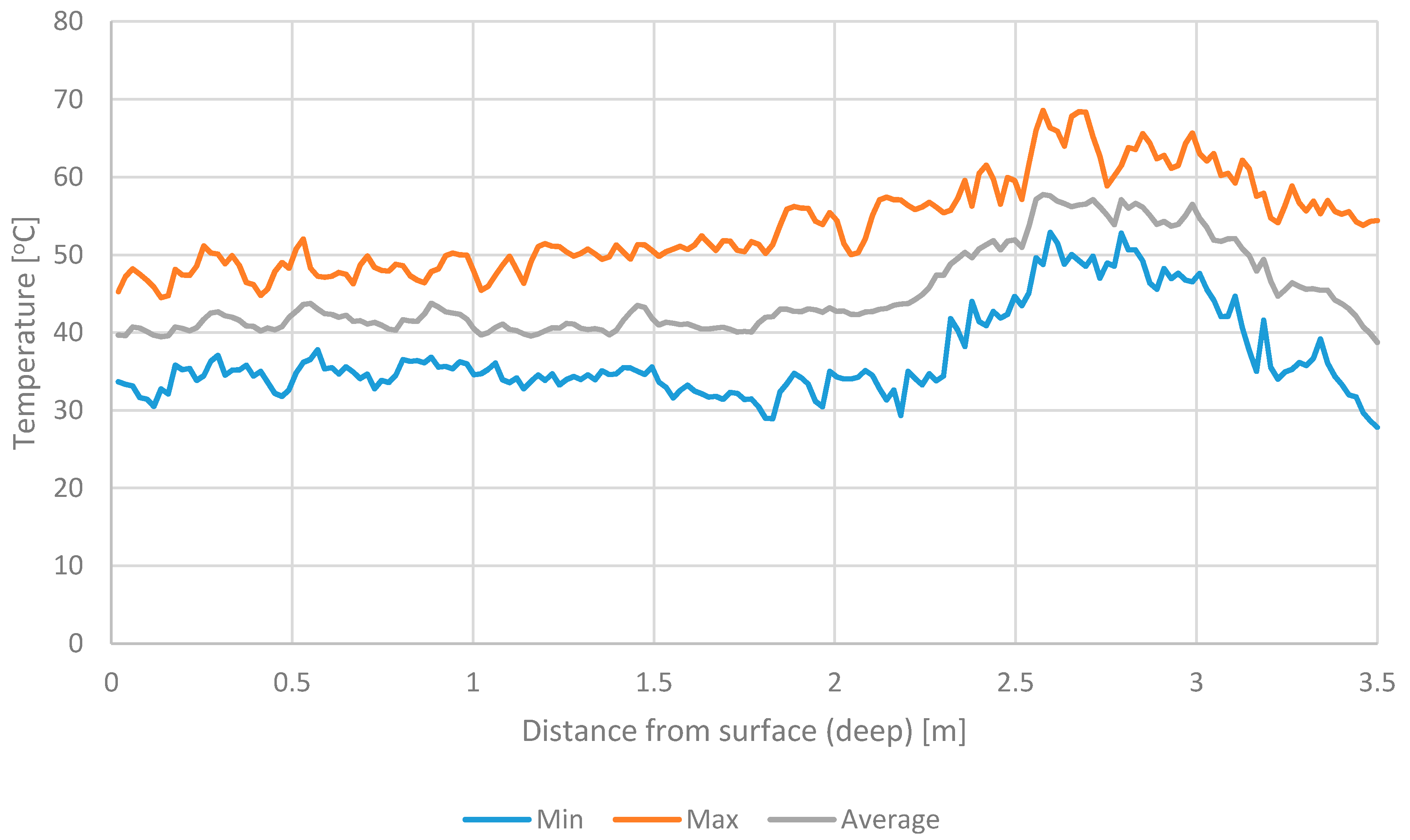
3.2. The Temperature of Materials Stored in the Windrows
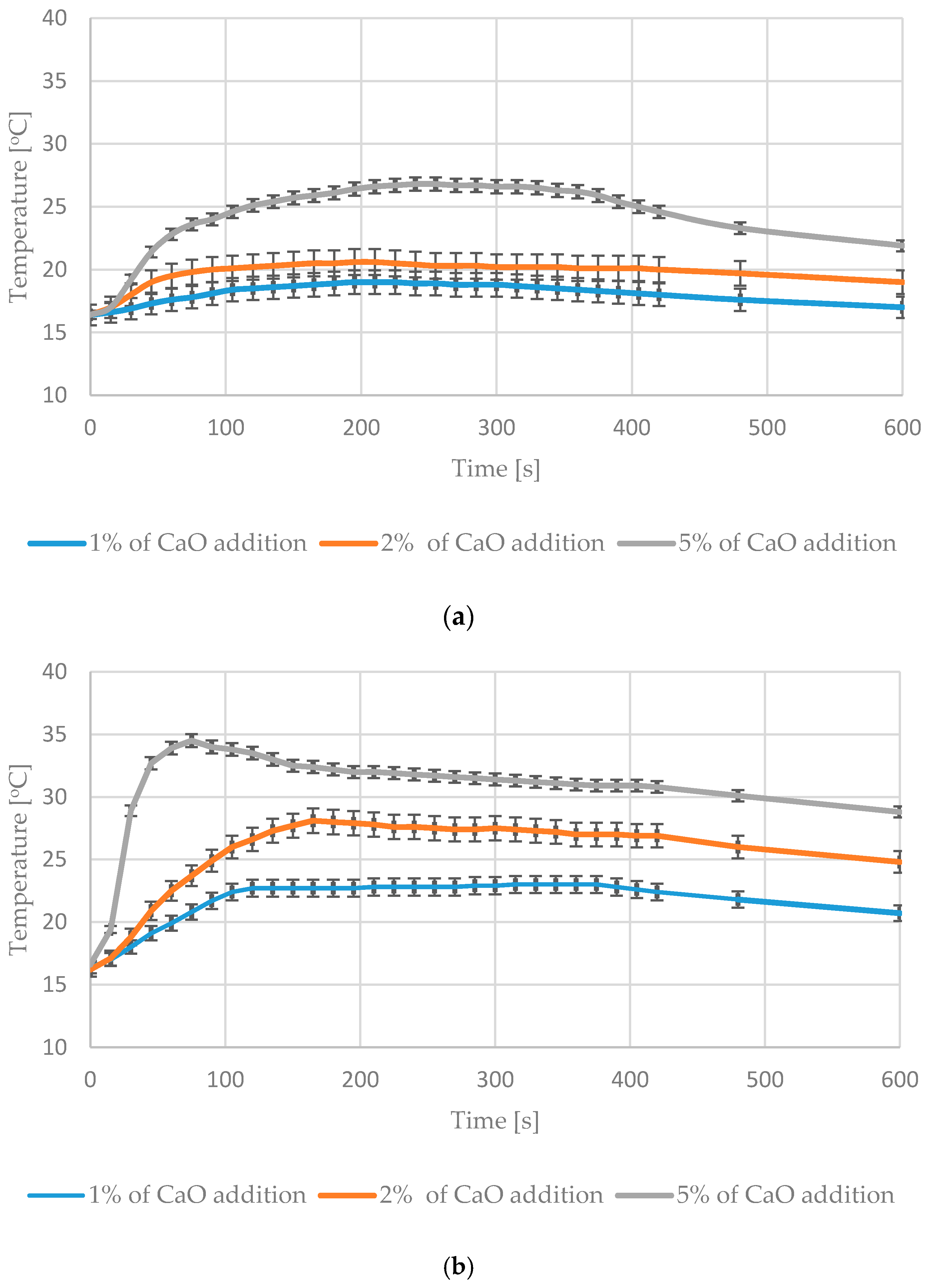
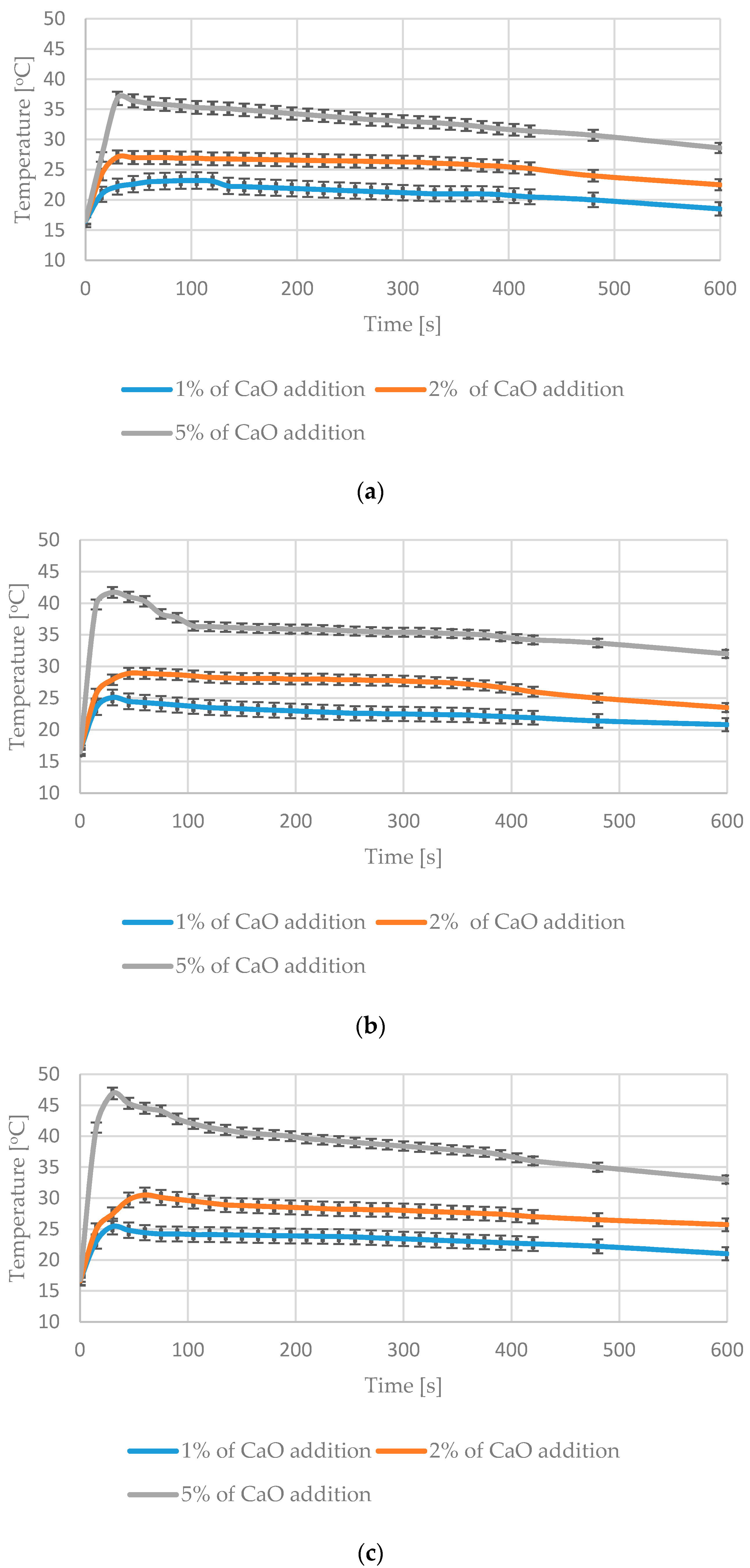
3.3. Impact of CaO Addition on the Temperature of the Analyzed Materials during the Mixing
3.4. Impact of CaO Addition on Moisture Content, pH, and Respiration Activity (AT4)
3.5. Influence of CaO Addition on the Number of Microorganisms
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sato, Y.; Ohata, H.; Inoue, A.; Ishihara, M.; Nakamura, S.; Fukuda, K.; Takayama, T.; Murakami, K.; Hiruma, S.; Yokoe, H. Application of Colloidal Dispersions of Bioshell Calcium Oxide (BiSCaO) for Disinfection. Polymers 2019, 11, 1991. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.F.; Santos, C.P.; Matos, A.M.; Cardoso, O.; Quina, M.J. Effect of Thermal Drying and Chemical Treatments with Wastes on Microbiological Contamination Indicators in Sewage Sludge. Microorganisms 2020, 8, 376. [Google Scholar] [CrossRef] [PubMed]
- Bień, D.J.; Bień, B. Utilisation of municipal sewage sludge by thermal methods in the face of storage disallowing. Inż. Ekolog. 2015, 45, 36–43. [Google Scholar] [CrossRef]
- Sato, Y.; Ishihara, M.; Nakamura, S.; Fukuda, K.; Takayama, T.; Hiruma, S.; Murakami, K.; Fujita, M.; Yokoe, H. Preparation and Application of Bioshell Calcium Oxide (BiSCaO) Nanoparticle-Dispersions with Bactericidal Activity. Molecules 2019, 24, 3415. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Selvam, A.; Lau, S.S.S.; Wong, J.W.C. Influence of lime and struvite on microbial community succession and odour emission during food waste composting. Bioresour. Technol. 2018, 247, 652–659. [Google Scholar] [CrossRef] [PubMed]
- Tatemoto, Y.; Bando, Y.; Yasuda, K.; Nakamura, M.; Azegami, M. Effect of CaO Addition on Rotted Material. J. Chem. Eng. Jpn. 1999, 32, 549–552. [Google Scholar] [CrossRef]
- Corella, J.; Toledo, J.M.; Molina, G. Performance of CaO and MgO for the hot gas clean up in gasification of chlorine-containing (RDF) feedstock. Bioresour. Technol. 2008, 99, 7539–7544. [Google Scholar] [CrossRef]
- Malinowski, M.; Wolny-Koładka, K. Investigation of the self-heating process of an alternative fuel derived from municipal solid waste. Proc. Ecopole 2015, 9, 256–261. [Google Scholar]
- Malinowski, M.; Wolny-Koładka, K. Microbiological and energetic assessment of the effects of the biodrying of fuel produced from waste. Ecol. Chem. Eng. 2017, 24, 551–564. [Google Scholar] [CrossRef][Green Version]
- Malinowski, M.; Sikora, J. Impact of biodegradable waste contents on technological properties of an alternative fuel. Proc. Ecopole 2014, 8, 223–230. [Google Scholar]
- Vasconcelos, C.; Silva, B. Insight on the Self-Ignition Behaviour of RDF Components; Energy and Environmental Engineering Research Group (ENVERG) of IBB/CERENA: Lisboa, Portugal, 2014; pp. 1–10. [Google Scholar]
- Hogland, W.; Marques, M. Physical, biological and chemical processes during storage and spontaneous combustion of waste fuel. Resour. Conserv. Recycl. 2003, 40, 53–69. [Google Scholar] [CrossRef]
- Yasuhara, A. Chemical consideration on spontaneous incineration accidents of refuse-derived fuels and exothermic reaction mechanism. J. Jpn. Soc. Saf. Eng. 2006, 45, 117–124. [Google Scholar]
- Yasuhara, A.; Amano, Y.; Shibamoto, T. Investigation of the self-heating and spontaneous ignition of refuse-derived fuel (RDF) during storage. Waste Manag. 2010, 30, 1161–1164. [Google Scholar] [CrossRef] [PubMed]
- Białowiec, A.; Micuda, M.; Szumny, A.; Łyczko, J.; Koziel, J.A. the Proof-of-the-Concept of Application of Pelletization for Mintigation of Volatile Organic Compounds Emissions from Carbonized Refuse-Derived Fuel. Materials 2019, 12, 1692. [Google Scholar] [CrossRef]
- Vaverková, M.D. Landfill impacts on the environment—Review. Geosciences 2019, 9, 431. [Google Scholar] [CrossRef]
- Szafranko, E. Selected Problems of the Environmental Impact Analysis of Investment Projects Based on Life Cycle Assessment Procedure. J. Ecol. Eng. 2019, 20, 87–94. [Google Scholar] [CrossRef]
- Wolny-Koładka, K.; Żukowski, W. Mixed municipal solid waste hygienisation for refuse-derived fuel production by ozonation in the novel configuration using fluidized bed and horizontal reactor. Waste Biomass Valor. 2019, 10, 575–583. [Google Scholar] [CrossRef]
- Dehghani, H.M. Effectiveness of ultrasound on the destruction of Escherichia coli. Am. J. Environ. Sci. 2005, 1, 187–189. [Google Scholar] [CrossRef][Green Version]
- Grzesik, K.; Malinowski, M. Life cycle Assessment of the mechanical—Biological treatment of mixed municipal waste in Miki Recycling, Krakow, Poland. Environ. Eng. Sci. 2017, 34, 207–220. [Google Scholar] [CrossRef]
- Gajewska, T.; Malinowski, M.; Szkoda, M. The Use of Biodrying to Prevent Self-Heating of Alternative Fuel. Materials 2019, 12, 3039. [Google Scholar] [CrossRef]
- Debicka, M.; Zygadło, M.; Latosinska, J. Investigations of bio-drying process of municipal solid waste. Ecol. Chem. Eng. A 2013, 20, 1461–1470. [Google Scholar]
- Wrobel, M.; Mudryk, K.; Jewiarz, M.; Knapczyk, A. Impact of raw material properties and agglomeration pressure on selected parmeters of granulates obtained from willow and black locust biomass. Eng. Rural Dev. 2018, 17, 1933–1938. [Google Scholar]
- Białowiec, A.; Micuda, M.; Koziel, J.A. Waste to Carbon: Densification of Torrefied Refuse-Derived Fuel. Energies 2018, 11, 3233. [Google Scholar] [CrossRef]
- Jewiarz, M.; Mudryk, K.; Wróbel, M.; Frączek, J.; Dziedzic, K. Parameters affecting RDF-based pellet quality. Energies 2020, 13, 910. [Google Scholar] [CrossRef]
- Knapczyk, A.; Francik, S.; Fraczek, J.; Slipek, Z. Analysis of research trends in production of solid biofuels. Eng. Rural Dev. 2019, 18, 1503–1509. [Google Scholar]
- Jewiarz, M.; Frączek, J.; Mudryk, K.; Wróbel, M.; Dziedzic, K. Analysis of MSW Potential in Terms of Processing into Granulated Fuels for Power Generation. In Renewable Energy Sources: Engineering, Technology, Innovation; Springer: Cham, Switzerland, 2018; pp. 661–670. [Google Scholar]
- Rahman, A.; Rasul, M.G.; Khan, M.M.K.; Sharma, S.C. Assessment of energy performance and emission control using alternative fuels in cement industry through a process model. Energies 2017, 10, 1996. [Google Scholar] [CrossRef]
- Hemidat, S.; Saidan, M.; Al-Zu’bi, S.; Irshidat, M.; Nassour, A.; Nelles, M. Potential utilization of RDF as an alternative fuel to be used in cement industry in Jordan. Sustainability 2019, 11, 5819. [Google Scholar] [CrossRef]
- Białowiec, A.; Pulka, J.; Stępień, P.; Manczarski, P.; Gołaszewski, J.; Adefeso, I.B.; Ikhu-Omoregbe, D.I.O.; Rabiu, A.M. Sustainable co-generation plant: Refuse-derived fuel gasification integrated with high temperature PEM fuel cell system. Waste Manag. 2013, 70, 91–100. [Google Scholar] [CrossRef]
- Grzesik, K.; Malinowski, M. Life cycle assessment of refuse-derived fuel production from mixed municipal waste. Energy Sources Part A Recovery Util. Environ. Eff. 2016, 38, 3150–3157. [Google Scholar] [CrossRef]
- Dziedzic, K.; Łapczyńska-Kordon, B.; Malinowski, M.; Niemiec, M.; Sikora, J. 2015. Impact of aerobic biostabilization and biodrying process of municipal solid waste on minimization of waste deposited in landfills. Chem. Proc. Eng. 2015, 36, 381–394. [Google Scholar] [CrossRef][Green Version]
- Famielec, S.; Gliniak, M.; Kapjor, A.; Łukasiewicz, M.; Malinowski, M. Thermographic evaluation of CaO additive on the process of waste hygienisation. Infrastruct. Ecol. Rural Areas. 2016, IV/4, 1857–1865. [Google Scholar]
- Deacon, L.; Pankhurst, L.; Liu, J.; Drew, G.H.; Hayes, E.T.; Jackson, S.; Longhurst, J.; Longhurst, P.; Pollard, S.; Tyrrel, S. Endotoxin emissions from commercial composting activities. Environ. Health. 2009, 8, S9. [Google Scholar] [CrossRef] [PubMed]
- European Committee for Standardization. Characterization of Waste—Sampling of Waste Materials—Framework for the Preparation and Application of a Sampling Plan; DD CEN/TS 15310-1:2006; European Committee for Standardization: Brussels, Belgium, 2010. [Google Scholar]
- European Committee for Standardization. Solid Recovered Fuels. Determination of Moisture Content Using the Oven Dry Method. Determination of Total Moisture by a Reference Method; DD CEN/TS 15414-1:2006; European Committee for Standardization: Brussels, Belgium, 2010. [Google Scholar]
- Informational Materials from WTW–Measurement and Analytical Technical Equipment (in Polish). Available online: http://wtw.pl/oferta-plik/102 (accessed on 15 May 2020).
- Kopeć, M.; Baran, A.; Mierzwa-Hersztek, M.; Gondek, K.; Chmiel, J.M. Effect of the addition of biochar and coffee grounds on the biological properties and ecotoxicity of composts. Waste Biomass Valor. 2018, 9, 1389–1398. [Google Scholar] [CrossRef]
- Mokrzycki, E.; Uliasz-Bocheńczyk, A.; Sarna, M. Use of alternative fuels in the Polish cement industry. Appl. Energy 2003, 74, 101–111. [Google Scholar] [CrossRef]
- Wróbel, M.; Frączek, J.; Mudryk, K.; Jewiarz, M.; Dziedzic, K. Conceptual Design of the RDF Granulation Line. In Renewable Energy Sources: Engineering, Technology, Innovation; Springer: Cham, Switzerland, 2018; pp. 813–821. [Google Scholar]
- Malinowski, M.; Wolny-Koładka, K.; Vaverkova, M. Effect of biochar addition on the OFMSW composting process under real conditions. Waste Manag. 2019, 4, 364–372. [Google Scholar] [CrossRef]
- Gliniak, M.; Grabowski, Ł.; Wołosiewicz-Głąb, M.; Polek, D. Influence of ozone aeration on toxic metal content and oxygen activity in green waste compost. J. Ecol. Eng. 2017, 18, 90–94. [Google Scholar] [CrossRef]
- Voberková, S.; Vaverková, M.D.; Burešová, A.; Adamcová, D.; Vršanská, M.; Kynický, J.; Brtnický, M.; Adam, V. Effect of inoculation with white-rot fungi and fungal consortium on the composting efficiency of municipal solid waste. Waste Manag. 2017, 61, 157–164. [Google Scholar] [CrossRef]
- Adani, F.; Baido, D.; Calcatera, E.; Genevini, P. The influence of biomass temperature on biostabilization-biodrying of municipal solid waste. Bioresour. Technol. 2002, 83, 173–179. [Google Scholar] [CrossRef]
- Adani, F.; Tambone, F.; Gotti, A. Biostabilization of municipal solid waste. Waste Manag. 2004, 24, 775–783. [Google Scholar] [CrossRef]
- Wong, J.W.C.; Fang, M. Effects of lime addition on sewage sludge composting process. Water Res. 2000, 34, 3691–3698. [Google Scholar] [CrossRef]
| Microorganisms Groups | Nutrient | Temperature of Incubation [°C] | Time of Incubation [h] |
|---|---|---|---|
| Vegetative bacteria | MPA agar, BTL | 37 | 24 |
| Spores bacteria | |||
| Mold fungi | malt extract agar—MEA, BTL | 28 | 120 |
| Actinobacteria | Pochon’s agar, BTL | 28 | 168 |
| Staphylococcus spp. | Chapman’s agar, BTL | 37 | 24 |
| E. coli | TBX agar, BTL | 44 | 24 |
| Salmonella spp. | SS agar, BTL | 37 | 24 |
| Shigella spp. | |||
| E. faecalis | Slanetz Bartley substrate, BTL | 37 | 48 |
| C. perfringens | agar with sulfate and cycloserine SC, BTL | 37 | 24 |
| Parameter | Unit | Variants | |
|---|---|---|---|
| RDF (Control Sample) | UFMSW (Control Sample) | ||
| Moisture content | % w/w | 20.2 ± 1.9 | 35.5 ± 2.0 |
| Bulk density | kg·m−3 | 141 ± 13 | 428 ± 33 |
| pH | - | 8.31 ± 0.12 | 7.54 ± 0.09 |
| AT4 | mgO2·g−1d.m | 11.2 ± 0.4 | 26.3 ± 0.7 |
| Ash content | % d.m. | 14.8 ± 0.6 | 43.5 ± 2.7 |
| Total carbon content | % d.m. | 48.7 ± 2.6 | 29.2 ± 5.2 |
| Sulphur content | % d.m. | 0.24 ± 0.06 | 0.68 ± 0.16 |
| Nitrogen content | % d.m. | 0.78 ± 0.12 | 0.89 ± 0.19 |
| Heat of combustion | MJ·kg−1 d.m. | 22.5 ± 1.3 | 10.9 ± 0.7 |
| Calorific value | MJ·kg−1 | 17.5 ± 0.8 | 6.0 ± 0.7 |
| As | mg·kg−1 d.m. | 10 ± 3 | 15 ± 3 |
| Ba | mg·kg−1 d.m. | 150 ± 11 | 170 ± 29 |
| Cd | mg·kg−1 d.m. | 2.2 ± 0.6 | 3.2 ± 0.3 |
| Cr | mg·kg−1 d.m. | 56.1 ± 16.0 | 22.6 ± 9.1 |
| Cu | mg·kg−1 d.m. | 110.2 ± 12.1 | 371.1 ± 62.6 |
| Zn | mg·kg−1 d.m. | 990 ± 160 | 1438 ± 280 |
| Hg | mg·kg−1 d.m. | 0.2 ± 0.1 | 0.6 ± 0.1 |
| Chlorides | mg·kg−1 d.m. | 1400 ± 56 | 3050 ± 106 |
| Sulphates | mg·kg−1 d.m. | 2860 ± 202 | 23660 ± 890 |
| Groups od Microorganisms | Variants | |
|---|---|---|
| RDF | UFMSW | |
| Vegetative bacteria | 350,000 | 636,418 |
| Spores bacteria | 1567 | 5207 |
| Mold fungi | 1687 | 3933 |
| Staphylococcus spp. | 17,380 | 17,355 |
| E. coli | 601.4 | 2091.6 |
| Salmonella spp. | 65 | 221.9 |
| E. faecalis | 25 | 133.6 |
| C. perfringens | 24.7 | 10.6 |
| Samples/The Initial Moisture Content | Moisture Content [% w/w] | pH | AT4 [mgO2·g−1d.m] |
|---|---|---|---|
| with 1% CaO addition | |||
| RDF/20% | 19.9 ± 0.9 | 10.40 ± 0.12 | 11.5 ± 0.7 |
| RDF/30% | 28.9 ± 2.9 | 12.19 ± 0.11 | 12.5 ± 1.1 |
| UFMSW/35% | 35.1 ± 2.2 | 10.17 ± 0.17 | 26.0 ± 1.2 |
| UFMSW/50% | 48.8 ± 3.1 | 12.22 ± 0.13 | 28.1 ± 0.8 |
| UFMSW/60% | 58.2 ± 2.0 | 12.19 ± 0.15 | 27.6 ± 2.6 |
| with 2% CaO addition | |||
| RDF/20% | 19.9 ± 1.1 | 11.80 ± 0.08 | 11.5 ± 0.9 |
| RDF/30% | 29.2 ± 1.7 | 12.11 ± 0.12 | 11.7 ± 1.7 |
| UFMSW/35% | 34.2 ± 2.6 | 11.24 ± 0.08 | 22.2 ± 2.2 |
| UFMSW/50% | 49.2 ± 2.4 | 12.11 ± 0.09 | 23.1 ± 3.0 |
| UFMSW/60% | 56.6 ± 3.1 | 12.16 ± 0.10 | 23.0 ± 2.3 |
| with 5% CaO addition | |||
| RDF/20% | 19.5 ± 1.2 | 11.86 ± 0.13 | 11.3 ± 0.6 |
| RDF/30% | 28.2 ± 3.1 | 12.35 ± 0.09 | 11.3 ± 0.9 |
| UFMSW/35% | 33.2 ± 2.8 | 12.39 ± 0.10 | 20.1 ± 1.3 |
| UFMSW/50% | 44.1 ± 2.1 | 12.34 ± 0.12 | 16.1 ± 2.0 |
| UFMSW/60% | 52.9 ± 2.2 | 12.48 ± 0.11 | 16.6 ± 2.2 |
| Samples/Initial Moisture Content | Vegetative Bacteria | Spores Bacteria | Mold Fungi | Staphylococcus spp. | E. coli | Salmonella spp. | E. faecalis | C. perfringens |
|---|---|---|---|---|---|---|---|---|
| with 1% CaO addition | ||||||||
| RDF/20% | 121132 c | 614 ab | 1999 a | 8626 b | 0 a | 0 a | 0.4 a | 0.8 a |
| RDF/30% | 300040 c | 738 ab | 1151 a | 11446 b | 1.8 a | 2.8 a | 18.7 a | 0.5 a |
| UFMSW/35% | 140284 c | 509 ab | 1365 a | 10319 b | 0 a | 0 a | 0.6 a | 0.6 a |
| UFMSW/50% | 254459 c | 738 ab | 1521 a | 11487 b | 2.9 a | 3.3 a | 19.9 a | 0 a |
| UFMSW/60% | 206801 c | 574 ab | 11539 a | 9793 a | 4.5 a | 1.6 a | 16.5 a | 0 a |
| with 2% CaO addition | ||||||||
| RDF/20% | 32873 b | 720 ab | 1332 a | 1950 a | 0 a | 0 a | 0 a | 0.5 a |
| RDF/30% | 22564 b | 117 a | 167 a | 1688 a | 5.7 a | 0.4 a | 1.2 a | 2.4 a |
| UFMSW/35% | 52604 b | 747 ab | 2140 a | 1969 a | 0 a | 0 a | 0 a | 0.4 a |
| UFMSW/50% | 25993 b | 543 ab | 150 a | 1512 a | 0 a | 0.4 a | 1.1 a | 5.4 a |
| UFMSW/60% | 31201 b | 108 ab | 170 a | 1497 a | 9.1 a | 0.5 a | 0.8 a | 1.4 a |
| with 5% CaO addition | ||||||||
| RDF/20% | 32873 b | 720 ab | 1332 a | 1950 a | 0 a | 0 a | 0 a | 0.5 a |
| RDF/30% | 22564 b | 117 a | 167 a | 1688 a | 5.7 a | 0.4 a | 1.2 a | 2.4 a |
| UFMSW/35% | 11681 a | 814 ab | 901 a | 2131 a | 0 a | 0.1 a | 0 a | 0 a |
| UFMSW/50% | 4136 a | 4012 b | 1753 a | 2203 a | 0.9 a | 0 a | 0 a | 0 a |
| UFMSW/60% | 1991 a | 123 a | 1041 a | 1114 a | 0 a | 0 a | 0 a | 0 a |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wolny-Koładka, K.; Malinowski, M.; Żukowski, W. Impact of Calcium Oxide on Hygienization and Self-Heating Prevention of Biologically Contaminated Polymer Materials. Materials 2020, 13, 4012. https://doi.org/10.3390/ma13184012
Wolny-Koładka K, Malinowski M, Żukowski W. Impact of Calcium Oxide on Hygienization and Self-Heating Prevention of Biologically Contaminated Polymer Materials. Materials. 2020; 13(18):4012. https://doi.org/10.3390/ma13184012
Chicago/Turabian StyleWolny-Koładka, Katarzyna, Mateusz Malinowski, and Witold Żukowski. 2020. "Impact of Calcium Oxide on Hygienization and Self-Heating Prevention of Biologically Contaminated Polymer Materials" Materials 13, no. 18: 4012. https://doi.org/10.3390/ma13184012
APA StyleWolny-Koładka, K., Malinowski, M., & Żukowski, W. (2020). Impact of Calcium Oxide on Hygienization and Self-Heating Prevention of Biologically Contaminated Polymer Materials. Materials, 13(18), 4012. https://doi.org/10.3390/ma13184012







