Heat-Resistant Polymer Composites Based on Ethylene Tetrafluoroethylene Mixed with Inorganic Polyoxides
Abstract
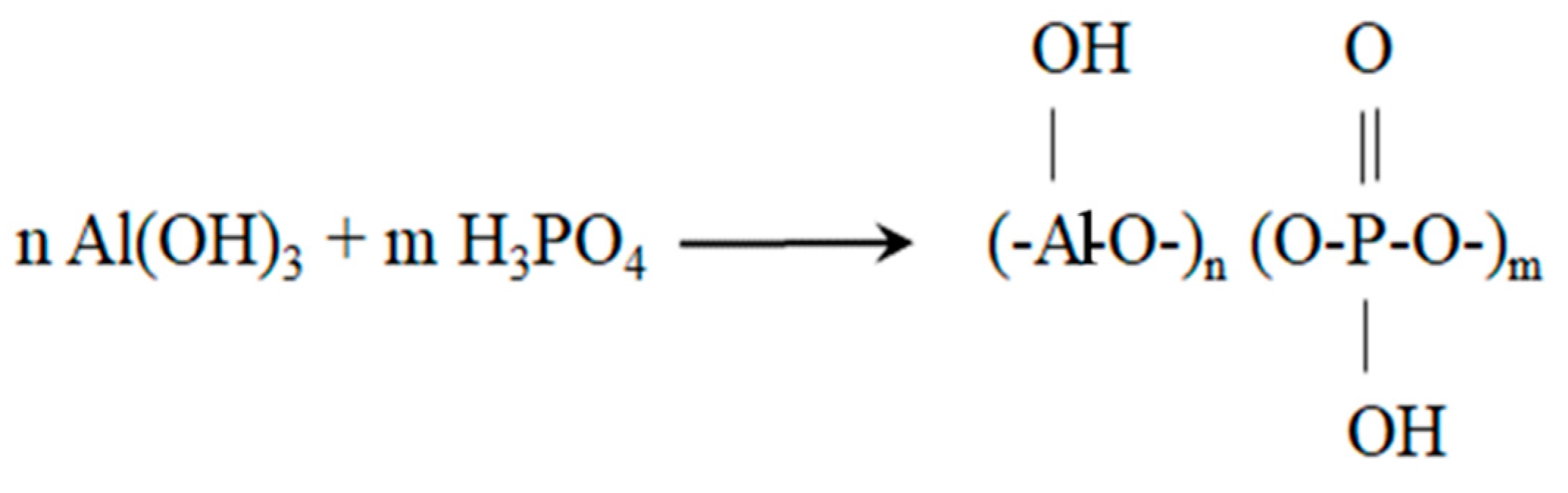
1. Introduction
2. Materials and Methods
3. Results and Discussion
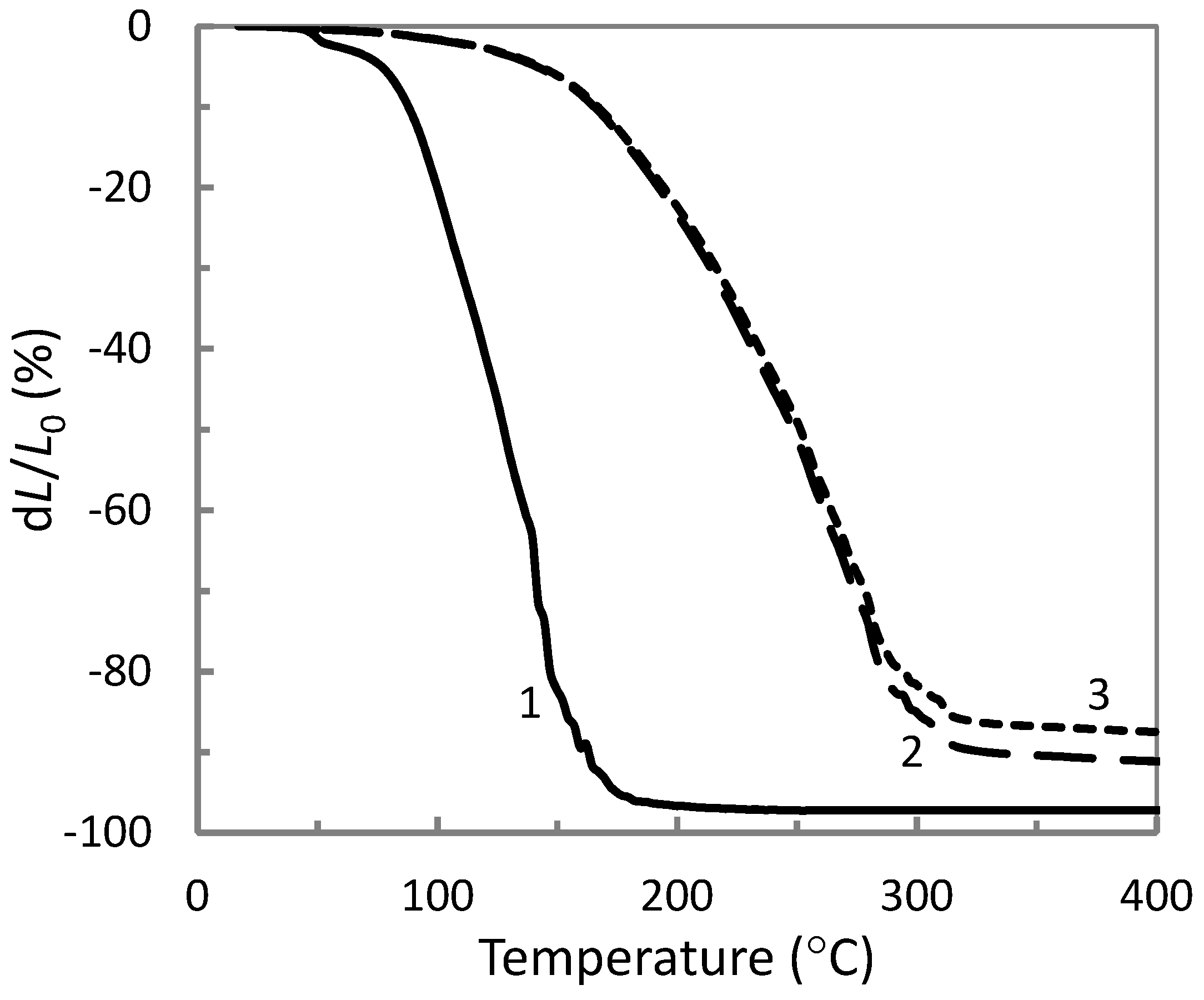
3.1. Processability
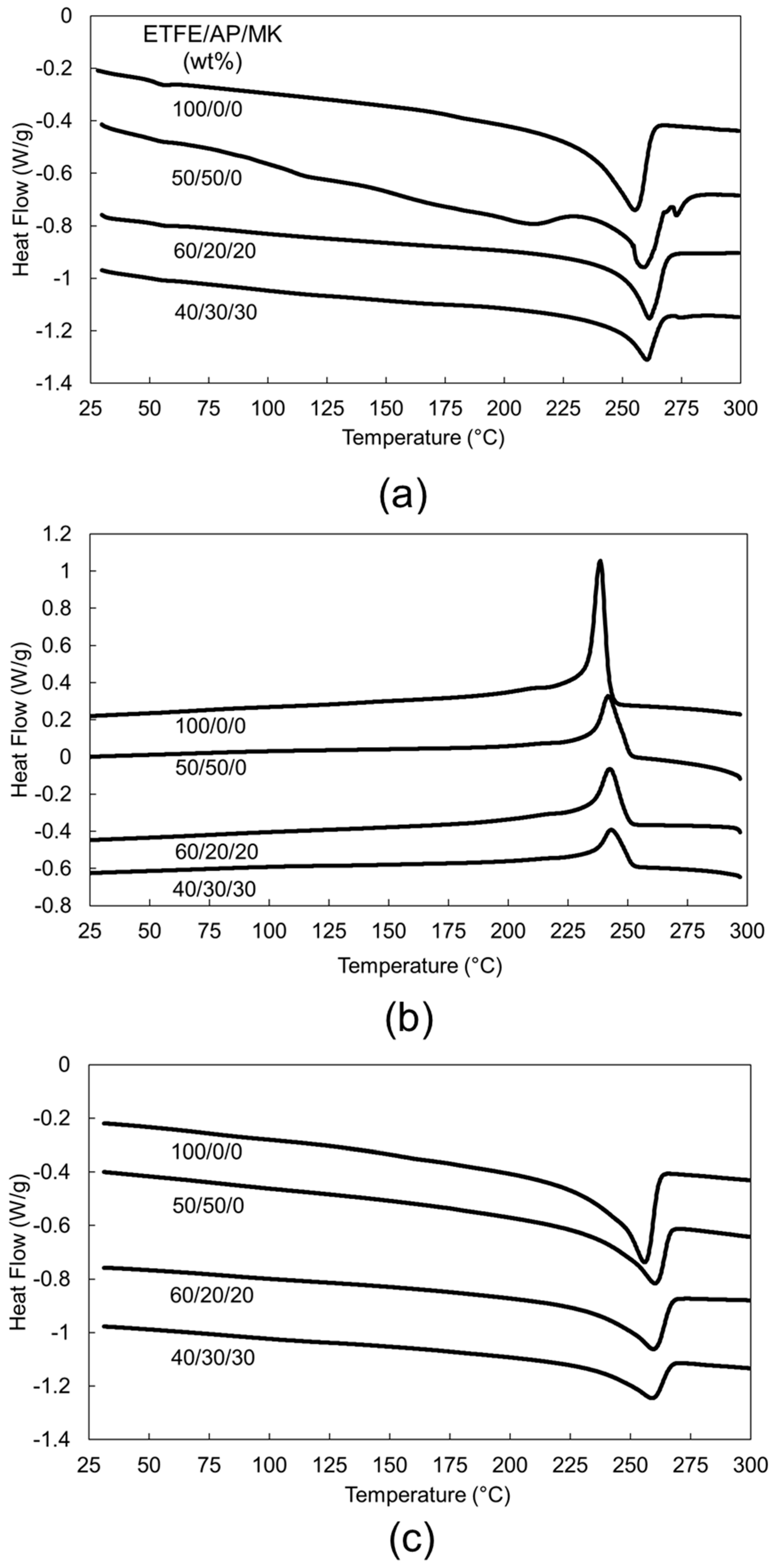
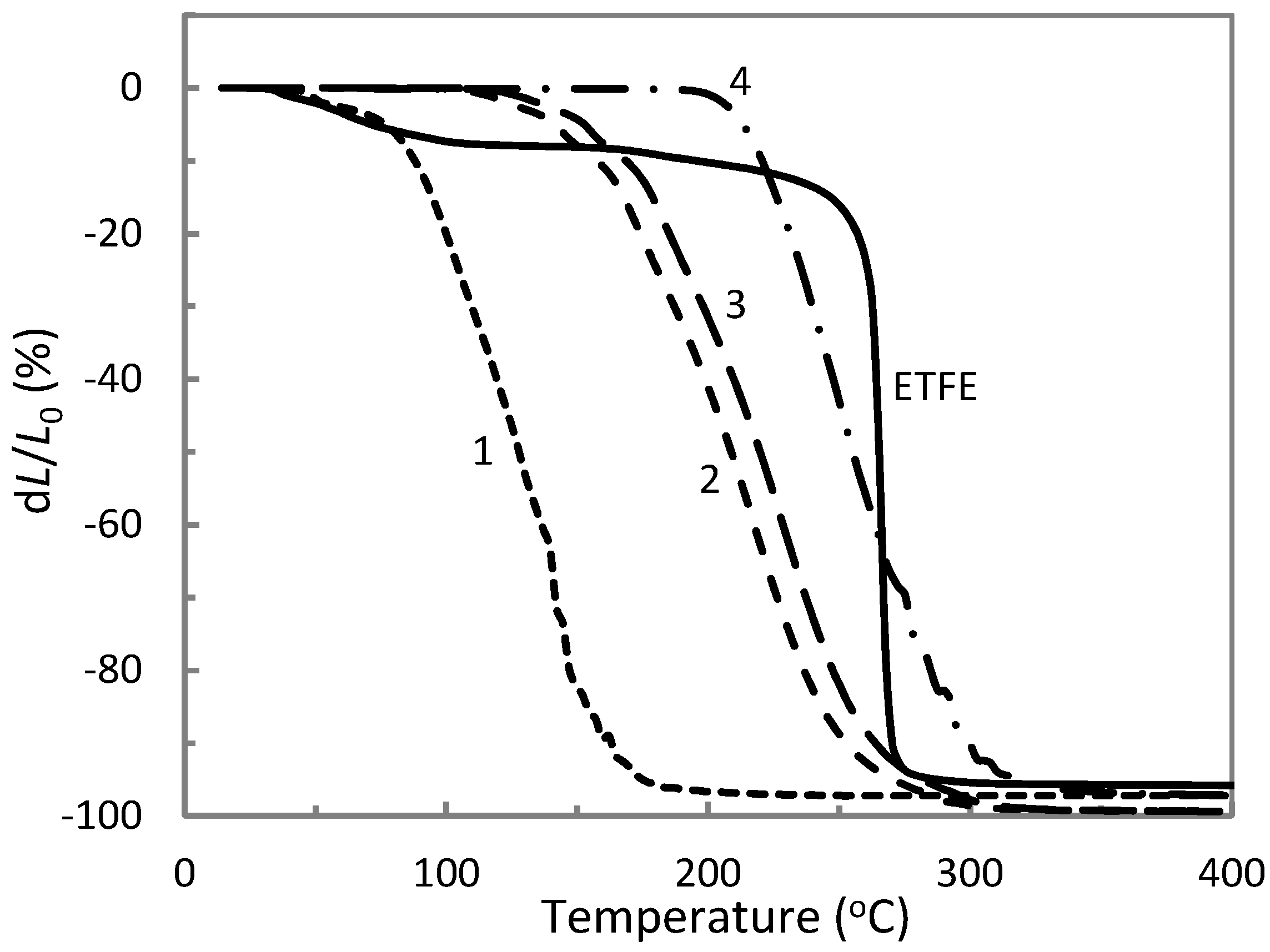
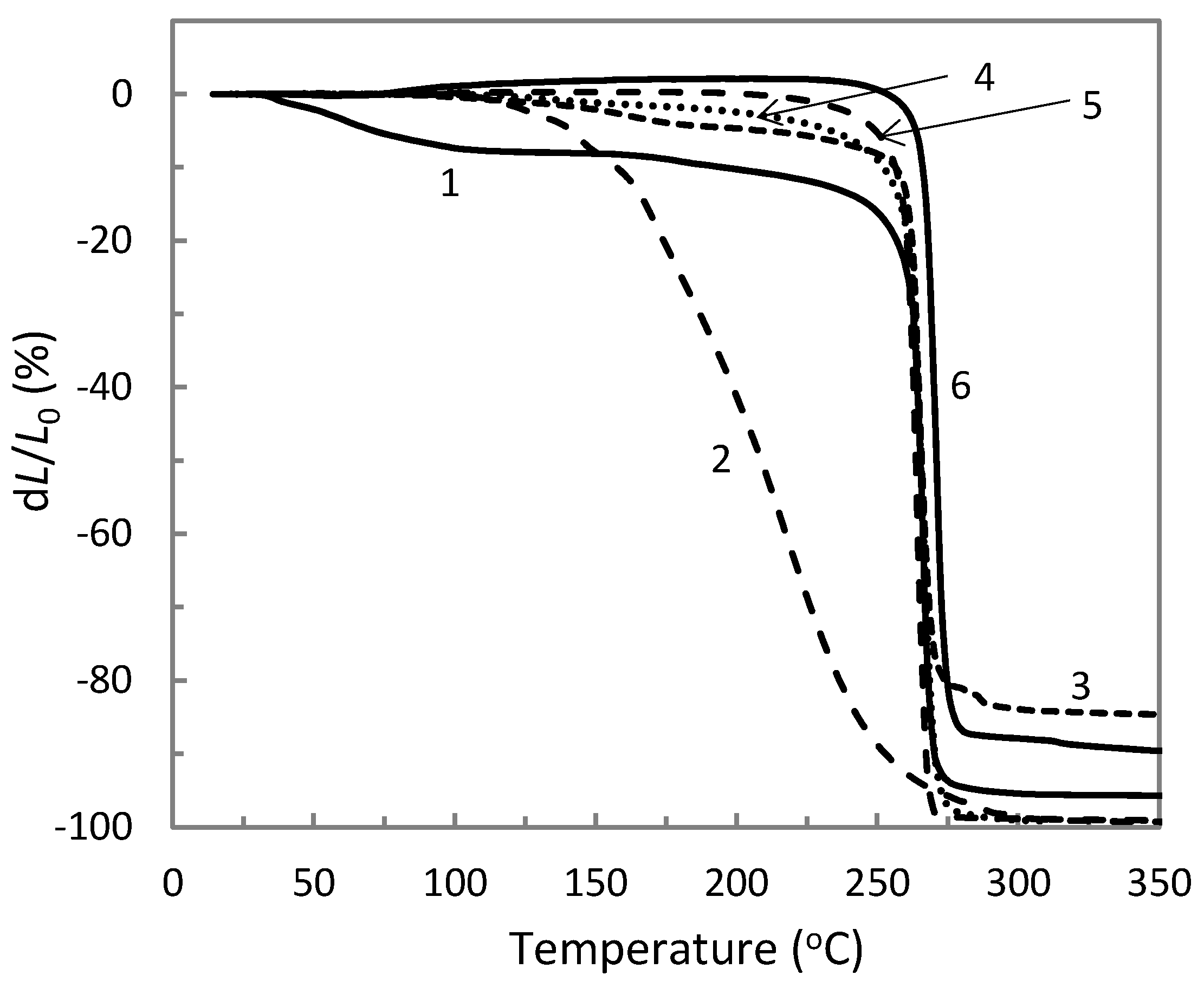
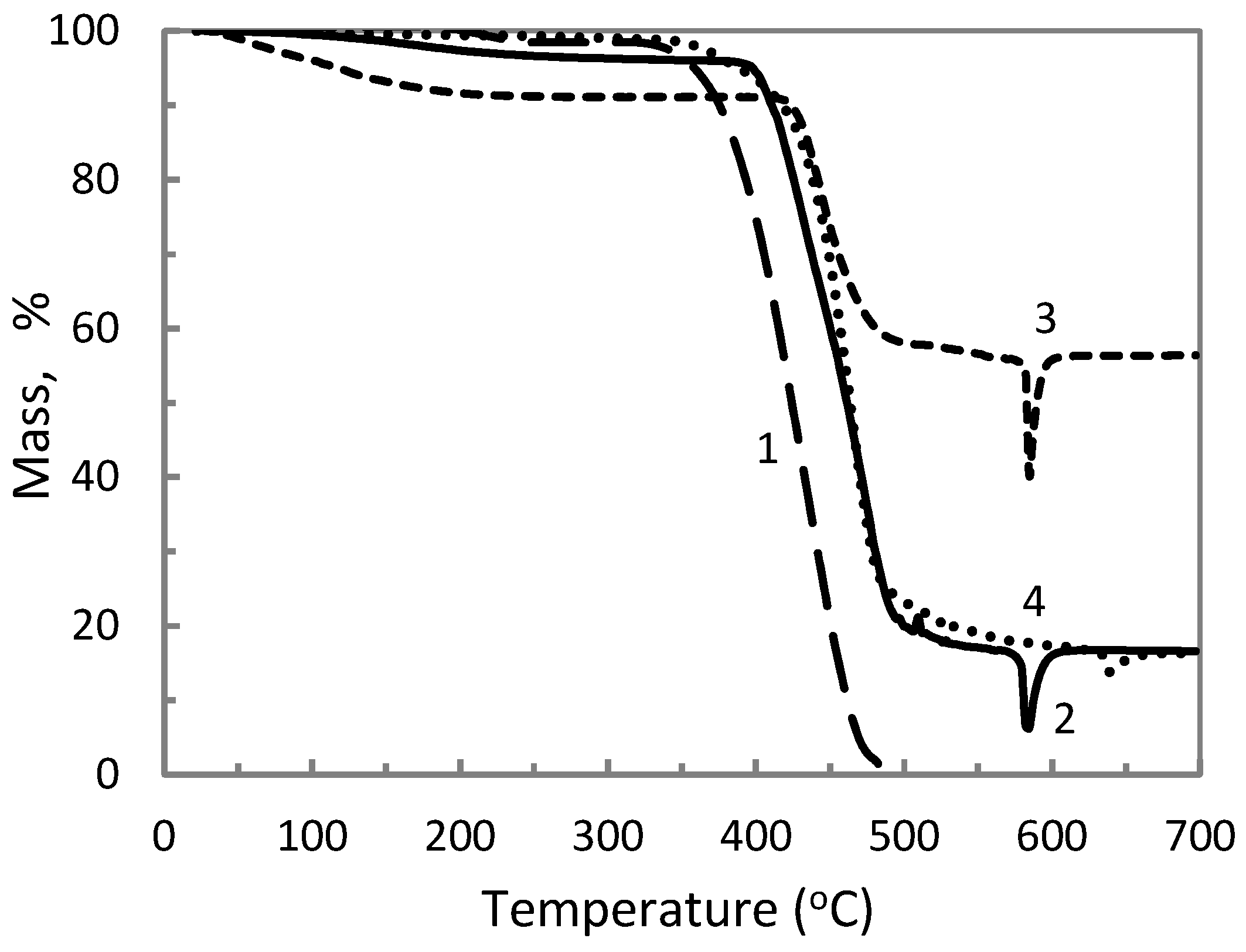
3.2. Thermal Properties
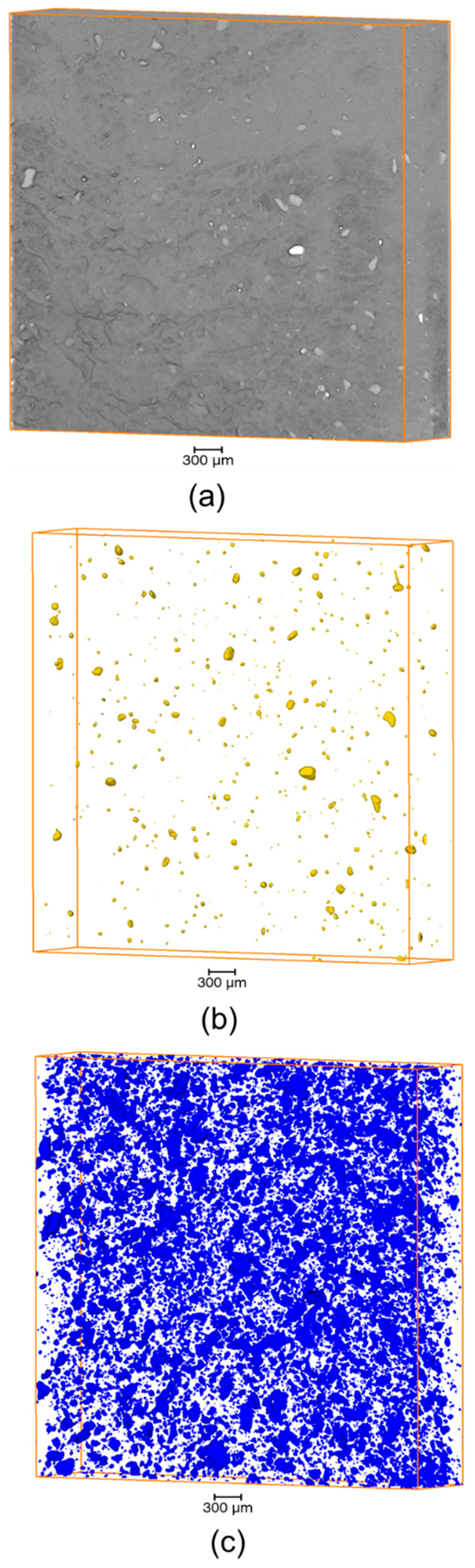
3.3. Microstructure
3.4. Mechanical Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ishizawa, J.; Mori, K. Space Environment Effects on Cross-linked ETFE Polymer. In Proceedings of the 11th International Symposium on Materials in the Space Environment, Aix-en-Provence, France, 15–18 September 2009. [Google Scholar]
- Oshima, A.; Ikeda, S.; Seguchi, T.; Tabata, Y. Temperature Effect on Radiation Induced Reactions in Ethylene and Tetrafluoroethylene Copolymer (ETFE). Radiat. Phys. Chem. 1997, 50, 519–522. [Google Scholar] [CrossRef]
- Zen, H.A.; Ribeiro, G.; Geraldes, A.N.; Souza, C.P.; Parra, D.F.; Lugão, A.B. Effect of Radiation Induced Crosslinking and Degradation of ETFE Films. Radiat. Phys. Chem. 2013, 84, 136–139. [Google Scholar] [CrossRef]
- Shaulov, A.Y.; Vladimirov, L.V.; Grachev, A.V.; Lalayan, V.V.; Nechvolodova, E.M.; Sakovich, R.A.; Stegno, E.V.; Tkachenko, L.A.; Patlazhan, S.A.; Berlin, A.A. Inorganic and Hybrid polymers and composites. Russ. J. Phys. Chem. B 2020, 14, 183–189. [Google Scholar] [CrossRef]
- Urman, K.; Otaigbe, J.U. New Phosphate Glass/Polymer Hybrids—Current Status and Future Prospects. Prog. Polym. Sci. 2007, 32, 1462–1498. [Google Scholar] [CrossRef]
- Adalja, S.B.; Otaigbe, J.U.; Thalacker, J. Glass-Polymer Melt Hybrids. I: Viscoelastic Properties of Novel Affordable Organic-Inorganic Polymer Hybrids. Polym. Eng. Sci. 2001, 41, 1055–1067. [Google Scholar] [CrossRef]
- Lalayan, V.M.; Stegno, E.V.; Grachev, A.V.; Ignat’eva, L.N.; Goncharuk, V.K.; Shaulov, A.Y.; Berlin, A.A.; Buznik, V.M. Composite Materials Based on Fluoropolymers and Oxyfluoride Glasses. Dokl. Chem. 2016, 468, 187–190. [Google Scholar] [CrossRef]
- Stegno, E.V.; Lalayan, V.M.; Grachev, A.V.; Vladimirov, L.V.; Nelyub, V.A.; Shaulov, A.Y.; Berlin, A.A. Characteristics of Hybrid Mixtures of Boron Polyoxide and Copolymer of Ethylene with Vinyl Acetate. Polym. Sci. Ser. D 2018, 11, 426–430. [Google Scholar] [CrossRef]
- Shaulov, A.Y.; Aliev, I.I.; Lyumpanova, A.Y.; Zarkhina, T.S.; Barashkova, I.I.; Vasserman, A.M.; Berlin, A.A. On the Structure of Composites Based on Mixtures of Boron Polyoxides with Polyethylene. Dokl. Phys. Chem. 2007, 413, 63–65. [Google Scholar] [CrossRef]
- Ilyin, S.O.; Malkin, A.Y.; Kulichikhin, V.G.; Shaulov, A.Y.; Stegno, E.V.; Berlin, A.A.; Patlazhan, S.A. Rheological Properties of Polyethylene/Metaboric Acid Thermoplastic Blends. Rheol. Acta 2014, 53, 467–475. [Google Scholar] [CrossRef]
- Stegno, E.V.; Lalayan, V.M.; Berezkina, N.G.; Grachev, A.V.; Shaulov, A.Y.; Berlin, A.A.; Patlazhan, S.A. Abnormal Mechanical Properties of Composites Based on Boron Oxide Oligomer/Polyethylene Blends. Polym. Compos. 2019, 40, 916–923. [Google Scholar] [CrossRef]
- Stegno, E.V.; Lalayan, V.M.; Grachev, A.V.; Vladimirov, L.V.; Berezkina, N.G.; Shaulov, A.Y.; Patlazhan, S.A.; Berlin, A.A. Orientation Effects in Hybrid-Polymer Mixtures. Polym. Sci. Ser. D 2018, 11, 209–214. [Google Scholar] [CrossRef]
- Shaulov, A.Y.; Lomakin, S.M.; Rakhimkulov, A.D.; Koverzanova, E.V.; Shchegolikhin, A.N.; Glushenko, P.B.; Shilkina, N.G.; Berlin, A.A. High-Temperature Thermal Degradation of Polyethylene in an Inorganic Polyoxide Matrix. Dokl. Phys. Chem. 2004, 398, 231–235. [Google Scholar] [CrossRef]
- Shaulov, A.Y.; Lomakin, S.M.; Zarkhina, T.S.; Rakhimkulov, A.D.; Shilkina, N.G.; Muravlev, Y.B.; Berlin, A.A. Carbonization of Poly(Vinyl Alcohol) in Blends with Boron Polyoxide. Dokl. Phys.Chem. 2005, 403, 154–158. [Google Scholar] [CrossRef]
- Walsby, N.; Sundholm, F.; Kallio, T.; Sundholm, G. Radiation-Grafted Ion-Exchange Membranes: Influence of the Initial Matrix on the Synthesis and Structure. J. Polym. Sci. A Polym. Chem. 2001, 39, 3008–3017. [Google Scholar] [CrossRef]
- Fu, S.-Y.; Feng, X.-Q.; Lauke, B.; Mai, Y.-W. Effects of Particle Size, Particle/Matrix Interface Adhesion and Particle Loading on Mechanical Properties of Particulate–Polymer Composites. Compos. Part B Eng. 2008, 39, 933–961. [Google Scholar] [CrossRef]
- Iuliano, M.; De Rosa, C.; Guerra, G.; Petraccone, V.; Corradini, P. Structural variations in ethylene-tetrafluoroethylene copolymers as a function of composition and temperature. Makromol. Chem. 1989, 190, 827–835. [Google Scholar] [CrossRef]
- Huang, Y.; Miranda, D.F.; Iacob, C.; Zhang, S.; Dan, Y.; Runt, J. Crystalline Microstructure and Dielectric Properties of Oriented Poly(Ethylene-Co-Tetrafluoroethylene). Polymer 2017, 113, 1–8. [Google Scholar] [CrossRef]
- Ono, Y.; Kakiage, M.; Yamanobe, T.; Yukawa, Y.; Higuchi, Y.; Kamiya, H.; Arai, K.; Uehara, H. Structural and Property Changes during Uniaxial Drawing of Ethylene–Tetrafluoroethylene Copolymer Films as Analyzed by in-Situ X-Ray Measurements. Polymer 2011, 52, 1172–1179. [Google Scholar] [CrossRef]
- Hsu, S.L.; Wu, Y.; Honeker, C.C.; Bravet, D.; Williams, D. A Melt Processable Composition and Method of Making. European Patent 2726553A2, 7 May 2014. [Google Scholar]
- Van Wazer, J.R. Phosphorus and Its Compounds, Vol.1—Chemistry; Interscience Publishers: New York, NY, USA, 1958; 954p. [Google Scholar]
- Sudakas, L.G. Phosphate Binding Systems; RIA Kuintet: St. Petersburg, Russia, 2008; 260p. (In Russian) [Google Scholar]
- Ray, S.; Cooney, R.P. Thermal Degradation of Polymer and Polymer Composites. In Handbook of Environmental Degradation of Materials; Elsevier: Amsterdam, The Netherlands, 2018; pp. 185–206. ISBN 978032352. [Google Scholar]
- Li, H.; Zuo, J.; Dong, B.; Xing, F. Effect of Lamellar Inorganic Fillers on the Properties of Epoxy Emulsion Cement Mortar. Int. J. Concr. Struct. Mater. 2020, 14, 18. [Google Scholar] [CrossRef]


| ETFE/AP/MK Composition (wt %) | First Heating | Cooling | Second Heating | ||
|---|---|---|---|---|---|
| Tm (°C) | Xc (wt %) | Tc (°C) | Tm (°C) | Xc (wt %) | |
| 100/0/0 | 255.3 ± 0.4 | 57.7 ± 2.8 | 238.8 ± 0.0 | 255.7 ± 0.1 | 56.2 ± 4.0 |
| 50/50/0 | 260.8 ± 2.1 | n.m. | 241.9 ± 0.2 | 259.9 ± 0.3 | 70.0 ± 7.6 |
| 60/20/20 | 261.2 ± 0.4 | 70.3 ± 1.3 | 242.7 ± 0.3 | 259.2 ± 0.2 | 70.2 ± 2.2 |
| 40/30/30 | 259.8 ± 0.6 | 65.9 ± 3.7 | 243.3 ± 0.1 | 258.5 ± 0.2 | 62.5 ± 1.0 |
| Materials | ETFE | AP (130 °C) | AP (260 °C) | AP (270 °C) | AP (280 °C) |
|---|---|---|---|---|---|
| Ts (°C) | 255 | 80 | 145 | 160 | 210 |
| Tf (°C) | 275 | 170 | 260 | 280 | 300 |
| ETFE/AP/MK Composition (wt %). | Total Filler Content (wt %) | Tonset (°C) |
|---|---|---|
| 100/0/0 | 0 | 381.5 |
| 70/30/0 | 30 | 406.8 |
| 50/50/0 | 50 | 427.6 |
| 60/20/20 | 40 | 459.4 |
| 40/30/30 | 60 | 438.2 |
| 55/0/45 | 45 | 420.0 |
| ETFE/AP/MK Composition (wt %) | Object of Interest | Mean Equivalent Diameter (µm) | Number of Objects per mm3 | Volume Fraction (vol%) | Theoretical Volume Fraction (vol%) |
|---|---|---|---|---|---|
| 50/50/0 | AP | 39.6 | 178.7 | 1.10 | 40.00 |
| 60/20/20 | AP | 30.2 | 26.2 | 0.11 | 15.60 |
| MK | 39.6 | 390.9 | 3.02 | 15.60 | |
| 40/30/30 | AP | 23.5 | 21.0 | 0.03 | 25.20 |
| MK | 30.3 | 1015.7 | 3.89 | 25.20 | |
| Pores | 212.0 | 5.6 | 6.36 | 0.00 |
| ETFE/AP/MK Composition (wt %) | E (GPa) | σ (MPa) | ε (%) |
|---|---|---|---|
| 100/0/0 | 0.4 | 23 | 185.1 |
| 70/30/0 | 0.5 | 21 | 14.0 |
| 50/50/0 | 0.6 | 17 | 4.5 |
| 60/20/20 | 1.6 | 27 | 28.5 |
| 40/30/30 | 1.7 | 21 | 2.6 |
| 55/0/45 | 1.2 | 30 | 49.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shaulov, A.; Addiego, F.; Eloy Federico, C.; Stegno, E.; Grachev, A.; Patlazhan, S. Heat-Resistant Polymer Composites Based on Ethylene Tetrafluoroethylene Mixed with Inorganic Polyoxides. Materials 2021, 14, 969. https://doi.org/10.3390/ma14040969
Shaulov A, Addiego F, Eloy Federico C, Stegno E, Grachev A, Patlazhan S. Heat-Resistant Polymer Composites Based on Ethylene Tetrafluoroethylene Mixed with Inorganic Polyoxides. Materials. 2021; 14(4):969. https://doi.org/10.3390/ma14040969
Chicago/Turabian StyleShaulov, Alexander, Frédéric Addiego, Carlos Eloy Federico, Elena Stegno, Andrei Grachev, and Stanislav Patlazhan. 2021. "Heat-Resistant Polymer Composites Based on Ethylene Tetrafluoroethylene Mixed with Inorganic Polyoxides" Materials 14, no. 4: 969. https://doi.org/10.3390/ma14040969
APA StyleShaulov, A., Addiego, F., Eloy Federico, C., Stegno, E., Grachev, A., & Patlazhan, S. (2021). Heat-Resistant Polymer Composites Based on Ethylene Tetrafluoroethylene Mixed with Inorganic Polyoxides. Materials, 14(4), 969. https://doi.org/10.3390/ma14040969







