Influence of Dry Milling on Phase Transformation of Sepiolite upon Alkali Activation: Implications for Textural, Catalytic and Sorptive Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods of Physico-Chemical Characterization
2.3. Catalytic Testing
2.4. Sorption of CO2
3. Results and Discussion
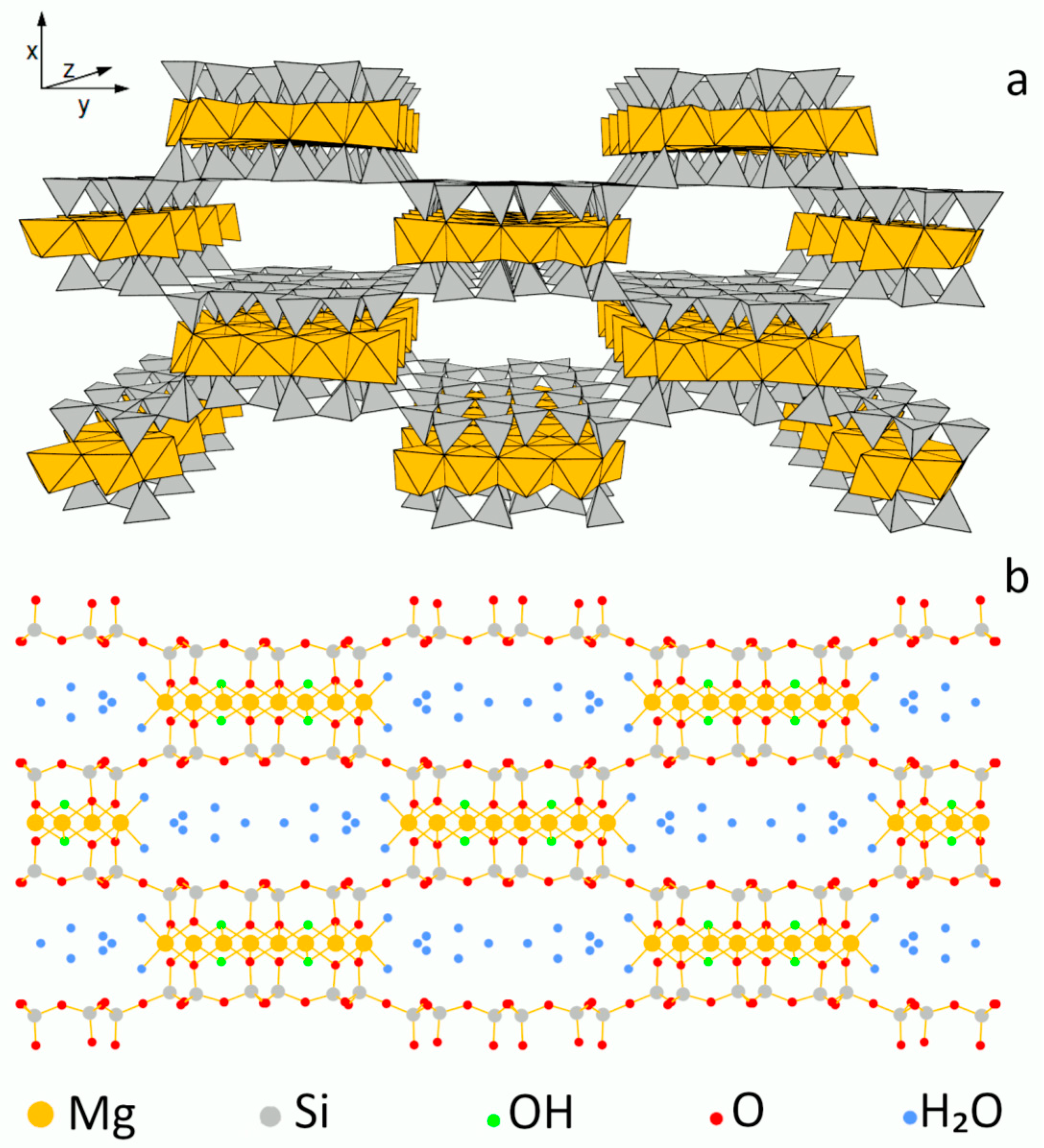
3.1. SEM Analysis
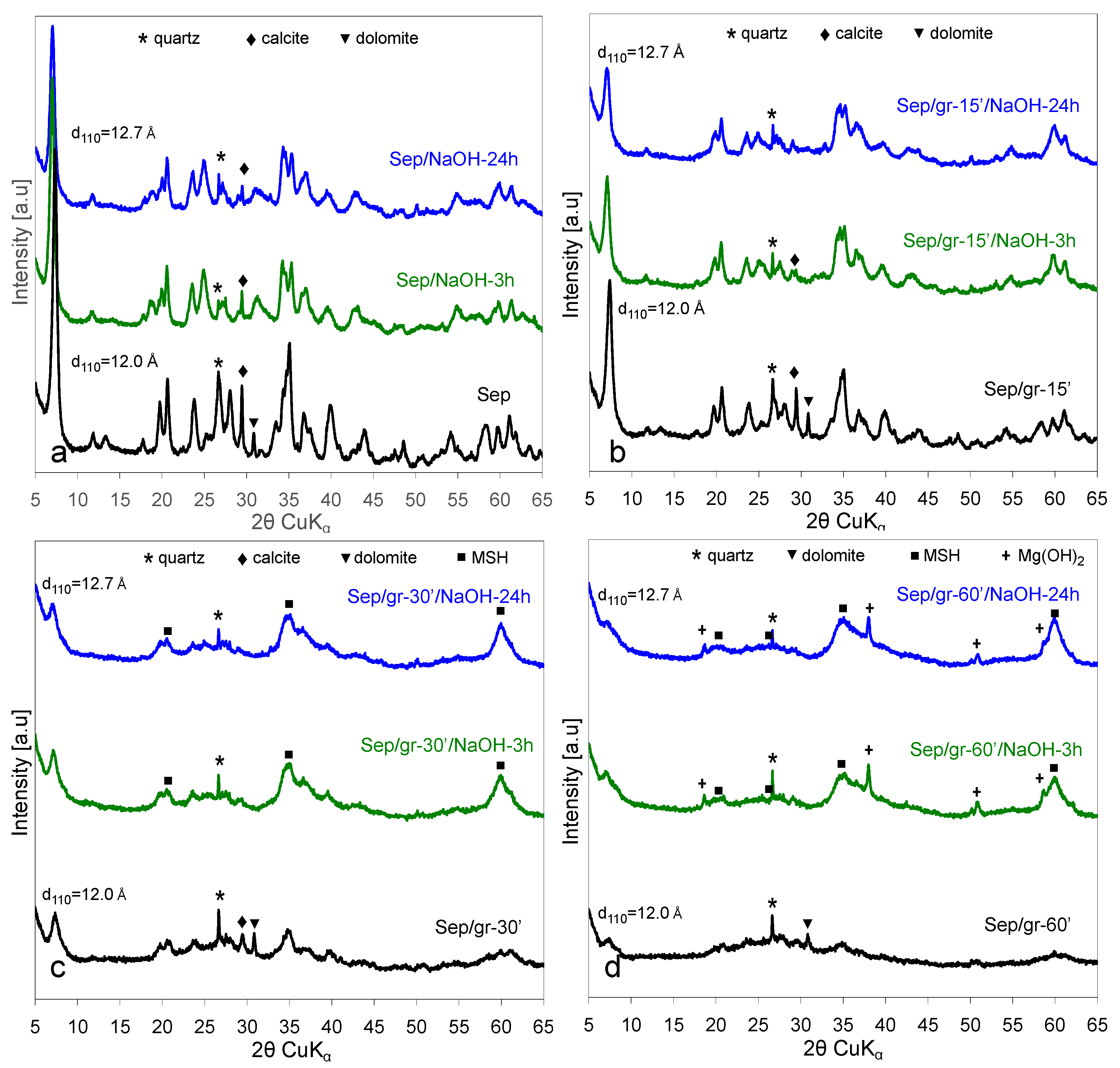
3.2. XRD Analysis
3.3. Chemical Composition
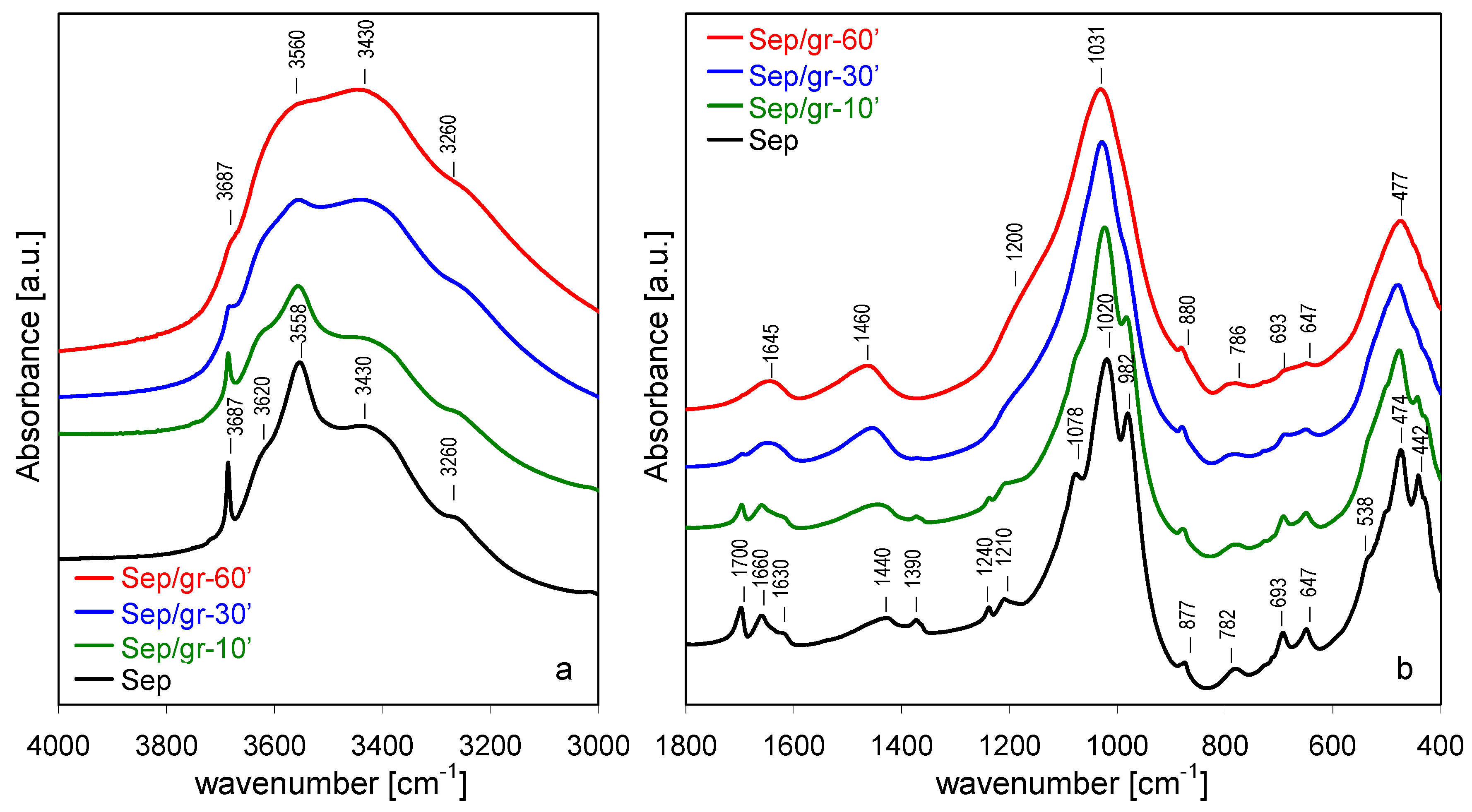
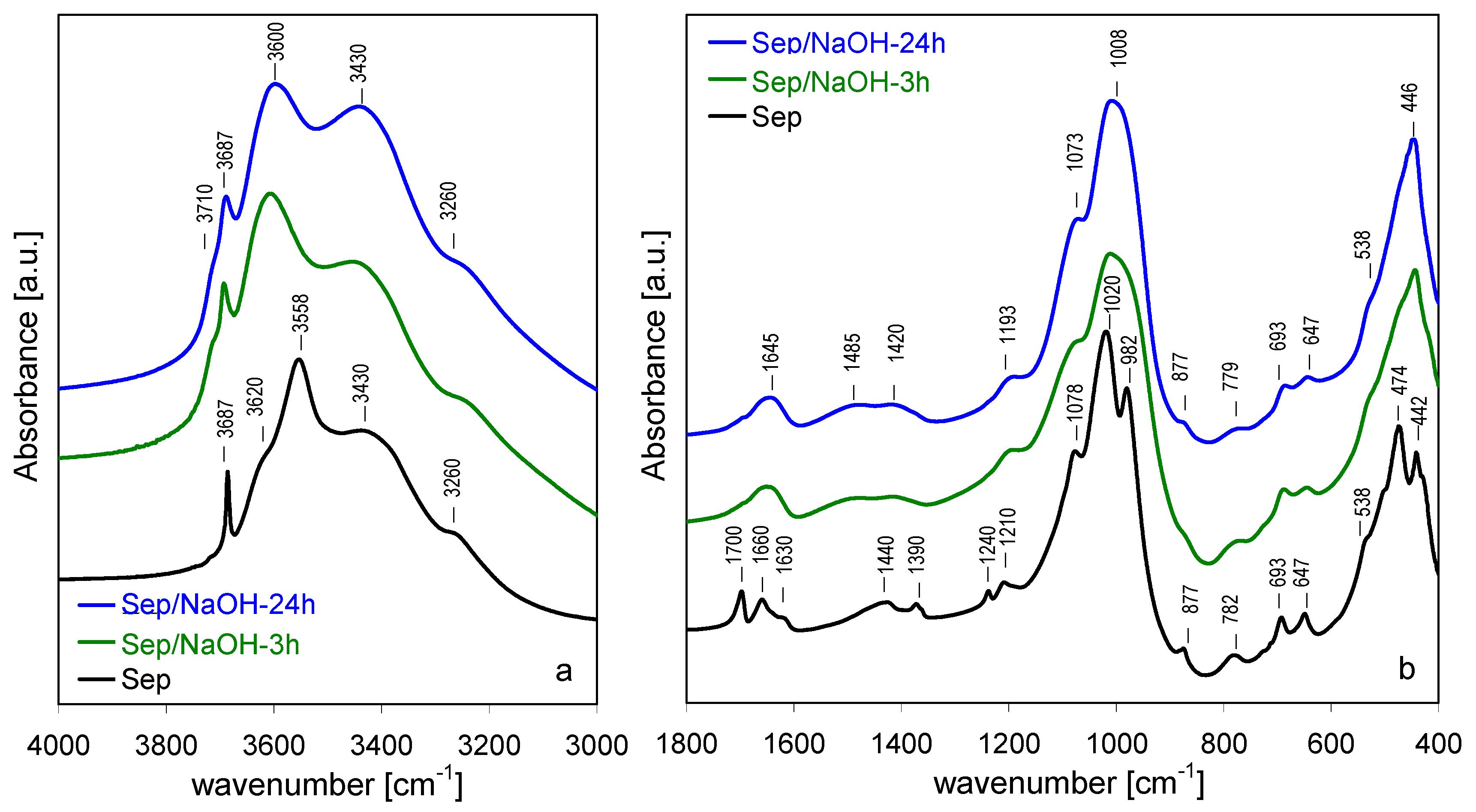
3.4. FTIR Spectroscopy
3.5. 29Si MAS NMR Spectroscopy
3.6. Textural Properties and Surface Basicity in Relation to Catalysis and Sorption
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Pérez-Rodríguez, J. Transformation of clay minerals on grinding: A review. In Applied Study of Cultural Heritage and Clays; Pérez-Rodríguez, J., Ed.; Servicio Publicaciones del CSIC: Madrid, Spain, 2003; pp. 425–444. [Google Scholar]
- Tole, K.; Habermehl-Cwirzen, K.; Cwirzen, A. Mechanochemical activation of natural clay minerals: An alternative to produce sustainable cementitious binders—Review. Miner. Petrol. 2019, 113, 449–462. [Google Scholar] [CrossRef]
- Komadel, P. Acid Activated Clays: Materials in Continuous Demand. Appl. Clay Sci. 2016, 131, 84–99. [Google Scholar] [CrossRef]
- Ono, Y.H.; Hattori, H. Solid Base Catalysis; Springer Series in Chemical Physics; Springer: Berlin/Heidelberg, Germany; Tokyo Institute of Technology Press: Tokyo, Japan, 2012. [Google Scholar]
- Avhad, M.R.; Marchetti, J.M. Innovation in solid heterogeneous catalysis for the generation of economically viable and ecofriendly biodiesel: A review. Catal. Rev. 2016, 58, 157–208. [Google Scholar] [CrossRef]
- Choi, S.; Drese, J.H.; Jones, C.W. Adsorbent materials for carbon dioxide capture from large anthropogenic point sources. ChemSusChem 2009, 2, 796–854. [Google Scholar] [CrossRef] [PubMed]
- Martín-Aranda, R.M.; Vicente-Rodríguez, M.A.; López-Pestaña, J.M.; López-Peinado, A.J.; Jerez, A.; de López-González, J.D.; Bañares-Muñoz, M.A. Application of Basic Clays in Microwave Activated Michael Additions: Preparation of N-substituted Imidazoles. J. Mol. Catal. A 1997, 124, 115–121. [Google Scholar] [CrossRef]
- Soetaredjo, F.E.; Ayucitra, A.; Ismadji, S.; Maukar, A.L. KOH/bentonite Catalysts for Transesterification of Palm Oil to Biodiesel. Appl. Clay Sci. 2011, 53, 341–346. [Google Scholar] [CrossRef]
- Boz, N.; Degirmenbasi, N.; Kalyon, D.M. Transesterification of Canola Oil to Biodiesel Using Calcium Bentonite Functionalized with K Compounds. Appl. Catal. B 2013, 138–139, 236–242. [Google Scholar] [CrossRef]
- Alves, H.J.; da Rocha, A.M.; Monteiro, M.R.; Moretti, C.; Cabrelon, M.D.; Schwengber, C.A.; Milinsk, M.C. Treatment of Clay with KF: New Solid Catalyst for Biodiesel Production. Appl. Clay Sci. 2014, 91, 98–104. [Google Scholar] [CrossRef]
- Olszówka, J.; Karcz, R.; Napruszewska, B.; Bielańska, E.; Dula, R.; Krzan, M.; Nattich-Rak, M.; Socha, R.P.; Klimek, A.; Bahranowski, K.; et al. Magnesium and/ or calcium-containing natural minerals as ecologically friendly catalysts for the Baeyer–Villiger oxidation of cyclohexanone with hydrogen peroxide. Appl. Catal. A 2016, 509, 52–65. [Google Scholar] [CrossRef]
- Walczyk, A.; Michalik, A.; Napruszewska, B.D.; Kryściak-Czerwenka, J.; Karcz, R.; Duraczyńska, D.; Socha, R.P.; Olejniczak, Z.; Gaweł, A.; Klimek, A.; et al. New insight into the phase transformation of sepiolite upon alkali activation: Impact on composition, structure, texture, and catalytic/sorptive properties. Appl. Clay Sci. 2020, 195, 105740. [Google Scholar] [CrossRef]
- Olszówka, J.E.; Karcz, R.; Michalik-Zym, A.; Napruszewska, B.D.; Bielańska, E.; Kryściak-Czerwenka, J.; Socha, R.P.; Nattich-Rak, M.; Krzan, M.; Klimek, A.; et al. Effect of grinding on the physico-chemical properties of Mg-Al hydrotalcite and its performance as a catalyst for Baeyer-Villiger oxidation of cyclohexanone. Catal. Today 2019, 333, 147–153. [Google Scholar] [CrossRef]
- Galan, E. Properties and Applications of Palygorskite-sepiolite. Clays Clay Miner. 1996, 31, 443–453. [Google Scholar] [CrossRef]
- Preisinger, A. X-ray Study of the Structure of Sepiolite. Clays Clay Miner. 1959, 6, 61–67. [Google Scholar] [CrossRef]
- Post, J.E.; Bish, D.L.; Heaney, P. Synchrotron Powder X-ray Diffraction Study of the Structure and Dehydration Behavior of Sepiolite. Am. Miner. 2007, 92, 91–97. [Google Scholar] [CrossRef]
- Giustetto, R.; Levy, D.; Wahyudi, O.; Ricchiardi, G.; Vitillo, J.G. Crystal Structure Refinement of a Sepiolite/Indigo Maya Blue Pigment Using Molecular Modelling and Synchrotron Diffraction. Eur. J. Miner. 2011, 23, 449–466. [Google Scholar] [CrossRef]
- Cornejo, J.; Hermosin, M.C. Structural alteration of sepiolite by dry grinding. Clay Miner. 1988, 23, 391–398. [Google Scholar] [CrossRef]
- Vucelic, D.; Simic, D.; Kovacevic, O.; Dojinovic, M.; Mitrovic, M. The effects of grinding on the physicochemical characteristics of white sepiolite from Golesh. J. Serb. Chem. Soc. 2003, 67, 197–211. [Google Scholar] [CrossRef]
- Kojdecki, M.A.; Bastida, J.; Pardo, P.; Amorós, P. Crystalline microstructure of sepiolite influenced by grinding. J. Appl. Crystallogr. 2005, 38, 888–899. [Google Scholar] [CrossRef]
- Maqueda, C.; dos Santos Afonso, M.; Morillo, E.; Torres Sánchez, R.M.; Perez-Sayago, M.; Undabeytia, T. Adsorption of diuron on mechanically and thermally treated montmorillonite and sepiolite. Appl. Clay Sci. 2013, 72, 175–183. [Google Scholar] [CrossRef]
- Boudriche, L.; Chamayou, A.; Calvet, R.; Hamdi, B.; Balard, H. Influence of different dry milling processes on the properties of an attapulgite clay, contribution of inverse gas chromatography. Powder Technol. 2014, 254, 352–363. [Google Scholar] [CrossRef]
- Karcz, R.; Olszówka, J.E.; Napruszewska, B.D.; Kryściak-Czerwenka, J.; Serwicka, E.M.; Klimek, A.; Bahranowski, K. Combined H2O2/nitrile/bicarbonate system for catalytic Baeyer-Villiger oxidation of cyclohexanone to ε-caprolactone over Mg-Al hydrotalcite catalysts. Catal. Commun. 2019, 132, 105821. [Google Scholar] [CrossRef]
- Opoczky, L. Fine Grinding and Agglomeration of Silicates. Powder Technol. 1977, 17, 1–7. [Google Scholar] [CrossRef]
- Anthony, J.W.; Bideaux, R.A.; Bladh, K.W.; Nichols, M.C. Handbook of Mineralogy; Mineralogical Society of America: Chantilly, VA, USA, 2001; Available online: http://www.handbookofmineralogy.org/ (accessed on 4 July 2020).
- Cole, W.F.A. Crystalline hydrated magnesium silicate formed in the breakdown of a concrete sea-wall. Nature 1953, 171, 354–355. [Google Scholar] [CrossRef]
- Bernard, E.; Lothenbach, B.; Rentsch, D.; Pochard, I.; Dauzères, A. Formation of magnesium silicate hydrates (M-S-H). Phys. Chem. Earth 2017, 99, 142–157. [Google Scholar] [CrossRef]
- Tonelli, M.; Martini, F.; Calucci, L.; Fratini, E.; Geppi, M.; Ridi, F.; Borsacchi, S.; Baglioni, P. Structural characterization of magnesium silicate hydrate: Towards the design of eco-sustainable cements. Dalton Trans. 2016, 45, 3294–3304. [Google Scholar] [CrossRef]
- Cornu, D.; Lin, L.; Daou, M.M.; Jaber, M.; Krafft, J.-M.; Herledan, V.; Laugel, G.; Millot, Y.; Lauron-Pernot, H. Influence of acid base properties of Mg based catalysts in transesterification. Role of Magnesium Silicate Hydrate formation. Catal. Sci. Technol. 2017, 7, 1701–1712. [Google Scholar] [CrossRef]
- Nied, D.; Enemark-Rasmussen, K.; L’Hopital, E.; Skibsted, J.; Lothenbach, B. Properties of magnesium silicate hydrates (M-S-H). Cem. Concr. Res. 2016, 79, 323–332. [Google Scholar] [CrossRef]
- Brew, D.R.M.; Glasser, F.P. Synthesis and characterisation of magnesium silicate hydrate gels. Cem. Concr. Res. 2005, 35, 85–98. [Google Scholar] [CrossRef]
- Bernard, E.; Lothenbach, B.; Pochard, I.; Cau-dit-Coumes, C. Alkali binding by magnesium silicate hydrates. J. Am. Ceram. Soc. 2019, 192, 6322–6336. [Google Scholar] [CrossRef]
- Roosz, C.; Grangeon, S.; Blanc, P.; Montouillout, V.; Lothenbach, B.; Henocq, P.; Giffaut, E.; Vieillard, P.; Gaboreau, S. Crystal structure of magnesium silicate hydrates (MSH): The relation with 2:1 Mg-Si phyllosilicates. Cem. Concr. Res. 2015, 73, 228–237. [Google Scholar] [CrossRef]
- Galan, E.; Castillo, A. Palygorskite-Sepiolite, Occurrence, Genesis and Uses. In Developments in Sedimentology; Singer, A., Galan, E., Eds.; Elsevier: Amsterdam, The Netherlands, 1984; Volume 37, pp. 87–124. [Google Scholar]
- Mulders, J.J.P.; Oelkers, E.H. An experimental study of sepiolite dissolution rates and mechanisms at 25 °C. Geochim. Cosmochim. Acta 2020, 270, 296–312. [Google Scholar] [CrossRef]
- Prost, R. Infrared Study of the Interactions Between the Different Kinds of Water Molecules Present in Sepiolite. Spectrochim. Acta 1975, 31A, 1497–1499. [Google Scholar] [CrossRef]
- Serna, C.; Ahlrichs, J.L.; Serratosa, J.M. Folding in Sepiolite Crystals. Clays Clay Miner. 1975, 23, 452–457. [Google Scholar] [CrossRef]
- Yariv, S. Infrared Evidence for the Occurrence of SiO Groups with Double-bond Character in Antigorite, Sepiolite and Palygorskite. Clay Miner. 1986, 21, 925–936. [Google Scholar] [CrossRef]
- Vicente-Rodríguez, M.A.; Suarez, M.; Bañares-Muñoz, M.A.; Lopez-Gonzalez, J.D.D. Comparative FT-IR Study of the Removal and Structural Modifications during Acid Silicates of Octahedral Cations Treatment of Several Silicates. Spectrochim. Acta 1996, 52, 1685–1694. [Google Scholar] [CrossRef]
- Frost, R.L.; Locos, O.B.; Ruan, J.; Kloprogge, J.T. Near-infrared and Mid-infrared Spectroscopic Study of Sepiolites and Palygorskites. Vib. Spectrosc. 2001, 27, 1–13. [Google Scholar] [CrossRef]
- Mora, M.; López, M.I.; Carmona, M.A.; Jiménez-Sanchidrián, C.; Ruiz, J.R. Study of the Thermal Decomposition of a Sepiolite by Mid- and Near-infrared Spectroscopies. Polyhedron 2010, 29, 3046–3051. [Google Scholar] [CrossRef]
- Bukas, V.J.; Tsampodimou, M.; Gionis, V.; Chryssikos, G.D. Synchronous ATR Infrared and NIR-spectroscopy Investigation of Sepiolite Upon Drying. Vib. Spectrosc. 2013, 68, 51–60. [Google Scholar] [CrossRef]
- Lescano, L.; Castillo, L.; Marfil, S.; Barbosa, S.; Maiza, P. Alternative Methodologies for Sepiolite Defibering. Appl. Clay Sci. 2014, 95, 378–382. [Google Scholar] [CrossRef]
- Madejová, J.; Gates, W.P.; Petit, S. IR spectra of clay minerals. In Infrared and Raman Spectroscopies of Clay Minerals. Developments in Clay Science; Gates, W.P., Klopproge, J.T., Madejová, J., Bergaya, F., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; Volume 8, pp. 107–149. [Google Scholar]
- Gunasekaran, S.; Anbalagan, G.; Pandi, S. Raman and Infrared Spectra of Carbonates of Calcite Structure. J. Raman Spectrosc. 2006, 37, 892–899. [Google Scholar] [CrossRef]
- Olszówka, J.E.; Karcz, R.; Bielańska, E.; Kryściak-Czerwenka, J.; Napruszewska, B.D.; Sulikowski, B.; Socha, R.P.; Bahranowski, K.; Olejniczak, Z.; Serwicka, E.M. New Insight into the Preferred Valency of Interlayer Anions in Hydrotalcite-like Compounds: The Effect of Mg/Al Ratio. Appl. Clay Sci. 2018, 155, 84–94. [Google Scholar] [CrossRef]
- McKeown, D.A.; Post, J.E.; Etz, E.S. Vibrational Analysis of Palygorskite and Sepiolite. Clays Clay Miner. 2002, 50, 667–680. [Google Scholar] [CrossRef]
- Mendelovici, E. Selective mechanochemical reactions on dry grinding structurally different silicates. J. Mater. Sci. Lett. 2001, 20, 81–83. [Google Scholar] [CrossRef]
- Gates, W.P.; Anderson, J.S.; Raven, M.D.; Churchman, G.J. Mineralogy of a bentonite from Miles, Queensland, Australia and characterisation of its acid activation products. Appl. Clay Sci. 2002, 20, 189–197. [Google Scholar] [CrossRef]
- Zhang, T.; Zou, J.; Wang, B.; Wu, Z.; Jia, Y.; Cheeseman, C.R. Characterization of Magnesium Silicate Hydrate (MSH) Gel Formed by Reacting MgO and Silica Fume. Materials 2018, 11, 909. [Google Scholar] [CrossRef]
- Walling, S.A.; Kinoshita, H.; Bernal, S.A.; Colliera, N.C.; Provis, J.L. Structure and properties of binder gels formed in the system Mg(OH)2–SiO2–H2O for immobilisation of Magnox sludge. Dalton Trans. 2015, 44, 8126–8137. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; Dai, Y.; Xu, Y. Hydration evolution of MgO-SiO2 slurries in the presence of sodium metasilicate. Ceram. Int. 2018, 44, 6626–6633. [Google Scholar] [CrossRef]
- Lippmaa, E.; Magi, M.; Samoson, A.; Engelhardt, G.; Grimmer, A.R. Structural studies of silicates by solid-state high-resolution 29Si NMR. J. Am. Chem. Soc. 1980, 102, 4489–4893. [Google Scholar] [CrossRef]
- D’Espinose de la Caillerie, J.B.; Fripiat, J.J. A Reassessment of the 29Si MAS-NMR Spectra of Sepiolite and Aluminated Sepiolite. Clay Miner. 1994, 29, 313–318. [Google Scholar] [CrossRef]
- Weir, M.R.; Kuang, W.; Facey, G.A.; Detellier, C. Solid-state Nuclear Magnetic Resonance Study of Sepiolite and Partially Dehydrated Sepiolite. Clays Clay Miner. 2002, 50, 240–247. [Google Scholar]
- Sanz, J.; Massiot, D. Nuclear magnetic resonance spectroscopy. In Handbook of Clay Science. Part B; Bergaya, F., Lagaly, G., Eds.; Elsevier: Amsterdam, The Netherlands; Oxford, UK, 2013; pp. 233–274. [Google Scholar]
- Bernard, E.; Lothenbach, B.; Chlique, C.; Wyrzykowski, M.; Dauzères, A.; Pochard, I.; Cau-Dit-Coumes, C. Characterization of magnesium silicate hydrate (M-S-H). Cem. Concr. Res. 2019, 116, 309–330. [Google Scholar] [CrossRef]
- Bautista, F.M.; Campelo, J.M.; Luna, D.; Marinas, J.M.; Quirós, R.A.; Romero, A.A. Screening of Amorphous Metal–phosphate Catalysts for the Oxidative Dehydrogenation of Ethylbenzene to Styrene. Appl. Catal. B Environ. 2007, 70, 611–620. [Google Scholar] [CrossRef]
- Nielsen, A.T.; Houlihan, W.J. The Aldol Condensation. Org. React. 1968, 16, 1–438. [Google Scholar]
- Kunkes, E.L.; Simonetti, D.A.; West, R.M.; Serrano-Ruiz, J.C.; Gärtner, C.A.; Dumesic, J.A. Catalytic Conversion of Biomass to Monofunctional Hydrocarbons and Targeted Liquid-fuel Classes. Science 2006, 322, 417–421. [Google Scholar] [CrossRef] [PubMed]
- Climent, M.J.; Corma, A.; Fornes, V.; Guil-Lopez, R.; Iborra, S. Aldol Condensations on Solid Catalysts: A Cooperative Effect between Weak Acid and Base Sites. Adv. Synth. Catal. 2002, 344, 1090–1096. [Google Scholar] [CrossRef]
- Snell, R.W.; Combs, E.; Shanks, B.H. Aldol Condensations Using Bio-oil Model Compounds: The Role of Acid–Base Bi-functionality. Top. Catal. 2010, 53, 1248–1253. [Google Scholar] [CrossRef]
- Brunelli, N.A.; Venkatasubbaiah, K.; Jones, C.W. Cooperative Catalysis with Acid−Base Bifunctional Mesoporous Silica: Impact of Grafting and Co-condensation Synthesis Methods on Material Structure and Catalytic Properties. Chem. Mater. 2012, 24, 2433–2442. [Google Scholar] [CrossRef]
- Labet, M.; Thielemans, W. Synthesis of polycaprolactone: A review. Chem. Soc. Rev. 2009, 38, 3484–3504. [Google Scholar] [CrossRef]
- Ten Brink, G.J.; Arends, I.W.C.E.; Sheldon, R.A. The Baeyer−Villiger Reaction: New Developments toward Greener Procedures. Chem. Rev. 2004, 104, 4105–4124. [Google Scholar] [CrossRef]
- Richardson, D.E.; Yao, H.; Frank, K.M.; Bennett, D.A. Equilibria, Kinetics, and Mechanism in the Bicarbonate Activation of Hydrogen Peroxide: Oxidation of Sulfides by Peroxymonocarbonate. J. Am. Chem. Soc. 2000, 122, 1729–1739. [Google Scholar] [CrossRef]
- Cecilia, J.A.; Vilarrasa-García, E.; Cavalcante, C.L.; Azevedo, D.C.S.; Franco, F.; Rodríguez-Castellón, E. Evaluation of Two Fibrous Clay Minerals (Sepiolite and Palygorskite) for CO2 Capture. J. Environ. Chem. Eng. 2018, 6, 4573–4587. [Google Scholar] [CrossRef]
- Chouikhi, N.; Cecilia, J.A.; Vilarrasa-García, E.; Besghaier, S.; Chlendi, M.; Franco Duro, F.I.; Rodriguez Castellon, E.; Bagane, M. CO2 Adsorption of Materials Synthesized from Clay Minerals: A Review. Minerals 2019, 9, 514. [Google Scholar] [CrossRef]
- Irani, M.; Fan, M.; Ismail, H.; Tuwati, A.; Dutcher, B.; Russell, A.G. Modified nanosepiolite as an inexpensive support of tetraethylenepentamine for CO2 sorption. Nano Energy 2015, 11, 235–246. [Google Scholar] [CrossRef]
- Yuan, M.; Gao, G.; Hu, X.; Luo, X.; Huang, Y.; Jin, B.; Liang, Z. Premodified Sepiolite Functionalized with Triethylenetetramine as an Effective and Inexpensive Adsorbent for CO2 Capture. Ind. Eng. Chem. Res. 2018, 57, 6189–6200. [Google Scholar] [CrossRef]
- Vilarrasa-García, E.; Cecilia, J.; Bastos-Neto, M.; Cavalcante, C.; Azevedo, D.; Rodríguez-Castellon, E. Microwave-assisted nitric acid; treatment of sepiolite and functionalization with polyethylenimine applied to CO2 capture and CO2/N2 separation. Appl. Surf. Sci. 2017, 410, 315–325. [Google Scholar] [CrossRef]





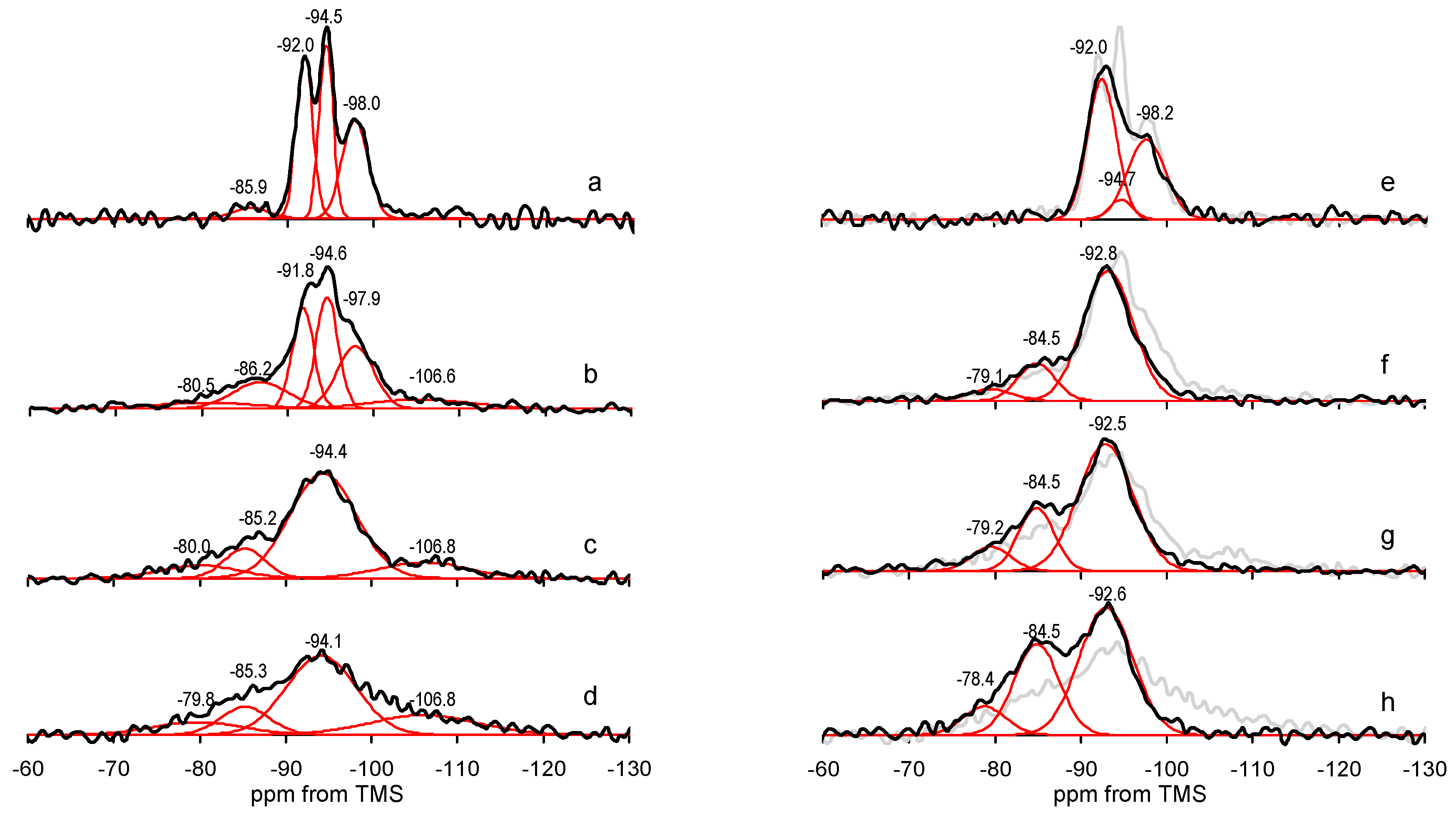
| Sample | SiO2 | MgO | Al2O3 | CaO | Fe2O3 | K2O | Na2O | LOI | Mg/Si | Mg Loss [%] | Si Loss [%] |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sep | 53.1 | 21.4 | 1.9 | 2.2 | 0.4 | 0.4 | 0.1 | 20.6 | 0.60 | - | - |
| Sep/NaOH-3 h | 49.6 | 21.9 | 2.0 | 2.2 | 0.4 | 0.4 | 3.8 | 19.7 | 0.65 | 0.3 | 9.1 |
| Sep/NaOH-24 h | 46.8 | 22.6 | 2.0 | 2.1 | 0.4 | 0.3 | 3.8 | 22.0 | 0.73 | 0.3 | 15.9 |
| Sep/gr-10’ | 54.2 | 22.0 | 2.1 | 2.3 | 0.4 | 0.5 | 0.1 | 18.6 | 0.59 | - | - |
| Sep/gr-10’/NaOH-3 h | 46.4 | 22.4 | 2.2 | 2.2 | 0.4 | 0.5 | 3.5 | 22.5 | 0.72 | - | - |
| Sep/gr-10’/NaOH-24 h | 46.0 | 23.3 | 2.1 | 2.2 | 0.4 | 0.2 | 4.0 | 21.9 | 0.75 | 0.2 | 21.1 |
| Sep/gr-30’ | 54.5 | 22.3 | 2.2 | 2.1 | 0.4 | 0.5 | 0.1 | 17.9 | 0.61 | - | - |
| Sep/gr-30’/NaOH-3 h | 46.0 | 26.1 | 2.4 | 2.6 | 0.5 | 0.3 | 2.2 | 19.9 | 0.85 | - | - |
| Sep/gr-30’/NaOH-24 h | 40.3 | 25.4 | 2.9 | 2.9 | 0.8 | 0 | 2.9 | 25.0 | 0.93 | 0.3 | 34.1 |
| Sep/gr-60’ | 55.4 | 21.8 | 2.0 | 2.5 | 0.4 | 0.5 | 0.2 | 17.3 | 0.59 | - | - |
| Sep/gr-60’/NaOH-3 h | 40.5 | 27.2 | 2.3 | 2.6 | 0.4 | 0 | 1.4 | 25.6 | 1.00 | - | - |
| Sep/gr-60’/NaOH-24 h | 38.5 | 28.2 | 2.5 | 2.7 | 0.4 | 0 | 1.7 | 26.0 | 1.09 | 0.3 | 43.8 |
| Sample | 29Si MAS NMR Parameter | Q1 | Q2 | Q3 | Q4 |
|---|---|---|---|---|---|
| Sep | center (ppm) | - | −85.9 | −92.0, −94.5, −98.0 | - |
| FWHM (ppm) | - | 4.9 | 2.2, 1.9, 3.5 | - | |
| intensity (%) | - | 4 | 34, 30, 32 | - | |
| Sep/NaOH-24 h | center (ppm) | - | - | −92.5, −94.7, −97.6 | - |
| FWHM (ppm) | - | - | 3.9, 3.1, 5.1 | - | |
| intensity (%) | - | - | 54, 6, 40 | - | |
| Sep/gr-10’ | center (ppm) | −80.5 | −86.2 | −91.8, −94.6, −97.9 | −106.6 |
| FWHM (ppm) | 10.3 | 5.7 | 3.2, 3.1, 5.1 | 11.2 | |
| intensity (%) | 7 | 13 | 23, 26, 22 | 9 | |
| Sep/gr-10’/NaOH-24 h | center (ppm) | −79.1 | −84.5 | −92.8 | - |
| FWHM (ppm) | 5.9 | 5.1 | 6.9 | - | |
| intensity (%) | 5 | 17 | 78 | - | |
| Sep/gr-30’ | center (ppm) | −80.0 | −85.2 | −94.4 | −106.8 |
| FWHM (ppm) | 10.1 | 5.5 | 9.1 | 11.8 | |
| intensity (%) | 9 | 11 | 67 | 13 | |
| Sep/gr-30’/NaOH-24 h | center (ppm) | −79.2 | −84.5 | −92.5 | - |
| FWHM (ppm) | 5.4 | 4.9 | 7.6 | - | |
| intensity (%) | 9 | 22 | 69 | - | |
| Sep/gr-60’ | center (ppm) | −79.8 | −85.3 | −94.1 | −106.8 |
| FWHM (ppm) | 12.0 | 6.7 | 9.5 | 14.5 | |
| intensity (%) | 11 | 13 | 55 | 21 | |
| Sep/gr-60’/NaOH-24 h | center (ppm) | −78.4 | −84.5 | −92.6 | - |
| FWHM (ppm) | 5.9 | 6.1 | 7.4 | - | |
| intensity (%) | 10 | 34 | 56 | - |
| Sample | SBET [m2g−1] | Vtot [cm3g−1] | Smicro [m2g−1] | Vmicro [cm3g−1] | Dav [Å] |
|---|---|---|---|---|---|
| Sep | 299 | 0.44 | 140 | 0.059 | 59 |
| Sep/NaOH-3 h | 108 | 0.35 | 12 | 0.005 | 131 |
| Sep/NaOH-24 h | 119 | 0.37 | 20 | 0.009 | 124 |
| Sep/gr-10’ | 182 | 0.37 | 85 | 0.052 | 82 |
| Sep/gr-10’/NaOH-3 h | 228 | 0.41 | 67 | 0.031 | 73 |
| Sep/gr-10’/NaOH-24 h | 223 | 0.39 | 54 | 0.025 | 69 |
| Sep/gr-30’ | 70 | 0.15 | 30 | 0.020 | 88 |
| Sep/gr-30’/NaOH-3 h | 266 | 0.30 | 63 | 0.030 | 45 |
| Sep/gr-30’/NaOH-24 h | 320 | 0.31 | 91 | 0.041 | 39 |
| Sep/gr-60’ | 31 | 0.09 | 10 | 0.008 | 115 |
| Sep/gr-60’/NaOH-3 h | 304 | 0.29 | 83 | 0.039 | 38 |
| Sep/gr-60’/NaOH-24 h | 328 | 0.34 | 63 | 0.030 | 41 |
| Sample | Basicity [μmolg−1]/[μmolm−2] | DAA Yield [mmolg−1] | ε-Caprolactone Yield [mmolg−1] | CO2 Sorption [mmolg−1] |
|---|---|---|---|---|
| Sep | 214 ± 11 / 0.7 | 0.44 ± 0.01 | 21.0 ± 0.6 | 1.5 ± 0.02 |
| Sep/NaOH-3 h | 1039 ± 16 / 9.6 | 8.64 ± 0.03 | 36.1 ± 1.0 | - |
| Sep/NaOH-24 h | 1090 ± 22 / 9.2 | 9.02 ± 0.03 | 36.9 ± 0.7 | 1.2 |
| Sep/gr-10’ | 265 ± 8 / 1.5 | 2.32 ± 0.01 | 17.2 ± 0.8 | 1.1 |
| Sep/gr-10’/NaOH-3 h | 833 ± 26 / 3.7 | 12.21 ± 0.03 | 25.5 ± 0.7 | - |
| Sep/gr-10’/NaOH-24 h | 790 ± 3 / 3.5 | 14.37 ± 0.07 | 31.9 ± 0.6 | 1.3 |
| Sep/gr-30’ | 167 ± 6 / 2.3 | 0.80 ± 0.01 | 16.8 ± 0.7 | 0.9 |
| Sep/gr-30’/NaOH-3 h | 983 ± 33 / 3.7 | 16.83 ± 0.05 | 23.1 ± 0.6 | - |
| Sep/gr-30’/NaOH-24 h | 1133 ± 29 / 3.5 | 20.62 ± 0.03 | 29.0 ± 0.9 | 1.9 |
| Sep/gr-60’ | 80 ± 4 / 2.9 | 0.53 ± 0.01 | 15.7 ± 1.0 | 0.7 |
| Sep/gr-60’/NaOH-3 h | 986 ± 33 / 3.2 | 18.11 ± 0.04 | 25.2 ± 0.6 | - |
| Sep/gr-60’/NaOH-24 h | 1185 ± 45 / 3.6 | 17.59 ± 0.05 | 25.6 ± 0.9 | 1.8 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Walczyk, A.; Karcz, R.; Kryściak-Czerwenka, J.; Napruszewska, B.D.; Duraczyńska, D.; Michalik, A.; Olejniczak, Z.; Tomczyk, A.; Klimek, A.; Bahranowski, K.; et al. Influence of Dry Milling on Phase Transformation of Sepiolite upon Alkali Activation: Implications for Textural, Catalytic and Sorptive Properties. Materials 2020, 13, 3936. https://doi.org/10.3390/ma13183936
Walczyk A, Karcz R, Kryściak-Czerwenka J, Napruszewska BD, Duraczyńska D, Michalik A, Olejniczak Z, Tomczyk A, Klimek A, Bahranowski K, et al. Influence of Dry Milling on Phase Transformation of Sepiolite upon Alkali Activation: Implications for Textural, Catalytic and Sorptive Properties. Materials. 2020; 13(18):3936. https://doi.org/10.3390/ma13183936
Chicago/Turabian StyleWalczyk, Anna, Robert Karcz, Joanna Kryściak-Czerwenka, Bogna D. Napruszewska, Dorota Duraczyńska, Alicja Michalik, Zbigniew Olejniczak, Anna Tomczyk, Agnieszka Klimek, Krzysztof Bahranowski, and et al. 2020. "Influence of Dry Milling on Phase Transformation of Sepiolite upon Alkali Activation: Implications for Textural, Catalytic and Sorptive Properties" Materials 13, no. 18: 3936. https://doi.org/10.3390/ma13183936
APA StyleWalczyk, A., Karcz, R., Kryściak-Czerwenka, J., Napruszewska, B. D., Duraczyńska, D., Michalik, A., Olejniczak, Z., Tomczyk, A., Klimek, A., Bahranowski, K., & Serwicka, E. M. (2020). Influence of Dry Milling on Phase Transformation of Sepiolite upon Alkali Activation: Implications for Textural, Catalytic and Sorptive Properties. Materials, 13(18), 3936. https://doi.org/10.3390/ma13183936





