CO2 Curing Efficiency for Cement Paste and Mortars Produced by a Low Water-to-Cement Ratio
Abstract
1. Introduction
2. Experimental Details
2.1. Materials
2.2. Sample Preparation
2.3. CO2 Curing and Successive Hydration
2.4. Measurements
3. Results
3.1. CO2 Uptake by Pressure Monitoring
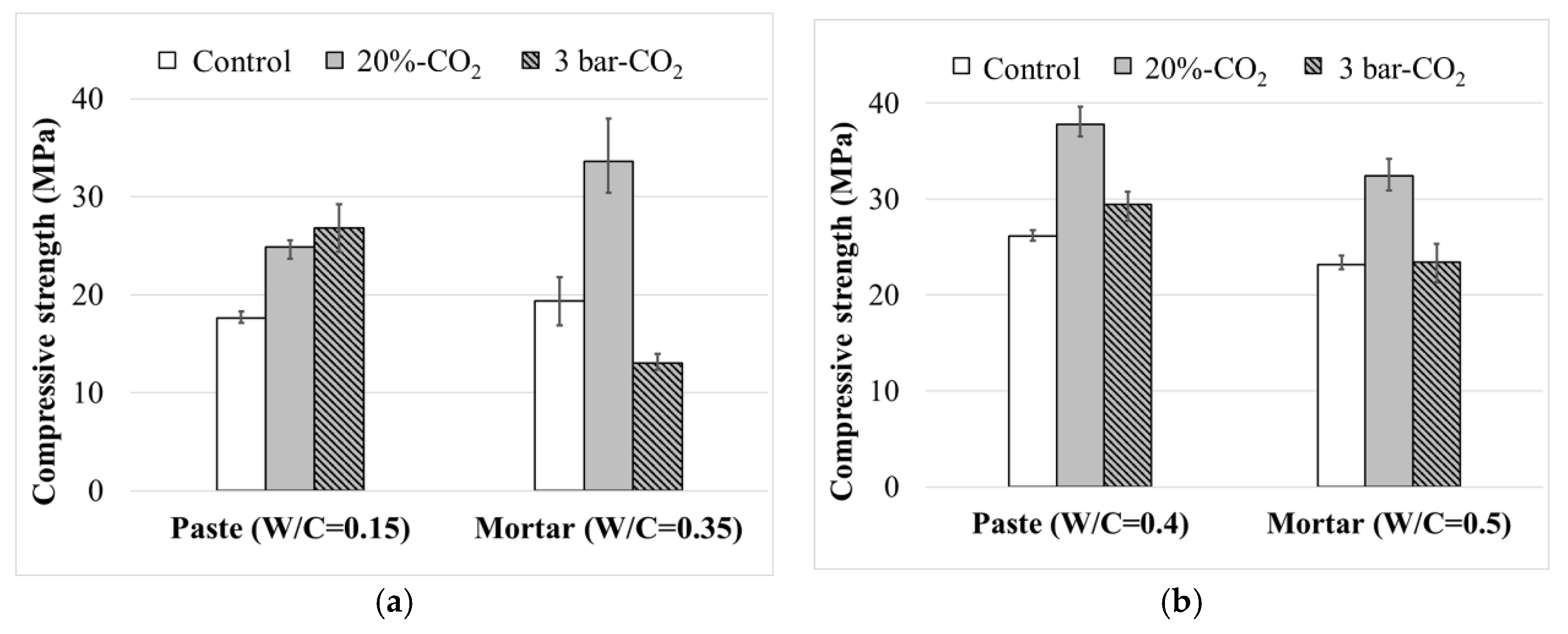
3.2. Compressive Strength
4. Discussions
4.1. Optimization for CO2 Curing Condition
4.2. Effect of Specimen Size
4.3. Effect of Colloidal Silica on CO2 Curing
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gartner, E.; Hirao, H. A review of alternative approaches to the reduction of CO2 emissions associated with the manufacture of the binder phase in concrete. Cem. Concr. Res. 2015, 78, 126–142. [Google Scholar] [CrossRef]
- Huang, C.-H.; Tan, C.-S. A Review: CO2 Utilization. Aerosol Air Qual. Res. 2014, 14, 480–499. [Google Scholar] [CrossRef]
- Klemm, W.A.; Berger, R.L. Accelerated curing of cementitious systems by carbon dioxide. Part I. Portland cement. Cem. Concr. Res. 1972, 2, 567–576. [Google Scholar] [CrossRef]
- Watanabe, K.; Yokozeki, K.; Ashizawa, R.; Sakata, N.; Morioka, M.; Sakai, E.; Daimon, M. High durability cementitious material with mineral admixtures and carbonation curing. Waste Manag. 2006, 26, 752–757. [Google Scholar] [CrossRef]
- Monkman, S.; Shao, Y. Carbonation curing of slag-cement concrete for binding CO2 and improving performance. J. Mater. Civ. Eng. 2010, 22, 296–304. [Google Scholar] [CrossRef]
- Rostami, V.; Shao, Y.; Boyd, A.J.; He, Z. Microstructure of cement paste subject to early carbonation curing. Cem. Concr. Res. 2012, 42, 186–193. [Google Scholar] [CrossRef]
- El-Hassan, H.; Shao, Y. Early carbonation curing of concrete masonry units with Portland limestone cement. Cem. Concr. Compos. 2015, 62, 168–177. [Google Scholar] [CrossRef]
- Young, J.F.; Berger, R.L.; Breese, J. Accelerated Curing of Compacted Calcium Silicate Mortars on Exposure to CO2. J. Am. Ceram. Soc. 1974, 57, 394–397. [Google Scholar] [CrossRef]
- Berger, R.L.; Young, J.F.; Leung, K. Acceleration of Hydration of Calcium Silicates by Carbon Dioxide Treatment. Nat. Phys. Sci. 1972, 240, 16–18. [Google Scholar] [CrossRef]
- Shaikh, F.U.A.; Supit, S.W.M. Mechanical and durability properties of high volume fly ash (HVFA) concrete containing calcium carbonate (CaCO3) nanoparticles. Constr. Build. Mater. 2014, 70, 309–321. [Google Scholar] [CrossRef]
- Mahoutian, M.; Ghouleh, Z.; Shao, Y. Carbon dioxide activated ladle slag binder. Constr. Build. Mater. 2014, 66, 214–221. [Google Scholar] [CrossRef]
- Monkman, S.; Shao, Y. Assessing the carbonation behavior of cementitious materials. J. Mater. Civ. Eng. 2006, 18, 768–776. [Google Scholar] [CrossRef]
- Shi, C.; He, F.; Wu, Y. Effect of pre-conditioning on CO2 curing of lightweight concrete blocks mixtures. Constr. Build. Mater. 2012, 26, 257–267. [Google Scholar] [CrossRef]
- Matsushita, F.; Aono, Y.; Shibata, S. Carbonation degree of autoclaved aerated concrete. Cem. Concr. Res. 2000, 30, 1741–1745. [Google Scholar] [CrossRef]
- Shi, C.; Wu, Y. Studies on some factors affecting CO2 curing of lightweight concrete products. Resour. Conserv. Recycl. 2008, 52, 1087–1092. [Google Scholar] [CrossRef]
- Zhang, D.; Li, V.C.; Ellis, B.R. Optimal Pre-hydration Age for CO2 Sequestration through Portland Cement Carbonation. ACS Sustain. Chem. Eng. 2018, 6, 15976–15981. [Google Scholar] [CrossRef]
- Tu, Z.; Guo, M.Z.; Poon, C.S.; Shi, C. Effects of limestone powder on CaCO3 precipitation in CO2 cured cement pastes. Cem. Concr. Compos. 2016, 72, 9–16. [Google Scholar] [CrossRef]
- Shao, Y.; Rostami, V.; He, Z.; Boyd, A.A. Accelerated carbonation of portland limestone cement. J. Mater. Civ. Eng. 2014, 26, 117–124. [Google Scholar] [CrossRef]
- Aly, M.; Hashmi, M.S.J.; Olabi, A.G.; Messeiry, M.; Abadir, E.F.; Hussain, A.I. Effect of colloidal nano-silica on the mechanical and physical behaviour of waste-glass cement mortar. Mater. Des. 2012, 33, 127–135. [Google Scholar] [CrossRef]
- Zhang, M.H.; Li, H. Pore structure and chloride permeability of concrete containing nano-particles for pavement. Constr. Build. Mater. 2011, 25, 608–616. [Google Scholar] [CrossRef]
- Givi, A.N.; Rashid, S.A.; Aziz, F.N.A.; Salleh, M.A.M. Investigations on the development of the permeability properties of binary blended concrete with nano-SiO2 particles. J. Compos. Mater. 2011, 45, 1931–1938. [Google Scholar] [CrossRef]
- Jo, B.W.; Kim, C.H.; Lim, J.H. Characteristics of cement mortar with nano-SiO 2 particles. ACI Mater. J. 2007, 104, 404–407. [Google Scholar] [CrossRef]
- ISO. ISO 679:2009 Cement—Test Methods—Determination of Strength; ISO: Geneva, Switzerland, 2009. [Google Scholar]
- ASTM C109. Standard Test Method for Compressive Strength of Hydraulic Cement Mortars (Using 2-in. or [50-mm] Cube Specimens); ASTM International: West Conshohocken, PA, USA, 2013. [Google Scholar] [CrossRef]
- Shi, C.; Liu, M.; He, P.; Ou, Z. Factors affecting kinetics of CO2 curing of concrete. J. Sustain. Cem. Mater. 2012, 1, 24–33. [Google Scholar] [CrossRef]
- Kashef-Haghighi, S.; Shao, Y.; Ghoshal, S. Mathematical modeling of CO2 uptake by concrete during accelerated carbonation curing. Cem. Concr. Res. 2015, 67, 1–10. [Google Scholar] [CrossRef]
- El-Hassan, H.; Shao, Y.; Ghouleh, Z. Effect of initial curing on carbonation of lightweight concrete masonry units. ACI Mater. J. 2013, 110, 441–450. [Google Scholar] [CrossRef]
- Bažant, Z.P. Size effect in blunt fracture: Concrete, rock, metal. J. Eng. Mech. 1984, 110, 518–535. [Google Scholar] [CrossRef]
- Kim, J.-K. Size effect in concrete specimens with dissimilar initial cracks. Mag. Concr. Res. 1990, 42, 233–238. [Google Scholar] [CrossRef]
- Yi, S.-T.; Yang, E.-I.; Choi, J.-C. Effect of specimen sizes, specimen shapes, and placement directions on compressive strength of concrete. Nucl. Eng. Des. 2006, 236, 115–127. [Google Scholar] [CrossRef]
- Lim, S.; Mondal, P. Effects of incorporating nanosilica on carbonation of cement paste. J. Mater. Sci. 2015, 50, 3531–3540. [Google Scholar] [CrossRef]
- Péra, J.; Husson, S.; Guilhot, B. Influence of finely ground limestone on cement hydration. Cem. Concr. Compos. 1999, 21, 99–105. [Google Scholar] [CrossRef]
- Sato, T.; Diallo, F. Seeding effect of nano-CaCO3 on the hydration of tricalcium silicate. Transp. Res. Rec. 2010, 2141, 61–67. [Google Scholar] [CrossRef]
- Jayapalan, A.R.; Lee, B.Y.; Fredrich, S.M.; Kurtis, K.E. Influence of additions of anatase TiO2 nanoparticles on early-age properties of cement-based materials. Transp. Res. Rec. 2010, 41–46. [Google Scholar] [CrossRef]
- Farahi, E.; Purnell, P.; Short, N.R. Supercritical carbonation of calcareous composites: Influence of curing. Cem. Concr. Compos. 2013, 43, 48–53. [Google Scholar] [CrossRef]
- Šavija, B.; Luković, M. Carbonation of cement paste: Understanding, challenges, and opportunities. Constr. Build. Mater. 2016, 117, 285–301. [Google Scholar] [CrossRef]
- Farahi, E.; Purnell, P.; Short, N.R. Supercritical carbonation of calcareous composites: Influence of mix design. Cem. Concr. Compos. 2013, 43, 12–19. [Google Scholar] [CrossRef]





| CaO | SiO2 | Al2O3 | Fe2O3 | SO3 | MgO | Na2O | K2O |
|---|---|---|---|---|---|---|---|
| 67.29 | 17.18 | 4.13 | 4.17 | 3.16 | 1.94 | 0.22 | 1.23 |
| Label | Fabrication Method | Mix Proportion (g) | |||
|---|---|---|---|---|---|
| Water | Cement | Sand | Colloidal Silica | ||
| Paste (W/C = 0.15) | Compacting † | 150 | 1000 | 0 | – |
| Mortar (W/C = 0.35) | 157.5 | 450 | 1350 | – | |
| Mortar (W/C = 0.35) with colloidal silica | 157.5 | 450 | 1350 | 18 | |
| Paste (W/C = 0.4) | Consolidating-in-mold ‡ | 400 | 1000 | 0 | – |
| Mortar (W/C = 0.5) | 225 | 450 | 1350 | – | |
| Mortar (W/C = 0.5) with colloidal silica | 225 | 450 | 1350 | 18 | |
| Label | Fabrication Time | Initial Curing for 24 h | Curing Until 28 Days | |
|---|---|---|---|---|
| 3 h | 21 h | |||
| Paste (W/C = 0.15) Mortar (W/C = 0.35) | 0.5 h | Moisture curing (Control) | ||
| 3 bar CO2 curing | Moisture curing | |||
| 20% concentration CO2 curing | ||||
| Paste (W/C = 0.4) Mortar (W/C = 0.5) | 24 h | Water curing (Control) | ||
| 3 bar CO2 curing | Water curing | |||
| 20% concentration CO2 curing | ||||
| Sample | CO2 Uptake at 3 h (%) | |
|---|---|---|
| 40-mm Cube | 25-mm Cube | |
| Paste compact (W/C = 0.15) | 17.54 | 15.80 |
| Paste sample (W/C = 0.4) | 1.43 | 0.80 |
| Mortar compact (W/C = 0.35) | 9.51 | – |
| Mortar sample (W/C = 0.5) | 2.60 | – |
| Mortar compact (W/C = 0.35) with colloidal silica | 14.85 | – |
| Mortar sample (W/C = 0.5) with colloidal silica | 3.11 | – |
| Sample | Demolding Time (h) | CO2 Uptake (%) |
|---|---|---|
| Paste (W/C = 0.4) | 6 | 0.76 |
| 12 | 1.31 | |
| 18 | 0.89 | |
| 21 | 0.74 | |
| Mortar (W/C = 0.5) | 6 | – |
| 12 | 3.06 | |
| 18 | 3.77 | |
| 21 | 2.74 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, S.H.; Jun, Y.; Shin, T.Y.; Kim, J.H. CO2 Curing Efficiency for Cement Paste and Mortars Produced by a Low Water-to-Cement Ratio. Materials 2020, 13, 3883. https://doi.org/10.3390/ma13173883
Han SH, Jun Y, Shin TY, Kim JH. CO2 Curing Efficiency for Cement Paste and Mortars Produced by a Low Water-to-Cement Ratio. Materials. 2020; 13(17):3883. https://doi.org/10.3390/ma13173883
Chicago/Turabian StyleHan, Seong Ho, Yubin Jun, Tae Yong Shin, and Jae Hong Kim. 2020. "CO2 Curing Efficiency for Cement Paste and Mortars Produced by a Low Water-to-Cement Ratio" Materials 13, no. 17: 3883. https://doi.org/10.3390/ma13173883
APA StyleHan, S. H., Jun, Y., Shin, T. Y., & Kim, J. H. (2020). CO2 Curing Efficiency for Cement Paste and Mortars Produced by a Low Water-to-Cement Ratio. Materials, 13(17), 3883. https://doi.org/10.3390/ma13173883







