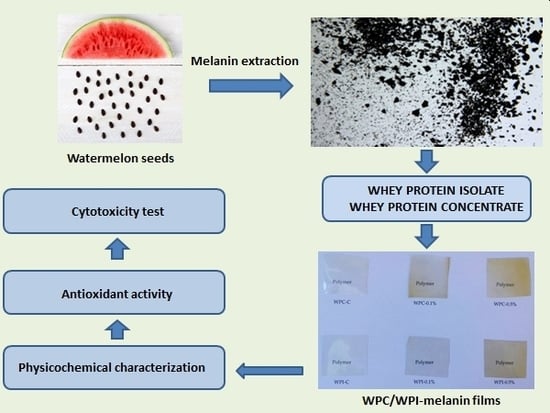
Whey Protein Concentrate/Isolate Biofunctional Films Modified with Melanin from Watermelon (Citrullus lanatus) Seeds
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Isolation, Purification and Preparation of Melanin Powder
2.3. Preparation of WPC and WPI Films
2.4. Determination of Moisture Content, Water Solubility and Swelling Ratio
2.5. Thickness, Mechanical, and Thermal Properties of WPC/WPI Films
2.6. The Water Vapour Transmission Rate (WVTR) of the Films
2.7. The Water Contact Angle (WCA)
2.8. Spectral Analysis
2.9. Color Analysis
2.10. Antioxidant Potential of the Films
2.10.1. Reducing Power
2.10.2. Free Radical Scavenging Activity
2.11. Evaluation of Cytotoxicity
2.12. Statistical Analyses
3. Results and Discussion
3.1. Hydrodynamic Properties (Moisture Content, Water Solubility and Swelling Ratio)
3.2. The Thickness, Mechanical and Thermal Properties
3.3. Color
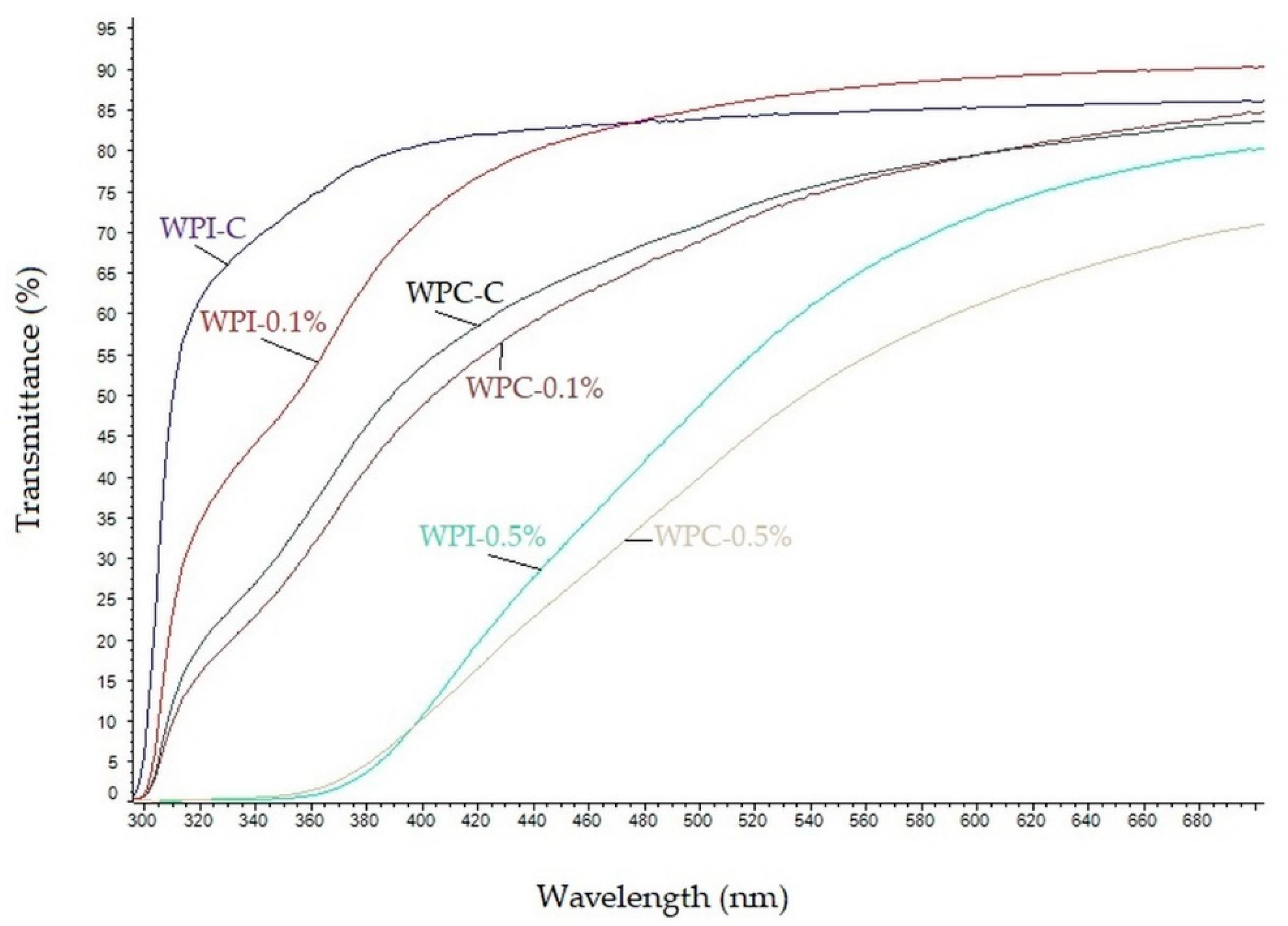
3.4. UV-Barrier Properties
3.5. Antioxidant Activity
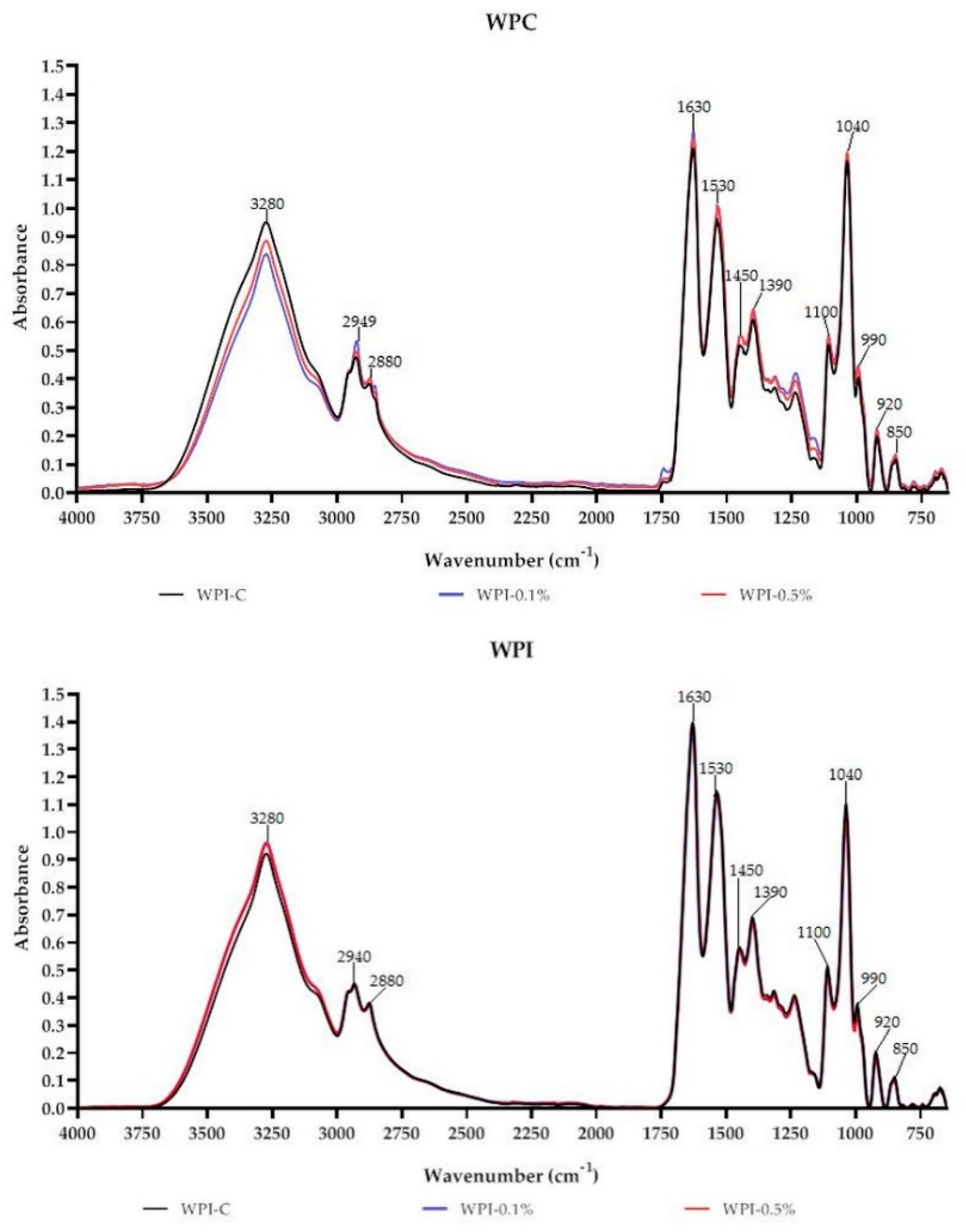
3.6. FT-IR
3.7. Water Contact Angle and WVTR
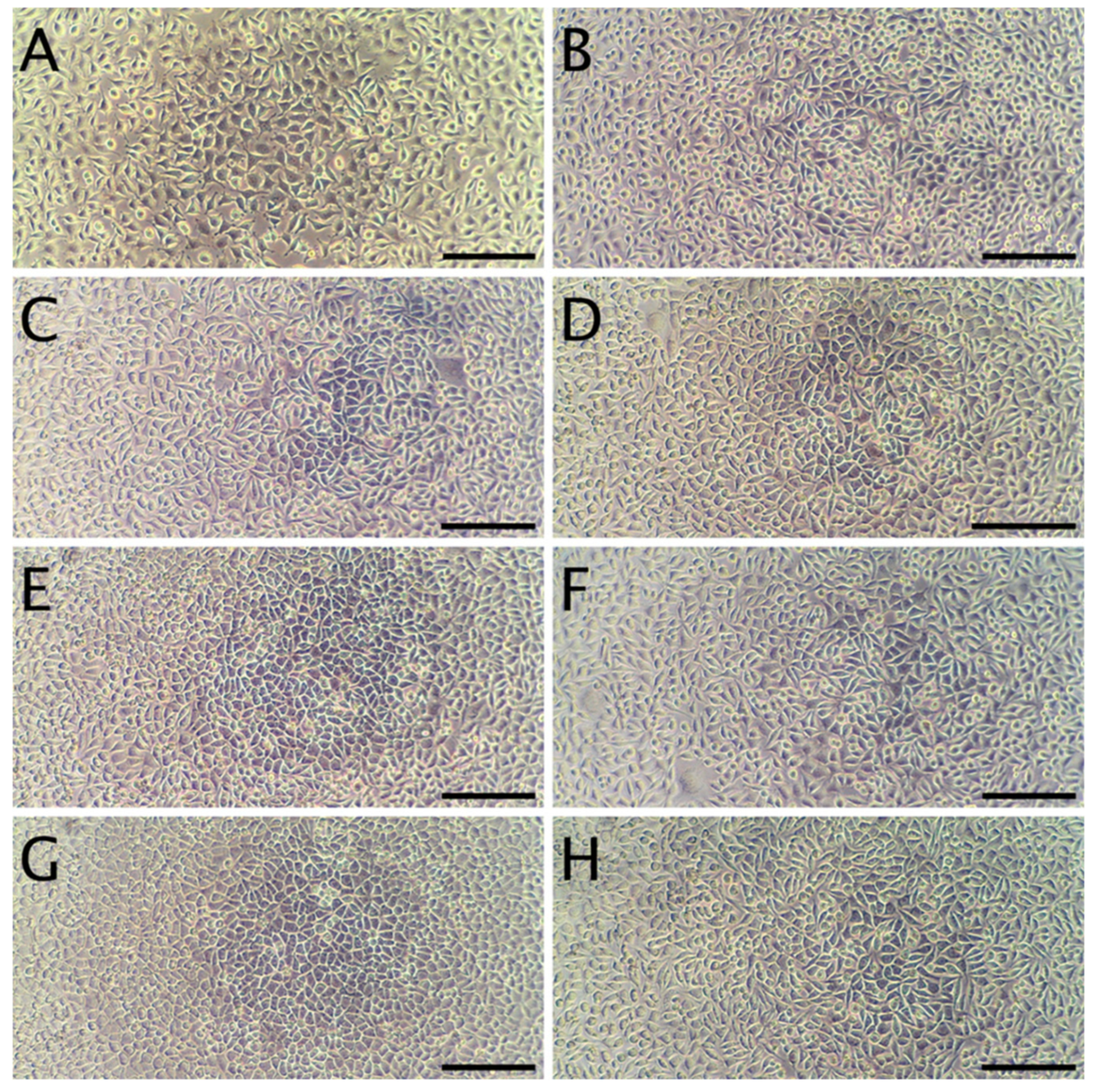
3.8. Cytotoxicity
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Avramescu, S.M.; Butean, C.; Popa, C.V.; Ortan, A.; Moraru, I.; Temocico, G. Edible and functionalized films/coatings—Performances and perspectives. Coatings 2020, 10, 687. [Google Scholar] [CrossRef]
- Dueñas, M.; García-Estévez, I. Agricultural and food waste: Analysis, characterization and extraction of bioactive compounds and their possible utilization. Foods 2020, 9, 817. [Google Scholar] [CrossRef] [PubMed]
- Drozłowska, E.; Łopusiewicz, Ł.; Mężyńska, M.; Bartkowiak, A. Valorization of flaxseed oil cake residual from cold-press oil production as a material for preparation of spray-dried functional powders for food applications as emulsion stabilizers. Biomolecules 2020, 10, 153. [Google Scholar] [CrossRef] [PubMed]
- Turon, X.; Venus, J.; Arshadi, M.; Koutinas, M.; Lin, C.S.K.; Koutinas, A. Food waste and byproduct valorization through bio-processing: Opportunities and challenges. BioResources 2014, 9, 5774–5777. [Google Scholar] [CrossRef]
- Maina, S.; Kachrimanidou, V.; Koutinas, A. A roadmap towards a circular and sustainable bioeconomy through waste valorization. Curr. Opin. Green Sustain. Chem. 2017, 8, 18–23. [Google Scholar] [CrossRef]
- Deshmukh, C.D.; Jain, A.; Tambe, M.S. Phytochemical and Pharmacological profile of Citrullus lanatus (THUNB). Biolife 2015, 483–488. [Google Scholar] [CrossRef]
- Mehra, M.; Pasricha, V.; Gupta, R.K. Estimation of nutritional, phytochemical and antioxidant activity of seeds of musk melon (Cucumis melo) and water melon (Citrullus lanatus) and nutritional analysis of their respective oils. J. Pharmacogn. Phytochem. 2015, 3, 98–102. [Google Scholar]
- Tabiri, B. Watermelon seeds as food: Nutrient composition, phytochemicals and antioxidant activity. Int. J. Nutr. Food Sci. 2016, 5, 139. [Google Scholar] [CrossRef]
- Seidu, K.T.; Otutu, O.L. Phytochemical composition and radical scavenging activities of watermelon (Citrullus lanatus) seed constituents. Croat. J. Food Sci. Technol. 2016, 8, 83–89. [Google Scholar] [CrossRef]
- Łopusiewicz, Ł. Antioxidant, antibacterial properties and the light barrier assessment of raw and purified melanins isolated from Citrullus lanatus (watermelon) seeds. Herba Pol. 2018, 64, 25–36. [Google Scholar] [CrossRef]
- Glagoleva, A.Y.; Shoeva, O.Y.; Khlestkina, E.K. Melanin pigment in plants: Current knowledge and future perspectives. Front. Plant Sci. 2020, 11. [Google Scholar] [CrossRef]
- Wani, A.A.; Sogi, D.S.; Singh, P.; Shivhare, U.S. Characterization and functional properties of watermelon (Citrullus lanatus) seed protein isolates and salt assisted protein concentrates. Food Sci. Biotechnol. 2011, 20, 877–887. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J.W. Carrageenan-based antimicrobial bionanocomposite films incorporated with ZnO nanoparticles stabilized by melanin. Food Hydrocoll. 2019, 90, 500–507. [Google Scholar] [CrossRef]
- Roy, S.; Van Hai, L.; Kim, H.C.; Zhai, L.; Kim, J. Preparation and characterization of synthetic melanin-like nanoparticles reinforced chitosan nanocomposite films. Carbohydr. Polym. 2020, 231, 115729. [Google Scholar] [CrossRef] [PubMed]
- Soazo, M.; Rubiolo, A.C.; Verdini, R.A. Effect of drying temperature and beeswax content on physical properties of whey protein emulsion films. Food Hydrocoll. 2011, 25, 1251–1255. [Google Scholar] [CrossRef]
- Łupina, K.; Kowalczyk, D.; Zięba, E.; Kazimierczak, W.; Mężyńska, M.; Basiura-Cembala, M.; Wiącek, A.E. Edible films made from blends of gelatin and polysaccharide-based emulsifiers—A comparative study. Food Hydrocoll. 2019. [Google Scholar] [CrossRef]
- Szymańska, M.; Karakulska, J.; Sobolewski, P.; Kowalska, U.; Grygorcewicz, B.; Böttcher, D.; Bornscheuer, U.T.; Drozd, R. Glycoside hydrolase (PelAh) immobilization prevents Pseudomonas aeruginosa biofilm formation on cellulose-based wound dressing. Carbohydr. Polym. 2020, 246. [Google Scholar] [CrossRef]
- Łopusiewicz, Ł.; Jędra, F.; Mizielińska, M. New poly(lactic acid) active packaging composite films incorporated with fungal melanin. Polymers 2018, 10, 386. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J.W. Agar-based antioxidant composite films incorporated with melanin nanoparticles. Food Hydrocoll. 2019, 94, 391–398. [Google Scholar] [CrossRef]
- Moghadam, M.; Salami, M.; Mohammadian, M.; Khodadadi, M.; Emam-Djomeh, Z. Development of antioxidant edible films based on mung bean protein enriched with pomegranate peel. Food Hydrocoll. 2020, 104, 105735. [Google Scholar] [CrossRef]
- Yang, M.; Li, L.; Yu, S.; Liu, J.; Shi, J. High performance of alginate/polyvinyl alcohol composite film based on natural original melanin nanoparticles used as food thermal insulating and UV–vis block. Carbohydr. Polym. 2020, 233, 115884. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Kim, H.C.; Kim, J.W.; Zhai, L.; Zhu, Q.Y.; Kim, J. Incorporation of melanin nanoparticles improves UV-shielding, mechanical and antioxidant properties of cellulose nanofiber based nanocomposite films. Mater. Today Commun. 2020, 24, 100984. [Google Scholar] [CrossRef]
- Schmid, M.; Merzbacher, S.; Müller, K. Time-dependent crosslinking of whey protein based films during storage. Mater. Lett. 2018, 215, 8–10. [Google Scholar] [CrossRef]
- Catarino, M.D.; Alves-Silva, J.M.; Fernandes, R.P.; Gonçalves, M.J.; Salgueiro, L.R.; Henriques, M.F.; Cardoso, S.M. Development and performance of whey protein active coatings with Origanum virens essential oils in the quality and shelf life improvement of processed meat products. Food Control 2017, 80, 273–280. [Google Scholar] [CrossRef]
- Yoshida, C.M.P.; Antunes, A.C.B.; Antunes, L.J.; Antunes, A.J. An analysis of water vapour diffusion in whey protein films. Int. J. Food Sci. Technol. 2003, 38, 595–601. [Google Scholar] [CrossRef]
- Pires, A.F.; Marnotes, N.G.; Bella, A.; Viegas, J.; Gomes, D.M.; Henriques, M.H.F.; Pereira, C.J.D. Use of ultrafiltrated cow’s whey for the production of whey cheese with Kefir or probiotics. J. Sci. Food Agric. 2020. [Google Scholar] [CrossRef]
- Agudelo-Cuartas, C.; Granda-Restrepo, D.; Sobral, P.J.A.; Castro, W. Determination of mechanical properties of whey protein films during accelerated aging: Application of FTIR profiles and chemometric tools. J. Food Process Eng. 2020. [Google Scholar] [CrossRef]
- Xu, R.; Liu, N.; Xu, X.; Kong, B. Antioxidative effects of whey protein on peroxide-induced cytotoxicity. J. Dairy Sci. 2011, 94, 3739–3746. [Google Scholar] [CrossRef]
- Owonubi, S.J.; Mukwevho, E.; Aderibigbe, B.A.; Revaprasadu, N.; Sadiku, E.R. Cytotoxicity and in vitro evaluation of whey protein-based hydrogels for diabetes mellitus treatment. Int. J. Ind. Chem. 2019, 10, 213–223. [Google Scholar] [CrossRef]
- Gunasekaran, S.; Xiao, L.; Ould Eleya, M.M. Whey protein concentrate hydrogels as bioactive carriers. J. Appl. Polym. Sci. 2006, 99, 2470–2476. [Google Scholar] [CrossRef]
- Kerasioti, E.; Stagos, D.; Priftis, A.; Aivazidis, S.; Tsatsakis, A.M.; Hayes, A.W.; Kouretas, D. Antioxidant effects of whey protein on muscle C2C12 cells. Food Chem. 2014, 155, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Solano, F. Melanins: Skin pigments and much more—Types, structural models, biological functions, and formation routes. New J. Sci. 2014, 2014, 498276. [Google Scholar] [CrossRef]
- Xu, C.; Chen, T.; Li, J.; Jin, M.; Ye, M. The structural analysis and its hepatoprotective activity of melanin isolated from Lachnum sp. Process Biochem. 2020, 90, 249–256. [Google Scholar] [CrossRef]
- Ghadge, V.; Kumar, P.; Singh, S.; Mathew, D.E.; Bhattacharya, S.; Nimse, S.B.; Shinde, P.B. Natural melanin produced by the endophytic Bacillus subtilis 4NP-BL Associated with the Halophyte Salicornia brachiata. J. Agric. Food Chem. 2020, 68, 6854–6863. [Google Scholar] [CrossRef]
- Di Mauro, E.; Camaggi, M.; Vandooren, N.; Bayard, C.; De Angelis, J.; Pezzella, A.; Baloukas, B.; Silverwood, R.; Ajji, A.; Pellerin, C.; et al. Eumelanin for nature-inspired UV-absorption enhancement of plastics. Polym. Int. 2019, 68, 984–991. [Google Scholar] [CrossRef]
- Caldas, M.; Santos, A.C.; Veiga, F.; Rebelo, R.; Reis, R.L.; Correlo, V.M. Melanin nanoparticles as a promising tool for biomedical applications—A review. Acta Biomater. 2020, 105, 26–43. [Google Scholar] [CrossRef]
- Bang, Y.J.; Shankar, S.; Rhim, J.W. Preparation of polypropylene/poly (butylene adipate-co-terephthalate) composite films incorporated with melanin for prevention of greening of potatoes. Packag. Technol. Sci. 2020, 1–9. [Google Scholar] [CrossRef]
- Łopusiewicz, Ł.; Jędra, F.; Bartkowiak, A. New active packaging films made from gelatin modified with fungal melanin. World Sci. News 2018, 101, 1–30. [Google Scholar]
- Łopusiewicz, Ł.; Jędra, F.; Bartkowiak, A. The application of melanin modified gelatin coatings for packaging and the oxidative stability of pork lard. World Sci. News 2018, 101, 108–119. [Google Scholar]
- Dong, W.; Wang, Y.; Huang, C.; Xiang, S.; Ma, P.; Ni, Z.; Chen, M. Enhanced thermal stability of poly(vinyl alcohol) in presence of melanin. J. Therm. Anal. Calorim. 2014, 115. [Google Scholar] [CrossRef]
- Kiran, G.S.; Jackson, S.A.; Priyadharsini, S.; Dobson, A.D.W.; Selvin, J. Synthesis of Nm-PHB (nanomelanin-polyhydroxy butyrate) nanocomposite film and its protective effect against biofilm-forming multi drug resistant Staphylococcus aureus. Sci. Rep. 2017, 7, 1–13. [Google Scholar] [CrossRef]
- Łopusiewicz, Ł.; Drozłowska, E.; Siedlecka, P.; Mężyńska, M.; Bartkowiak, A.; Sienkiewicz, M.; Zielińska-Bliźniewska, H.; Kwiatkowski, P. Development, characterization, and bioactivity of non-dairy kefir-like fermented beverage based on flaxseed oil cake. Foods 2019, 8, 544. [Google Scholar] [CrossRef] [PubMed]
- Bishai, M.; De, S.; Adhikari, B.; Banerjee, R. A comprehensive study on enhanced characteristics of modified polylactic acid based versatile biopolymer. Eur. Polym. J. 2014, 54, 52–61. [Google Scholar] [CrossRef]
- Ye, M.; Wang, Y.; Guo, G.Y.; He, Y.L.; Lu, Y.; Ye, Y.W.; Yang, Q.H.; Yang, P.Z. Physicochemical characteristics and antioxidant activity of arginine-modified melanin from Lachnum YM-346. Food Chem. 2012, 135, 2490–2497. [Google Scholar] [CrossRef] [PubMed]
- ISO 10993-5. Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity; International Organization for Standardization: Geneva, Switzerland, 2009. [Google Scholar]
- Riss, T.L.; Moravec, R.A.; Niles, A.L.; Duellman, S.; Benink, H.A.; Worzella, T.J.; Minor, L. Cell Viability Assays; Sittampalam, G.S., Grossman, A., Brimacombe, K., Arkin, M., Auld, D., Austin, C.P., Baell, J., Bejcek, B., Caaveiro, J.M.M., Chung, T.D.Y., et al., Eds.; Bethesda: Rockville, MD, USA, 2004. [Google Scholar]
- Jayaramudu, T.; Varaprasad, K.; Kim, H.C.; Kafy, A.; Kim, J.W.; Kim, J. Calcinated tea and cellulose composite films and its dielectric and lead adsorption properties. Carbohydr. Polym. 2017, 171, 183–192. [Google Scholar] [CrossRef]
- Łupina, K.; Kowalczyk, D.; Drozłowska, E. Polysaccharide/gelatin blend films as carriers of ascorbyl palmitate—A comparative study. Food Chem. 2020, 333. [Google Scholar] [CrossRef]
- Ebaid, H.; Salem, A.; Sayed, A.; Metwalli, A. Whey protein enhances normal inflammatory responses during cutaneous wound healing in diabetic rats. Lipids Health Dis. 2011, 10, 235. [Google Scholar] [CrossRef]
- Fitzmaurice, S.D.; Sivamani, R.K.; Isseroff, R.R. Antioxidant therapies for wound healing: A clinical guide to currently commercially available products. Skin Pharmacol. Physiol. 2011, 24, 113–126. [Google Scholar] [CrossRef]
- Garraud, O.; Hozzein, W.N.; Badr, G. Wound healing: Time to look for intelligent, “natural” immunological approaches? BMC Immunol. 2017, 18. [Google Scholar] [CrossRef]
- Coltelli, M.; Aliotta, L.; Gigante, V.; Bellusci, M.; Cinelli, P.; Bugnicourt, E.; Schmid, M.; Staebler, A.; Lazzeri, A. Preparation and compatibilization of PBS/Whey protein isolate based blends. Molecules 2020, 25, 3313. [Google Scholar] [CrossRef]
- Al-Tayib, O.A.; Elbadwi, S.M.; Bakhiet, A.O. Cytotoxicity assay for herbal melanin derived from Nigella sativa seeds using in vitro cell lines. IOSR J. Humanit. Soc. Sci. 2017, 22, 43. [Google Scholar] [CrossRef]

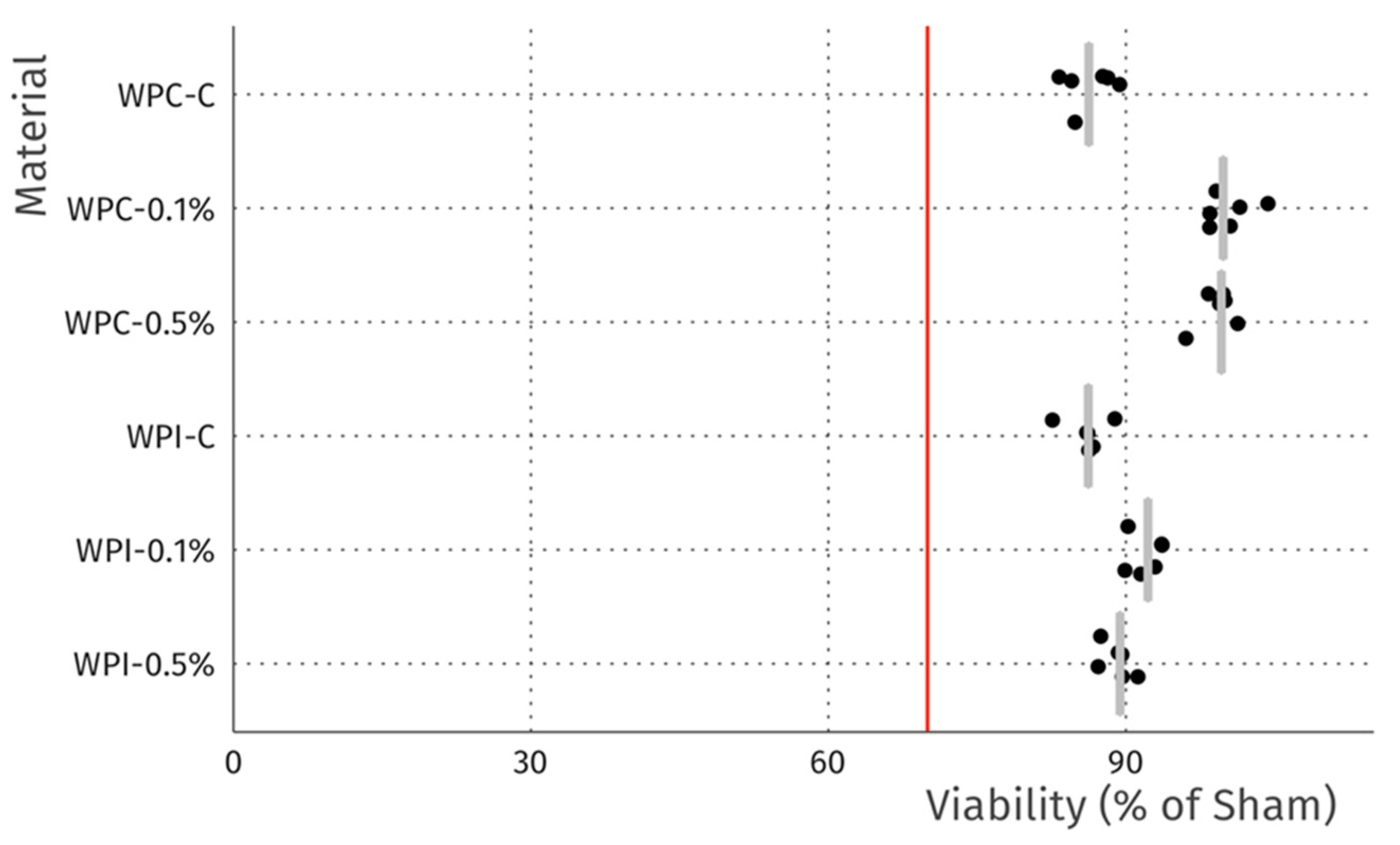
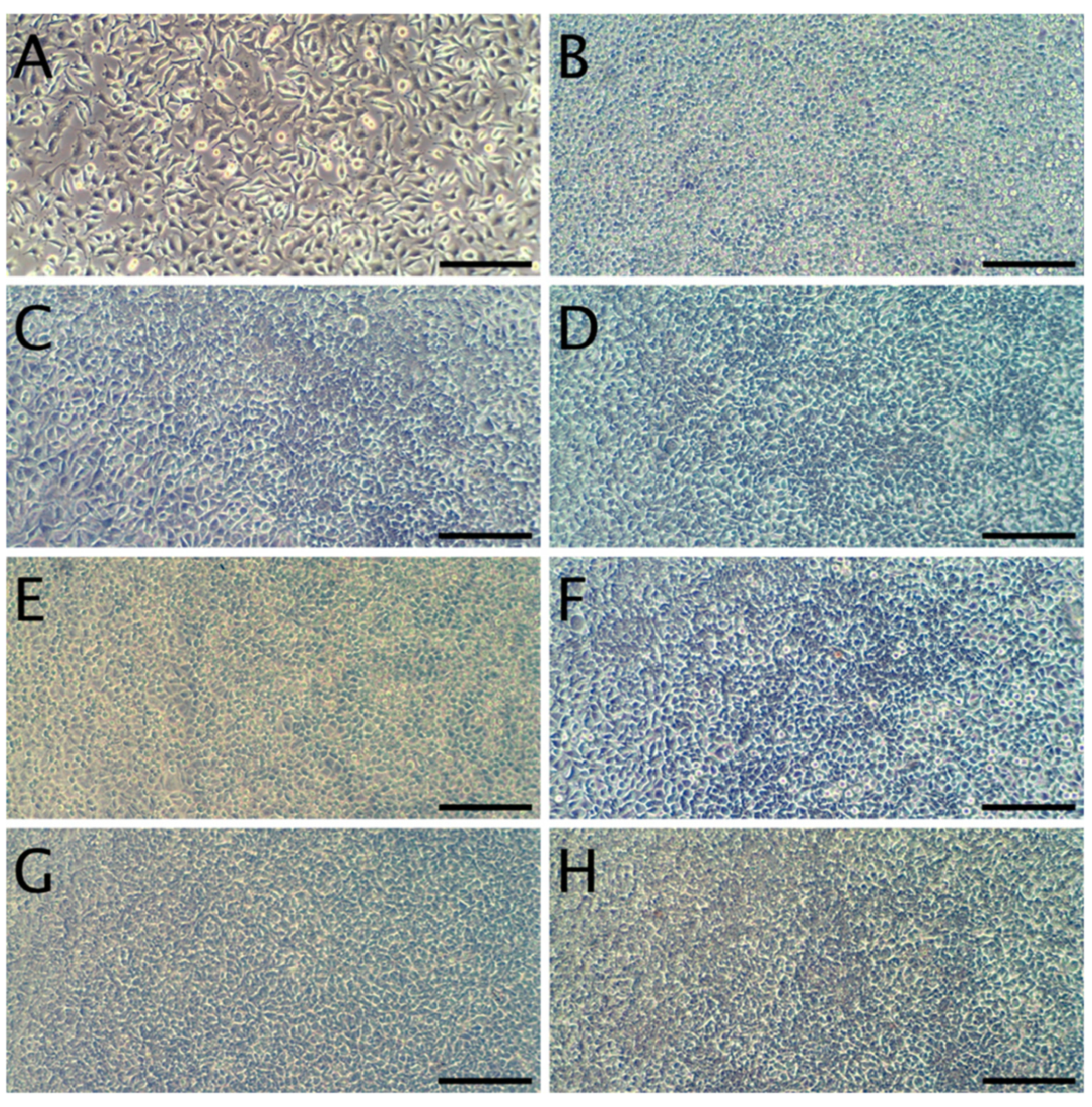
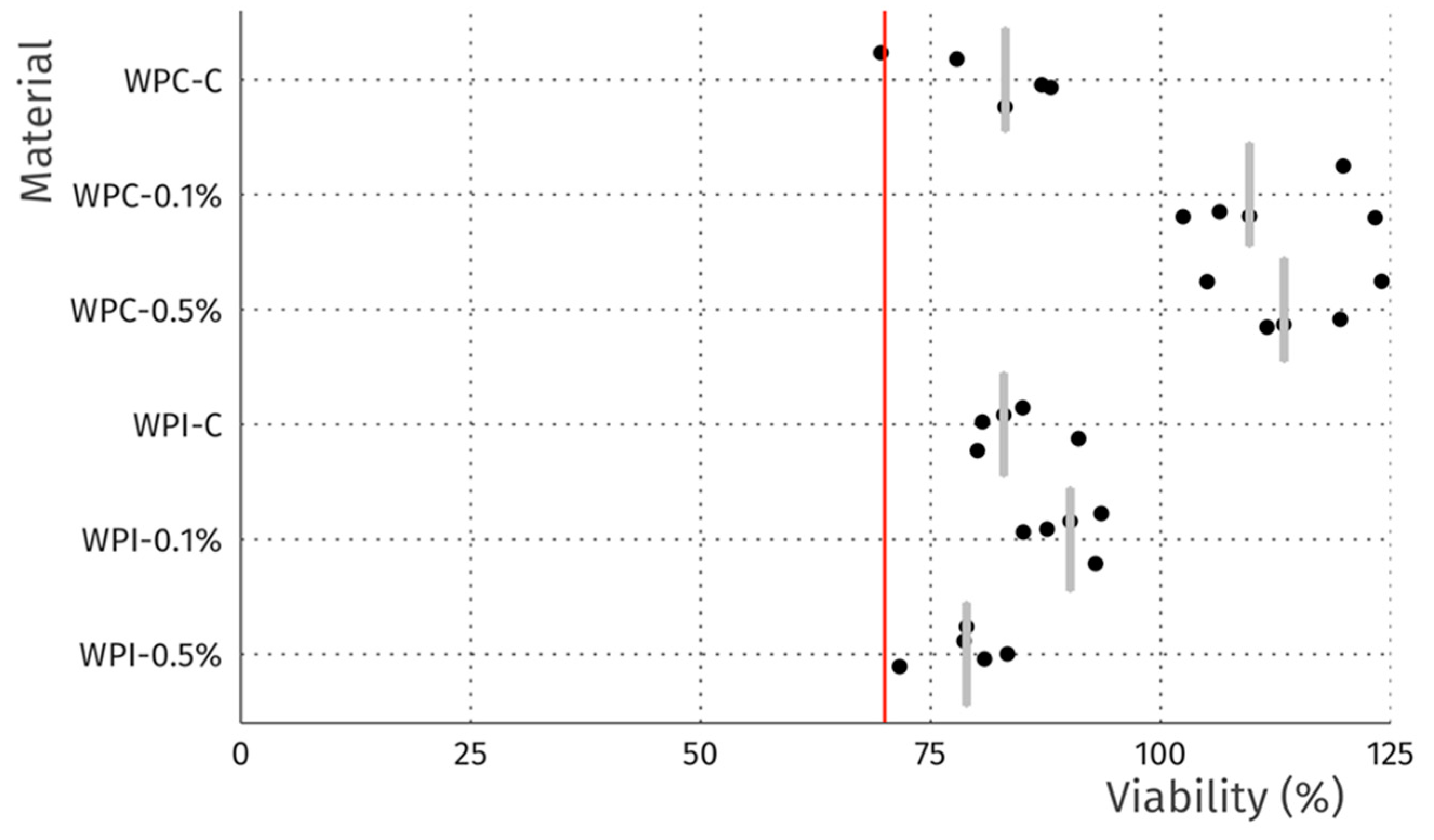
| Sample | MC (%) | WS (%) | SR (%) |
|---|---|---|---|
| WPC-C | 27.16 ± 1.31 b | 65.90 ± 6.12 a | 324.30 ± 68.92 a |
| WPC-0.1 | 27.55 ± 0.25 b | 55.31 ± 4.29 b | 331.47 ± 18.62 a |
| WPC-0.5 | 31.60 ± 0.56 a | 45.02 ± 2.98 c | 469.47 ± 21.69 b |
| WPI-C | 24.31 ± 0.51 a | 70.57 ± 5.24 a | 75.85 ± 8.58 a |
| WPI-0.1 | 26.78 ± 0.38 b | 64.25 ± 3.54 a,b | 111.94 ± 11.69 b |
| WPI-0.5 | 27.92 ± 0.06 b | 57.08 ± 6.37 b | 147.58 ± 18.04 c |
| Sample | Thickness (mm) | TS (MPa) | EB (%) | Tm (°C) | ΔHm (J/g) |
|---|---|---|---|---|---|
| WPC-C | 0.18 ± 0.02 a | 4.11 ± 0.36 a | 18.32 ± 1.28 a | 98.17 | −68.83 |
| WPC-0.1 | 0.18 ± 0.04 a | 4.61 ± 0.45 b | 12.66 ± 1.28 b | 99.57 | −62.50 |
| WPC-0.5 | 0.18 ± 0.01 a | 4.87 ± 1.04 b | 11.14 ± 1.08 b | 106.90 | −59.30 |
| WPI-C | 0.14 ± 0.02 a | 4.50 ± 1.02 a | 16.22 ± 0.62 a | 103.71 | −162.24 |
| WPI-0.1 | 0.16 ± 0.01 a | 5.51 ± 1.34 a | 14.88 ± 2.12 a,b | 106.35 | −125.92 |
| WPI-0.5 | 0.16 ± 0.04 a | 6.13 ± 0.41 b | 13.96 ± 1.08 b | 108.21 | −96.91 |
| Sample | L* | a* | b* | ΔE | YI |
|---|---|---|---|---|---|
| WPC-C | 86.42 ± 0.91 a | −0.51 ± 0.10 a | 11.74 ± 1.73 a | used as standard | 19.41 ± 3.08 a |
| WPC-0.1 | 85.84 ± 1.18 a | −0.44 ± 0.17 a | 13.96 ± 2.24 a | 2.29 ± 0.85 a | 23.23 ± 4.10 a |
| WPC-0.5 | 80.83 ± 1.27 b | 0.90 ± 0.56 b | 25.73 ± 3.26 b | 15.13 ± 0.13 b | 45.48 ± 7.18 b |
| WPI-C | 90.57 ± 0.11 a | −0.85 ± 0.07 a | 4.64 ± 0.47 a | used as standard | 7.32 ± 0.74 a |
| WPI-0.1 | 89.38 ± 0.61 b | −0.72 ± 0.08 a,b | 7.77 ± 1.22 b | 3.35 ± 1.53 a | 12.42 ± 1.87 b |
| WPI-0.5 | 86.41 ± 0.64 c | −0.66 ± 0.10 b | 16.14 ± 1.78 c | 12.23 ± 1.08 b | 26.68 ± 3.14 c |
| Sample | RP (700 nm) | DPPH (%) | ABTS (%) | O2− (%) | ·OH (%) |
|---|---|---|---|---|---|
| WPC-C | 0.661 ± 0.245 a | 37.70 ± 1.67 a | 72.03 ± 5.78 a | 26.80 ± 1.61 a | 31.68 ± 3.21 a |
| WPC-0.1 | 0.863 ± 0.122 a | 49.87 ± 0.13 b | 76.86 ± 6.13 a,b | 29.07 ± 1.17 a,b | 33.45 ± 0.30 a |
| WPC-0.5 | 0.924 ± 0.069 b | 63.68 ± 2.69 c | 85.18 ± 1.74 b | 38.11 ± 0.31 b | 39.08 ± 0.14 b |
| WPI-C | 0.740 ± 0.192 a | 58.76 ± 0.10 a | 60.94 ± 4.50 a | 27.96 ± 3.45 a | 32.33 ± 0.69 a |
| WPI-0.1 | 1.110 ± 0.111 b | 65.45 ± 0.20 b | 95.83 ± 1.52 b | 33.48 ± 5.78 b | 33.91 ± 0.27 b |
| WPI-0.5 | 1.186 ± 0.060 b | 72.70 ± 0.43 c | 96.87 ± 0.48 b | 38.19 ± 1.85 c | 37.47 ± 0.05 c |
| Sample | WCA (°) | WVTR (g/(m2 × Day)) |
|---|---|---|
| WPC-C | 27.67 ± 0.47 a | 1712.64 ± 7.46 a |
| WPC-0.1 | 18.00 ± 0.00 b | 1599.23 ± 5.01 b |
| WPC-0.5 | 14.33 ± 0.47 c | 1483.53 ± 5.49 c |
| WPI-C | 45.00 ± 0.00 a | 1618.57 ± 6.23 a |
| WPI-0.1 | 33.00 ± 0.00 b | 1566.70 ± 7.14 b |
| WPI-0.5 | 31.00 ± 0.00 c | 1490.49 ± 5.37 b |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Łopusiewicz, Ł.; Drozłowska, E.; Trocer, P.; Kostek, M.; Śliwiński, M.; Henriques, M.H.F.; Bartkowiak, A.; Sobolewski, P. Whey Protein Concentrate/Isolate Biofunctional Films Modified with Melanin from Watermelon (Citrullus lanatus) Seeds. Materials 2020, 13, 3876. https://doi.org/10.3390/ma13173876
Łopusiewicz Ł, Drozłowska E, Trocer P, Kostek M, Śliwiński M, Henriques MHF, Bartkowiak A, Sobolewski P. Whey Protein Concentrate/Isolate Biofunctional Films Modified with Melanin from Watermelon (Citrullus lanatus) Seeds. Materials. 2020; 13(17):3876. https://doi.org/10.3390/ma13173876
Chicago/Turabian StyleŁopusiewicz, Łukasz, Emilia Drozłowska, Paulina Trocer, Mateusz Kostek, Mariusz Śliwiński, Marta H. F. Henriques, Artur Bartkowiak, and Peter Sobolewski. 2020. "Whey Protein Concentrate/Isolate Biofunctional Films Modified with Melanin from Watermelon (Citrullus lanatus) Seeds" Materials 13, no. 17: 3876. https://doi.org/10.3390/ma13173876
APA StyleŁopusiewicz, Ł., Drozłowska, E., Trocer, P., Kostek, M., Śliwiński, M., Henriques, M. H. F., Bartkowiak, A., & Sobolewski, P. (2020). Whey Protein Concentrate/Isolate Biofunctional Films Modified with Melanin from Watermelon (Citrullus lanatus) Seeds. Materials, 13(17), 3876. https://doi.org/10.3390/ma13173876