Design and Evaluation of 223Ra-Labeled and Anti-PSMA Targeted NaA Nanozeolites for Prostate Cancer Therapy–Part I
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Synthesis of NaA Nanozeolites
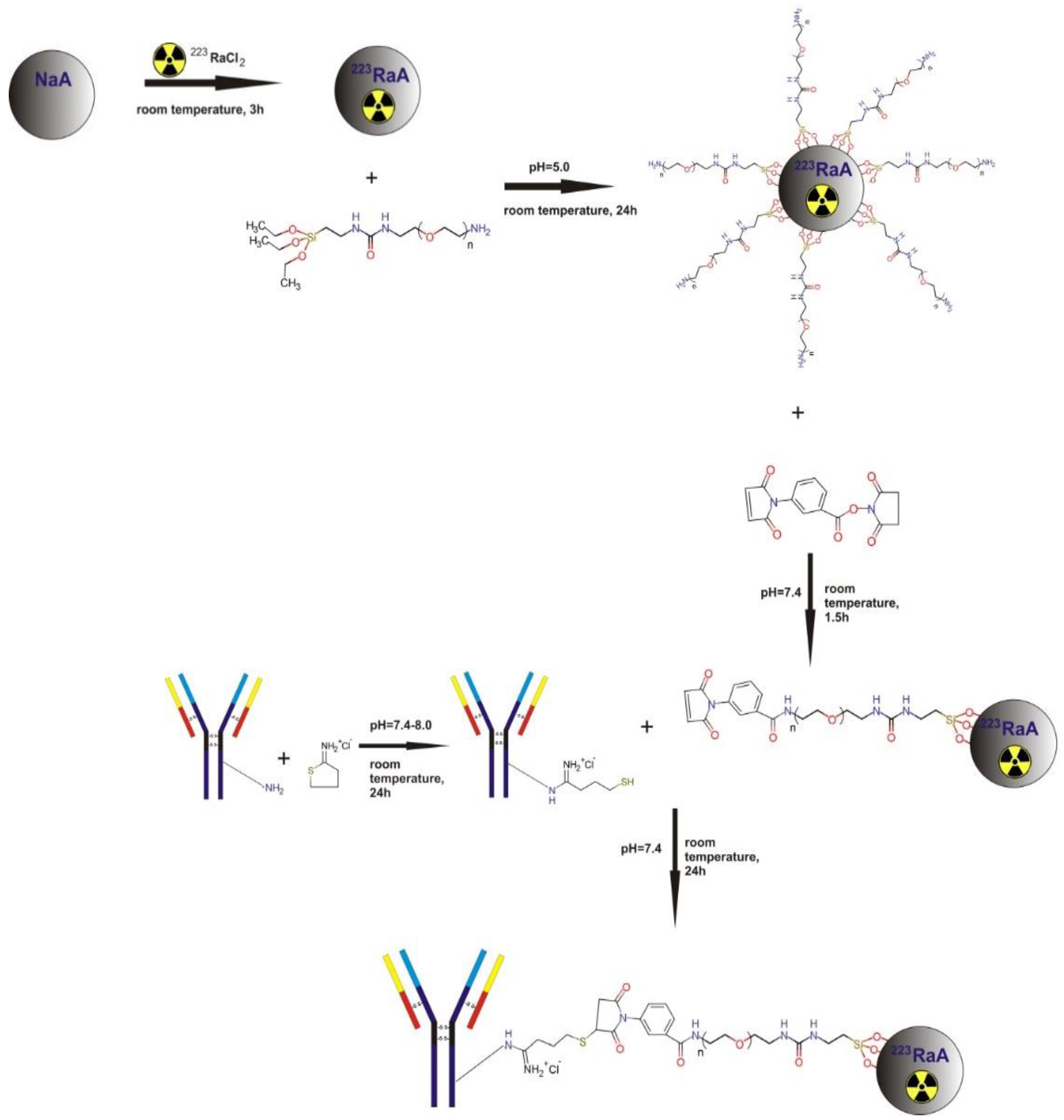
2.3. Radiolabeling of NaA Nanozeolite with 223Ra Radionuclide
2.4. Surface Modification of NaA Nanozeolites with Silane–PEG
2.5. Conjugation of Anti-PSMA Antibody to NaA-Silane-PEG Nanozeolite
2.6. High-Resolution Scanning Electron Microscopy (HR-SEM) with Energy Dispersive X-ray Spectroscopy (EDS)
2.7. Transmission Electron Microscopy (TEM)
2.8. XRD Diffraction Measurements
2.9. Fourier Transform Infrared Spectroscopy (FTIR) Analysis
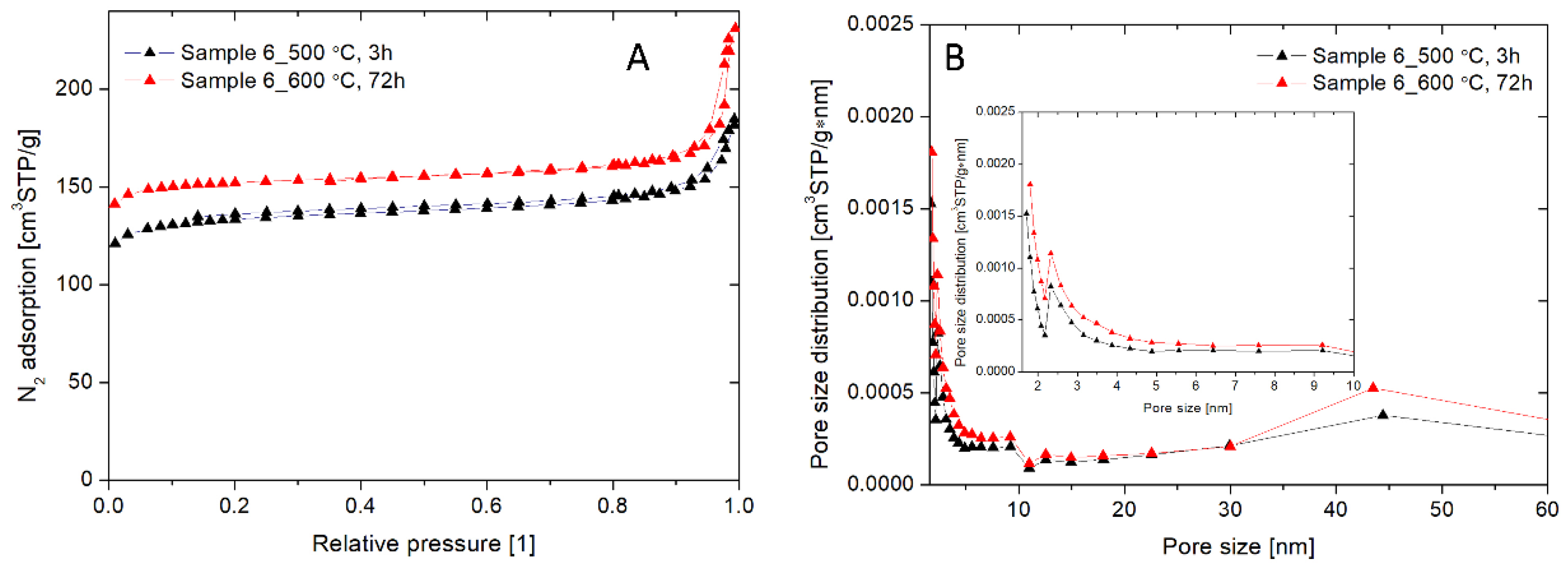
2.10. The Brunauer, Emmett, and Teller (BET) Measurements
2.11. Nanoparticle Tracking Analysis (NTA)
2.12. Zeta Potential and Polydispersity Index Measurements by Dynamic Light Scattering (DLS) Method
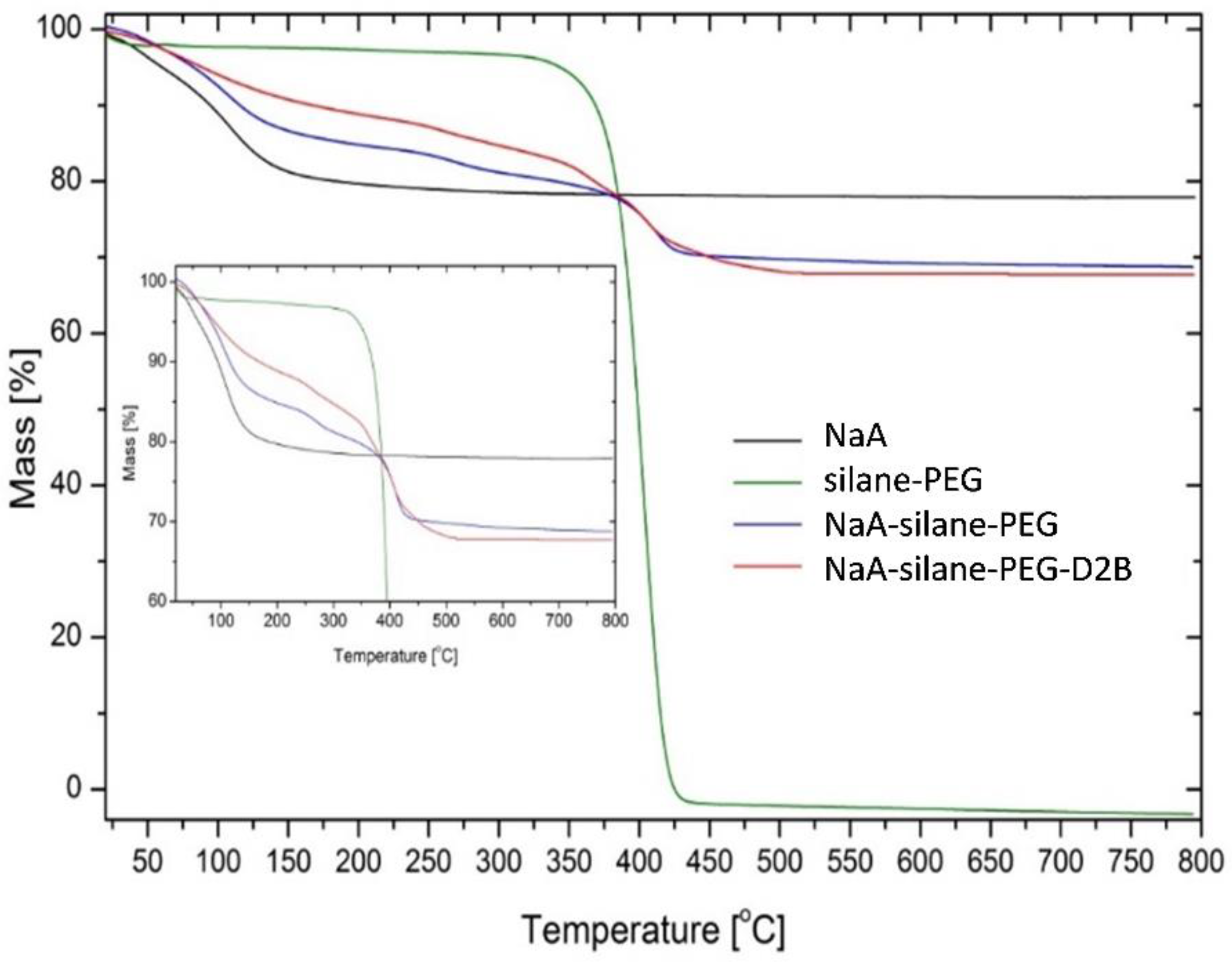
2.13. Thermogravimetric Analysis
2.14. Analysis of the Stability of Radioimmunoconjugate
2.15. Cell Cultures
2.16. Determination of the Number of Attached Anti-PSMA Antibodies Per NaA Nanozeolite
2.17. Binding Specificity and Internalization Properties of 131I-D2B-PEG-Silane-NaA
2.18. Measurements of Metabolic Activity by the MTT Assay
2.19. Statistical Analysis
3. Results and Discussion
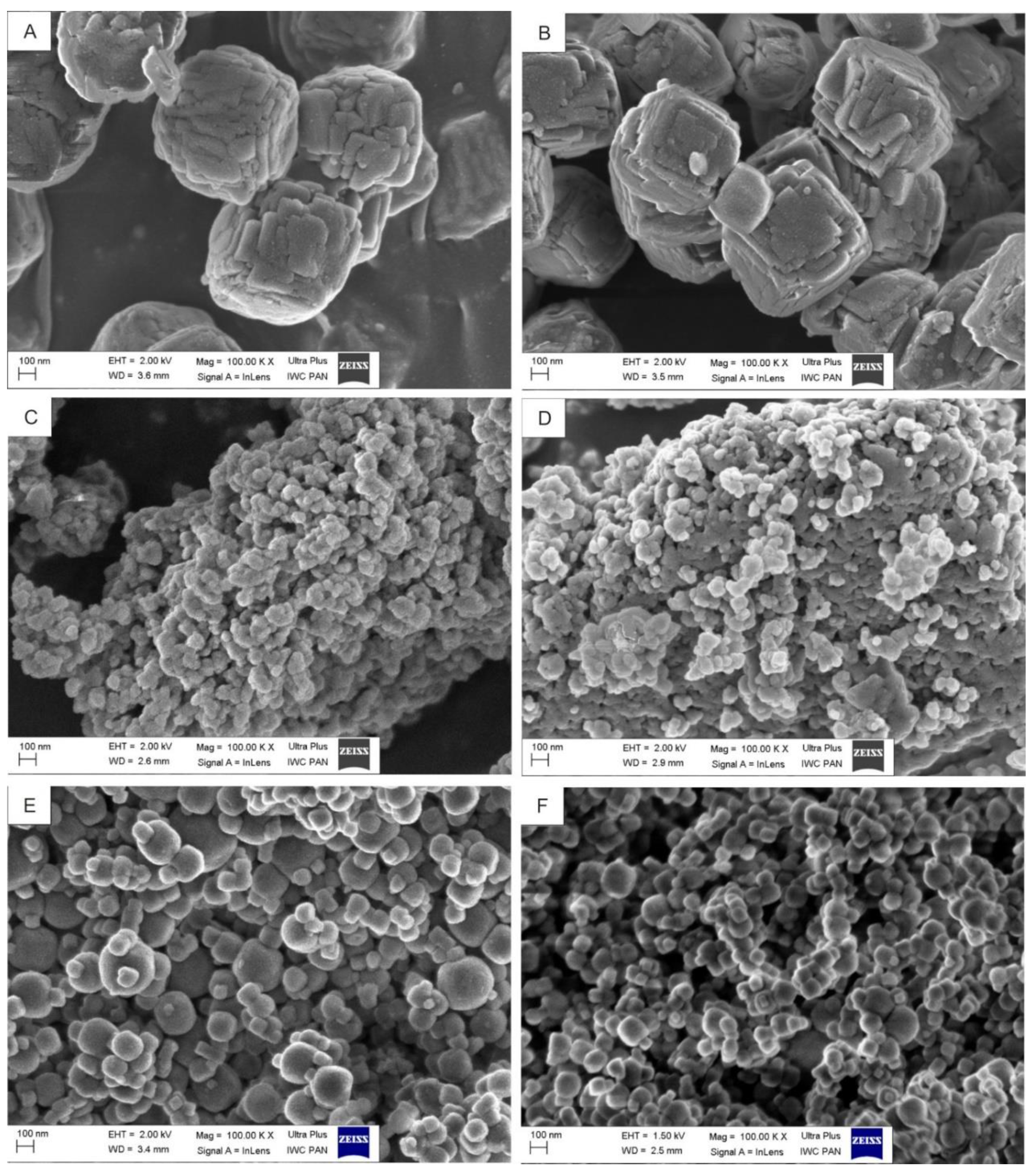
3.1. Influence of Gel Composition and Synthesis Conditions on the Size, Shape, and Crystallinity of Synthesized Zeolites
3.2. Physicochemical Characteristics of the Chosen NaA Nanozeolite (Sample 6)
3.3. Physicochemical Characteristics of the NaA Nanozeolite Modified with Silane-PEG and Anti-PSMA Antibody D2B
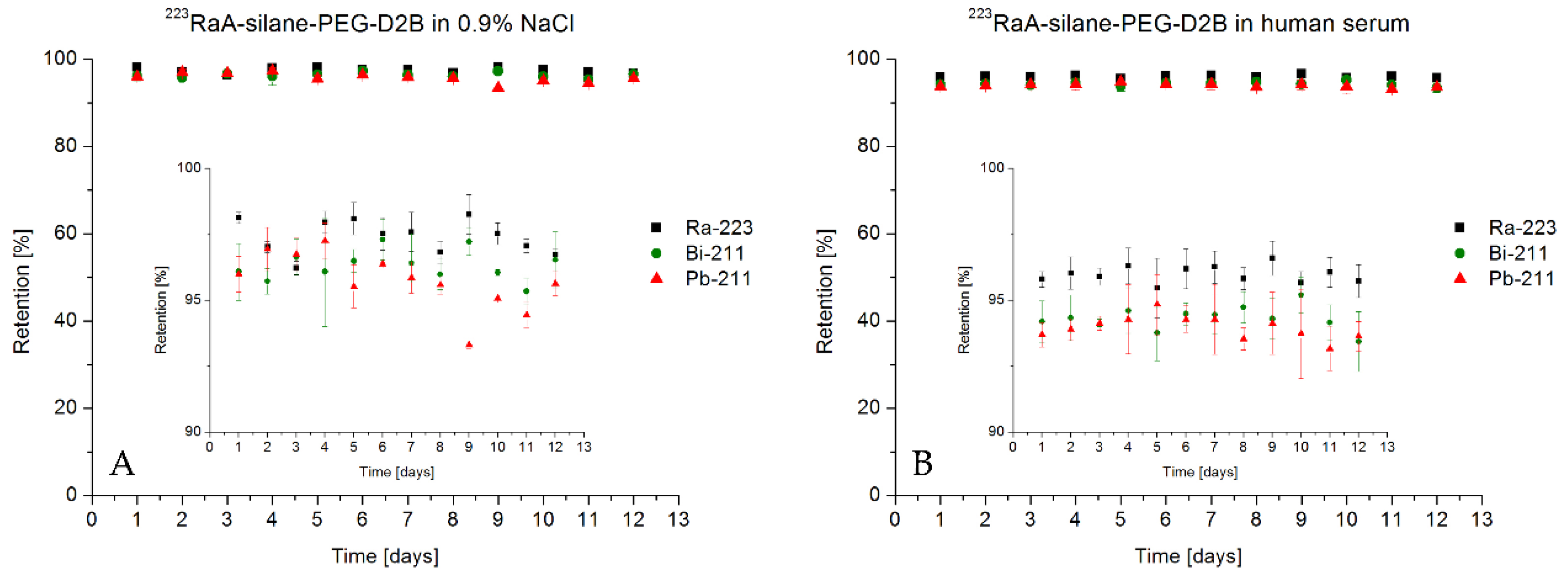
3.4. Stability of Radioimmunoconjugate 223RaA-Silane-PEG-D2B
3.5. The Average Number of Attached Anti-PSMA D2B mAbs Per NaA Nanoparticle
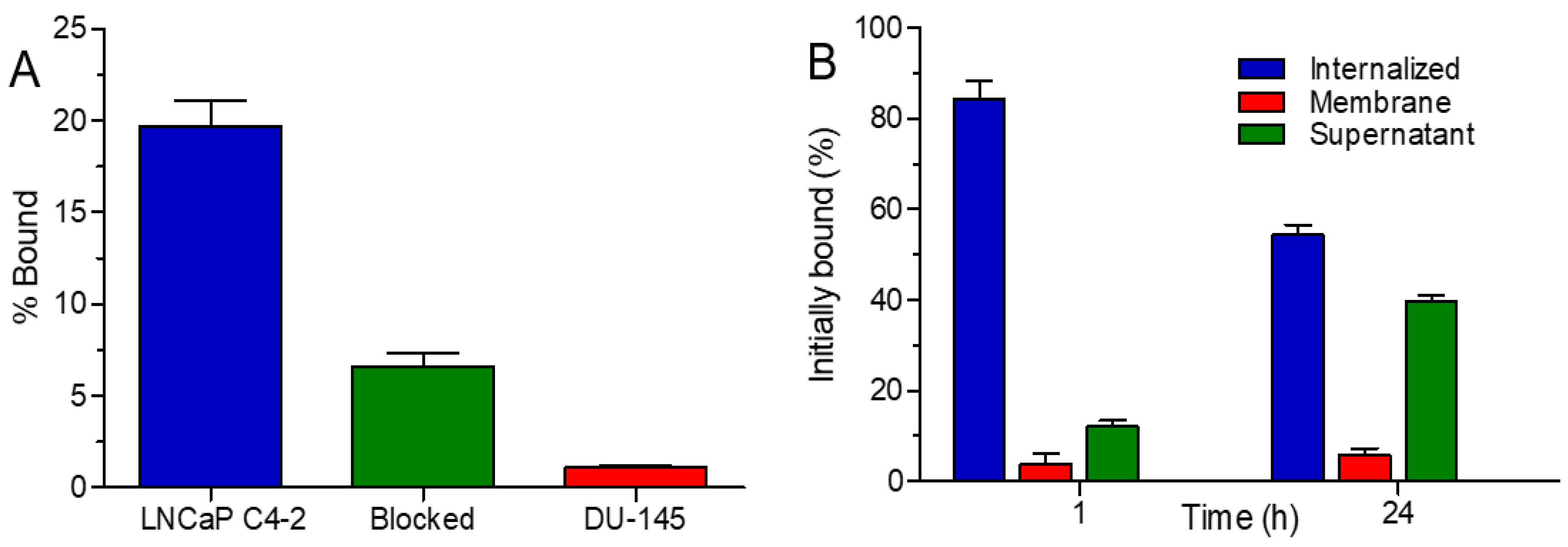
3.6. Binding Specificity and Internalization Properties of 131I-D2B-PEG-Silane-NaA
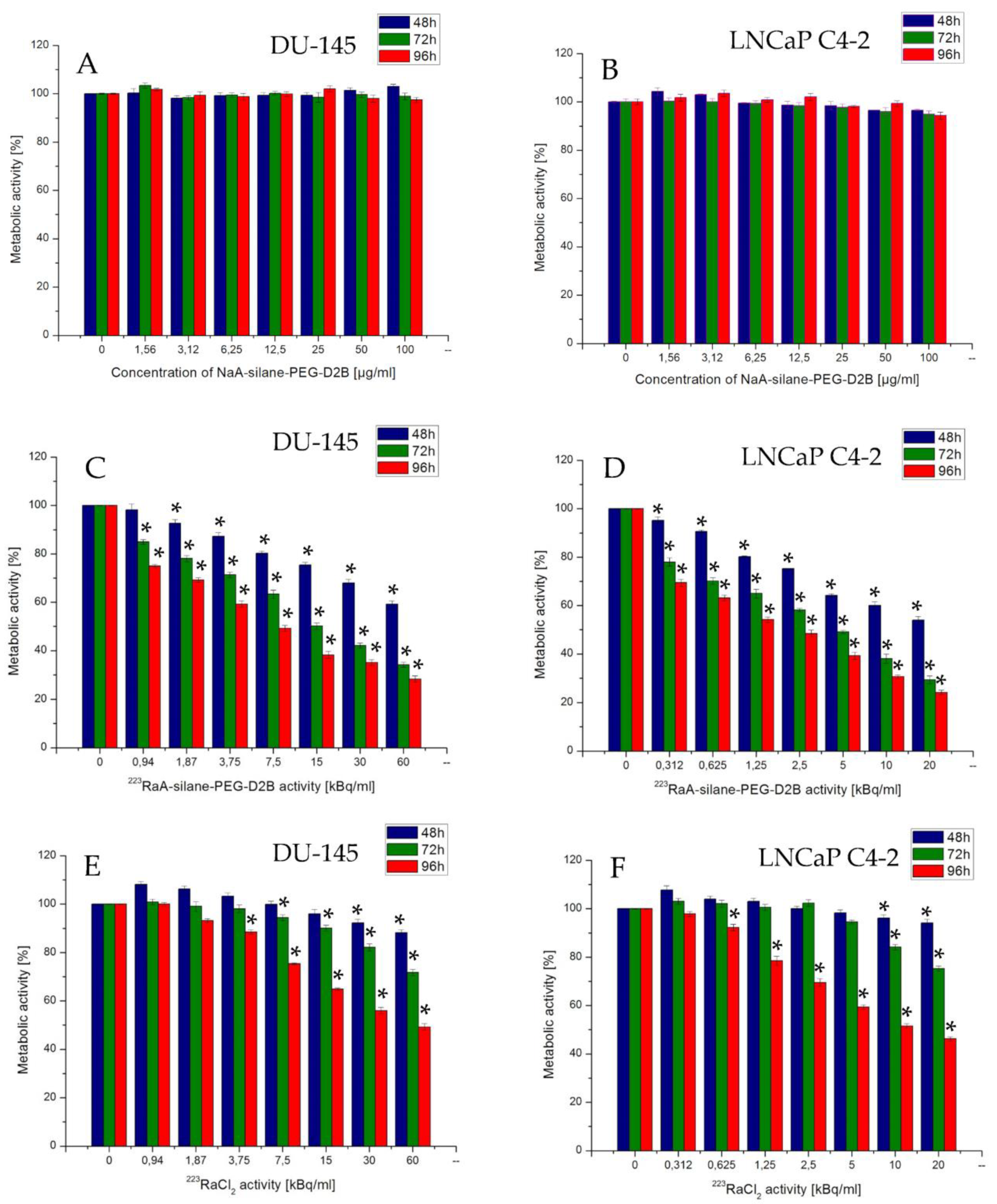
3.7. Effect of the Synthesized Compounds on Cells’ Metabolic Activity
4. Conclusions
5. Patents
Author Contributions
Funding
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Testa, U.; Castelli, G.; Pelosi, E. Cellular and molecular mechanisms underlying prostate cancer development: Therapeutic implications. Medicines 2019, 6, 82. [Google Scholar] [CrossRef]
- Nuhn, P.; De Bono, J.S.; Fizazi, K.; Freedland, S.J.; Grilli, M.; Kantoff, P.W.; Sonpavde, G.; Sternberg, C.N.; Yegnasubramanian, S.; Antonarakis, E.S. Update on systemic prostate cancer therapies: Management of metastatic castration-resistant prostate cancer in the era of precision oncology. Eur. Urol. 2019, 75, 88–99. [Google Scholar] [CrossRef] [PubMed]
- Moreira, D.M.; Howard, L.E.; Sourbeer, K.N.; Amarasekara, H.S.; Chow, L.C.; Cockrell, D.C.; Pratson, C.L.; Hanyok, B.T.; Aronson, W.J.; Kane, C.J.; et al. Predicting time from metastasis to overall survival in castration-resistant prostate cancer: Results from SEARCH. Clin. Genitourin. Cancer 2017, 15, 60–66.e2. [Google Scholar] [CrossRef] [PubMed]
- Wojewódzka, M.; Lankoff, A.; Dusińska, M.; Brunborg, G.; Czerwińska, J.; Iwaneńko, T.; Stepkowski, T.; Szumiel, I.; Kruszewski, M. Treatment with silver nanoparticles delays repair of X-ray induced DNA damage in HepG2 cells. Nukleonika 2011, 56, 29–33. [Google Scholar]
- Troyer, J.K.; Beckett, M.L.; Wright, G.L. Location of prostate-specific membrane antigen in the LNCaP prostate carcinoma cell line. Prostate 1997, 30, 232–242. [Google Scholar] [CrossRef]
- Silver, D.A.; Pellicer, I.; Fair, W.R.; Heston, W.D.W.; Cordon-Cardo, C. Prostate-specific membrane antigen expression in normal and malignant human tissues. Clin. Cancer Res. 1997, 3, 81–85. [Google Scholar]
- Chang, S.S. Monoclonal antibodies and prostate-specific membrane antigen. Curr. Opin. Investig. Drugs 2004, 5, 611–615. [Google Scholar]
- Haffner, M.C.; Kronberger, I.E.; Ross, J.S.; Sheehan, C.E.; Zitt, M.; Mühlmann, G.; Öfner, D.; Zelger, B.; Ensinger, C.; Yang, X.J.; et al. Prostate-specific membrane antigen expression in the neovasculature of gastric and colorectal cancers. Hum. Pathol. 2009, 40, 1754–1761. [Google Scholar] [CrossRef]
- Paschalis, A.; Sheehan, B.; Riisnaes, R.; Rodrigues, D.N.; Gurel, B.; Bertan, C.; Ferreira, A.; Lambros, M.B.K.; Seed, G.; Yuan, W.; et al. Prostate-specific membrane antigen heterogeneity and DNA repair defects in prostate cancer. Eur. Urol. 2019, 76, 469–478. [Google Scholar] [CrossRef]
- Perico, M.E.; Grasso, S.; Brunelli, M.; Martignoni, G.; Munari, E.; Moiso, E.; Fracasso, G.; Cestari, T.; Naim, H.Y.; Bronte, V.; et al. Prostate-specific membrane antigen (PSMA) assembles a macromolecular complex regulating growth and survival of prostate cancer cells “in vitro” and correlating with progression “in vivo”. Oncotarget 2016, 7, 74189–74202. [Google Scholar] [CrossRef]
- Kaittanis, C.; Andreou, C.; Hieronymus, H.; Mao, N.; Foss, C.A.; Eiber, M.; Weirich, G.; Panchal, P.; Gopalan, A.; Zurita, J.; et al. Prostate-specific membrane antigen cleavage of vitamin B9 stimulates oncogenic signaling through metabotropic glutamate receptors. J. Exp. Med. 2018. [Google Scholar]
- Liu, H.; Rajasekaran, A.K.; Moy, P.; Xia, Y.; Kim, S.; Navarro, V.; Rahmati, R.; Bander, N.H. Constitutive and antibody-induced internalization of prostate-specific membrane antigen. Cancer Res. 1998, 58, 4055–4060. [Google Scholar]
- DiPippo, V.A.; Olson, W.C.; Nguyen, H.M.; Brown, L.G.; Vessella, R.L.; Corey, E. Efficacy studies of an antibody-drug conjugate PSMA-ADC in patient-derived prostate cancer xenografts. Prostate 2015, 75, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Tykvart, J.; Navrátil, V.; Sedlák, F.; Corey, E.; Colombatti, M.; Fracasso, G.; Koukolík, F.; Bařinka, C.; Šácha, P.; Konvalinka, J. Comparative analysis of monoclonal antibodies against prostate-specific membrane antigen (PSMA). Prostate 2014, 74, 1674–1690. [Google Scholar] [CrossRef] [PubMed]
- Nevedomskaya, E.; Baumgart, S.J.; Haendler, B. Recent advances in prostate cancer treatment and drug discovery. Int. J. Mol. Sci. 2018, 19, 1359. [Google Scholar] [CrossRef] [PubMed]
- Kratochwil, C.; Bruchertseifer, F.; Giesel, F.L.; Weis, M.; Verburg, F.A.; Mottaghy, F.; Kopka, K.; Apostolidis, C.; Haberkorn, U.; Morgenstern, A. 225Ac-PSMA-617 for PSMA-targeted a-radiation therapy of metastatic castration-resistant prostate cancer. J. Nucl. Med. 2016, 57, 1941–1944. [Google Scholar] [CrossRef]
- Kim, Y.-S.; Brechbiel, M.W. An overview of targeted alpha therapy. Tumour Biol. 2012, 33, 573–590. [Google Scholar] [CrossRef]
- Kratochwil, C.; Haberkorn, U.; Giesel, F.L. Radionuclide therapy of metastatic prostate cancer. Semin. Nucl. Med. 2019, 49, 313–325. [Google Scholar] [CrossRef]
- Yadav, M.P.; Ballal, S.; Tripathi, M.; Damle, N.A.; Sahoo, R.K.; Seth, A.; Bal, C. 177Lu-DKFZ-PSMA-617 therapy in metastatic castration resistant prostate cancer: Safety, efficacy, and quality of life assessment. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 81–91. [Google Scholar] [CrossRef]
- Kratochwil, C.; Haberkorn, U.; Giesel, F.L. 225Ac-PSMA-617 for therapy of prostate cancer. Semin. Nucl. Med. 2020, 50, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Sgouros, G.; Roeske, J.C.; Mcdevitt, M.R.; Palm, S.; Allen, B.J.; Fisher, D.R.; Brill, A.B.; Song, H.; Howell, R.W.; Akabani, G. MIRD Pamphlet No. 22 (abridged): Radiobiology and dosimetry of α -particle emitters for targeted radionuclide therapy. J. Nucl. Med. 2017, 51, 311–328. [Google Scholar] [CrossRef] [PubMed]
- de Kruijff, R.M.; Wolterbeek, H.T.; Denkova, A.G. A critical review of alpha radionuclide therapy-how to deal with recoiling daughters? Pharmaceuticals 2015, 8, 321–336. [Google Scholar] [CrossRef] [PubMed]
- Kozempel, J.; Mokhodoeva, O.; Vlk, M. Progress in targeted alpha-particle therapy. What we learned about recoils release from in vivo generators. Molecules 2018, 23, 581. [Google Scholar] [CrossRef]
- Sathekge, M.; Knoesen, O.; Meckel, M.; Modiselle, M.; Vorster, M.; Marx, S. 213Bi-PSMA-617 targeted alpha-radionuclide therapy in metastatic castration-resistant prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 1099–1100. [Google Scholar] [CrossRef]
- Engle, J.W. The production of Ac-225. Curr. Radiopharm. 2018, 11, 173–179. [Google Scholar] [CrossRef]
- Henriksen, G.; Fisher, D.R.; Roeske, J.C.; Bruland, Ø.S.; Larsen, R.H. Targeting of osseous sites with α-emitting 223Ra: Comparison with the β-emitter 89Sr in mice. J. Nucl. Med. 2003, 44, 252–259. [Google Scholar]
- Henriksen, G.; Schoultz, B.W.; Michaelsen, T.E.; Bruland, S.; Larsen, R.H. Sterically stabilized liposomes as a carrier for α-emitting radium and actinium radionuclides. Nucl. Med. Biol. 2004, 31, 441–449. [Google Scholar] [CrossRef]
- Jonasdottir, T.J.; Fisher, D.R.; Borrebæk, J.; Bruland, Ø.S.; Larsen, R.H. First in vivo evaluation of liposome-encapsulated 223Ra as a potential alpha-particle-emitting cancer therapeutic agent. Anticancer Res. 2006, 26, 2841–2848. [Google Scholar]
- Mokhodoeva, O.; Vlk, M.; Málková, E.; Kukleva, E.; Mičolová, P.; Štamberg, K.; Šlouf, M.; Dzhenloda, R.; Kozempel, J. Study of 223Ra uptake mechanism by Fe3O4 nanoparticles: Towards new prospective theranostic SPIONs. J. Nanoparticle Res. 2016, 18, 301. [Google Scholar] [CrossRef]
- Suchánková, P.; Kukleva, E.; Štamberg, K.; Nykl, P.; Vlk, M.; Kozempel, J. Study of 223Ra uptake mechanism on hydroxyapatite and titanium dioxide nanoparticles as a function of ph. RSC Adv. 2020, 10, 3659–3666. [Google Scholar] [CrossRef]
- Reissig, F.; Zarschler, K.; Hübner, R.; Pietzsch, H.; Kopka, K.; Mamat, C. Sub-10 nm radiolabeled barium sulfate nanoparticles as carriers for theranostic applications and targeted alpha therapy. ChemistryOpen 2020, 9, 797–805. [Google Scholar] [CrossRef] [PubMed]
- Westrøm, S.; Malenge, M.; Jorstad, I.S.; Napoli, E.; Bruland, Ø.S.; Bønsdorff, T.B.; Larsen, R.H. Ra-224 labeling of calcium carbonate microparticles for internal α-therapy: Preparation, stability, and biodistribution in mice. J. Label. Compd. Radiopharm. 2018, 61, 472–486. [Google Scholar] [CrossRef] [PubMed]
- Piotrowska, A.; Leszczuk, E.; Bruchertseifer, F.; Morgenstern, A.; Bilewicz, A. Functionalized NaA nanozeolites labeled with224,225Ra for targeted alpha therapy. J. Nanoparticle Res. 2013, 15, 2082. [Google Scholar] [CrossRef] [PubMed]
- Piotrowska, A.; Męczyńska-Wielgosz, S.; Majkowska-Pilip, A.; Koźmiński, P.; Wójciuk, G.; Cędrowska, E.; Bruchertseifer, F.; Morgenstern, A.; Kruszewski, M.; Bilewicz, A. Nanozeolite bioconjugates labeled with 223Ra for targeted alpha therapy. Nucl. Med. Biol. 2017, 47, 10–18. [Google Scholar] [CrossRef]
- Jafari, M.; Nouri, A.; Kazemimoghadam, M.; Mohammadi, T. Investigations on hydrothermal synthesis parameters in preparation of nanoparticles of LTA zeolite with the aid of TMAOH. Powder Technol. 2013, 237, 442–449. [Google Scholar] [CrossRef]
- Hermanson, G.T.; Książki, G. Bioconjugate Technique. Available online: https://books.google.pl/books?hl=pl&lr=&id=6aO-207lhdgC&oi=fnd&pg=PP1&ots=aK7IB3MpMT&sig=e2Jxi8NlpoTZIDVvB8mjY8iE5-E&redir_esc=y#v=onepage&q&f=false (accessed on 22 June 2020).
- Frigerio, B.; Fracasso, G.; Luison, E.; Cingarlini, S.; Mortarino, M.; Coliva, A.; Seregni, E.; Bombardieri, E.; Zuccolotto, G.; Rosato, A.; et al. A single-chain fragment against prostate specific membrane antigen as a tool to build theranostic reagents for prostate cancer. Eur. J. Cancer 2013, 49, 2223–2232. [Google Scholar] [CrossRef]
- Loiola, A.R.; Andrade, J.C.R.A.; Sasaki, J.M.; da Silva, L.R.D. Structural analysis of zeolite NaA synthesized by a cost-effective hydrothermal method using kaolin and its use as water softener. J. Colloid Interface Sci. 2012, 367, 34–39. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of gases in multimolecular layers. J. Am. Chem. Soc. 1938, 60, 309. [Google Scholar] [CrossRef]
- Cędrowska, E.; Pruszyński, M.; Gawęda, W.; Zuk, M.; Krysiński, P.; Bruchertseifer, F.; Morgenstern, A.; Karageorgou, M.A.; Bouziotis, P.; Bilewicz, A. Trastuzumab conjugated superparamagnetic iron oxide nanoparticles labeled with 225AC as a perspective tool for combined α-radioimmunotherapy and magnetic hyperthermia of HER2-positive breast cancer. Molecules 2020, 25, 1025. [Google Scholar] [CrossRef]
- Pruszyński, M.; Łyczko, M.; Bilewicz, A.; Zalutsky, M.R. Stability and in vivo behavior of Rh[16aneS4-diol]211At complex: A potential precursor for astatine radiopharmaceuticals. Nucl. Med. Biol. 2015, 42, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Tsotsalas, M.M.; Kopka, K.; Luppi, G.; Wagner, S.; Law, M.P.; Schäfers, M.; De Cola, L. Encapsulating 111in in nanocontainers for scintigraphic imaging: Synthesis, characterization, and in vivo biodistribution. ACS Nano 2010, 4, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Męczyńska-Wielgosz, S.; Piotrowska, A.; Majkowska-Pilip, A.; Bilewicz, A.; Kruszewski, M. Effect of surface functionalization on the cellular uptake and toxicity of nanozeolite A. Nanoscale Res. Lett. 2016, 11, 123. [Google Scholar] [CrossRef] [PubMed]
- Mintova, S.; Olson, N.H.; Valtchev, V.; Bein, T. Mechanism of zeolite a nanocrystal growth from colloids at room temperature. Science 1999, 283, 958–960. [Google Scholar] [CrossRef]
- Mintova, S.; Bein, T. Nanosized zeolite films for vapor-sensing applications. Microporous Mesoporous Mater. 2001, 50, 159–166. [Google Scholar] [CrossRef]
- Grosse, Y.; Baan, R.; Straif, K.; Secretan, B.; El Ghissassi, F.; Cogliano, V.; Cantor, K.P.; Falconer, I.R.; Levallois, P.; Verger, P.; et al. Carcinogenicity of nitrate, nitrite, and cyanobacterial peptide toxins. Lancet Oncol. 2006, 7, 628–629. [Google Scholar] [CrossRef]
- Yang, G.; Zhang, X.; Liu, S.; Yeung, K.L.; Wang, J. A novel method for the assembly of nano-zeolite crystals on porous stainless steel microchannel and then zeolite film growth. J. Phys. Chem. Solids 2007, 68, 26–31. [Google Scholar] [CrossRef]
- Biemmi, E.; Bein, T. Assembly of nanozeolite monolayers on the gold substrates of piezoelectric sensors. Langmuir 2008, 24, 11196–11202. [Google Scholar] [CrossRef]
- Jawor, A.; Jeong, B.H.; Hoek, E.M.V. Synthesis, characterization, and ion-exchange properties of colloidal zeolite nanocrystals. J. Nanoparticle Res. 2009, 11, 1795–1803. [Google Scholar] [CrossRef]
- Mirfendereski, S.M. Synthesis of zeolite NaA nano-crystals: Effect of synthesis parameters on crystallinity and crystal size. Iran. J. Chem. Eng. 2019, 16, 22–38. [Google Scholar]
- Cundy, C.S.; Lowe, B.M.; Sinclair, D.M. Crystallisation of zeolitic molecular sieves: Direct measurements of the growth behaviour of single crystals as a function of synthesis conditions. Faraday Discuss. 1993, 95, 235–252. [Google Scholar] [CrossRef]
- Guo, Q.; Huang, D.; Kou, X.; Cao, W.; Li, L.; Ge, L.; Li, J. Synthesis of disperse amorphous SiO2 nanoparticles via sol-gel process. Ceram. Int. 2017, 43, 192–196. [Google Scholar] [CrossRef]
- Velmurugan, P.; Shim, J.; Lee, K.J.; Cho, M.; Lim, S.S.; Seo, S.K.; Cho, K.M.; Bang, K.S.; Oh, B.T. Extraction, characterization, and catalytic potential of amorphous silica from corn cobs by sol-gel method. J. Ind. Eng. Chem. 2015, 29, 298–303. [Google Scholar] [CrossRef]
- Made Joni, I.; Vanitha, M.; Panatarani, C.; Faizal, F. Dispersion of amorphous silica nanoparticles via beads milling process and their particle size analysis, hydrophobicity and anti-bacterial activity. Adv. Powder Technol. 2020, 31, 370–380. [Google Scholar] [CrossRef]
- Brar, T.; France, P.; Smirniotis, P.G. Control of crystal size and distribution of zeolite A. Ind. Eng. Chem. Res. 2001, 40, 1133–1139. [Google Scholar] [CrossRef]
- Fahlke, B.; Starke, P.; Seefeld, V.; Wieker, W.; Wendlandt, K.P. On the intermediates in zeolite Y synthesis. Zeolites 1987, 7, 209–213. [Google Scholar] [CrossRef]
- Reinoso, D.; Adrover, M.; Pedernera, M. Green synthesis of nanocrystalline faujasite zeolite. Ultrason. Sonochem. 2018, 42, 303–309. [Google Scholar] [CrossRef]
- Bronić, J.; Subotlć, B.; Śmit, I.; Despotovlć, L.J.A. Influence of gel ageing on zeolite nucleation processes. In Studies in Surface Science and Catalysis; Elsevier Inc.: Amsterdam, The Netherlands, 1988; Volume 37, pp. 107–114. [Google Scholar]
- Gora, L.; Streletzky, K.; Thompson, R.W.; Phillies, G.D.J. Study of the effects of initial-bred nuclei on zeolite NaA crystallization by quasi-elastic light scattering spectroscopy and electron microscopy. Zeolites 1997, 19, 98–106. [Google Scholar] [CrossRef]
- Zhang, X.; Tang, D.; Jiang, G. Synthesis of zeolite NaA at room temperature: The effect of synthesis parameters on crystal size and its size distribution. Adv. Powder Technol. 2013, 24, 689–696. [Google Scholar] [CrossRef]
- Zhang, X.Q.; Xu, X.; Bertrand, N.; Pridgen, E.; Swami, A.; Farokhzad, O.C. Interactions of nanomaterials and biological systems: Implications to personalized nanomedicine. Adv. Drug Deliv. Rev. 2012, 64, 1363–1384. [Google Scholar] [CrossRef]
- Bahari, L.A.S.; Hamishehkar, H. The impact of variables on particle size of solid lipid nanoparticles and nanostructured lipid carriers; A comparative literature review. Adv. Pharm. Bull. 2016, 6, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Treacy, M.M.J.; Higgins, J.B. Collection of Simulated XRD Powder Patterns for Zeolites, 5th Revised ed.; Elsevier: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Fotovat, F.; Kazemian, H.; Kazemeini, M. Synthesis of Na-A and faujasitic zeolites from high silicon fly ash. Mater. Res. Bull. 2009, 44, 913–917. [Google Scholar] [CrossRef]
- Fruijtier-Pölloth, C. The safety of synthetic zeolites used in detergents. Arch. Toxicol. 2009, 83, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Kruk, M.; Jaroniec, M. Gas adsorption characterization of ordered organic-inorganic nanocomposite materials. Chem. Mater. 2001, 13, 3169–3183. [Google Scholar] [CrossRef]
- Chanda, N.; Kan, P.; Watkinson, L.D.; Shukla, R.; Zambre, A.; Carmack, T.L.; Engelbrecht, H.; Lever, J.R.; Katti, K.; Fent, G.M.; et al. Radioactive gold nanoparticles in cancer therapy: Therapeutic efficacy studies of GA-198AuNP nanoconstruct in prostate tumor-bearing mice. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 201–209. [Google Scholar] [CrossRef]
- Palchetti, S.; Colapicchioni, V.; Digiacomo, L.; Caracciolo, G.; Pozzi, D.; Capriotti, A.L.; La Barbera, G.; Laganà, A. The protein corona of circulating PEGylated liposomes. Biochim. Biophys. Acta Biomembr. 2016, 1858, 189–196. [Google Scholar] [CrossRef]
- Lankoff, A.; Sandberg, W.J.; Wegierek-Ciuk, A.; Lisowska, H.; Refsnes, M.; Sartowska, B.; Schwarze, P.E.; Meczynska-Wielgosz, S.; Wojewodzka, M.; Kruszewski, M. The effect of agglomeration state of silver and titanium dioxide nanoparticles on cellular response of HepG2, A549 and THP-1 cells. Toxicol. Lett. 2012, 208, 197–213. [Google Scholar] [CrossRef]
- Mehn, D.; Caputo, F.; Rösslein, M.; Calzolai, L.; Saint-Antonin, F.; Courant, T.; Wick, P.; Gilliland, D. Larger or more? Nanoparticle characterisation methods for recognition of dimers. RSC Adv. 2017, 7, 27747–27754. [Google Scholar] [CrossRef]
- Nguyen, V.H.; Lee, B.J. Protein corona: A new approach for nanomedicine design. Int. J. Nanomed. 2017, 12, 3137–3151. [Google Scholar] [CrossRef]
- Lu, G.W.; Gao, P. Emulsions and microemulsions for topical and transdermal drug delivery. In Handbook of Non-Invasive Drug Delivery Systems; Elsevier: Amsterdam, The Netherlands, 2010; pp. 59–94. [Google Scholar]
- Samimi, S.; Maghsoudnia, N.; Eftekhari, R.B.; Dorkoosh, F. Lipid-based nanoparticles for drug delivery systems. In Characterization and Biology of Nanomaterials for Drug Delivery: Nanoscience and Nanotechnology in Drug Delivery; Elsevier: Amsterdam, The Netherlands, 2018; pp. 47–76. ISBN 9780128140321. [Google Scholar]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of particle size and polydispersity index on the clinical applications of lipidic nanocarrier systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef]
- Bikit, I.; Mrdja, D.; Bikit, K.; Grujic, S.; Knezevic, D.; Forkapic, S.; Kozmidis-Luburic, U. Radon adsorption by zeolite. Radiat. Meas. 2015, 72, 70–74. [Google Scholar] [CrossRef]
- Holzwarth, U.; Ojea Jimenez, I.; Calzolai, L. A random walk approach to estimate the confinement of α-particle emitters in nanoparticles for targeted radionuclide therapy. EJNMMI Radiopharm. Chem. 2018, 3, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Kim, J.A.; Na, H.-Y.; Cho, S.-E.; Park, S.; Jung, S.-C.; Suh, S.H. Globotriaosylceramide induces lysosomal degradation of endothelial KCa3.1 in fabry disease. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 81–89. [Google Scholar] [CrossRef]
- Pruszynski, M.; Koumarianou, E.; Vaidyanathan, G.; Revets, H.; Devoogdt, N.; Lahoutte, T.; Lyerly, H.K.; Zalutsky, M.R. Improved tumor targeting of anti-her2 nanobody through n-succinimidyl 4-guanidinomethyl-3-iodobenzoate radiolabeling. J. Nucl. Med. 2014, 55, 650–656. [Google Scholar] [CrossRef]
- El-Awady, R.A.; Dikomey, E.; Dahm-Daphi, J. Radiosensitivity of human tumour cells is correlated with the induction but not with the repair of DNA double-strand breaks. Br. J. Cancer 2003, 89, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Gudkov, A.V.; Komarova, E.A. The role of p53 in determining sensitivity to radiotherapy. Nat. Rev. Cancer 2003, 3, 117–129. [Google Scholar] [CrossRef]
- Gaupel, A.C.; Wang, W.L.W.; Mordan-McCombs, S.; Yu Lee, E.C.; Tenniswood, M. Xenograft, Transgenic, and Knockout Models of Prostate Cancer; Elsevier: Amsterdam, The Netherlands, 2013; ISBN 9780124158948. [Google Scholar]
- John-Aryankalayil, M.; Palayoor, S.T.; Makinde, A.Y.; Cerna, D.; Simone, C.B.; Falduto, M.T.; Magnuson, S.R.; Coleman, C.N. Fractionated radiation alters oncomir and tumor suppressor miRNAs in human prostate cancer cells. Radiat. Res. 2012, 178, 105. [Google Scholar] [CrossRef]
- McIlwrath, A.J.; Vasey, P.A.; Ross, G.M.; Brown, R. Cell cycle arrests and radiosensitivity of human tumor cell lines: Dependence on wild-type p53 for radiosensitivity. Cancer Res. 1994, 54, 3718–3722. [Google Scholar]
- Alsbeih, G.; Torres, M.; Al-Harbi, N.; Alsubael, M. Loss of wild-type Trp53 protein in mouse fibroblasts leads to increased radioresistance with consequent decrease in repair of potentially lethal damage. Radiat. Res. 2004, 161, 185–192. [Google Scholar] [CrossRef]
- Mirzayans, R.; Andrais, B.; Scott, A.; Wang, Y.W.; Murray, D. Ionizing radiation-induced responses in human cells with differing TP53 status. Int. J. Mol. Sci. 2013, 14, 22409–22435. [Google Scholar] [CrossRef]
- Ouyang, D.-Y.; Xu, L.-H.; He, X.-H.; Zhang, Y.-T.; Zeng, L.-H.; Cai, J.-Y.; Ren, S. Autophagy is differentially induced in prostate cancer LNCaP, DU145 and PC-3 cells via distinct splicing profiles of ATG5. Autophagy 2013, 9, 20–32. [Google Scholar] [CrossRef] [PubMed]
- Elgqvist, J.; Timmermand, O.V.; Larsson, E.; Strand, S.E. Radiosensitivity of prostate cancer cell lines for irradiation from beta particle-emitting radionuclide 177Lu compared to alpha particles and gamma rays. Anticancer Res. 2016, 36, 103–109. [Google Scholar] [PubMed]
- Taylor, R.M.; Severns, V.; Brown, D.C.; Bisoffi, M.; Sillerud, L.O. Prostate cancer targeting motifs: Expression of α vβ 3, neurotensin receptor 1, prostate specific membrane antigen, and prostate stem cell antigen in human prostate cancer cell lines and xenografts. Prostate 2012, 72, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.; Wang, X.; Klein, E.; Heston, W.D.W. Novel role of prostate-specific membrane antigen in suppressing prostate cancer invasiveness. Cancer Res. 2005, 65, 727–731. [Google Scholar]

| Sample | Gel Composition | Reaction Time (h) | Crystallization Temperature (°C) | Crystallization Time (h) |
|---|---|---|---|---|
| 1 | 1.11 Na2O:0.16 Al2O3:1 SiO2:85.97 H2O: 2.21 TMAOH | 48 * | 100 | 24 |
| 2 | 96 * | 100 | 5 | |
| 3 | 48 * | 40 | 24 | |
| 4 | 48 * | 60 | 24 | |
| 5 | 0.16 Na2O:1 Al2O3:6 SiO2:350 H2O: 14.56 TMAOH | 96 * | 100 | 24 |
| 6 | 96 # | 100 | 24 |
| Sample | Average Crystal Size (nm) | Crystal Size Distribution (nm) | Crystallinity (%) |
|---|---|---|---|
| 1 | 651.40 ± 163.24 | 600–800 | 80 |
| 2 | 549.80 ± 132.03 | 500–700 | 65 |
| 3 | 88.72 ± 46.12 | 30–100 | Amorphous |
| 4 | 130.33 ± 44.75 | 50–150 | 30 |
| 5 | 240.63 ± 92.52 | 50–300 | 94 |
| 6 | 120.24 ± 28.33 | 50–150 | 95 |
| Time [h] | Temperature [°C] | SBET [m2/g] | Dave [nm] | Vt [cm3/g] | Vmicro [cm3/g] | Microporosity [%] |
|---|---|---|---|---|---|---|
| 3 | 500 | 428.52 | 2.58 | 0.27 | 0.179 | 64.80 |
| 72 | 600 | 485.03 | 2.88 | 0.35 | 0.217 | 62.14 |
| Sample | Hydrodynamic Diameter [nm] | Zeta (ζ) Potential [mV] | Polydispersity Index |
|---|---|---|---|
| NaA | 196.7 ± 53.5 * 206.0 ± 40.3 # | −40.3 ± 2.11 | 0.292 ± 1.21 |
| NaA-silane PEG | 216.9 ± 56.62 * 217.1 ± 34.22 # | −43.1 ± 2.45 | 0.202 ± 1.01 |
| NaA-silane-PEG-D2B | 225.1 ± 48.61 * 226.1 ± 44.21 # | −45.08 ± 1.97 | 0.186 ± 1.04 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Czerwińska, M.; Fracasso, G.; Pruszyński, M.; Bilewicz, A.; Kruszewski, M.; Majkowska-Pilip, A.; Lankoff, A. Design and Evaluation of 223Ra-Labeled and Anti-PSMA Targeted NaA Nanozeolites for Prostate Cancer Therapy–Part I. Materials 2020, 13, 3875. https://doi.org/10.3390/ma13173875
Czerwińska M, Fracasso G, Pruszyński M, Bilewicz A, Kruszewski M, Majkowska-Pilip A, Lankoff A. Design and Evaluation of 223Ra-Labeled and Anti-PSMA Targeted NaA Nanozeolites for Prostate Cancer Therapy–Part I. Materials. 2020; 13(17):3875. https://doi.org/10.3390/ma13173875
Chicago/Turabian StyleCzerwińska, Malwina, Giulio Fracasso, Marek Pruszyński, Aleksander Bilewicz, Marcin Kruszewski, Agnieszka Majkowska-Pilip, and Anna Lankoff. 2020. "Design and Evaluation of 223Ra-Labeled and Anti-PSMA Targeted NaA Nanozeolites for Prostate Cancer Therapy–Part I" Materials 13, no. 17: 3875. https://doi.org/10.3390/ma13173875
APA StyleCzerwińska, M., Fracasso, G., Pruszyński, M., Bilewicz, A., Kruszewski, M., Majkowska-Pilip, A., & Lankoff, A. (2020). Design and Evaluation of 223Ra-Labeled and Anti-PSMA Targeted NaA Nanozeolites for Prostate Cancer Therapy–Part I. Materials, 13(17), 3875. https://doi.org/10.3390/ma13173875








