Fast Response Isopropanol Sensing Properties with Sintered BiFeO3 Nanocrystals
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of BiFeO3 Nanocrystals
2.2. Characterization
2.3. Fabrication and Measurement of Gas Sensors
3. Results and Discussion
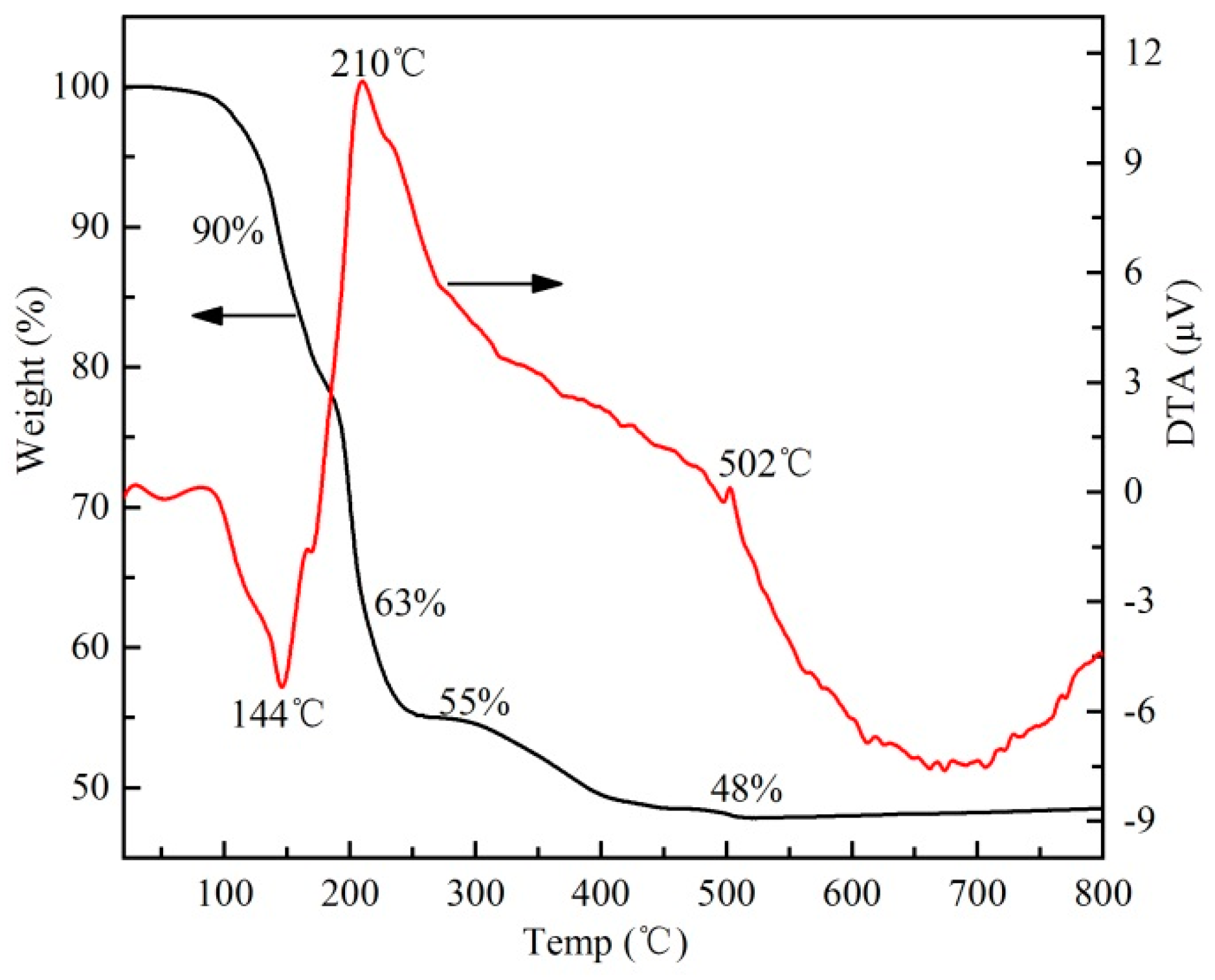
3.1. Growth Analysis
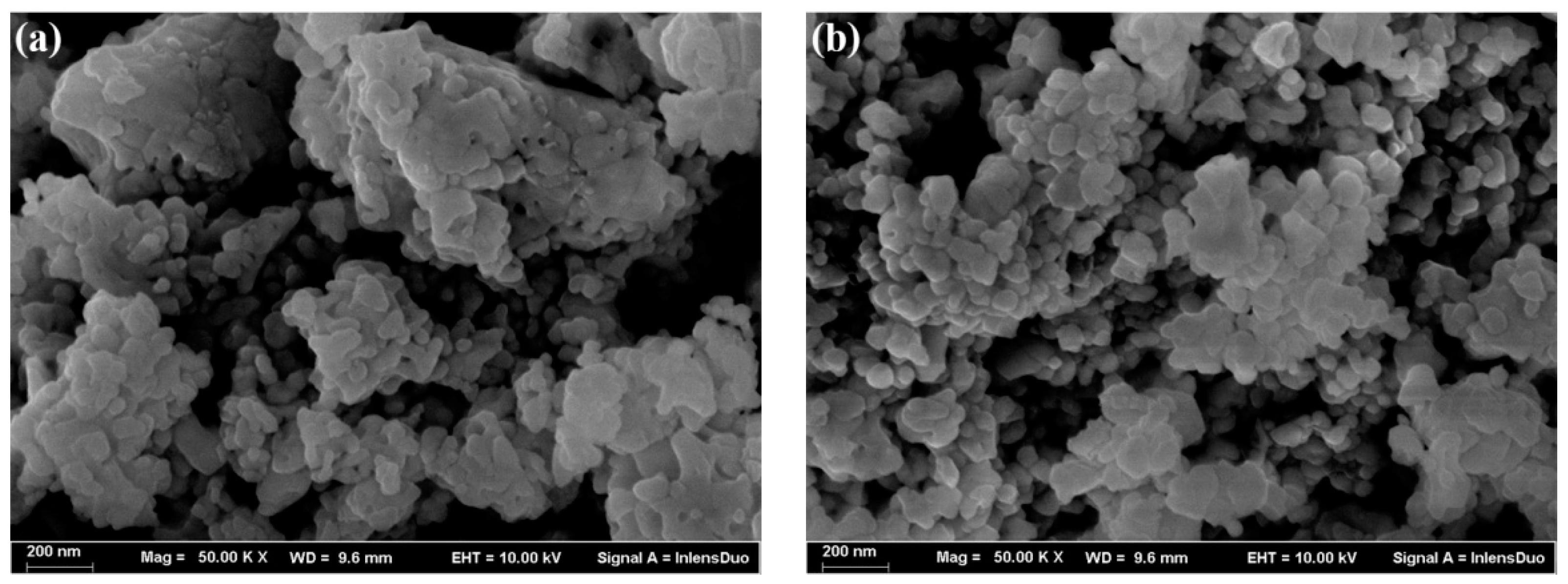
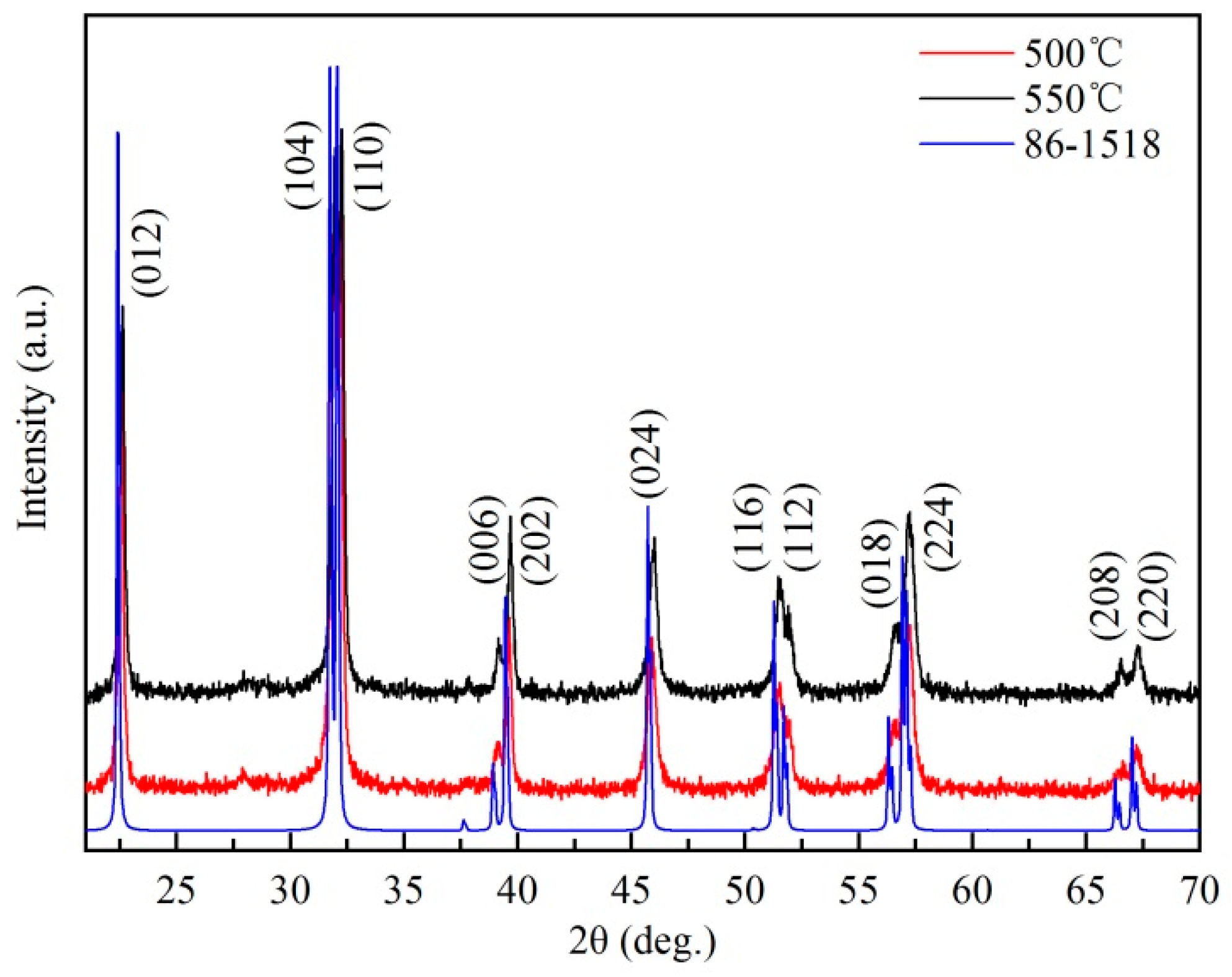
3.2. Characterization
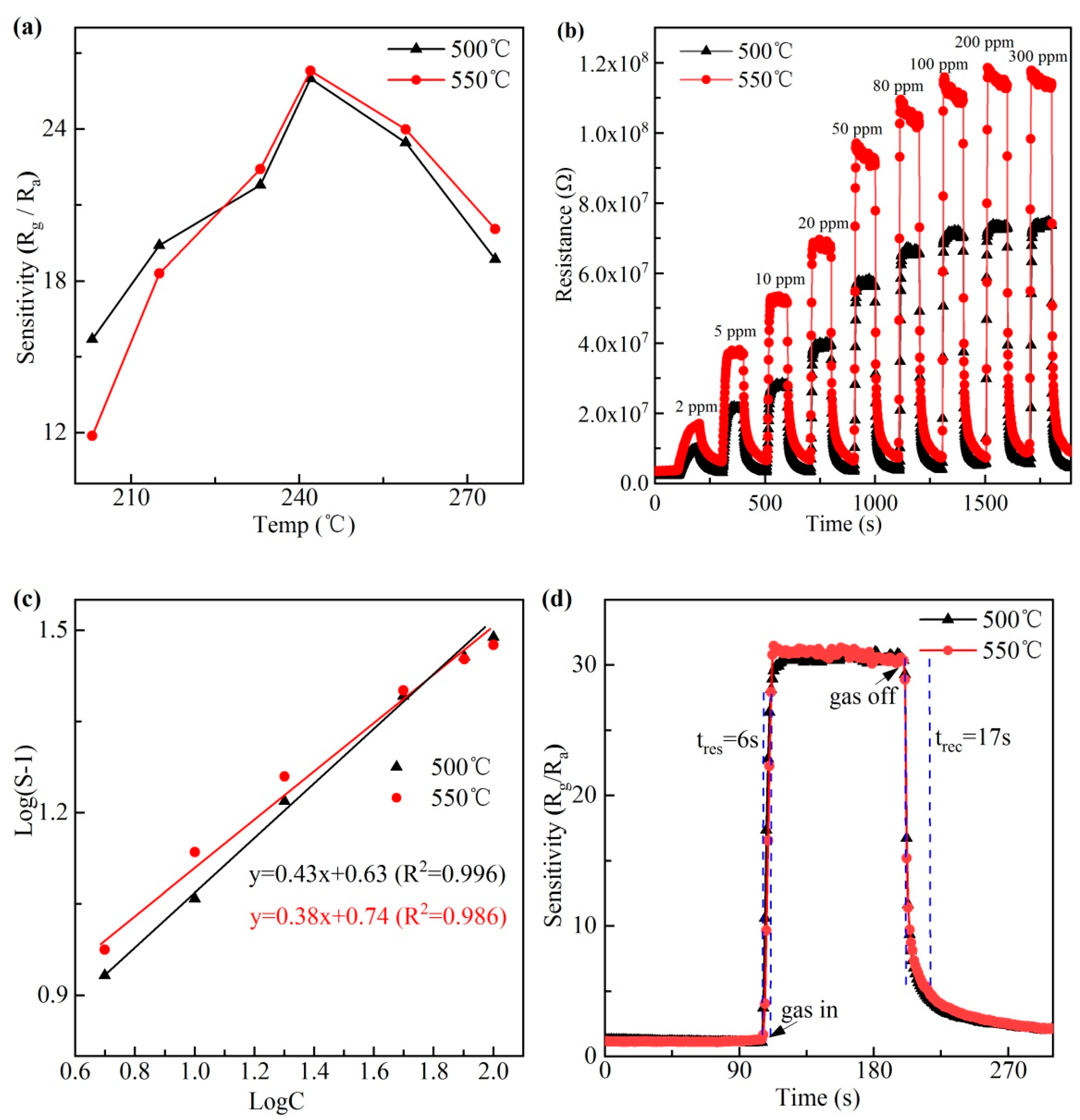
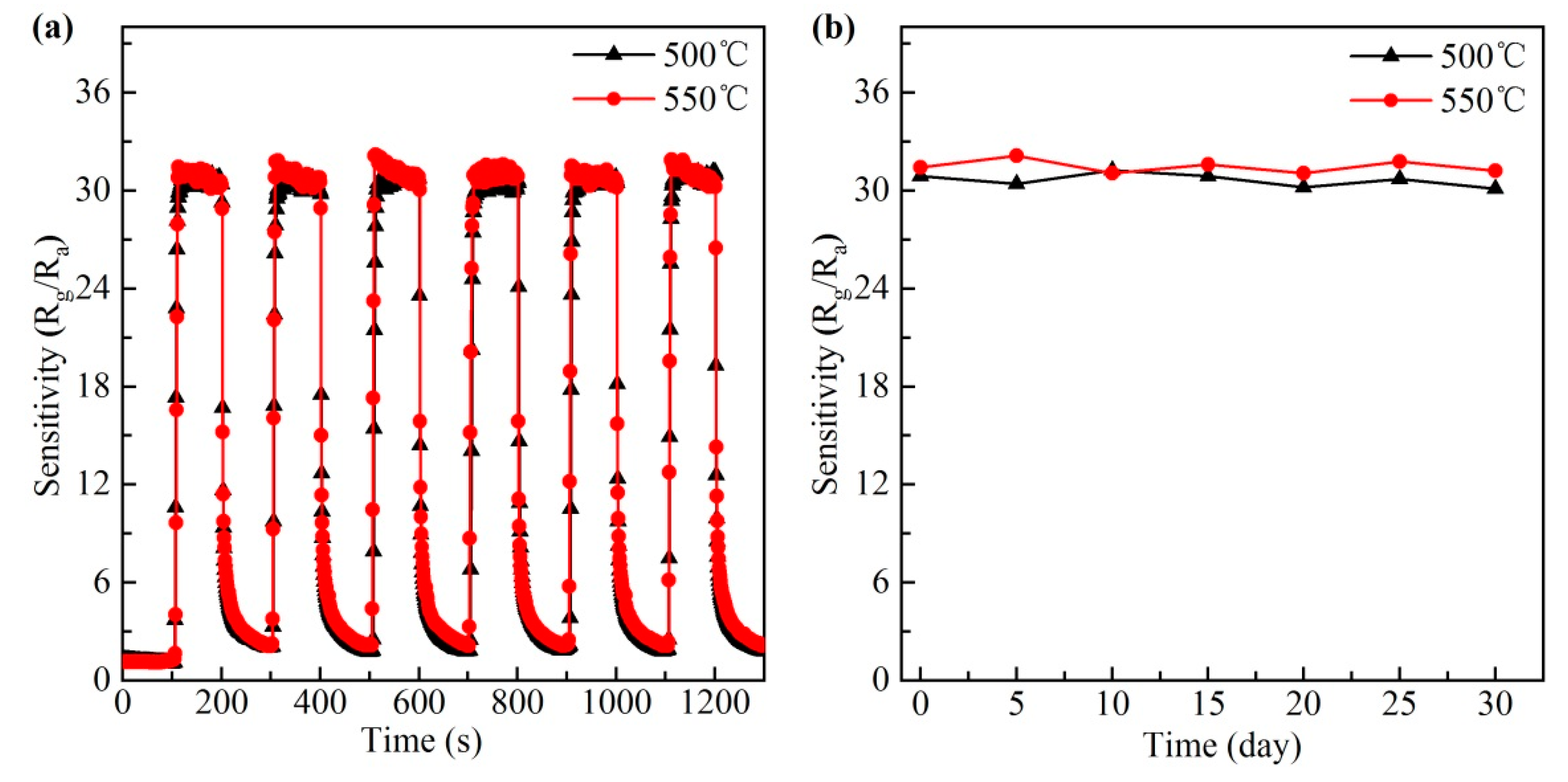
3.3. Gas Sensing Performance
4. Conclusions
- Pure BiFeO3 nanocrystals have been successfully fabricated by a simple wet chemical method. BiFeO3 nanocrystals sintered at 500 and 550 °C and the prepared gas sensors display almost the same performance.
- At the optimum working temperature of 240 °C, the fabricated sensor shows excellent isopropanol gas sensing properties with a high gas sensitivity of 31 exposed to 100 ppm isopropanol, fast response and recovery time (6 and 17 s), nice stability, and good selectivity to isopropanol.
- The sensor shows a perfect linear relationship between sensitivity and concentration in the range of 2–100 ppm at 240 °C and reaches the saturation point when the concentration is over 100 ppm. In addition, the measurement accuracy is of 1 ppm level. Therefore, the isopropanol gas sensor based on BiFeO3 nanocrystals can realize precise detection under 100 ppm concentration ranges and early warning over 100 ppm.
- The mechanism analysis reveals that the adsorbed oxygen ions may be mainly composed of O2.
- Conclusions above show that BiFeO3 nanocrystals are the superior candidate for a gas sensing application toward isopropanol detection.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jayababu, N.; Poloju, M.; Shruthi, J.; Reddy, M.V.R. NiO decorated CeO2 nanostructures as room temperature isopropanol gas sensors. RSC Adv. 2019, 9, 13765–13775. [Google Scholar] [CrossRef]
- Cai, X.; Hu, D.; Deng, S.; Han, B.; Wang, Y.; Wu, J.; Wang, Y. Isopropanol sensing properties of coral-like ZnO-CdO composites by flash preparation via self-sustained decomposition of metalorganic complexes. Sens. Actuators B Chem. 2014, 198, 402–410. [Google Scholar] [CrossRef]
- Jin, W.; Dong, B.; Chen, W.; Zhao, C.; Mai, L.; Dai, Y. Synthesis and gas sensing properties of Fe2O3 nanoparticles activated V2O5 nanotubes. Sens. Actuators B Chem. 2010, 145, 211–215. [Google Scholar] [CrossRef]
- Al-Ghamdi, A.A.; Al-Hazmi, F.; Al-Tuwirqi, R.M.; Alnowaiser, F.; Al-Hartomy, O.A.; El-Tantawy, F.; Yakuphanoglu, F. Synthesis, magnetic and ethanol gas sensing properties of semiconducting magnetite nanoparticles. Solid State Sci. 2013, 19, 111–116. [Google Scholar] [CrossRef]
- Song, C.; Li, C.; Yin, Y.; Xiao, J.; Zhang, X.; Song, M.; Dong, W. Preparation and gas sensing properties of partially broken WO3 nanotubes. Vacuum 2015, 114, 13–16. [Google Scholar] [CrossRef]
- Jin, C.; Kim, H.; Park, S.; Kim, H.W.; Lee, S.; Lee, C. Enhanced ethanol gas sensing properties of SnO2 nanobelts functionalized with Au. Ceram. Int. 2012, 38, 6585–6590. [Google Scholar] [CrossRef]
- Lian, X.-x.; Li, Y.; Lv, T.; Zou, Y.-l.; An, D.; Zhang, N. Preparation of ZnO Nanoparticles by Combustion Method and Their Gas Sensing Properties. Electron. Mater. Lett. 2016, 12, 24–31. [Google Scholar] [CrossRef]
- Zhu, Z.; Kao, C.-T.; Wu, R.-J. A highly sensitive ethanol sensor based on Ag@TiO2 nanoparticles at room temperature. Appl. Surf. Sci. 2014, 320, 348–355. [Google Scholar] [CrossRef]
- Jia, X.; Lian, D.; Shi, B.; Dai, R.; Li, C.; Wu, X. Facile synthesis of α-Fe2O3@graphene oxide nanocomposites for enhanced gas-sensing performance to ethanol. J. Mater. Sci. Mater. Electron. 2017, 28, 12070–12079. [Google Scholar] [CrossRef]
- Chen, N.; Li, X.; Wang, X.; Yu, J.; Wang, J.; Tang, Z.; Akbar, S.A. Enhanced room temperature sensing of Co3O4-intercalated reduced graphene oxide based gas sensors. Sens. Actuators B Chem. 2013, 188, 902–908. [Google Scholar] [CrossRef]
- Li, Y.-y.; Yu, H.; Yang, Y.; Dong, X.-t. Fabrication of 3D ordered mesoporous ball-flower structures ZnO material with the excellent gas sensitive property. Sens. Actuators B Chem. 2019, 300, 127050. [Google Scholar] [CrossRef]
- Zhang, R.; Ma, S.Y.; Zhang, Q.X.; Zhu, K.M.; Tie, Y.; Pei, S.T.; Wang, B.J.; Zhang, J.L. Highly sensitive formaldehyde gas sensors based on Ag doped Zn2SnO4/SnO2 hollow nanospheres. Mater. Lett. 2019, 254, 178–181. [Google Scholar] [CrossRef]
- Wang, B.; Yu, Q.; Zhang, S.; Wang, T.; Sun, P.; Chuai, X.; Lu, G. Gas sensing with yolk-shell LaFeO3 microspheres prepared by facile hydrothermal synthesis. Sens. Actuators B Chem. 2018, 258, 1215–1222. [Google Scholar] [CrossRef]
- Aparna, T.K.; Sivasubramanian, R. FeTiO3 nanohexagons based electrochemical sensor for the detection of dopamine in presence of uric acid. Mater. Chem. Phys. 2019, 233, 319–328. [Google Scholar] [CrossRef]
- Jun, A.; Kim, J.; Shin, J.; Kim, G. Perovskite as a Cathode Material: A Review of its Role in Solid-Oxide Fuel Cell Technology. ChemElectroChem 2016, 3, 511–530. [Google Scholar] [CrossRef]
- Zhang, L.; Li, X.; Wang, F.; Wang, T.; Shi, W. Colossal electroresistance and magnetoresistance effect in polycrystalline perovskite cobaltites Nd1−xSrxCoO3 (x = 0.1, 0.2, 0.3). Mater. Res. Bull. 2013, 48, 1088–1092. [Google Scholar] [CrossRef]
- Costa, L.V.; Deus, R.C.; Foschini, C.R.; Longo, E.; Cilense, M.; Simões, A.Z. Experimental evidence of enhanced ferroelectricity in Ca doped BiFeO3. Mater. Chem. Phys. 2014, 144, 476–483. [Google Scholar] [CrossRef]
- Moubah, R.; Rousseau, O.; Colson, D.; Artemenko, A.; Maglione, M.; Viret, M. Photoelectric Effects in Single Domain BiFeO3 Crystals. Adv. Funct. Mater. 2012, 22, 4814–4818. [Google Scholar] [CrossRef]
- Mocherla, P.S.V.; Karthik, C.; Ubic, R.; Rao, M.S.R.; Sudakar, C. Tunable bandgap in BiFeO3 nanoparticles: The role of microstrain and oxygen defects. Appl. Phys. Lett. 2013, 103, 022910. [Google Scholar] [CrossRef]
- Lim, J.S.; Lee, J.H.; Park, H.-S.; Gao, R.; Koo, T.Y.; Martin, L.W.; Ramesh, R.; Yang, C.-H. Ultrafast collective oxygen-vacancy flow in Ca-doped BiFeO3. NPG Asia Mater. 2018, 10, 943–955. [Google Scholar] [CrossRef]
- Wang, S.; Chen, D.; Niu, F.; Zhang, N.; Qin, L.; Huang, Y. Hydrogenation-induced surface oxygen vacancies in BiFeO3 nanoparticles for enhanced visible light photocatalytic performance. J. Alloys Compd. 2016, 688, 399–406. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, W.; Wang, C.; Ma, J.; Ning, L.; Fan, H. Ag modified bismuth ferrite nanospheres as a chlorine gas sensor. RSC Adv. 2018, 8, 33156–33163. [Google Scholar] [CrossRef]
- Dziubaniuk, M.; Bujakiewicz-Korońska, R.; Suchanicz, J.; Wyrwa, J.; Rękas, M. Application of bismuth ferrite protonic conductor for ammonia gas detection. Sens. Actuators B Chem. 2013, 188, 957–964. [Google Scholar] [CrossRef]
- Waghmare, S.D.; Jadhav, V.V.; Gore, S.K.; Yoon, S.-J.; Ambade, S.B.; Lokhande, B.J.; Mane, R.S.; Han, S.-H. Efficient gas sensitivity in mixed bismuth ferrite micro (cubes) and nano (plates) structures. Mater. Res. Bull. 2012, 47, 4169–4173. [Google Scholar] [CrossRef]
- Das, S.; Rana, S.; Mursalin, S.M.; Rana, P.; Sen, A. Sonochemically prepared nanosized BiFeO3 as novel SO2 sensor. Sens. Actuators B Chem. 2015, 218, 122–127. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, H.; Dong, S.; Han, R.; Liu, X.; Wang, Y.; Li, S.; Bu, Q.; Li, X.; Xiang, J. A fast response & recovery acetone gas sensor based on BiFeO3 nanomaterials with high sensitivity and low detection limit. J. Mater. Sci. Mater. Electron. 2018, 29, 2193–2200. [Google Scholar]
- Tong, T.; Chen, J.; Jin, D.; Cheng, J. Preparation and gas sensing characteristics of BiFeO3 crystallites. Mater. Lett. 2017, 197, 160–162. [Google Scholar] [CrossRef]
- Dong, G.; Fan, H.; Tian, H.; Fang, J.; Li, Q. Gas-sensing and electrical properties of perovskite structure p-type barium-substituted bismuth ferrite. RSC Adv. 2015, 5, 29618–29623. [Google Scholar] [CrossRef]
- Yu, X.; Wang, Y.; Hu, Y.; Cao, C.; Chan, H.L.W. Gas-Sensing Properties of Perovskite BiFeO3 Nanoparticles. J. Am. Ceram. Soc. 2009, 92, 3105–3107. [Google Scholar] [CrossRef]
- Poghossian, A.S.; Abovian, H.V.; Avakian, P.B.; Mkrtchian, S.H.; Haroutunian, V.M. Bismuth Ferrites: New Materials for Semiconductor Gas Sensors. Sens. Actuators B Chem. 1991, 4, 545–549. [Google Scholar] [CrossRef]
- Zou, C.W.; Wang, J.; Xie, W. Synthesis and enhanced NO2 gas sensing properties of ZnO nanorods/TiO2 nanoparticles heterojunction composites. J. Colloid Interface Sci. 2016, 478, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Ke, H.; Jia, D.; Wang, W.; Zhou, Y. Low-temperature synthesis of BiFeO3 nanopowders via a sol–gel method. J. Alloys Compd. 2009, 472, 473–477. [Google Scholar] [CrossRef]
- Li, Z.; Cheng, L.; Zhang, S.; Wang, Z.; Fu, C. Enhanced photocatalytic and magnetic recovery performance of Co-doped BiFeO3 based on MOFs precursor. J. Solid State Chem. 2019, 279, 120978. [Google Scholar] [CrossRef]
- Hu, Z.; Chen, D.; Wang, S.; Zhang, N.; Qin, L.; Huang, Y. Facile synthesis of Sm-doped BiFeO3 nanoparticles for enhanced visible light photocatalytic performance. Mater Sci Eng B Solid State Mater Adv Technol. 2017, 220, 1–12. [Google Scholar] [CrossRef]
- Sun, L.; Qin, H.; Wang, K.; Zhao, M.; Hu, J. Structure and electrical properties of nanocrystalline La1-xBaxFeO3 for gas sensing application. Mater. Chem. Phys. 2011, 125, 305–308. [Google Scholar] [CrossRef]
- Fan, F.; Tang, P.; Wang, Y.; Feng, Y.; Chen, A.; Luo, R.; Li, D. Facile synthesis and gas sensing properties of tubular hierarchical ZnO self-assembled by porous nanosheets. Sens. Actuators B Chem. 2015, 215, 231–240. [Google Scholar] [CrossRef]
- Bai, S.L.; Guo, T.; Li, D.Q.; Luo, R.X.; Chen, A.F.; Liu, C.C. Intrinsic sensing properties of the flower-like ZnO nanostructures. Sens. Actuators B Chem. 2013, 182, 747–754. [Google Scholar] [CrossRef]
- Hu, J.; Gao, F.; Zhao, Z.; Sang, S.; Li, P.; Zhang, W.; Zhou, X.; Chen, Y. Synthesis and characterization of Cobalt-doped ZnO microstructures for methane gas sensing. Appl. Surf. Sci. 2016, 363, 181–188. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, H.; Xu, J.; Wei, J.; Zhang, Y. Fast Response Isopropanol Sensing Properties with Sintered BiFeO3 Nanocrystals. Materials 2020, 13, 3829. https://doi.org/10.3390/ma13173829
Xu H, Xu J, Wei J, Zhang Y. Fast Response Isopropanol Sensing Properties with Sintered BiFeO3 Nanocrystals. Materials. 2020; 13(17):3829. https://doi.org/10.3390/ma13173829
Chicago/Turabian StyleXu, Hongxiang, Junhua Xu, Junlin Wei, and Yamei Zhang. 2020. "Fast Response Isopropanol Sensing Properties with Sintered BiFeO3 Nanocrystals" Materials 13, no. 17: 3829. https://doi.org/10.3390/ma13173829
APA StyleXu, H., Xu, J., Wei, J., & Zhang, Y. (2020). Fast Response Isopropanol Sensing Properties with Sintered BiFeO3 Nanocrystals. Materials, 13(17), 3829. https://doi.org/10.3390/ma13173829



