The Corrosion Features of Q235B Steel under Immersion Test and Electrochemical Measurements in Desulfurization Solution
Abstract
1. Introduction
2. Experiment
2.1. Desulfurization Solution Analysis
2.2. Immersion Test
2.3. Electrochemical Measurements
2.4. Morphologies and Component Analysis
3. Results and Discussion
3.1. Composition of the Desulfurization Solution
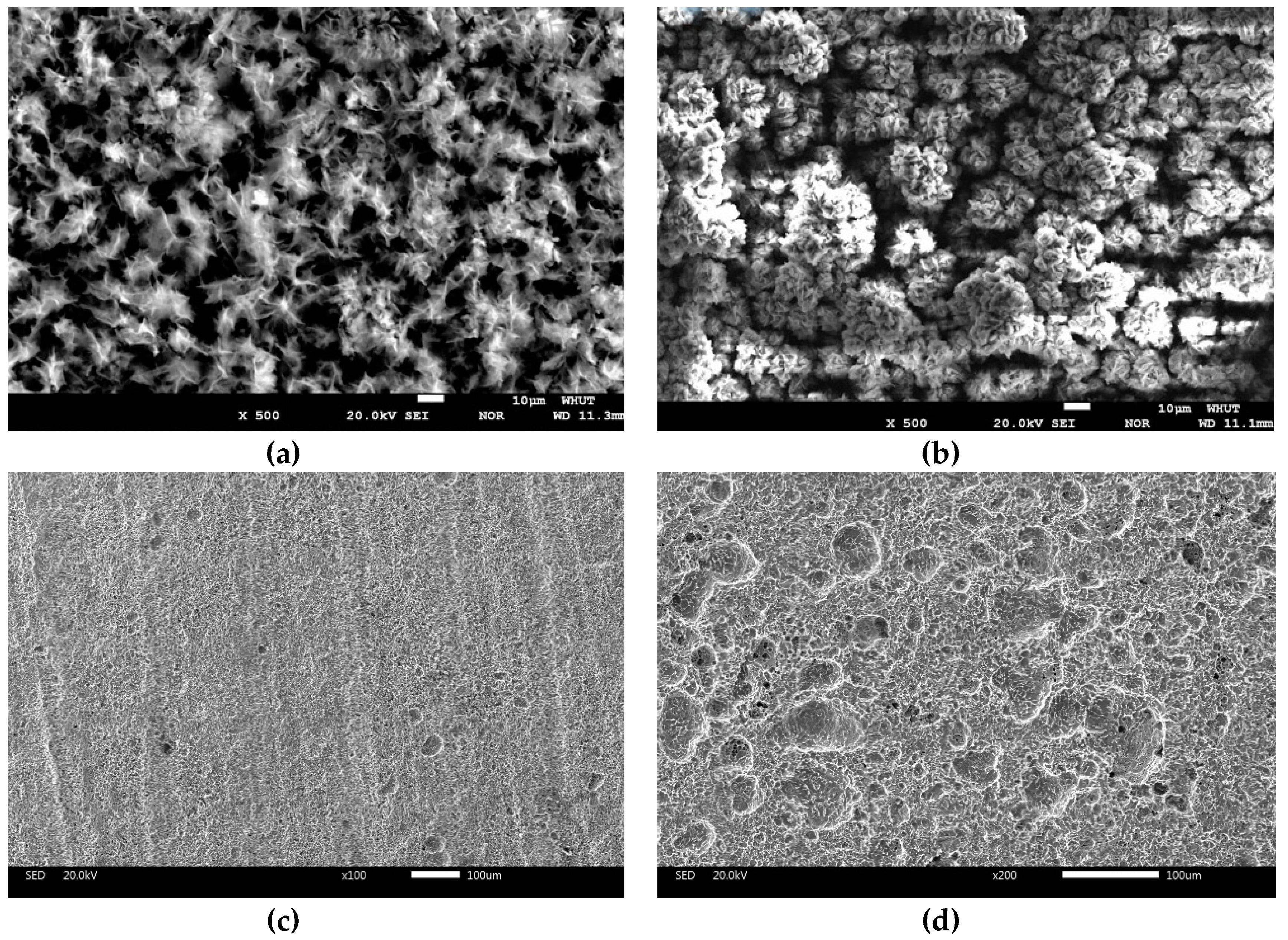
3.2. Corrosion Morphology
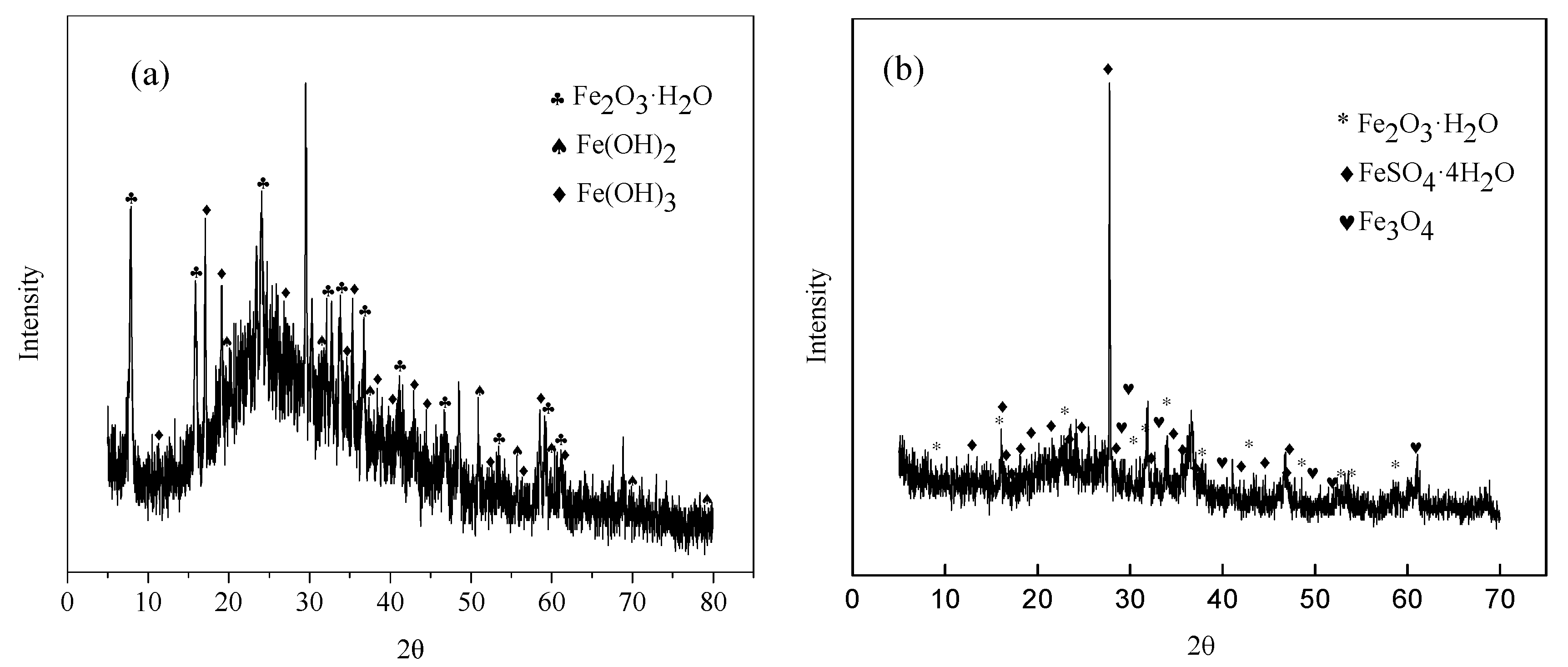
3.3. Corrosion Products Analysis
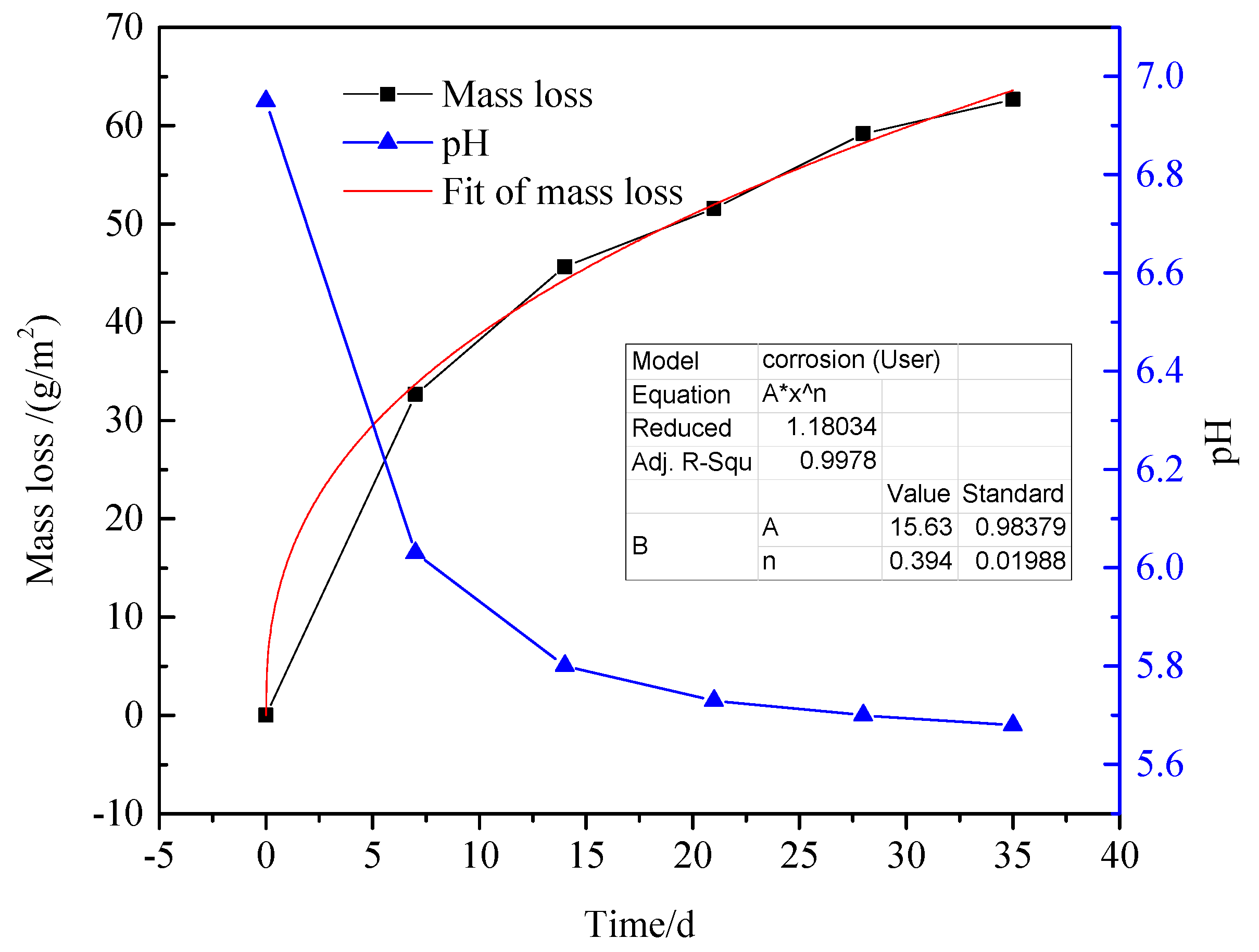
3.4. Mass Loss and Corrosion Rate
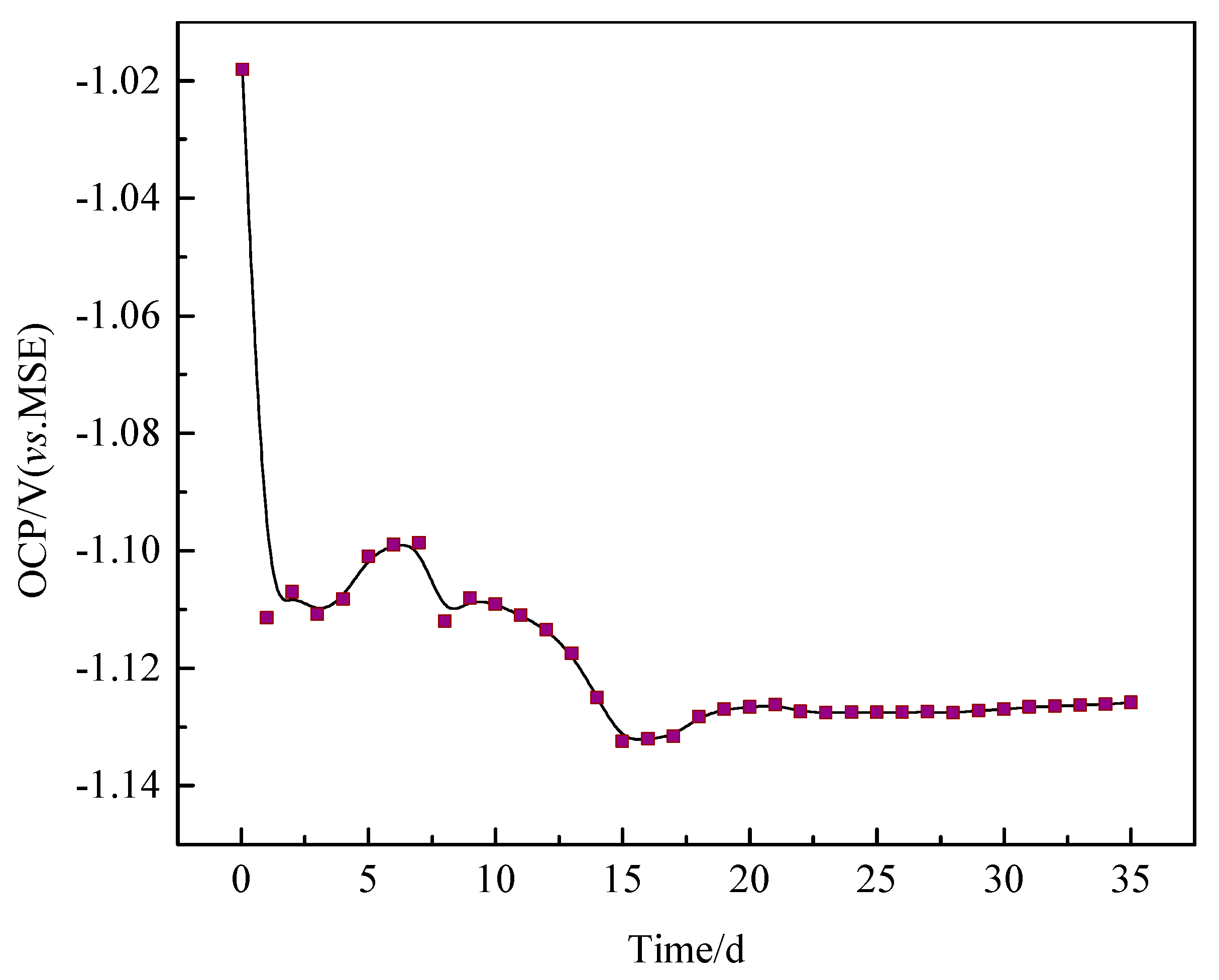
3.5. Open Circuit Potential Measurements
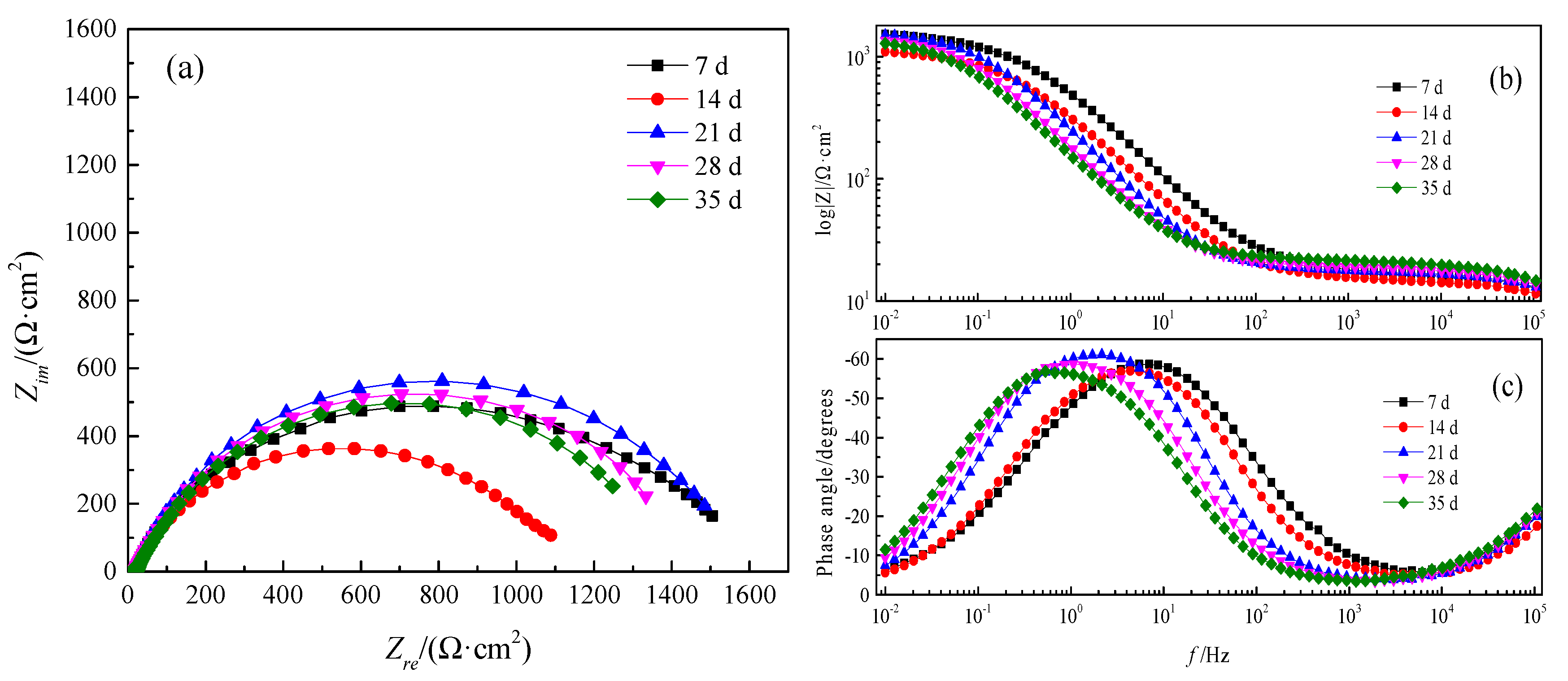
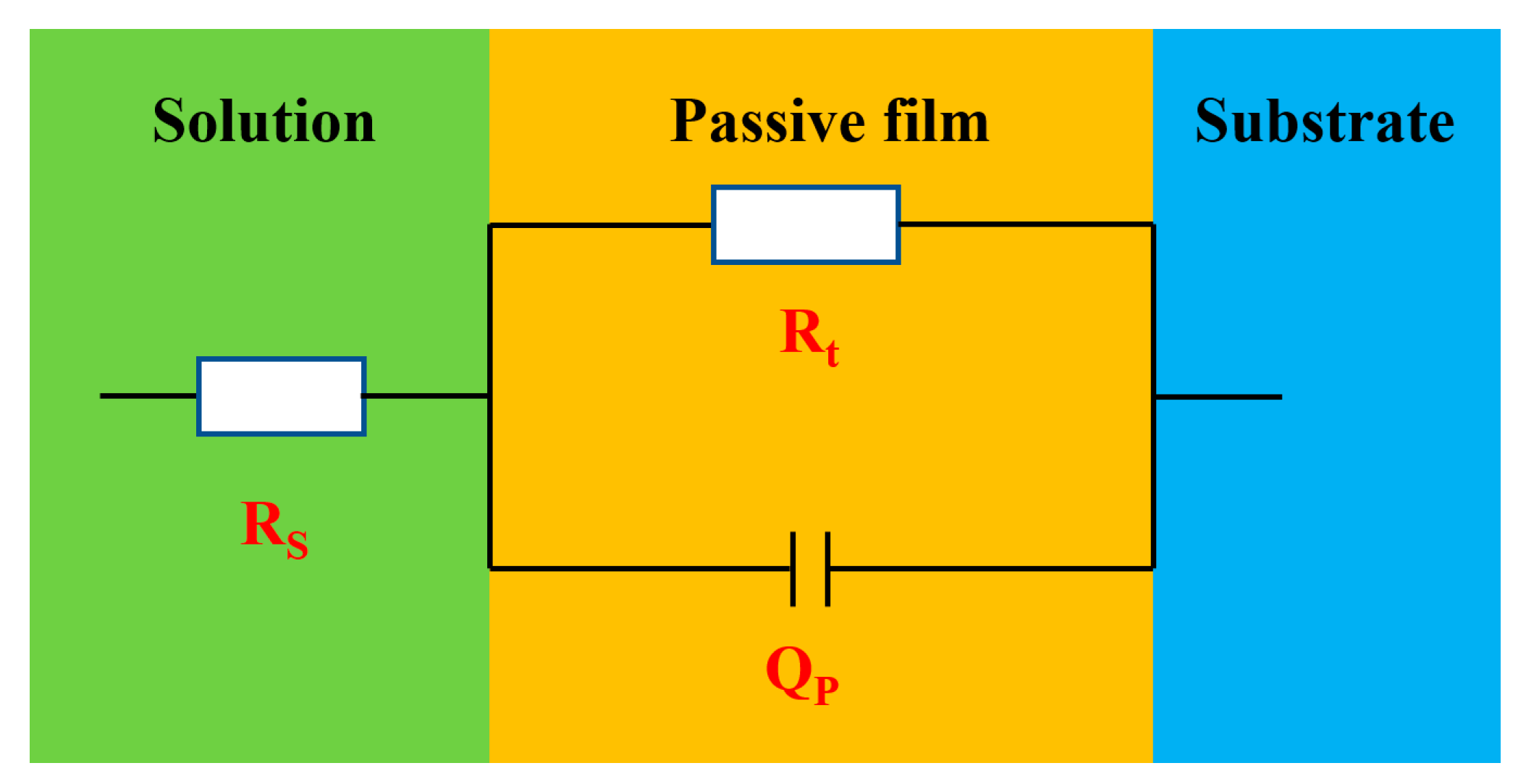
3.6. Electrochemical Impedance Spectroscopy
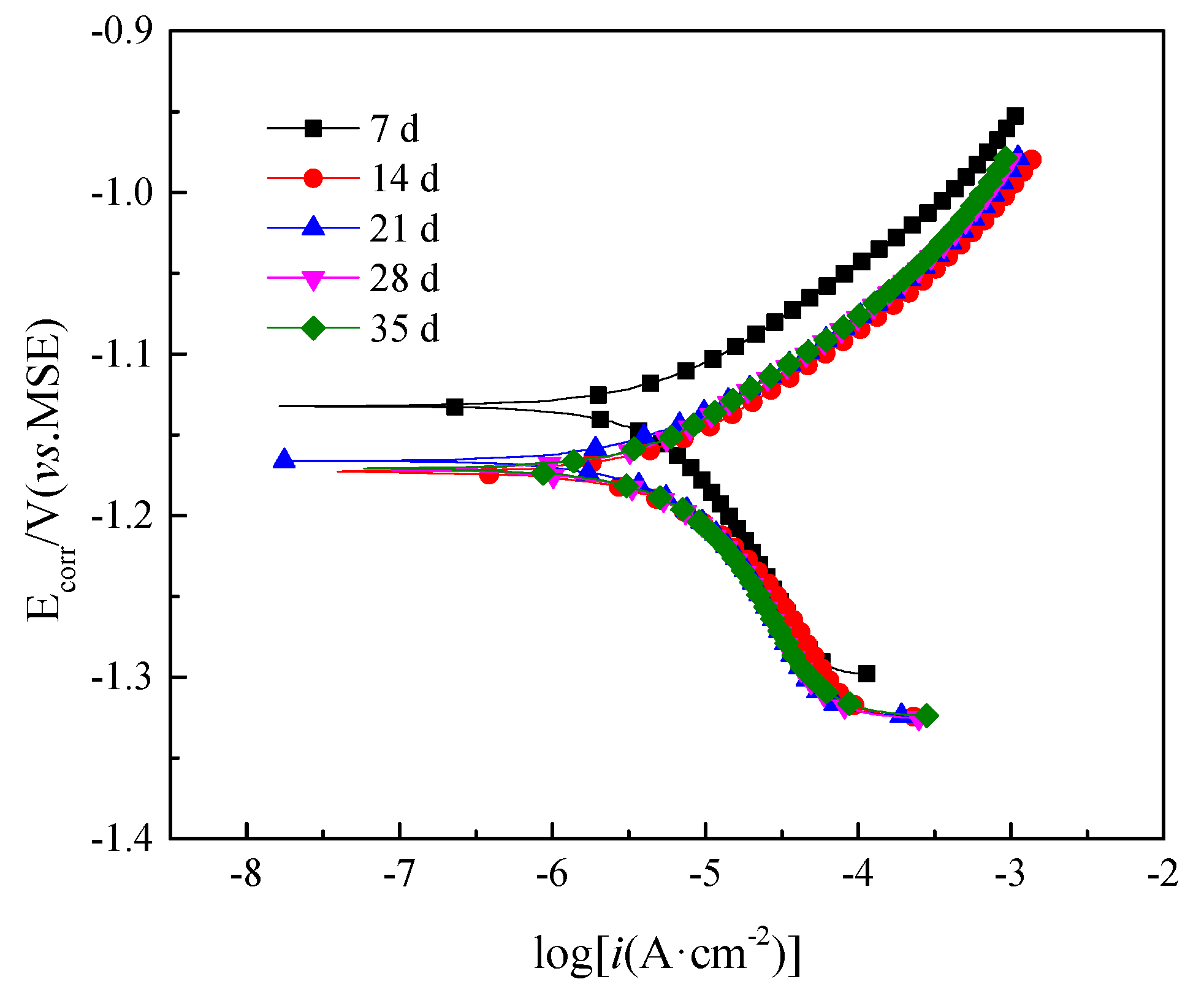
3.7. Polarization Curves
3.8. The Corrosion Mechanism of Q235B Steel
4. Conclusions
- (1)
- There was scale sediments attached to the surface of the steel. For 7 days of immersion, the sediments patches were connected and distributed on the matrix surface. The compactness of the sediments increased with time. The initial corrosion products was composed of Fe(OH)2, Fe(OH)3, and Fe2O3·H2O, the end corrosion products consisted of Fe2O3·H2O, FeSO4·4H2O, and Fe3O4.
- (2)
- For immersion test, the Vcorr reduced gradually with 35 days of immersion. However, the results of electrochemical measurement showed that the Vcorr was fluctuant in reality.
- (3)
- In the initial immersion stage (7 days), the primary corrosion type was general corrosion, pitting corrosion was slight and dispersed under the sediments. In the later stage of corrosion (35 days), the cyclic regeneration mechanism of acid, induced by oxidation hydrolysis of FeSO4, aggravated the pitting corrosion.
- (4)
- Though the sediments attached to the steel surface could inhibit corrosion, pitting corrosion under the sediments would bring about more serious damage (leak of pipeline and increase of equipment fault rate) thus should be given more attention.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Martínez, A.H. Study of exhaust gascleaning systems for vessels to fulfill IMO III in 2016. Univ. Politcnica Catalunya 2011, 5, 37. [Google Scholar]
- Anttila, M.; Hämäläinen, R.; Tuominiemi, S. Method and an Equipment for Reducing the Sulphur Dioxide Emissions of a Marine Engine. U.S. 20070798720, 16 May 2007. [Google Scholar]
- Zidar, M. Gas-liquid equilibrium-operational diagram: Graphical presentation of absorption of SO2 in the NaOH−SO2−H2O system taking place within a laboratory absorber. Ind. Eng. Chem. Res. 2000, 39, 3042. [Google Scholar] [CrossRef]
- Bandyopadhyay, A.; Biswas, M.N. Modeling of SO2 scrubbing in spray towers. Sci. Total Environ. 2007, 383, 25. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.F.; Shih, S.M. Effects of flue gas components on the reaction of Ca(OH)2 with SO2. Ind. Eng. Chem. Res. 2006, 45, 8765. [Google Scholar] [CrossRef]
- Machmudah, S.; Zulhijah, R.; Setyawan, H.; Kanda, H.; Goto, M. Magnetite thin film on mild steel formed by hydrothermal electrolysis for corrosion prevention. Chem. Eng. J. 2015, 268, 76–85. [Google Scholar] [CrossRef]
- Cheng, Q.; Tao, B.; Liu, S.; Zhang, W.; Liu, X.; Li, W.; Liu, Q. Corrosion behavior of Q235B steel in sediments water from crude oil. Corros. Sci. 2016, 111, 61–71. [Google Scholar] [CrossRef]
- Yu, Q.; Dong, C.; Fang, Y.; Fang, Y.; Xiao, K.; Guo, C.; He, G.; Li, X. Atmospheric corrosion of Q235 carbon steel and Q450 weathering steel in Turpan, China. J. Iron Steel Res. Int. 2016, 23, 1061–1070. [Google Scholar] [CrossRef]
- Liu, G.; Zhang, Y.; Ni, Z.; Huang, R. Corrosion behavior of steel submitted to chloride and sulphate ions in simulated concrete pore solution. Constr. Build. Mater. 2016, 115, 1–5. [Google Scholar] [CrossRef]
- Boah, J.K.; Somuah, S.K.; LeBlanc, P. Electrochemical behavior of steel in saturated calcium hydroxide solution containing Cl-, SO42-, and CO32- Ions. Corrosion 1990, 46, 153–158. [Google Scholar] [CrossRef]
- Xu, P.; Jiang, L.; Guo, M.; Zha, J.; Chen, L.; Chen, C.; Xu, N. Influence of sulfate salt type on passive film of steel in simulated concrete pore solution. Constr. Build. Mater. 2019, 223, 352–359. [Google Scholar] [CrossRef]
- Huang, B.; Yang, G.H. Research progress of ship tail gas gas cleaning desulfurization denitration and PM removal equipment. Chem. Ind. Eng. Prog. 2013, 32, 2826. [Google Scholar]
- Persaud, S.Y.; Carcea, A.G.; Newman, R.C. An electrochemical study assisting the interpretation of acid sulfate stress corrosion cracking of NiCrFe alloys. Corros. Sci. 2015, 90, 383–391. [Google Scholar] [CrossRef]
- Revie, R.W.; Uhlig, H.H. Treatment of water and steam systems. In Corrosion and Corrosion Control: An Introduction to Corrosion Science and Engineering, 4th ed.; John Wiley & Sons Inc.: New Jersey, NY, USA, 2009; pp. 317–332. [Google Scholar]
- Kuang, W.; Mathews, J.A.; Macdonald, D.D. The effect of Anodamine on the corrosion behavior of 1018 mild steel in deionized water: I. Immersion and polarization tests. Electrochim. Acta 2014, 127, 79–85. [Google Scholar] [CrossRef]
- Oliveira, N.; Guastaldi, A. Electrochemical behavior of Ti–Mo alloys applied as biomaterial. Corros. Sci. 2008, 50, 938–945. [Google Scholar] [CrossRef]
- Cai, B.P.; Liu, Y.H.; Tian, X.J.; Wang, F.; Li, H.; Ji, R. An experimental study of crevice corrosion behaviour of 316L stainless steel in artificial seawater. Corros. Sci. 2010, 52, 3235–3242. [Google Scholar] [CrossRef]
- Zheng, L.; Neville, A. Corrosion Behavior of Type 316L Stainless Steel in Hydraulic Fluid and Hydraulic Fluid/Seawater for Subsea Applications. Corrosion 2009, 65, 145–153. [Google Scholar] [CrossRef]
- Cheng, Q.; Song, S.; Song, L.; Hou, B. Effect of Relative Humidity on the Initial Atmospheric Corrosion Behavior of Zinc during Drying. J. Electrochem. Soc. 2013, 160, C380–C389. [Google Scholar] [CrossRef]
- Cheng, Q.; Chen, Z. The cause analysis of the incomplete semi-circle observed in high frequency region of EIS obtained from TEL-covered pure copper. Int. J. Electrochem. 2013, 8, 8282–8290. [Google Scholar]
- Wu, Y.H.; Liu, T.M.; Sun, C.; Xu, J.; Yu, C.K. Effects of simulated acid rain on corrosion behaviour of Q235 steel in acidic soil. Corros. Eng. Sci. Technol. 2010, 45, 136–141. [Google Scholar] [CrossRef]
- Gonzalez, J.E.G.; Mirza-Rosca, J.C. Study of the corrosion behavior of titanium and some of its alloys for biomedical and dental implant applications. J. Electroanal. Chem. 1999, 471, 109–115. [Google Scholar] [CrossRef]
- Sheng, X.; Ting, Y.P.; Pehkonen, S.O. The influence of sulphate-reducing bacteria biofilm on the corrosion of stainless steel AISI 316. Corros. Sci. 2007, 49, 2159–2176. [Google Scholar] [CrossRef]
- Mohammadloo, H.E.; Sarabi, A.A.; Sabbagh, A.A.; Salimi, R.; Sameie, H. The effect of solution temperature and pH on corrosion performance and morphology of nanoceramic-based conversion thin film. Mater. Corros. 2014, 64, 535–543. [Google Scholar] [CrossRef]
- Medhashree, H.; Shetty, A.N. Electrochemical investigation on the effects of sulfate ion concentration, temperature and medium pH on the corrosion behavior of Mg–Al–Zn–Mn alloy in aqueous ethylene glycol. J. Magnes. Alloys 2017, 5, 64–73. [Google Scholar] [CrossRef]
- Zou, Y.; Wang, J.; Zheng, Y. Electrochemical techniques for determining corrosion rate of rusted steel in seawater. Corros. Sci. 2011, 53, 208–216. [Google Scholar] [CrossRef]
- Sánchez-Tovar, R.; Montañés, M.T.; García-Antón, J. The effect of temperature on the galvanic corrosion of the copper/AISI 304 pair in LiBr solutions under hydrodynamic conditions. Corros. Sci. 2010, 52, 722–733. [Google Scholar] [CrossRef]
- Li, D.G.; Wang, J.D.; Chen, D.R. Influence of pH value on the structure and electronic property of the passive film on 316L SS in the simulated cathodic environment of proton exchange membrane fuel cell (PEMFC). Int. J. Hydrog. Energy 2014, 39, 20105–20115. [Google Scholar] [CrossRef]
- Dong, Z.H.; Shi, W.; Guo, X.P. Initiation and repassivation of pitting corrosion of steel in carbonated concrete pore solution. Corros. Sci. 2011, 53, 1322–1330. [Google Scholar] [CrossRef]
- Hubbard, A. Corrosion and Corrosion Control: An Introduction to Corrosion Science and Engineering, 4th ed.; Revie, R.W., Uhlig, H.H., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2008; 490p, Journal of Colloid & Interface Science, 2008, 328, 463–463. [Google Scholar]
- Stratmann, M.; Streckel, H. On the atmospheric corrosion of metals which are covered with thin electrolyte layers—I. Verification of the experimental technique. Corros. Sci. 1990, 30, 715–734. [Google Scholar] [CrossRef]
- Yan, M.C.; Sun, C.; Xu, J.; Dong, J.; Ke, W. Role of Fe oxides in corrosion of pipeline steel in a red clay soil. Corros. Sci. 2014, 80, 309–317. [Google Scholar] [CrossRef]
- Reffass, M.; Sabot, R.; Jeannin, M.; Berziou, C.; Refait, P. Effects of NO2− ions on localised corrosion of steel in NaHCO3 + NaCl electrolytes. Electrochim. Acta 2007, 52, 7599–7606. [Google Scholar] [CrossRef]
- Hao, L.; Zhang, S.; Dong, J.; Ke, W. Evolution of corrosion of MnCuP weathering steel submitted to wet/dry cyclic tests in a simulated coastal atmosphere. Corros. Sci. 2012, 58, 175–180. [Google Scholar] [CrossRef]
- Wang, J.H.; Wei, F.I.; Chang, Y.S.; Shih, H. The corrosion mechanisms of steel and weathering steel in SO2 polluted atmospheres. Mater. Chem. Phys. 1997, 47, 1–8. [Google Scholar] [CrossRef]
- González, J.; Miranda, J.; Otero, E.; Feliu, S. Effect of electrochemically reactive rust layers on the corrosion of steel in a Ca(OH)2 solution. Corros. Sci. 2007, 49, 436–448. [Google Scholar] [CrossRef]
| C | Al | Si | P | S | Mn | Fe |
|---|---|---|---|---|---|---|
| 0.15 | 0.184 | 0.128 | 0.017 | 0.013 | 0.218 | bal |
| Content | Chemical Formula | Value |
|---|---|---|
| Na+ | 2785.5 | |
| K+ | 540.7 | |
| Mg2+ | 4.7 | |
| Ca2+ | 26.2 | |
| Zn2+ | 90.2 | |
| Ionic concentration (mg L−1) | SO42− | 15,300.3 |
| SO32− | 9580.5 | |
| NH4+ | 224.6 | |
| NO3− | 3.4 | |
| NO2− | 0.3 | |
| Organics concentration (mg L−1) | PAHs | 158.7 |
| Suspended solids concentration (mg L−1) | 2519.5 | |
| pH | 6.95 ± 0.08 |
| Time/d | 7 | 14 | 21 | 28 | 35 |
|---|---|---|---|---|---|
| Vcorr/(mm/a) | 0.22 | 0.15 | 0.11 | 0.10 | 0.08 |
| ΔVcorr/(mm/a) | — | 0.07 | 0.04 | 0.01 | 0.02 |
| Time/d | Rs/(Ω·cm2) | Qdl/(F·cm−2) | n | Rt/(Ω·cm2) |
|---|---|---|---|---|
| 7 | 14.03 ± 0.15 | (4.48 ± 0.03) × 10−4 | 0.7474 ± 0.008 | 1092 ± 2.6 |
| 14 | 15.68 ± 0.13 | (7.82 ± 0.05) × 10−4 | 0.7588 ± 0.005 | 1224 ± 3.1 |
| 21 | 16.63 ± 0.11 | (9.01 ± 0.07) × 10−4 | 0.7988 ± 0.003 | 1545 ± 1.8 |
| 28 | 17.77 ± 0.13 | (1.29 ± 0.08) × 10−4 | 0.7784 ± 0.005 | 1469 ± 2.5 |
| 35 | 20.01 ± 0.09 | (1.61 ± 0.05) × 10−4 | 0.7619 ± 0.006 | 1430 ± 2.7 |
| 7 days | 14 days | 21 days | 28 days | 35 days | |
|---|---|---|---|---|---|
| Ecorr V | −1.133 | −1.174 | −1.167 | −1.172 | −1.171 |
| icorr μA/cm2 | 7.231 | 8.074 | 6.690 | 7.532 | 7.459 |
| Rp Ω·cm2 | 3607 | 3231 | 3899 | 3497 | 3463 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gong, P.; Zhang, G.; Chen, J. The Corrosion Features of Q235B Steel under Immersion Test and Electrochemical Measurements in Desulfurization Solution. Materials 2020, 13, 3783. https://doi.org/10.3390/ma13173783
Gong P, Zhang G, Chen J. The Corrosion Features of Q235B Steel under Immersion Test and Electrochemical Measurements in Desulfurization Solution. Materials. 2020; 13(17):3783. https://doi.org/10.3390/ma13173783
Chicago/Turabian StyleGong, Peng, Guangxu Zhang, and Jian Chen. 2020. "The Corrosion Features of Q235B Steel under Immersion Test and Electrochemical Measurements in Desulfurization Solution" Materials 13, no. 17: 3783. https://doi.org/10.3390/ma13173783
APA StyleGong, P., Zhang, G., & Chen, J. (2020). The Corrosion Features of Q235B Steel under Immersion Test and Electrochemical Measurements in Desulfurization Solution. Materials, 13(17), 3783. https://doi.org/10.3390/ma13173783




