Modelling and Experimental Investigation on the Settling Rate of Kaolinite Particles in Non-Ideal Sedimentation Stage under Constant Gravity
Abstract
1. Introduction
2. The Constant-Gravity Non-Ideal Sedimentation Model
3. Materials and Methods
3.1. Observation Test of Small Particle Precipitation Characteristics
- Weigh 8 g of Malaysian kaolinite, and make it into a 100 mL kaolinite suspension in the beaker.
- Use an S10 hand-held homogenizer (Shanghai Huxi Industrial Co., LTD, Shanghai, China) to shear the suspension at high speed. At a speed of 5000 r/s, the suspension continuously shears at high speed for 60 s to disperse the kaolinite into a single particle. To prevent the damage of the polymer structure, a polymer flocculant is added after the shear dispersion process.
- Slowly pour the prepared suspension into the 100 mL measuring cylinder, and keep the scale at 100 mL using a glue head dropper.
- Start timing after shaking, and observe and record the thickness of the supernatant and sediment. Data (suspension level scale and sediment thickness) were recorded every 12 h for 35 days.
- Take about 2 mL of the diluted suspension sample, insert the electrode and pass it through the alternating current. Under a microscope (Shanghai, China), observe that the soil particles in the suspension will migrate back and forth in the horizontal direction.
- When the migration process tends to be stable (the particles move back and forth in the same region), take a screenshot and read the corresponding horizontal and vertical coordinates of the soil particles in the two screenshots before and after, and calculate the zeta potential on the surface of the soil particles at this time.
3.2. Settlement Rate Test Experiment
- The prepared suspensions of each experimental group were cut with a homogenizer at high speed so that the kaolinite particles can be distributed in a single particle and in uniform contact with the flocculant, which were then evenly poured into a measuring cylinder with a capacity of 5000 mL.
- During the measurement of the soil particle concentration, the sludge concentration meter was used to measure the concentration at each 500 mL scale, and the sensor was stationary at the corresponding scale for about 2 min before each reading. The data were recorded after the sensor reading had been stabilized.
4. Results
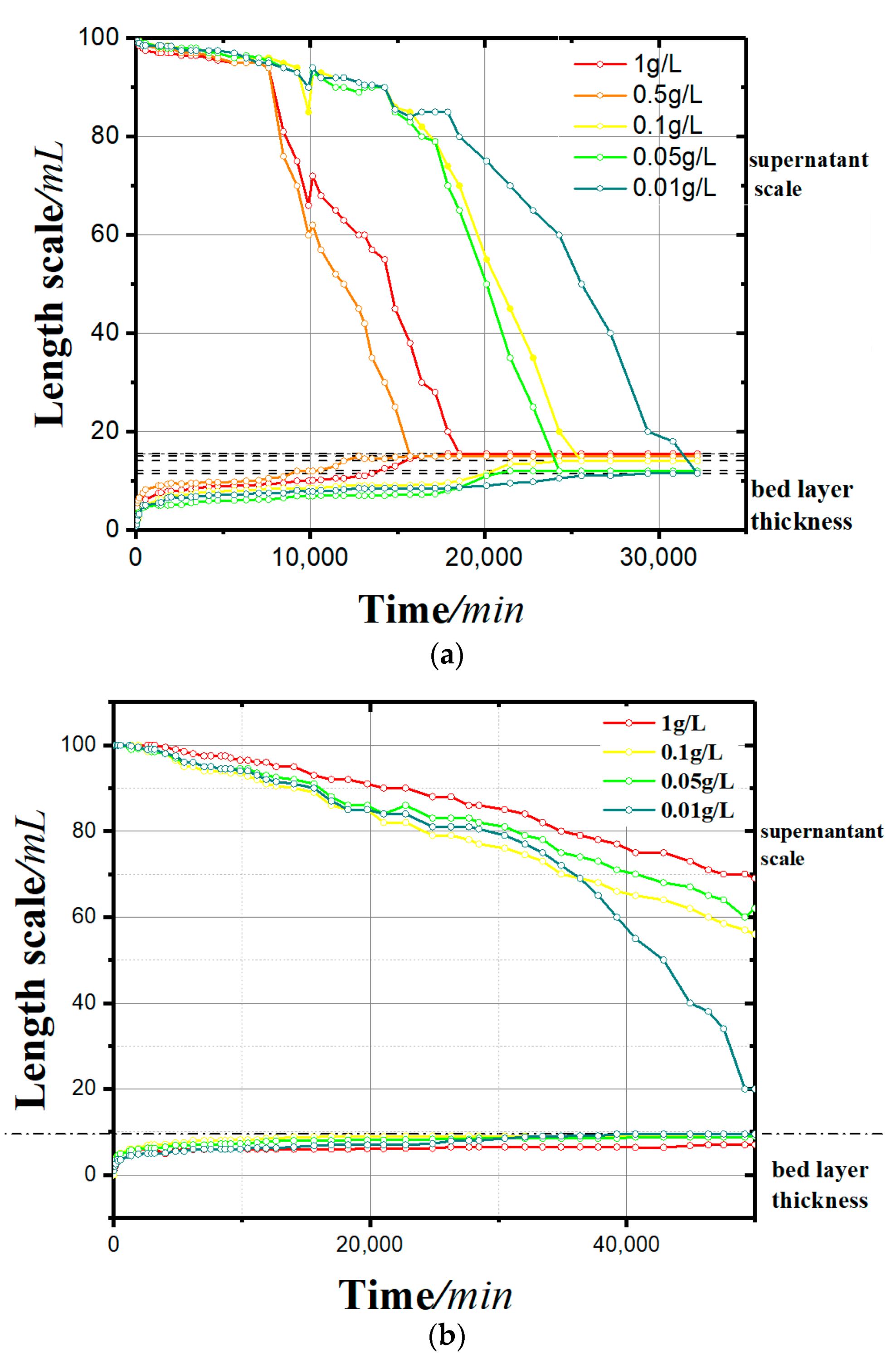
- In the sedimentation curves shown in Figure 4, the deposition process is reflected by both the supernatant calibration curve and the bed calibration curve. At the beginning of the flocculation settling, the scale of supernatant was 100 and that of the bed was 0. With the continuous process of flocculation settling, the scale of the supernatant decreased gradually, and that of the bed rose slowly. When the deposition process was completed, the two scales tended toward the same value.
- It can be seen from the sedimentation curves in Figure 4a,b that in the experimental group with soluble starch as the flocculant, the flocculation and precipitation process of kaolinite significantly intensified with the increase of soluble starch. In the experimental group with apple pectin as the added flocculant, the flocculation and precipitation of kaolinite slowed down with the increase in apple pectin.
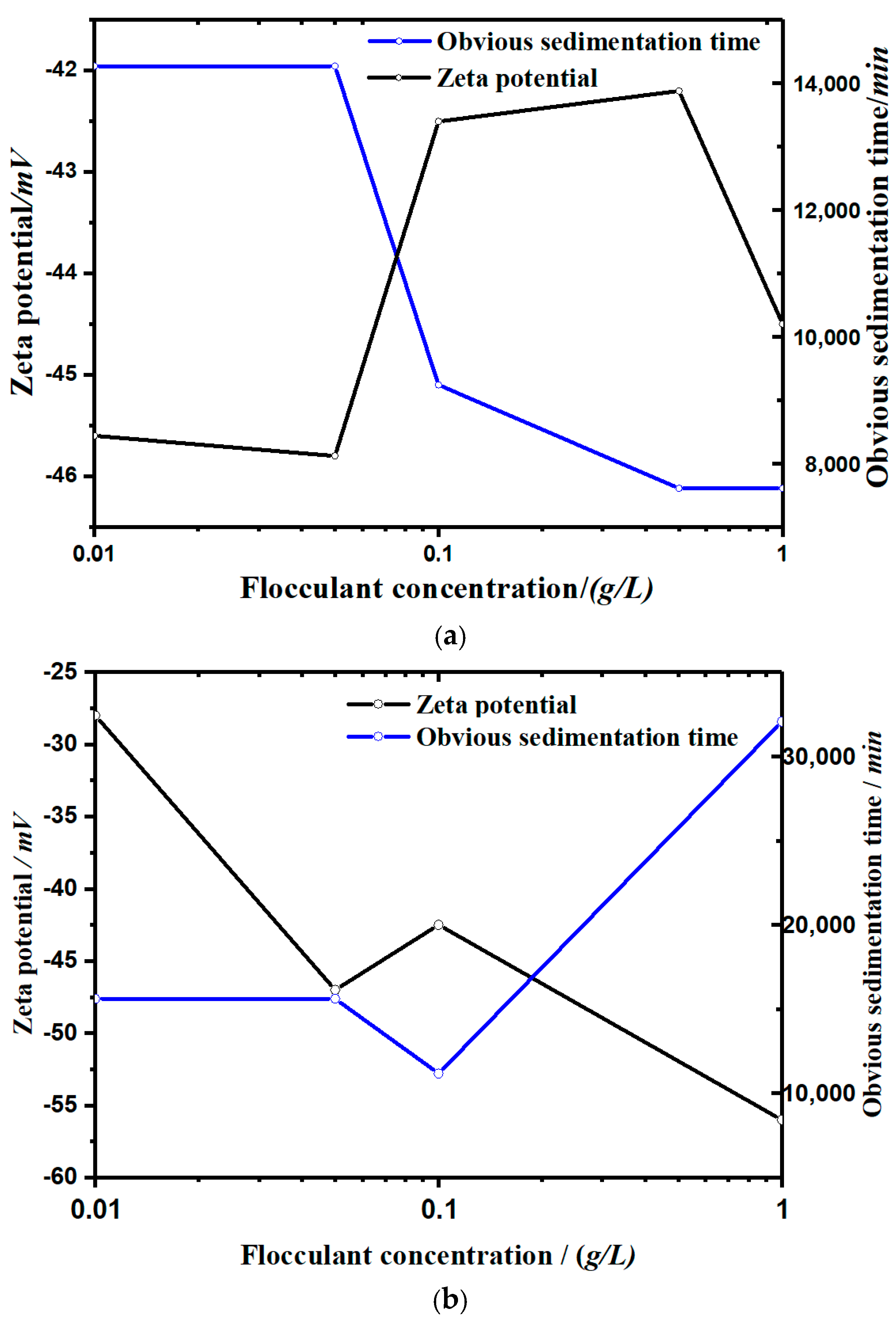
- It can be seen from Figure 5 that the settling rate of the kaolinite particles was negatively correlated with the zeta potential, and the types of flocculants added affected the change of zeta potential with the concentration. The zeta potential of the soluble starch group increased with the addition of the flocculant. The zeta potential value in the experimental group of apple pectin was inversely proportional to the amount of added flocculant. The zeta potential of the NaCl control group increased significantly with the increase of NaCl.
5. Case Analysis
6. Discussion
7. Conclusions
- Compared with the calculation method of the settling rate based on Stokes’ law, the model proposed in this paper avoids a series of difficulties, such as uneven suspension and the difficult observation of the boundary line between supernatant and suspension, and it can more accurately and intuitively obtain the variation law of concentration at different depths.
- Because of the characteristics of the added flocculant, different flocculants have different flocculation effects on the kaolinite particles. For example, after soluble starch is dissolved in water, the intermolecular hydrogen bond breaks and gelatinization occurs. This reaction promotes the flocculation of kaolinite particles, to some extent. After the apple pectin was dissolved in water, the amino functional groups in the macromolecules weakly ionized, and the generated hydroxide ions further reduced the zeta potential in the aqueous system and slowed the flocculation process as well as the precipitation of soil particles.
- The zeta potential reflects the electrification of the surface of small particles, and the more neutral the charge, the more likely it is to flocculate and settle. However, because the different flocculants have different flocculation mechanisms—for example, the gelatinization of soluble starch dissolved in water will not significantly change the electrification of the surface of small particles—the zeta potential has limitations regarding its control of the flocculant flocculation capacity.
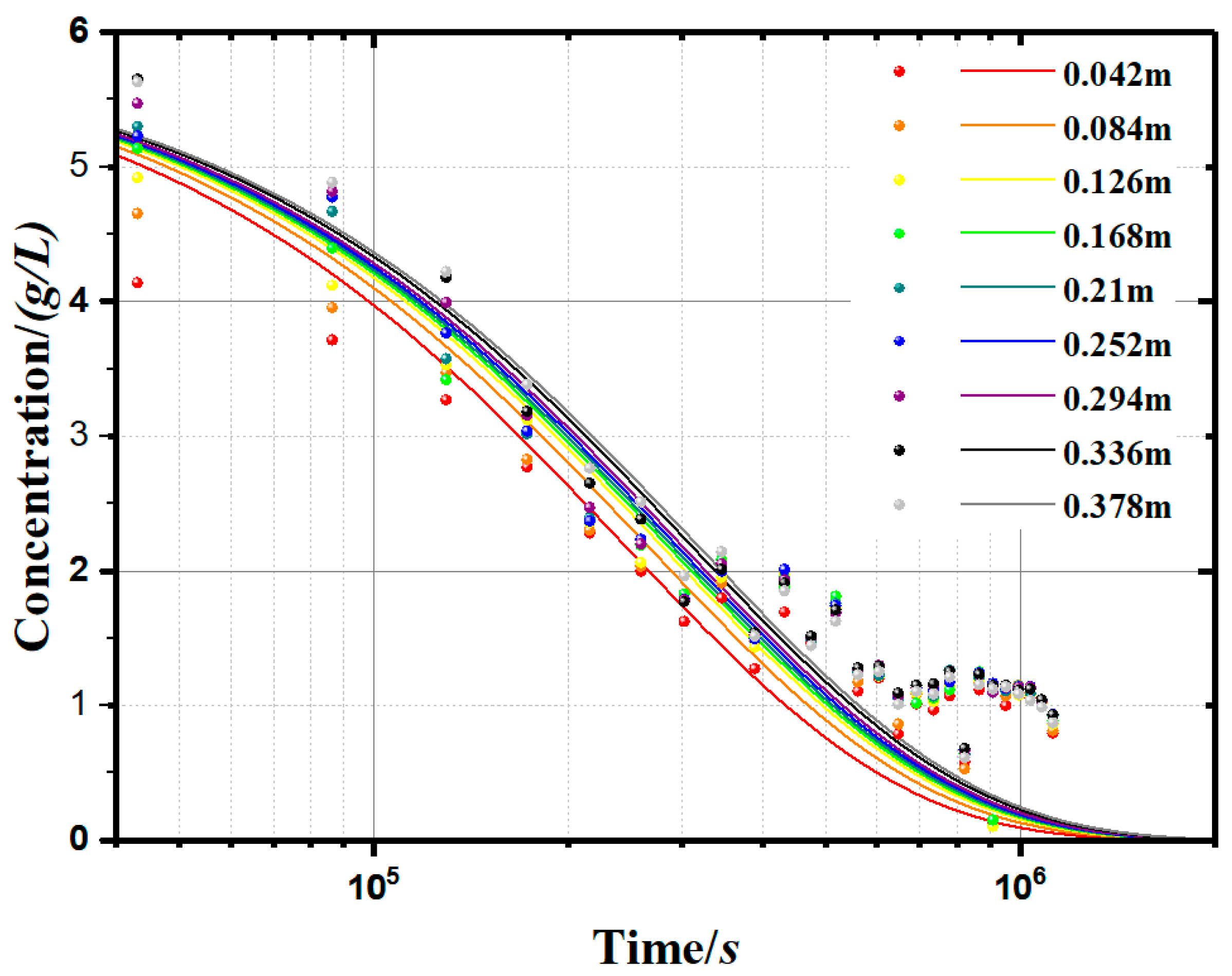
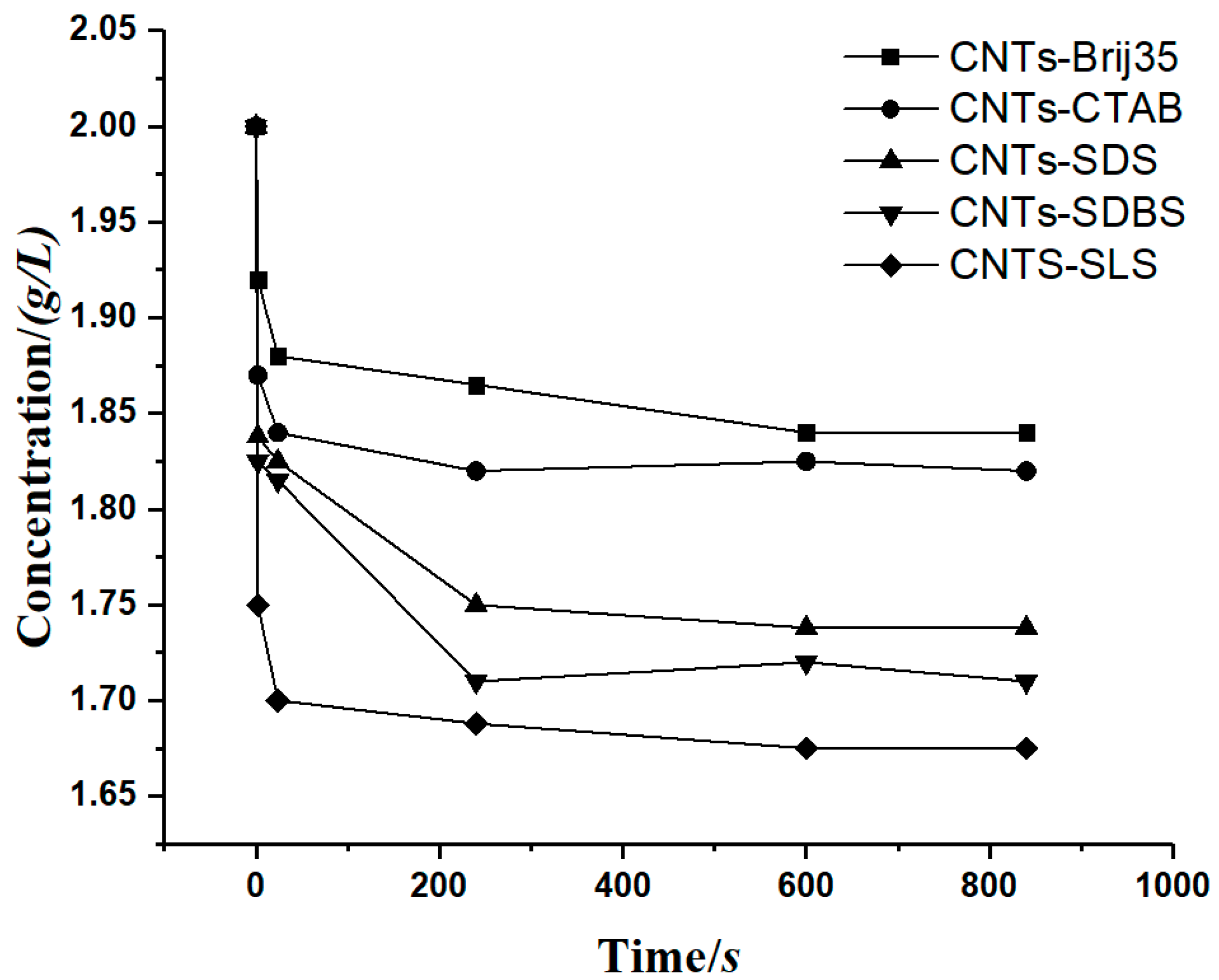
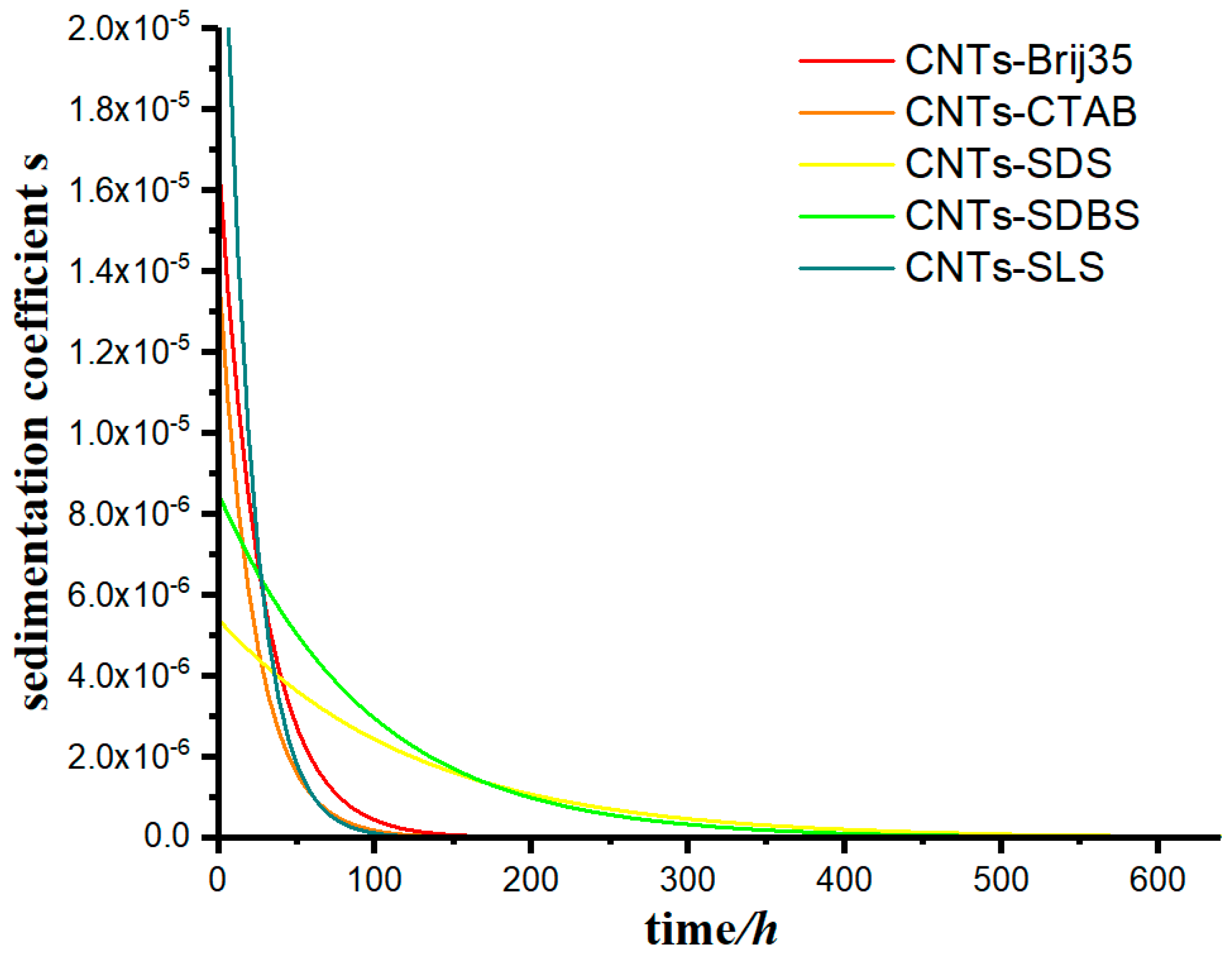
- The accuracy of the model was verified. As shown in Table 2, the value of the R-square ranges from 0.93296 to 0.96644, which has a high goodness of fit. This model can be used to calculate the case of carbon nanotubes suspension sedimentation, and the results are in good agreement with the conclusions of that study. It was proven that the model has a high application value in the study of sedimentary characteristics under a constant-gravity environment.
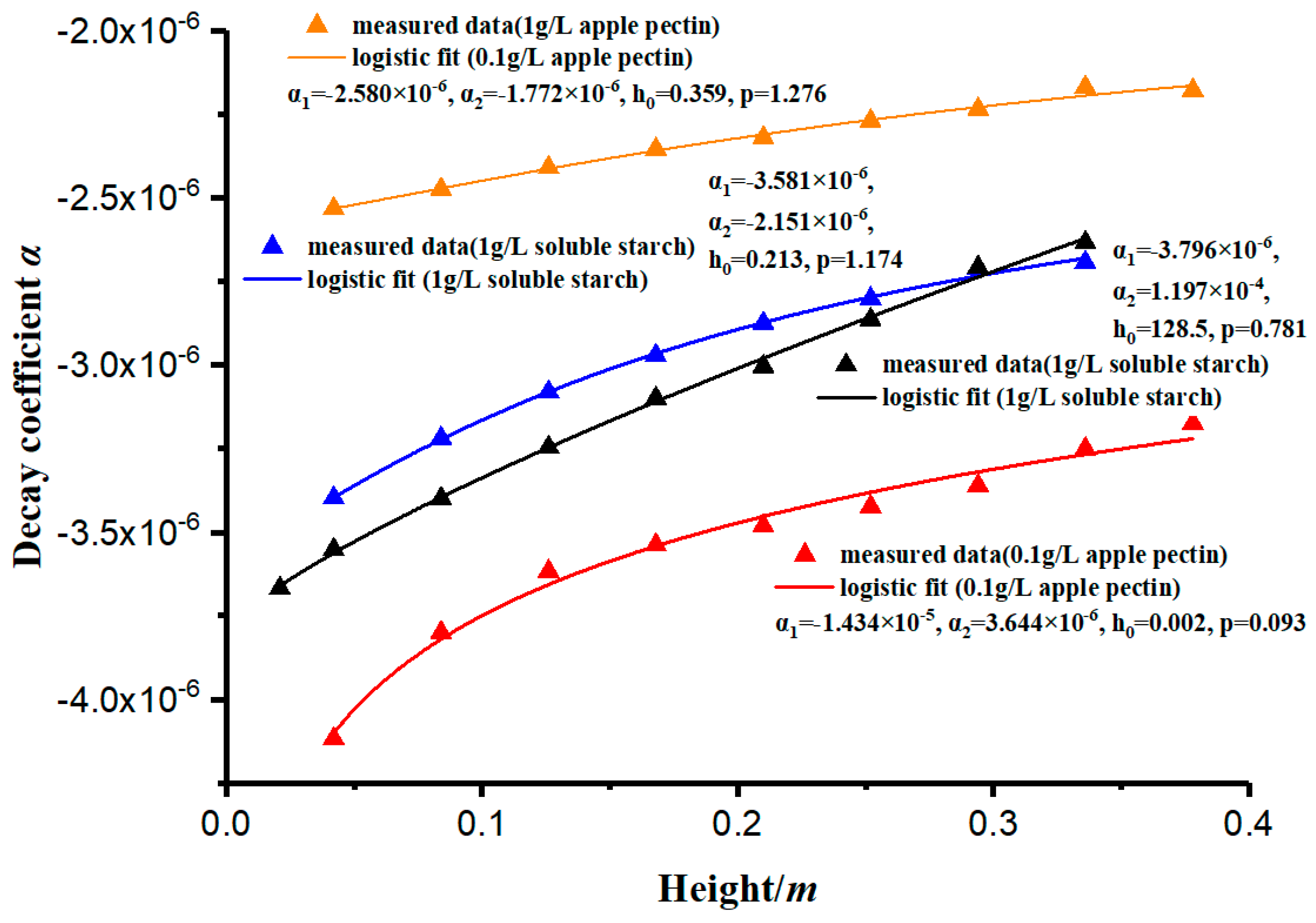
- Two important parameters were proposed in this paper—sedimentation coefficient s and decay coefficient α. Sedimentation coefficient s reflects the settling rate of kaolinite at any height in the measuring cylinder, and its value depends on the position in the measuring cylinder and the effect of the flocculant on the aggregate flocculation of kaolinite particles. It was found that sedimentation coefficient s increases with the increase in depth in the measuring cylinder. Decay coefficient α is a parameter that characterizes the material properties of the flocculant, and the coefficient depends on the physicochemical properties of the flocculant itself. Furthermore, the model can obtain the performance parameters of various additional flocculants and is helpful for the further study of the precipitation characteristics of small particles.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Adachi, Y.; Kawashima, Y.T.; Ghazali, M.E.B. Rheology and Sedimentation of Aqueous Suspension of Na-montmorillonite in the Very Dilute Domain. KONA Powder Part. J. 2020, 37, 145–165. [Google Scholar] [CrossRef]
- Zbik, M.S.; Song, Y.F.; Frost, R.L. Kaolinite flocculation induced by scemite addition-a transmission X-ray microscopic study. J. Colloid Interface Sci. 2010, 349, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Azam, S. Effect of Composition and Morphology on self-weight settling of Laterite Ore Slurries. Geotech. Geol. Eng. 2011, 30, 107–118. [Google Scholar] [CrossRef]
- Baird, J.C.; Walz, J.Y. The effects of added nanoparticles on aqueous kaolinite suspensions. I. Structural effects. J. Colloid Interface Sci. 2006, 297, 161–169. [Google Scholar] [CrossRef]
- Chandler, R.J. Stiff sedimentary clays: Geological origins and engineering properties. Geotechnique 2001, 60, 891–902. [Google Scholar] [CrossRef]
- Chang, M.Y.; Juang, R.S. Adsorption of tannic acid, humic acid, and dyes from water using the composite of chitosan and activated clay. J. Colloid Interface Sci. 2004, 278, 18–25. [Google Scholar] [CrossRef]
- Du, J.; Morris, G.; Pushkarova, R.A.; St, C.; Smart, R. Effect of surface structure of kaolinite on aggregation, settling rate, and bed density. Langmuir 2010, 26, 13227–13235. [Google Scholar] [CrossRef]
- Hawlader, B.C.; Muhunthan, B.; Imai, G. State-dependent constitutive model and numerical solution of self-weight consolidation. Geotechnique 2008, 58, 133–141. [Google Scholar] [CrossRef]
- Imai, G. Settling Behavior of Clay Suspension. Soils Found. 1980, 20, 61–77. [Google Scholar] [CrossRef]
- Liu, D.; Peng, Y. Reducing the entrainment of clay minerals in flotation using tap and saline water. Powder Technol. 2014, 253, 216–222. [Google Scholar] [CrossRef]
- Mpofu, P.; Addai-Mensah, J.; Ralston, J. Flocculation and dewatering behavior of smectite dispersions: Effect of polymer structure type. Miner. Eng. 2004, 17, 411–423. [Google Scholar] [CrossRef]
- McFarlane, A.; Bremmell, K.; Addai-Mensah, J. Improved dewatering behavior of clay minerals dispersions via interfacial chemistry and particle interactions optimization. J. Colloid Interface Sci. 2006, 293, 116–127. [Google Scholar] [CrossRef] [PubMed]
- McFarlane, A.; Yeap, K.Y.; Bremmell, K.; Addai-Mensah, J. The influence of flocculant adsorption kinetics on the dewaterability of kaolinite and smectite clay mineral dispersions. Colloids Surfaces A Physicochem. Eng. Asp. 2008, 317, 39–48. [Google Scholar] [CrossRef]
- McFarlane, A.J.; Bremmell, K.E.; Addai-Mensah, J. Optimising the dewatering behavior of clay tailings through interfacial and controlled shear. Powder Technol. 2005, 160, 27–34. [Google Scholar] [CrossRef]
- Mpofu, P.; Addai-Mensah, J.; Ralston, J. Investigation of the effect of polymer structure type on flocculation, rheology and dewatering behavior of kaolinite dispersions. Int. J. Miner. Process. 2003, 71, 247–268. [Google Scholar] [CrossRef]
- Mpofu, P.; Addai-Mensah, J.; Ralston, J. Temperature influence of nonionic polyethylene on flocculation and dewatering behavior of kaolinite dispersions. J. Colloid Interface Sci. 2004, 271, 145–156. [Google Scholar] [CrossRef]
- Nasser, M.S.; James, A.E. Effect of polyacrylamide polymers on floc size and rheological behavior of kaolinite suspensions. Colloids Surfaces A Physicochem. Eng. Asp. 2007, 301, 311–322. [Google Scholar] [CrossRef]
- Wu, C.; Chern, J. Experiment and Simulation of Sludge Batch Settling Curves: A wave approach. Ind. Eng. Chem. Res. 2006, 45, 2026–2031. [Google Scholar] [CrossRef]
- Brostow, W.; Pal, S.; Singh, P.P. A model of flocculation. Mater. Lett. 2007, 61, 4381–4384. [Google Scholar] [CrossRef]
- Brostow, W.; Lobland, H.H.; Pal, S.; Singh, R.P. Polymer flocculants for wastewater and industrial effluent treatment. J. Mater. Educ. 2009, 31, 157–166. [Google Scholar]
- Cao, J.; Kang, X.; Bate, B. Microscopic and physicochemical studies of polymer-modified kaolinite suspensions. Colloids Surf. A Physicochem. Eng. Asp. 2018, 554, 16–26. [Google Scholar] [CrossRef]
- Kang, X.; Xia, Z.; Wang, J.; Yang, W. A new approach to batch sedimentation and estimate the settling velocity, solid volume fraction, and floc size kaolinite in concentrated solutions. Colloids Surf. A Physicochem. Eng. Asp. 2019, 579, 123647. [Google Scholar] [CrossRef]
- Kang, X.; Bate, B. Shear wave velocity and its anisotropy of polymer modified mixtures. J. Geotech. Geoenviron. Eng. 2016, 142, 04016068. [Google Scholar] [CrossRef]
- Michaels, A.S.; Bolger, J.C. Settling rates and sediment volumes of flocculated kaolin suspensions. Ind. Eng. Chem. Fundam. 1962, 1, 24–33. [Google Scholar] [CrossRef]
- Solovyova, A.; Schuck, P.; Costenaro, L.; Ebel, C. Non-Ideality by Sedimentation Velocity of Halophilic Malate Dehydrogenase in Complex Solvents. Biophys. J. 2001, 81, 1868–1880. [Google Scholar] [CrossRef]
- Chaturvedi, S.K.; Ma, J.; Brown, P.H.; Zhao, H.; Schuck, P. Measuring macromolecular size distributions and interactions at high concentrations by sedimentation velocity. Nat. Commun. 2018, 9, 1–9. [Google Scholar] [CrossRef]
- Kaya, A.; Oren, A.H.; Yukselen, Y. Settling Behavior and Zeta Potential of Kaolinite in Aqueous Media. In Proceedings of the Thirteenth (2003) International Offshore and Polar Engineering Conference, Honolulu, HI, USA, 25–30 May 2003. [Google Scholar]
- Imai, G. Experimental Studies on Sedimentation Mechanism and Sediment Formation of Clay Materials. Soils Found. 1981, 21, 7–20. [Google Scholar] [CrossRef]
- Hu, C.Y.; Liao, X.N.; Li, W.K.; Zhang, R.F.; Xiang, J.H.; Li, M.S.; Duo, S.W.; Li, F.Y. Suspension of Carbon Nanotubes Prepared by Non-covalent Functionalization with Surfactants. Chin. J. Process. Eng. 2010, 10, 190–194. [Google Scholar]





| Experimental Group | Kaolinite/g | Deionized Water/L | Flocculant |
|---|---|---|---|
| 1 | 30 | 5 | 0.1 g/L soluble starch |
| 2 | 30 | 5 | 1 g/L soluble starch |
| 3 | 30 | 5 | 0.1 g/L apple pectin |
| 4 | 30 | 5 | 1 g/L apple pectin |
| 5 | 30 | 5 | 0.1 mol/L NaCl |
| Height/Flocculant | 0.021 m/Soluble Starch (1 g/L) | 0.336 m/Soluble Starch (1 g/L) | 0.378 m/Apple Pectin (0.1 g/L) |
|---|---|---|---|
| R2 | 0.966 | 0.979 | 0.933 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Kang, X.; Peng, C. Modelling and Experimental Investigation on the Settling Rate of Kaolinite Particles in Non-Ideal Sedimentation Stage under Constant Gravity. Materials 2020, 13, 3785. https://doi.org/10.3390/ma13173785
Wang J, Kang X, Peng C. Modelling and Experimental Investigation on the Settling Rate of Kaolinite Particles in Non-Ideal Sedimentation Stage under Constant Gravity. Materials. 2020; 13(17):3785. https://doi.org/10.3390/ma13173785
Chicago/Turabian StyleWang, Jianfu, Xin Kang, and Chunyin Peng. 2020. "Modelling and Experimental Investigation on the Settling Rate of Kaolinite Particles in Non-Ideal Sedimentation Stage under Constant Gravity" Materials 13, no. 17: 3785. https://doi.org/10.3390/ma13173785
APA StyleWang, J., Kang, X., & Peng, C. (2020). Modelling and Experimental Investigation on the Settling Rate of Kaolinite Particles in Non-Ideal Sedimentation Stage under Constant Gravity. Materials, 13(17), 3785. https://doi.org/10.3390/ma13173785





