Solution-Blown Aligned Nanofiber Yarn and Its Application in Yarn-Shaped Supercapacitor
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
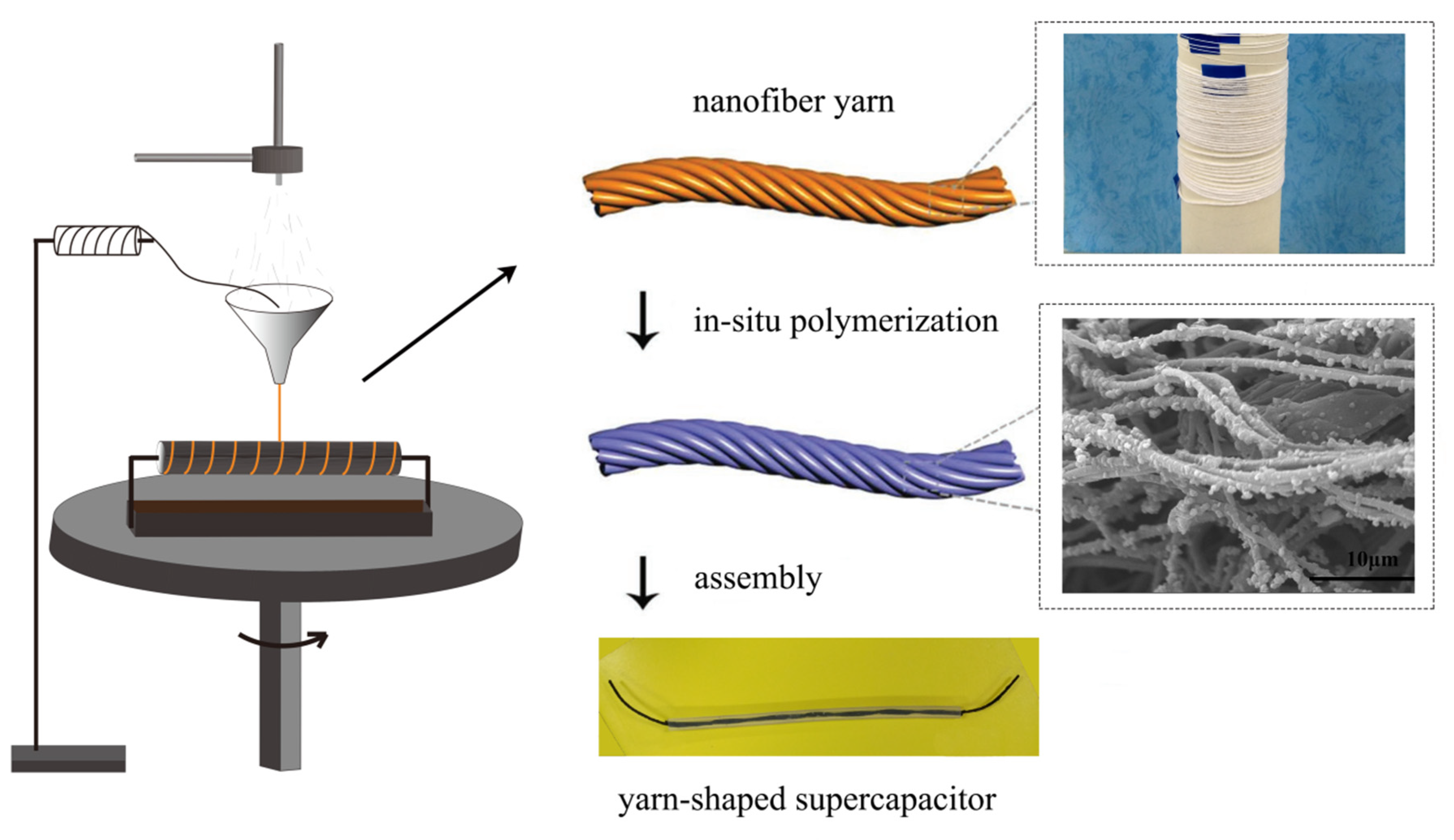
2.2. Preparation of Carbon Fiber Bundles @ Polyacrylonitrile Nanofiers (CFs@PAN NFs)
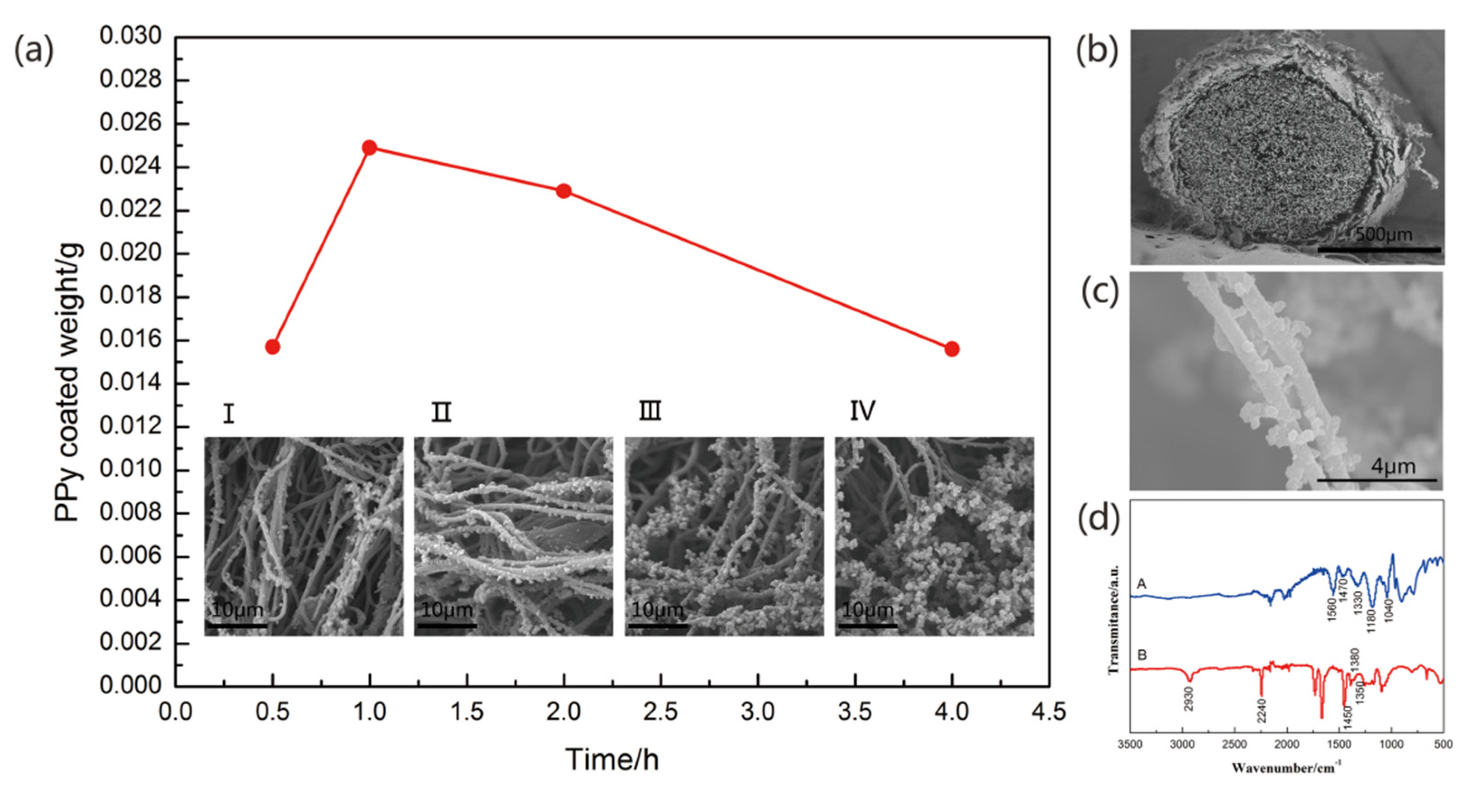
2.3. Preparation of Carbon Fiber Bundles@Polyacrylonitrile Nanofibers@polypyrrole (CFs@PAN NFs@PPy)
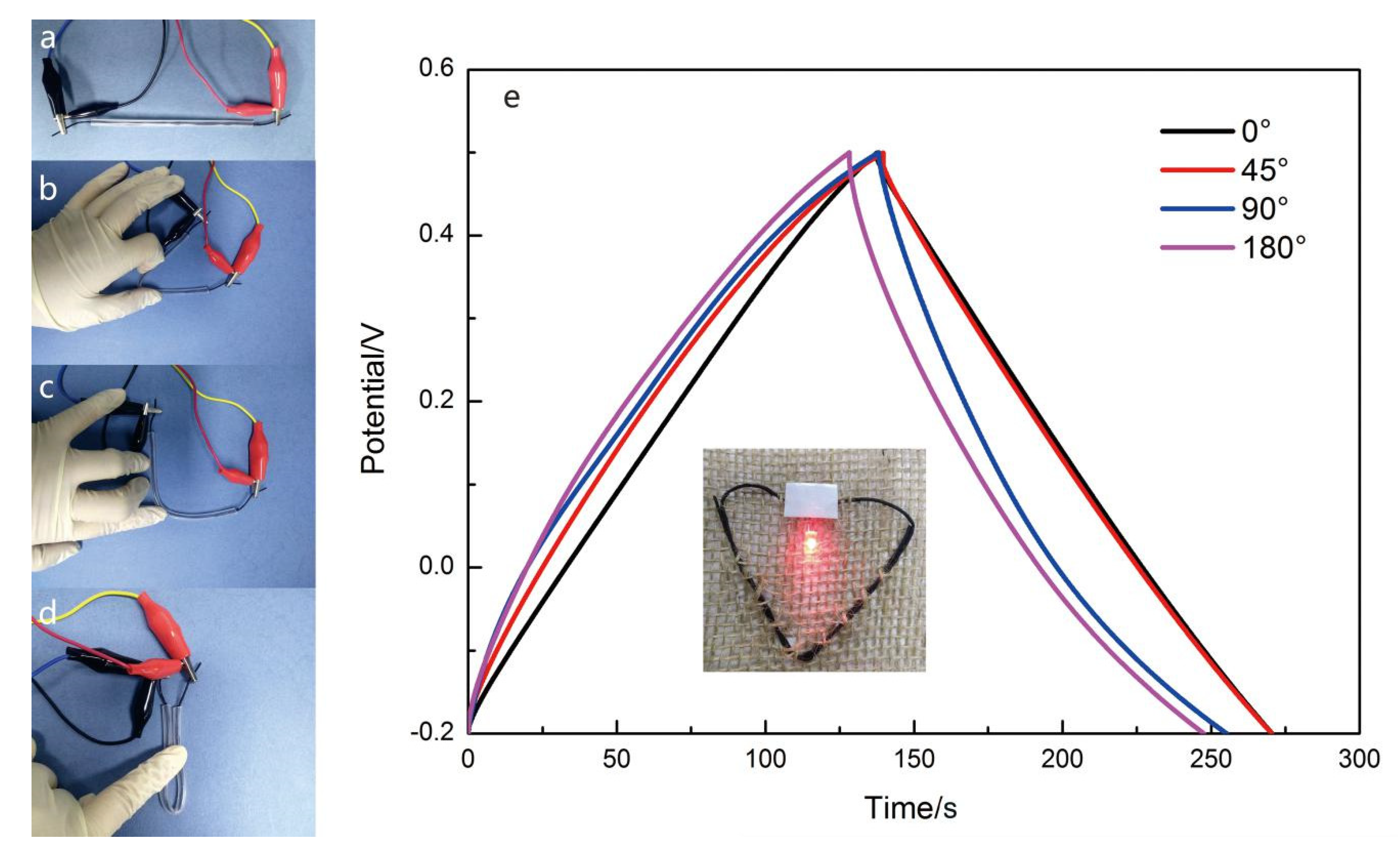
2.4. Assembly of the Yarn-Shaped Supercapacitor
2.5. Characterization
2.6. Measurements
3. Resullts and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ra, E.J.; Raymundo-Pinero, E.; Lee, Y.H.; Beguin, F. High power supercapacitors using polyacrylonitrile-based carbon nanofiber paper. Carbon 2009, 47, 2984–2992. [Google Scholar] [CrossRef]
- Dai, S.G.; Liu, Z.; Zhao, B.T.; Zeng, J.H.; Hu, H.; Zhang, Q.B.; Chen, D.C.; Qu, C.; Dang, D.; Liu, M.L. A High-Performance Supercapacitor Electrode Based on N-doped Porous Graphene. J. Power Sources 2018, 387, 43–48. [Google Scholar] [CrossRef]
- Gwon, H.; Kim, H.S.; Lee, K.U.; Seo, D.H.; Park, Y.C.; Lee, Y.S.; Ahn, B.T.; Kang, K. Flexible energy storage devices based on graphene paper. Energy Environ. Sci. 2011, 4, 1277–1283. [Google Scholar] [CrossRef]
- Shi, S.J.; Zhuang, X.P.; Cheng, B.W.; Wang, X.Q. Solution blowing of ZnO nanoflake-encapsulated carbon nanofibers as electrodes for supercapacitors. J. Mater. Chem. A 2013, 1, 13779–13788. [Google Scholar] [CrossRef]
- Wei, W.T.; Chen, W.H.; Ding, L.Y.; Cui, S.Z.; Mi, L.W. Construction of hierarchical three-dimensional interspersed flower-like nickel hydroxide for asymmetric supercapacitors. Nano Res. 2017, 10, 3726–3742. [Google Scholar] [CrossRef]
- Guo, Y.; Shi, Z.Q.; Chen, M.M.; Wang, C.Y. Hierarchical porous carbon derived from sulfonated pitch for electrical double layer capacitors. J. Power Sources 2014, 252, 235–243. [Google Scholar] [CrossRef]
- Zeng, W.; Shu, L.; Li, Q.; Chen, S.; Wang, F.; Tao, X.M. Fiber-Based Wearable Electronics: A Review of Materials, Fabrication, Devices, and Applications. Adv. Mater. 2014, 26, 5310–5336. [Google Scholar] [CrossRef]
- Zheng, X.Y.; Luo, J.Y.; Lv, W.; Wang, D.W.; Yang, Q.H. Two-Dimensional Porous Carbon: Synthesis and Ion-Transport Properties. Adv. Mater. 2015, 27, 5388–5395. [Google Scholar] [CrossRef]
- Peng, C.; Hu, D.; Chen, G.Z. Theoretical specific capacitance based on charge storage mechanisms of conducting polymers:Comment on Vertically oriented arrays of polyaniline nanorods and their super electrochemical properties. Chem. Commun. 2011, 47, 4105–4107. [Google Scholar] [CrossRef]
- Bobacka, J.; Lewenstam, A.; Ivaska, A. Electrochemical impedance spectroscopy of oxidized poly(3,4-ethylenedioxythiophene) film electrodes in aqueous solutions. J. Electroanal. Chem. 2000, 489, 17–27. [Google Scholar] [CrossRef]
- Zang, J.F.; Bao, S.J.; Li, C.M. Well-aligned cone-shaped nanostructure of polypyrrole/RuO2 and its electrochemical supercapacitor. J. Phys. Chem. C 2008, 112, 14843–14847. [Google Scholar] [CrossRef]
- Randriamahazaka, H.; Plesse, C.; Teyssie, D.; Chevrot, C. Relaxation kinetics of poly(3, 4-ethylenedioxythiophene) in 1-ethyl-3-methylimidazolium bis((trifluoromethyl)sulfonyl)amide ionic liquid during potential step experiments. Electrochim. Acta 2015, 50, 1515–1522. [Google Scholar] [CrossRef]
- Sun, J.F.; Huang, Y.; Fu, C.X.; Wang, Z.Y.; Huang, Y.; Zhu, M.S.; Zhi, C.Y.; Hu, H. High-performance stretchable yarn supercapacitor based on PPy@CNTs@urethane elastic fiber core spun yarn. Nano Energy 2016, 27, 230–237. [Google Scholar] [CrossRef]
- Zhang, C.J.; Chen, Z.Q.; Rao, W.D.; Fan, L.L.; Xia, Z.G.; Xu, W.L.; Xu, J. A high-performance all-solid-state yarn supercapacitor based on polypyrrole-coated stainless steel/cotton blended yarns. Cellulose 2019, 26, 1169–1181. [Google Scholar] [CrossRef]
- Xu, Q.; Fan, L.L.; Yuan, Y.; Wei, C.Z.; Bai, Z.K.; Xu, J. All-solid-state yarn supercapacitors based on hierarchically structured bacterial cellulose nanofiber-coated cotton yarns. Cellulose 2016, 23, 3987–3997. [Google Scholar] [CrossRef]
- Mulayim, B.B.; Goktepe, F. Analysis of polyacrylonitrile nanofiber yarn formation in electrospinning by using a conical collector and two oppositely charged nozzles. J. Text. Inst. 2020, 74, 1–11. [Google Scholar] [CrossRef]
- Yu, Y.X.; Tan, Z.K.; Zhang, Z.B.; Liu, G.B. Microstructural evolution and mechanical investigation of hot stretched graphene oxide reinforced polyacrylonitrile nanofiber yarns. Polym. Adv. Technol. 2020, 31, 1935–1945. [Google Scholar] [CrossRef]
- Qi, K.; Wang, H.B.; You, X.L.; Tao, X.J.; Li, M.Y.; Zhou, Y.M.; Zhang, Y.M.; He, J.X.; Shao, W.L.; Cui, S.Z. Core-sheath nanofiber yarn for textile pressure sensor with high pressure sensitivity and spatial tactile acuity. J. Colloid. Interf. Sci. 2020, 561, 93–103. [Google Scholar] [CrossRef]
- Huang, L.B.; Xie, X.X.; Huang, H.; Zhu, J.; Yu, J.R.; Wang, Y.; Hu, Z.M. Electrospun polyamide-6 nanofiber for hierarchically structured and multi-responsive actuator. Sens. Actuators A Phys. 2020, 302, 111793. [Google Scholar] [CrossRef]
- Ravandi, S.A.H.; Mehrara, S.; Sadrjahani, M.; Haghi, A.K. Tunable wicking behavior via titanium oxide embedded in polyacrylonitrile nanofiber strings of yarn. Polym. Bull. 2020, 77, 307–322. [Google Scholar] [CrossRef]
- Zhuang, X.P.; Jia, K.F.; Cheng, B.W.; Feng, X.; Shi, S.J.; Zhang, B. Solution blowing of continuous carbon nanofiber yarn and its electrochemical performance for supercapacitors. Chem. Eng. J. 2014, 237, 308–311. [Google Scholar] [CrossRef]
- Jia, K.F.; Zhuang, X.P.; Cheng, B.W. Solution blown aligned carbon nanofiber yarn as supercapacitor electrode. J. Mater. Sci. 2013, 24, 4769–4773. [Google Scholar] [CrossRef]
- Mao, N.; Chen, W.C.; Meng, J.; Li, Y.Y.; Zhang, K.; Qin, X.H.; Zhang, H.N.; Zhang, C.Y.; Qiu, Y.P.; Wang, S.R. Enhanced electrochemical properties of hiererchically sheath-core aligned carbon nanofibers coated carbon fiber yarn electrode-based supercapacitor via polyaniline nanowire arry modification. J. Power Sources 2018, 399, 406–413. [Google Scholar] [CrossRef]
- Wang, H.; Li, X.X.; Li, X.J. Self-Assembly DBS Nanofibrils on Solution-Blown Nanofibers as Hierarchical Ion-Conducting Pathway for Direct Methanol Fuel Cells. Polymers 2018, 10, 1037. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Li, D.P.; Chen, L.N.; Si, P.C.; Feng, J.K.; Zhang, L.; Li, Y.H.; Lou, J.; Ci, L.J. Core-shell structured carbon nanofibers yarn@polypyrrole@graphene for high performance all-solid-state fiber supercapacitors. Carbon 2018, 138, 264–270. [Google Scholar] [CrossRef]
- Xu, R.Q.; Guo, F.M.; Cui, X.; Zhang, L.; Wang, K.L.; Wei, J.Q. High performance carbon nanotube based fiber-shaped supercapacitors using redox additives of polypyrrole and hydroquinone. J. Mater. Chem. A 2015, 3, 22353–22360. [Google Scholar] [CrossRef]
- Sun, W.; Chen, X.Y. Fabrication and tests of a novel three dimensional micro supercapacitor. Microelecctron. Eng. 2009, 86, 1307–1310. [Google Scholar] [CrossRef]
- Ding, X.T.; Zhao, Y.; Hu, C.G.; Hu, Y.; Dong, Z.L.; Chen, N.; Zhang, Z.P.; Qu, L.T. Spinning fabrication of graphene/polypyrrole composite fibers for all-solid state, flexible fibriform supercapacitors. J. Mater. Chem. A 2014, 2, 12355–12360. [Google Scholar] [CrossRef]
- Changhoon, S.; Junyeong, Y.; Kayeon, K.; Yu, R.J.; Heun, P.; Hanchan, L.; Geumbee, L.; Seung, Y.O.; Jeong, S.H. High performance wire-type supercapacitor with PPy/CNT-ionic liquid/AuNP/carbon fiber electrode and ionic liquid based electrolyte. Carbon 2019, 144, 639–648. [Google Scholar]
- Ma, Y.; Wang, Q.F.; Liang, X.; Zhang, D.H.; Miao, M.H. Wearable supercapacitors based on conductive cotton yarns. J. Mater. Sci. 2018, 53, 14586–14597. [Google Scholar] [CrossRef]
- Huang, Y.; Hu, H.; Huang, Y.; Zhu, M.S.; Meng, W.J.; Pei, Z.X.; Liu, C.; Zhi, C.Y. From industrially weavable and knittable highly conductive yarns to large wearable energy storage textiles. ACS Nano 2015, 9, 4766–4775. [Google Scholar] [CrossRef] [PubMed]




| Electrode Materials | Areal Capacitance | Energy Density | Power Density | References |
|---|---|---|---|---|
| (mF cm−2) | (μWh cm−2) | (μW cm−2) | ||
| PPy@CNTs@urethane elastic fiber | 67 | 6.13 | 133 | [13] |
| PPy/SS/cotton | 344 | 36.2 | 135 | [14] |
| PPy/BC | 76.6 | 16.9 | 10.9 | [15] |
| PPy/CNT-ionic liquid/AuNP/carbon fiber | 38.49 | 24.7 | 3520 | [29] |
| PEDOT:PSS-PPy 50 wt% SSF/cotton yarn | 1368.2 | 160 | - | [30] |
| PPy/MnO2/rGO | 103 | 9.2 | 1330 | [31] |
| CFs@10 wt%-PAN NFs@1 h-PPy | 353 | 48 | 247 | This work |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.; Mao, Z.; Zheng, R.; Liu, H.; Shi, L. Solution-Blown Aligned Nanofiber Yarn and Its Application in Yarn-Shaped Supercapacitor. Materials 2020, 13, 3778. https://doi.org/10.3390/ma13173778
Yang J, Mao Z, Zheng R, Liu H, Shi L. Solution-Blown Aligned Nanofiber Yarn and Its Application in Yarn-Shaped Supercapacitor. Materials. 2020; 13(17):3778. https://doi.org/10.3390/ma13173778
Chicago/Turabian StyleYang, Jingjing, Zhaofei Mao, Ruiping Zheng, Hao Liu, and Lei Shi. 2020. "Solution-Blown Aligned Nanofiber Yarn and Its Application in Yarn-Shaped Supercapacitor" Materials 13, no. 17: 3778. https://doi.org/10.3390/ma13173778
APA StyleYang, J., Mao, Z., Zheng, R., Liu, H., & Shi, L. (2020). Solution-Blown Aligned Nanofiber Yarn and Its Application in Yarn-Shaped Supercapacitor. Materials, 13(17), 3778. https://doi.org/10.3390/ma13173778




