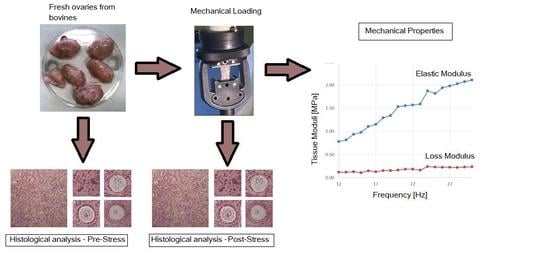
Dynamic Characterization of the Biomechanical Behaviour of Bovine Ovarian Cortical Tissue and Its Short-Term Effect on Ovarian Tissue and Follicles
Abstract
1. Introduction
2. Materials and Methods
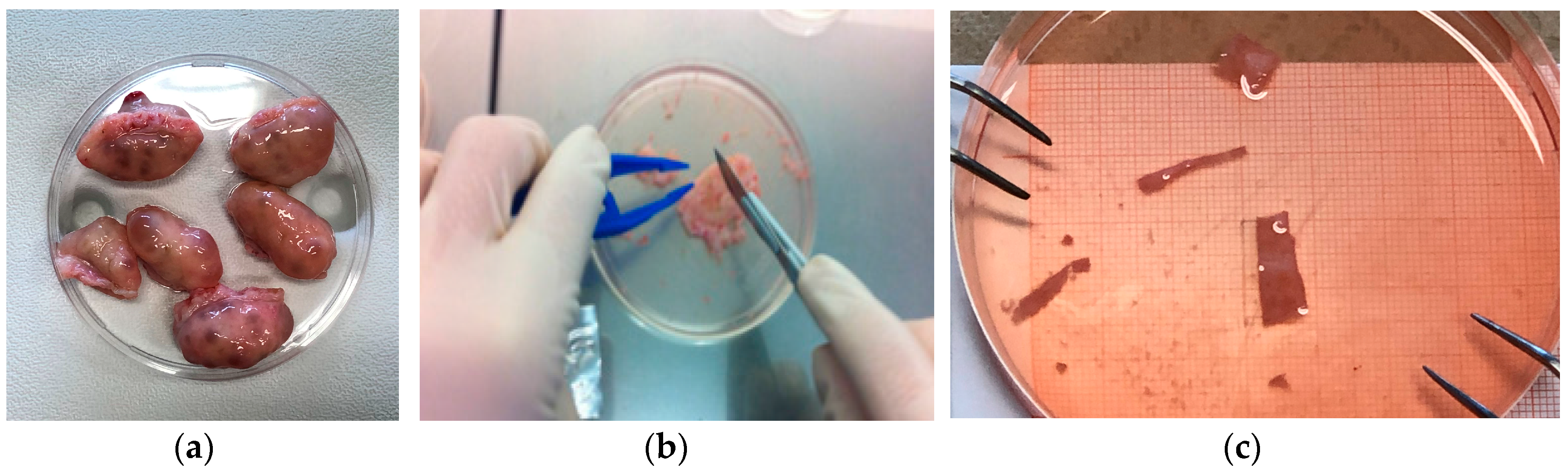
2.1. Materials
2.2. Mechanical Characterization
2.3. Histologic Characterization
2.4. Statistical Analysis
3. Results
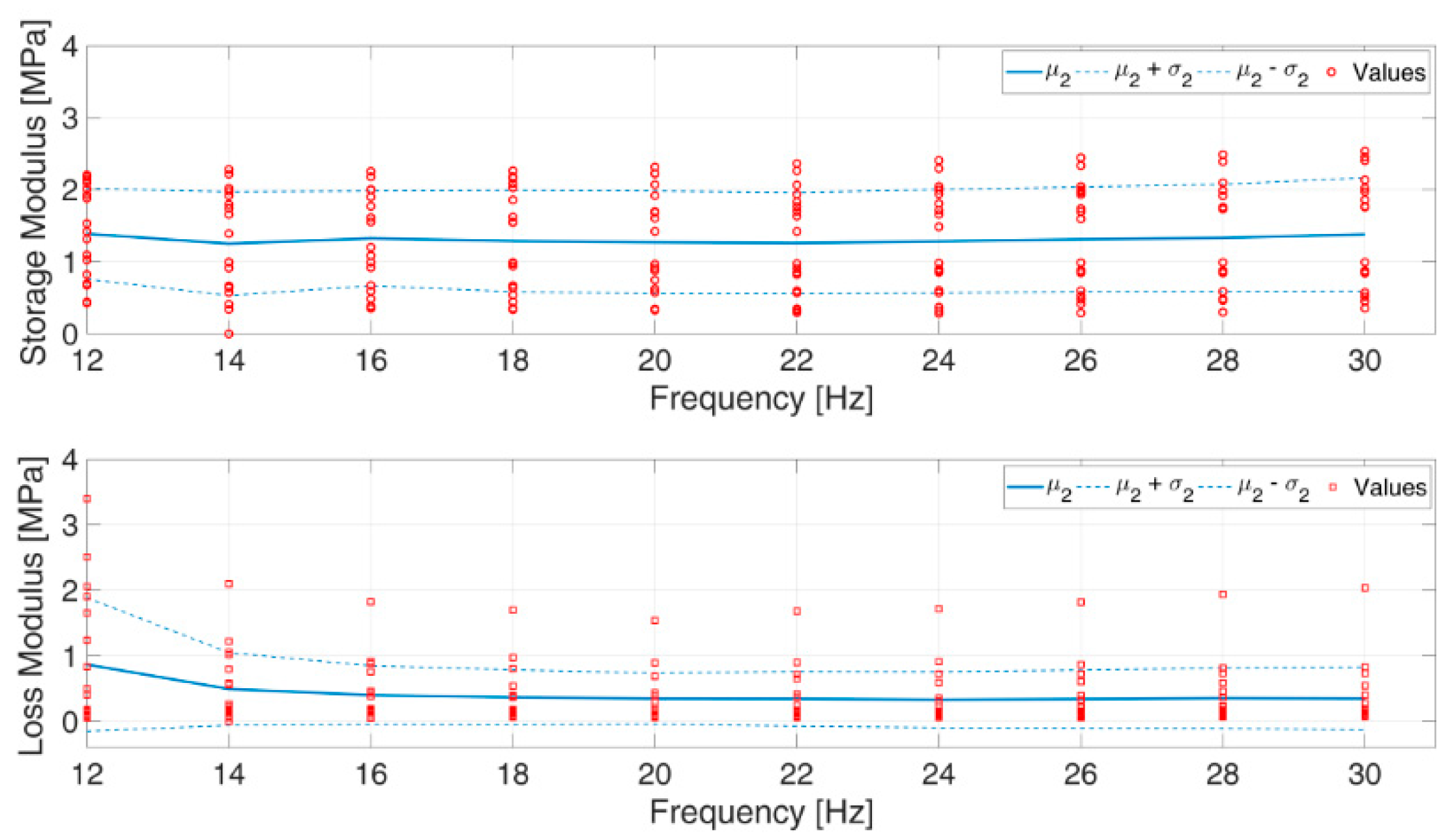
3.1. Mechanical Characterization
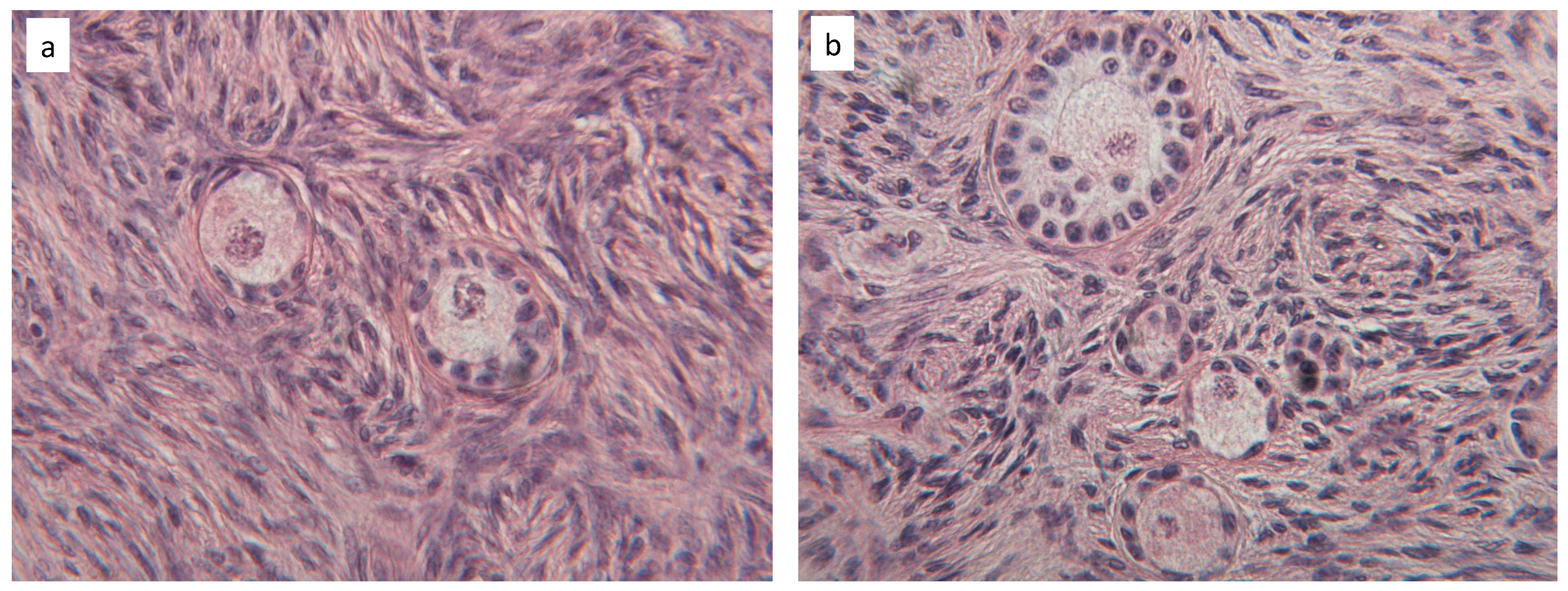
3.2. Tissue Characterization
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- WHO World Health Organisation. World report on disability. Lancet 2011, 377, 1977. [Google Scholar] [CrossRef]
- Donnez, J.; Dolmans, M.-M. Fertility Preservation in Women. N. Engl. J. Med. 2018, 378, 400–401. [Google Scholar] [PubMed]
- Donnez, J.; Silber, S.; Andersen, C.Y.; Demeestere, I.; Piver, P.; Meirow, D.; Pellicer, A.; Dolmans, M.M. Children born after autotransplantation of cryopreserved ovarian tissue. A review of 13 live births. Ann. Med. 2011, 43, 437–450. [Google Scholar] [CrossRef] [PubMed]
- Dolmans, M.M.; Luyckx, V.; Donnez, J.; Andersen, C.Y.; Greve, T. Risk of transferring malignant cells with transplanted frozen-thawed ovarian tissue. Fertil. Steril. 2013, 99, 1514–1522. [Google Scholar] [CrossRef] [PubMed]
- Abir, R.; Aviram, A.; Feinmesser, M.; Stein, J.; Yaniv, I.; Parnes, D.; Ben-Haroush, A.; Meirow, D.; Rabizadeh, E.; Fisch, B. Ovarian minimal residual disease in chronic myeloid leukaemia. Reprod. Biomed. Online 2014, 28, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Telfer, E.E.; McLaughlin, M.; Ding, C.; Thong, K.J. A Two-Step Serum-Free Culture System Supports Development of Human Oocytes From Primordial Follicles in the Presence of Activin. Hum. Reprod. 2008, 23, 1151–1158. [Google Scholar] [CrossRef]
- Laronda, M.M.; Duncan, F.E.; Hornick, J.E.; Xu, M.; Pahnke, J.E.; Whelan, K.A.; Shea, L.D.; Woodruff, T.K. Alginate encapsulation supports the growth and differentiation of human primordial follicles within ovarian cortical tissue. J. Assist. Reprod. Genet. 2014, 31, 1013–1028. [Google Scholar] [CrossRef]
- Hornick, J.E.; Duncan, F.E.; Shea, L.D.; Woodruff, T.K. Isolated primate primordial follicles require a rigid physical environment to survive and grow in vitro. Hum. Reprod. 2012, 27, 1801–1810. [Google Scholar] [CrossRef]
- O’Brien, M.J.; Pendola, J.K.; Eppig, J.J. A Revised Protocol for In Vitro Development of Mouse Oocytes from Primordial Follicles Dramatically Improves Their Developmental Competence1. Biol. Reprod. 2003, 68, 1682–1686. [Google Scholar] [CrossRef]
- Telfer, E.E.; Zelinski, M.B. Ovarian follicle culture: Advances and challenges for human and nonhuman primates. Fertil. Steril. 2013, 99, 1523–1533. [Google Scholar] [CrossRef]
- McLaughlin, M.; Albertini, D.F.; Wallace, W.H.B.; Anderson, R.A.; Telfer, E.E. Metaphase II oocytes from human unilaminar follicles grown in a multistep culture system. Mol. Hum. Reprod. 2018, 24, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Desai, N.; Alex, A.; AbdelHafez, F.; Calabro, A.; Goldfarb, J.; Fleischman, A.; Falcone, T. Three-dimensional in vitro follicle growth: Overview of culture models, biomaterials, design parameters and future directions. Reprod. Biol. Endocrinol. 2010, 8, 119. [Google Scholar] [CrossRef] [PubMed]
- Catapano, G.; Fragomeni, G.; Falvo D’Urso Labate, G.; De Napoli, L.; Barbato, V.; Di Nardo, M.; Costanzo, V.; Capriglione, T.; Gualtieri, R.; Talevi, R. Do Bioreactor Designs with More Efficient Oxygen Supply to Ovarian Cortical Tissue Fragments Enhance Follicle Viability and Growth In Vitro? Processes 2019, 7, 450. [Google Scholar] [CrossRef]
- Talevi, R.; Sudhakaran, S.; Barbato, V.; Merolla, A.; Braun, S.; Nardo, M.D.; Costanzo, V.; Ferraro, R.; Iannantuoni, N.; Catapano, G.; et al. Is oxygen availability a limiting factor for in vitro folliculogenesis? PLoS ONE 2018, 13, e0192501. [Google Scholar] [CrossRef]
- Riehl, B.D.; Park, J.H.; Kwon, I.K.; Lim, J.Y. Mechanical stretching for tissue engineering: Two-dimensional and three-dimensional constructs. Tissue Eng. Part B Rev. 2012, 18, 288–300. [Google Scholar] [CrossRef]
- Shah, J.S.; Sabouni, R.; Cayton Vaught, K.C.; Owen, C.M.; Albertini, D.F.; Segars, J.H. Biomechanics and mechanical signaling in the ovary: A systematic review. J. Assist. Reprod. Genet. 2018, 35, 1135–1148. [Google Scholar] [CrossRef]
- West, E.R.; Xu, M.; Woodruff, T.K.; Shea, L.D. Physical properties of alginate hydrogels and their effects on in vitro follicle development. Biomaterials 2007, 28, 4439–4448. [Google Scholar] [CrossRef]
- Kawamura, K.; Cheng, Y.; Suzuki, N.; Deguchi, M.; Sato, Y.; Takae, S.; Ho, C.H.; Kawamura, N.; Tamura, M.; Hashimoto, S.; et al. Hippo signaling disruption and Akt stimulation of ovarian follicles for infertility treatment. Proc. Natl. Acad. Sci. USA 2013, 110, 17474–17479. [Google Scholar] [CrossRef]
- Kawashima, I.; Kawamura, K. Regulation of follicle growth through hormonal factors and mechanical cues mediated by Hippo signaling pathway. Syst. Biol. Reprod. Med. 2018, 64, 3–11. [Google Scholar] [CrossRef]
- Telfer, E.E.; McLaughlin, M. WO2014043835A1-Method for Maturation of Oocytes In Vitro-Google Patents. Available online: https://patents.google.com/patent/WO2014043835A1/en (accessed on 1 July 2020).
- Aldieri, A.; Terzini, M.; Bignardi, C.; Zanetti, E.M.; Audenino, A.L. Implementation and validation of constitutive relations for human dermis mechanical response. Med. Biol. Eng. Comput. 2018, 56, 2083–2093. [Google Scholar] [CrossRef]
- Yang, X.; Church, C.C. A simple viscoelastic model for soft tissues in the frequency range 6-20 MHz. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2006, 53, 1404–1411. [Google Scholar] [CrossRef] [PubMed]
- Talevi, R.; Barbato, V.; Mollo, V.; Fiorentino, I.; De Stefano, C.; Guarino, F.M.; Gualtieri, R. Replacement of sodium with choline in slow-cooling media improves human ovarian tissue cryopreservation. Reprod. Biomed. Online 2013, 27, 381–389. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gougeon, A. Regulation of ovarian follicular development in primates: Facts and hypotheses. Endocr. Rev. 1996, 17, 121–155. [Google Scholar] [CrossRef] [PubMed]
- Van Wezel, I.L.; Rodgers, R.J. Morphological Characterization of Bovine Primordial Follicles and their Environment in Vivo. Biol. Reprod. 1996, 55, 1003–1011. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; West, E.; Shea, L.D.; Woodruff, T.K. Identification of a Stage-Specific Permissive In Vitro Culture Environment for Follicle Growth and Oocyte Development1. Biol. Reprod. 2006, 75, 916–923. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Pribenszky, C.S.; Molnár, M.; Zhang, X.; Yang, H.; Kuwayama, M.; Pedersen, A.M.; Villemoes, K.; Bolund, L.; Vajta, G. High hydrostatic pressure: A new way to improve in vitro developmental competence of porcine matured oocytes after vitrification. Reproduction 2008, 135, 13–17. [Google Scholar] [CrossRef]
- Connolly, J.M.; Kane, M.T.; Quinlan, L.R.; Hynes, A.C. Enhancing oxygen delivery to ovarian follicles by three different methods markedly improves growth in serum-containing culture medium. Reprod. Fertil. Dev. 2019, 31, 1339–1352. [Google Scholar] [CrossRef]
- Mondragon, E.; Yoshida, K.; Kristin Myers, M. Characterizing the Biomechanical and Biochemical Properties of Mouse Uterine Tissue. Columbia Undergrad. Sci. J. 2013, 7, 10–17. [Google Scholar]
- Omari, E.A.; Varghese, T.; Kliewer, M.A.; Harter, J.; Hartenbach, E.M. Dynamic and quasi-static mechanical testing for characterization of the viscoelastic properties of human uterine tissue. J. Biomech. 2015, 48, 1730–1736. [Google Scholar] [CrossRef]
- Callejas, A.; Gomez, A.; Melchor, J.; Riveiro, M.; Massó, P.; Torres, J.; López-López, M.T.; Rus, G. Performance study of a torsional wave sensor and cervical tissue characterization. Sensors 2017, 17, 2078. [Google Scholar] [CrossRef]
- Walles, B.; Owman, C.; Sjöberg, N.O. Contraction of the ovarian follicle induced by local stimulation of its sympathetic nerves. Brain Res. Bull. 1982, 9, 757–760. [Google Scholar] [CrossRef]
- Holzapfel, G.A. Biomechanics of Soft Tissue. In The Handbook of Material Behavior Models; Academic Press Inc.: San Diego, CA, USA, 2001; pp. 1057–1071. [Google Scholar]
- DeWall, R.J.; Varghese, T.; Kliewer, M.A.; Harter, J.M.; Hartenbach, E.M. Compression-dependent viscoelastic behavior of human cervix tissue. Ultrason. Imaging 2010, 32, 214–228. [Google Scholar] [CrossRef] [PubMed]
- House, M.; Kaplan, D.L.; Socrate, S. Relationships Between Mechanical Properties and Extracellular Matrix Constituents of the Cervical Stroma During Pregnancy. Semin. Perinatol. 2009, 33, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Haslene-Hox, H.; Ovel, E.; Woie, K.; Salvesen, H.B.; Tenstad, O.; Wiig, H. Distribution volumes of macromolecules in human ovarian and endometrial cancers—Effects of extracellular matrix structure. Am. J. Physiol. Heart Circ. Physiol. 2015, 308, H18–H28. [Google Scholar] [CrossRef] [PubMed]
- Baah-Dwomoh, A.; McGuire, J.; Tan, T.; De Vita, R. Mechanical Properties of Female Reproductive Organs and Supporting Connective Tissues: A Review of the Current State of Knowledge. Appl. Mech. Rev. 2016, 68, 060801. [Google Scholar] [CrossRef]


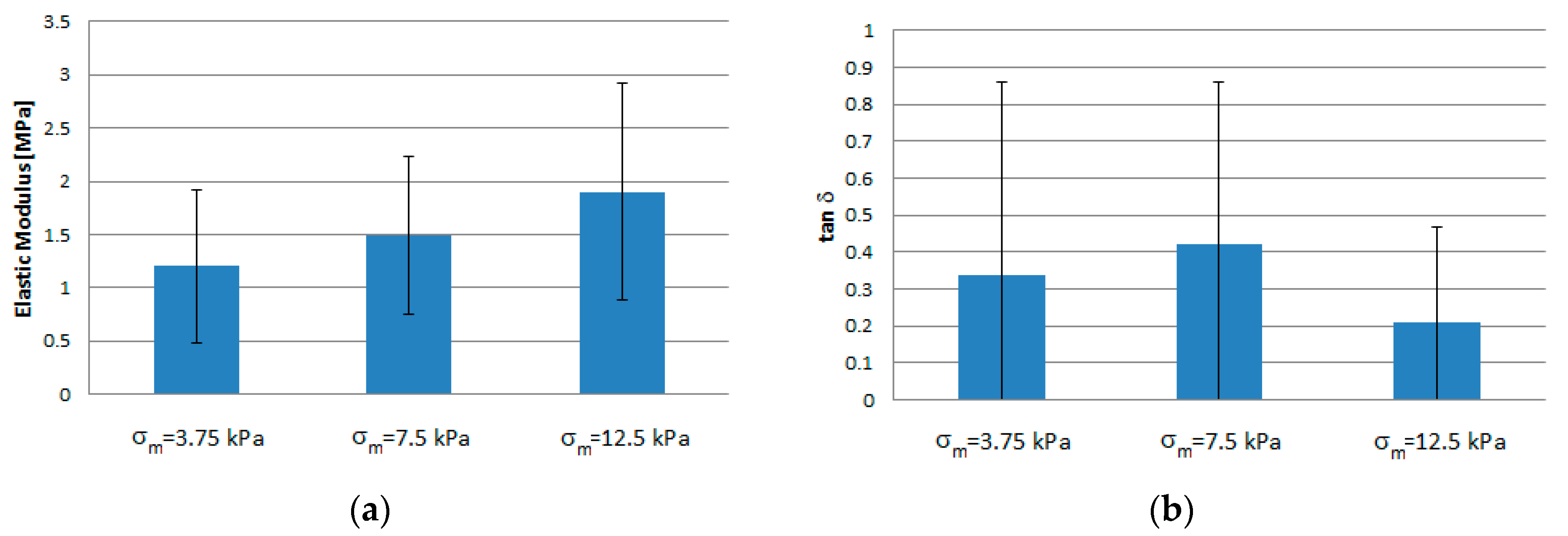



| Average Stress σm [kPa] | Average Ee [MPa] | tan δ [-] | Creep Rate [%/s−1] |
|---|---|---|---|
| 3.75 | 1.20 ± 0.72 | 0.34 ± 0.52 | 0.72 ± 0.17 |
| 7.50 | 1.49 ± 0.74 | 0.42 ± 0.44 | |
| 12.50 | 1.90 ± 1.02 | 0.21 ± 0.26 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pascoletti, G.; Di Nardo, M.; Fragomeni, G.; Barbato, V.; Capriglione, T.; Gualtieri, R.; Talevi, R.; Catapano, G.; Zanetti, E.M. Dynamic Characterization of the Biomechanical Behaviour of Bovine Ovarian Cortical Tissue and Its Short-Term Effect on Ovarian Tissue and Follicles. Materials 2020, 13, 3759. https://doi.org/10.3390/ma13173759
Pascoletti G, Di Nardo M, Fragomeni G, Barbato V, Capriglione T, Gualtieri R, Talevi R, Catapano G, Zanetti EM. Dynamic Characterization of the Biomechanical Behaviour of Bovine Ovarian Cortical Tissue and Its Short-Term Effect on Ovarian Tissue and Follicles. Materials. 2020; 13(17):3759. https://doi.org/10.3390/ma13173759
Chicago/Turabian StylePascoletti, Giulia, Maddalena Di Nardo, Gionata Fragomeni, Vincenza Barbato, Teresa Capriglione, Roberto Gualtieri, Riccardo Talevi, Gerardo Catapano, and Elisabetta M. Zanetti. 2020. "Dynamic Characterization of the Biomechanical Behaviour of Bovine Ovarian Cortical Tissue and Its Short-Term Effect on Ovarian Tissue and Follicles" Materials 13, no. 17: 3759. https://doi.org/10.3390/ma13173759
APA StylePascoletti, G., Di Nardo, M., Fragomeni, G., Barbato, V., Capriglione, T., Gualtieri, R., Talevi, R., Catapano, G., & Zanetti, E. M. (2020). Dynamic Characterization of the Biomechanical Behaviour of Bovine Ovarian Cortical Tissue and Its Short-Term Effect on Ovarian Tissue and Follicles. Materials, 13(17), 3759. https://doi.org/10.3390/ma13173759