Challenges and Opportunities for Integrating Dealloying Methods into Additive Manufacturing
Abstract
1. Introduction
2. Dealloying
2.1. Dealloying Techniques
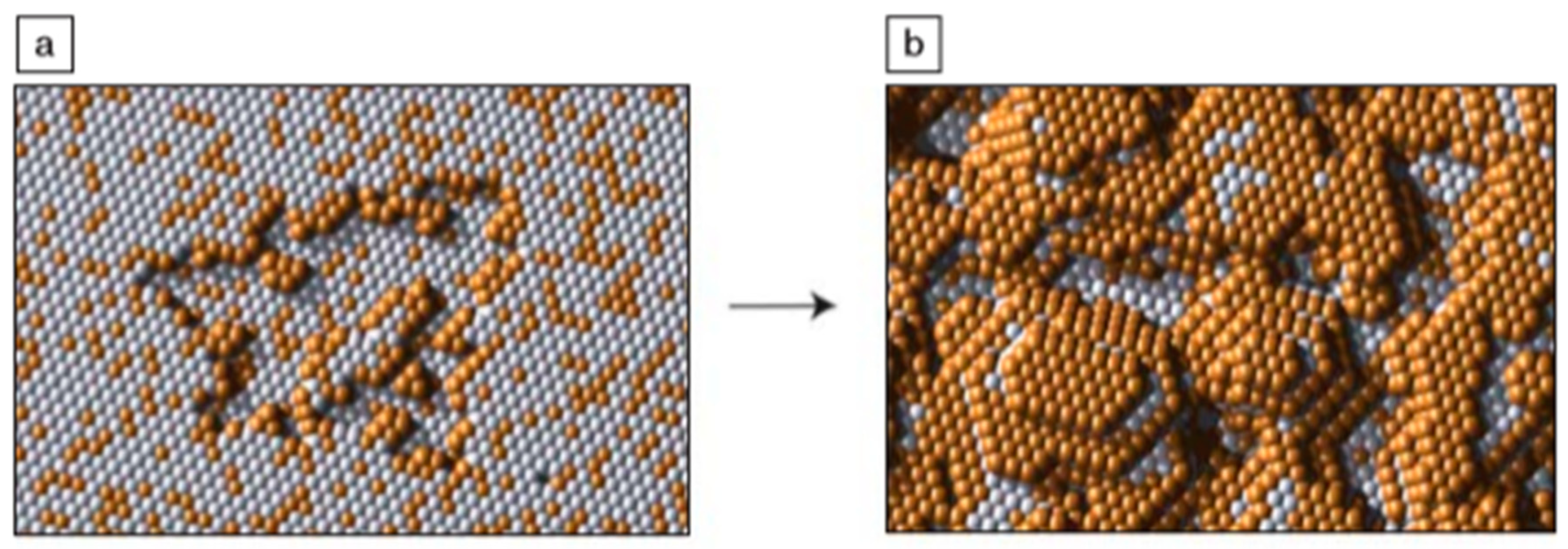
2.1.1. Electrochemical Dealloying
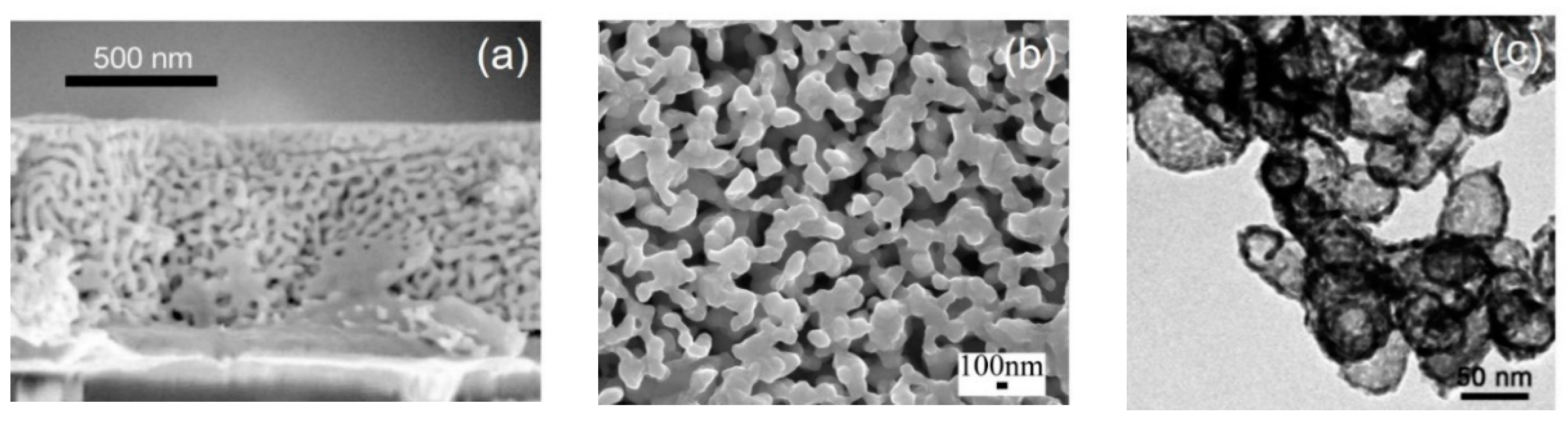
2.1.2. Liquid Metal Dealloying
2.1.3. Solid-State Dealloying
2.1.4. Vapor Phase Dealloying
2.2. Applications of Dealloyed Materials
3. Additive Manufacturing
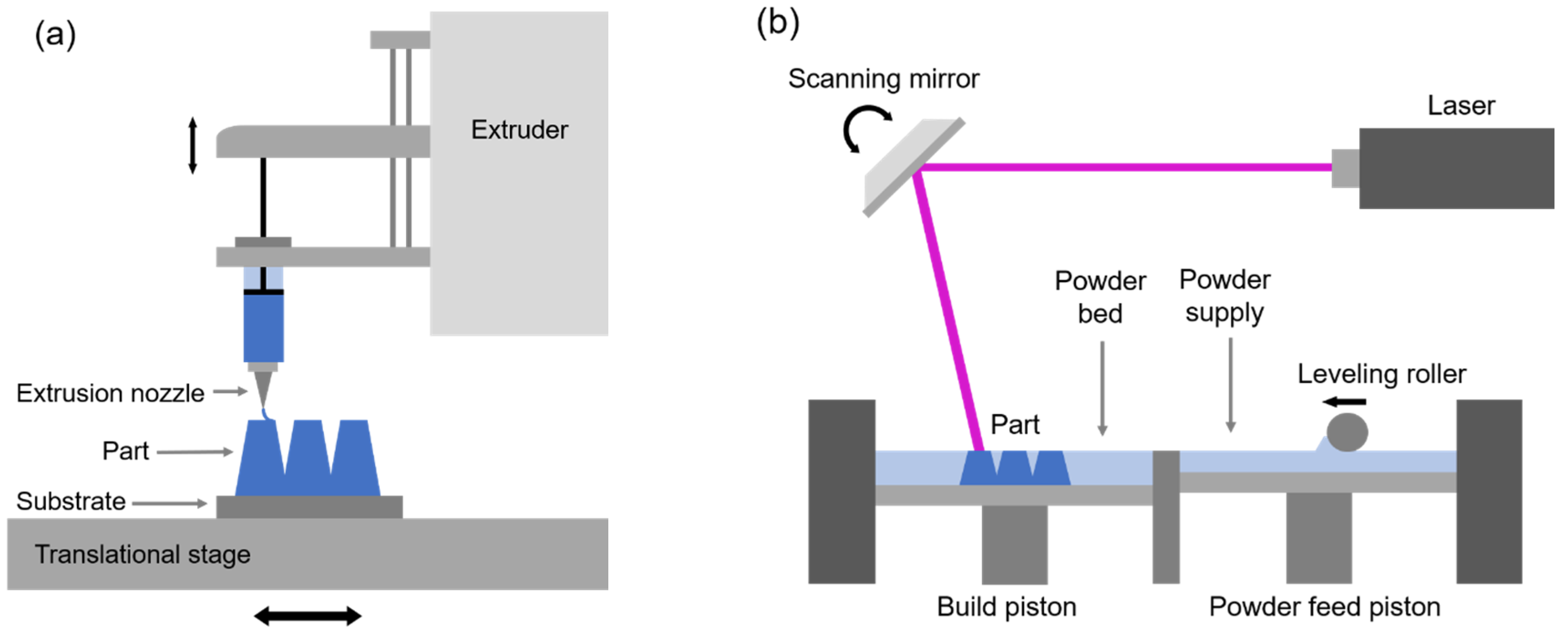
3.1. Direct Ink Writing
3.2. Selective Laser Melting/Sintering
4. Dealloying and Additive Manufacturing
4.1. Porous Materials with Hierarchical Porosity
4.2. The Matrix of Possibilities for Hierarchical Structures
4.3. Metal–Metal Nanocomposites
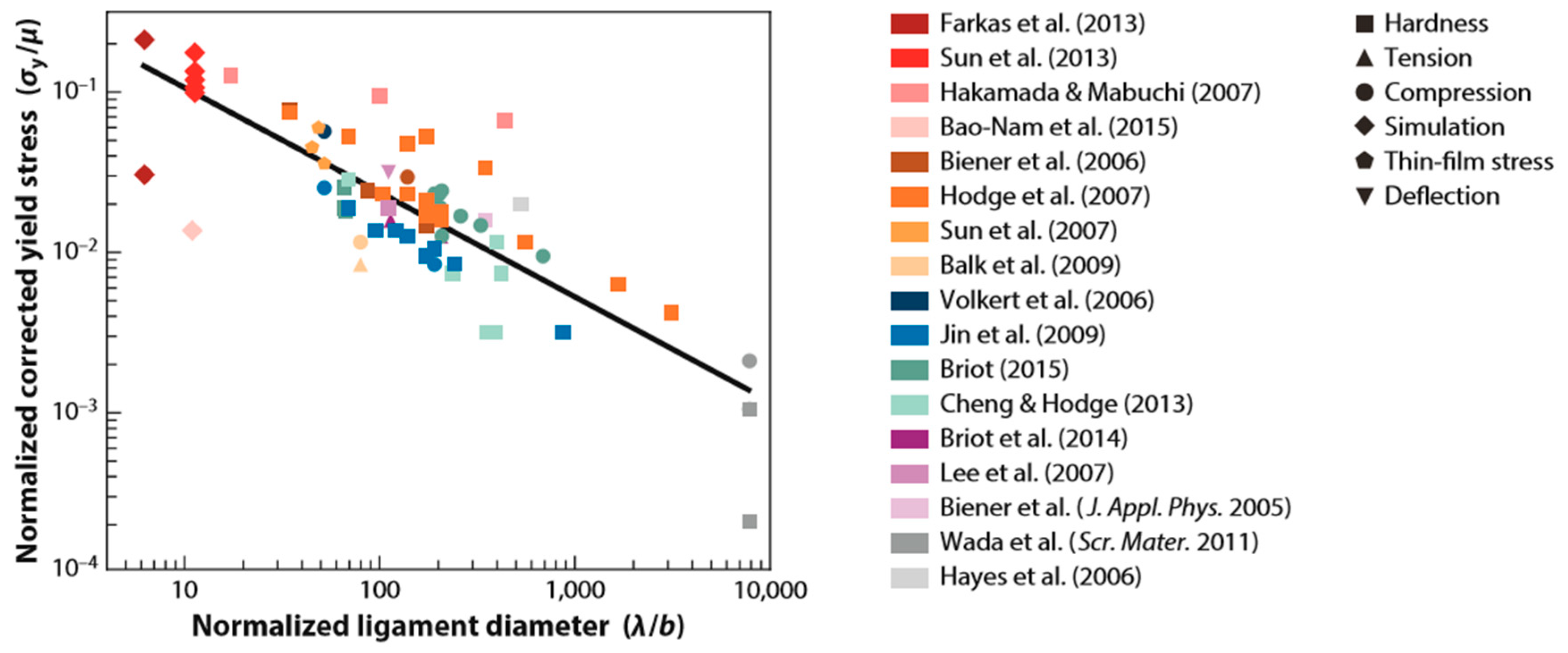
4.3.1. Kinetics of the Interface Velocity
4.3.2. Concentration Gradients in the Dissolution Medium
4.3.3. Coarsening behind the Dissolution Front
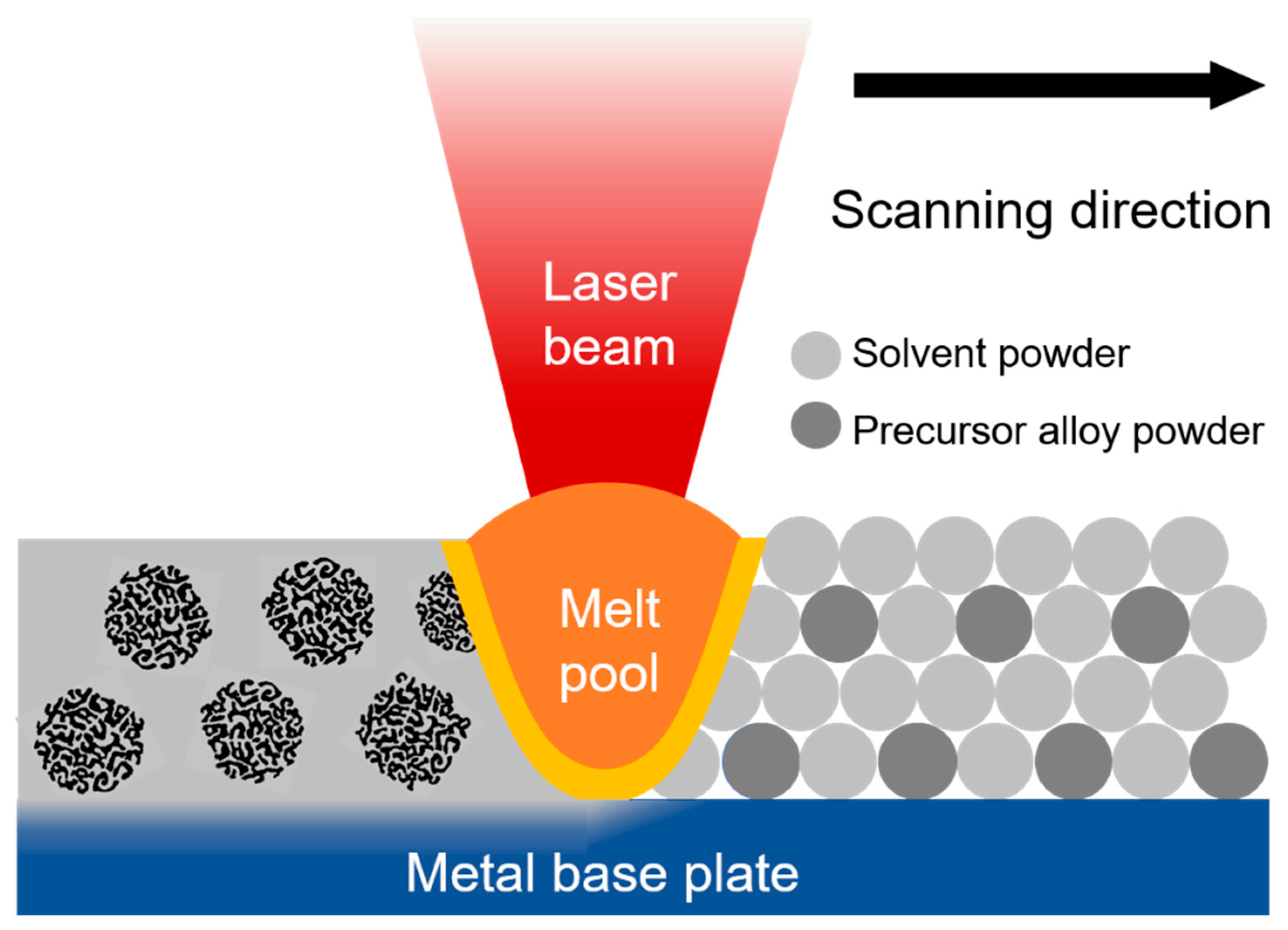
4.4. Challenges Associated with Dealloying Integrated with Laser-Based Processing
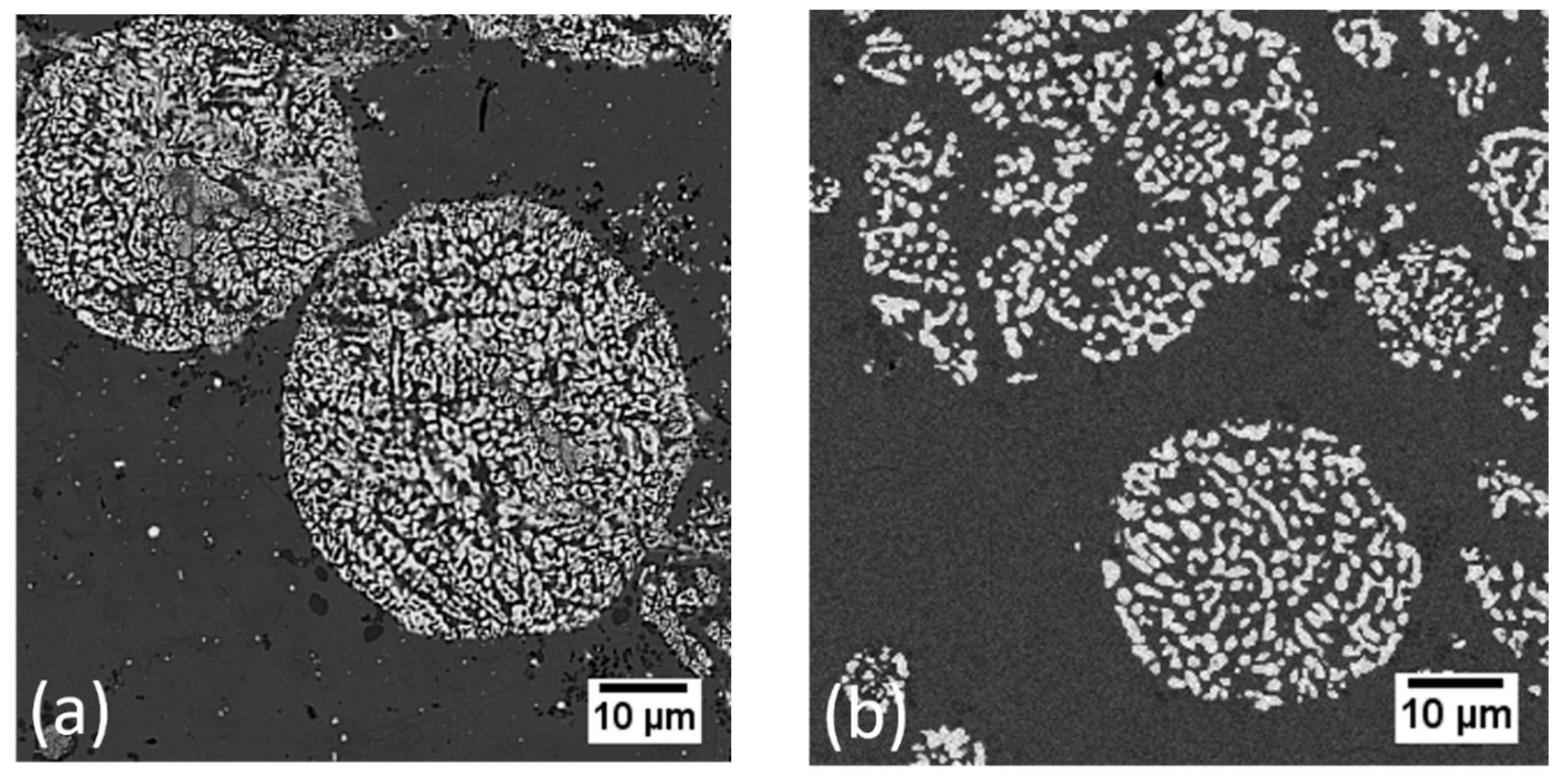
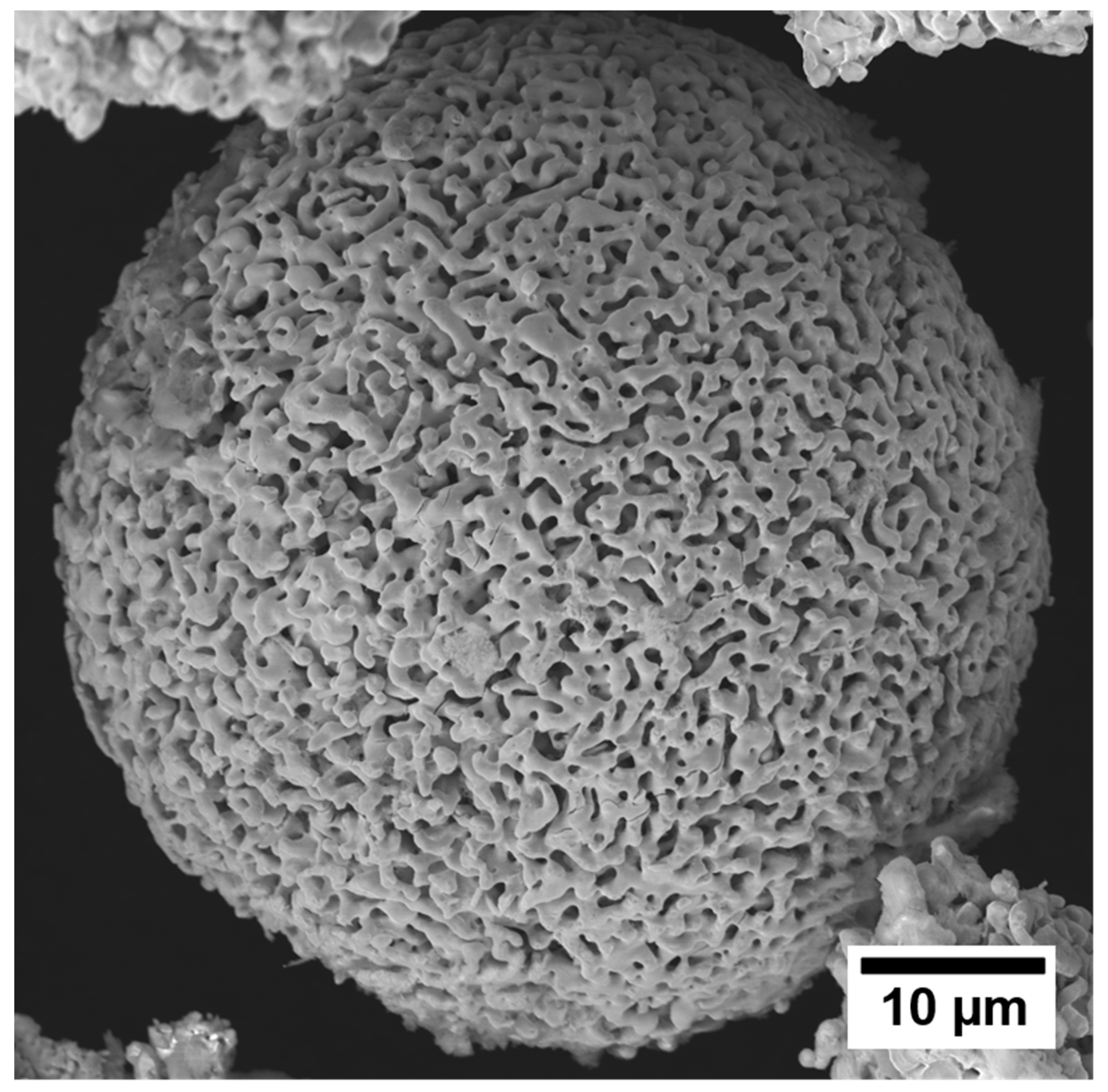
5. Standalone Porous Powders
Bulk Porous Structures via Sintering
6. Summary and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bourell, D.L. Perspectives on Additive Manufacturing. Annu. Rev. Mater. Res. 2016, 46, 1–18. [Google Scholar] [CrossRef]
- McCue, I.; Benn, E.; Gaskey, B.; Erlebacher, J. Dealloying and Dealloyed Materials. Annu. Rev. Mater. Res. 2016, 46, 263–286. [Google Scholar] [CrossRef]
- Erlebacher, J.; Seshadri, R. Hard Materials with Tunable Porosity. MRS Bull. 2009, 34, 561–568. [Google Scholar] [CrossRef]
- Zeng, Y.; Gaskey, B.; Benn, E.; McCue, I.; Greenidge, G.; Livi, K.; Zhang, X.; Jiang, J.; Erlebacher, J. Electrochemical dealloying with simultaneous phase separation. Acta Mater. 2019, 171, 8–17. [Google Scholar] [CrossRef]
- Fujita, T.; Guan, P.; McKenna, K.; Lang, X.; Hirata, A.; Zhang, L.; Tokunaga, T.; Arai, S.; Yamamoto, Y.; Tanaka, N.; et al. Atomic origins of the high catalytic activity of nanoporous gold. Nat. Mater. 2012, 11, 775–780. [Google Scholar] [CrossRef] [PubMed]
- Erlebacher, J.; Aziz, M.J.; Karma, A.; Dimitrov, N.; Sieradzki, K. Evolution of nanoporosity in dealloying. Nature 2001, 410, 450–453. [Google Scholar] [CrossRef] [PubMed]
- Qi, Z.; Zhao, C.; Wang, X.; Lin, J.; Shao, W.; Zhang, Z.; Bian, X. Formation and Characterization of Monolithic Nanoporous Copper by Chemical Dealloying of Al−Cu Alloys. J. Phys. Chem. C 2009, 113, 6694–6698. [Google Scholar] [CrossRef]
- Xu, C.; Li, Y.-Y.; Tian, F.; Ding, Y. Dealloying to Nanoporous Silver and Its Implementation as a Template Material for Construction of Nanotubular Mesoporous Bimetallic Nanostructures. Chem. Phys. Chem. 2010, 11, 3320–3328. [Google Scholar] [CrossRef]
- Pickering, H.W.; Wagner, C. Electrolytic Dissolution of Binary Alloys Containing a Noble Metal. J. Electrochem. Soc. 1967, 114, 698–706. [Google Scholar] [CrossRef]
- Pickering, H.W.; Byrne, P.J. On Preferential Anodic Dissolution of Alloys in the Low-Current Region and the Nature of the Critical Potential. J. Electrochem. Soc. 1971, 118, 209–215. [Google Scholar] [CrossRef]
- Snyder, J.; Fujita, T.; Chen, M.; Erlebacher, J. Oxygen reduction in nanoporous metal–ionic liquid composite electrocatalysts. Nat. Mater. 2010, 9, 904–907. [Google Scholar] [CrossRef] [PubMed]
- Hakamada, M.; Mabuchi, M. Fabrication of nanoporous palladium by dealloying and its thermal coarsening. J. Alloy. Compd. 2009, 479, 326–329. [Google Scholar] [CrossRef]
- Smith, A.; Tran, T.; Wainwright, M. Kinetics and mechanism of the preparation of Raney® copper. J. Appl. Electrochem. 1999, 29, 1085–1094. [Google Scholar] [CrossRef]
- Sun, L.; Chien, C.-L.; Searson, P.C. Fabrication of Nanoporous Nickel by Electrochemical Dealloying. Chem. Mater. 2004, 16, 3125–3129. [Google Scholar] [CrossRef]
- Snyder, J.; Livi, K.; Erlebacher, J.; Hamamoto, K.; Fujishiro, Y.; Awano, M. Dealloying Silver/Gold Alloys in Neutral Silver Nitrate Solution: Porosity Evolution, Surface Composition, and Surface Oxides. J. Electrochem. Soc. 2008, 155, C464. [Google Scholar] [CrossRef]
- Harrison, J.; Wagner, C. The attack of solid alloys by liquid metals and salt melts. Acta Met. 1959, 7, 722–735. [Google Scholar] [CrossRef]
- Wada, T.; Yubuta, K.; Inoue, A.; Kato, H. Dealloying by metallic melt. Mater. Lett. 2011, 65, 1076–1078. [Google Scholar] [CrossRef]
- Wada, T.; Setyawan, A.D.; Yubuta, K.; Kato, H. Nano- to submicro-porous β-Ti alloy prepared from dealloying in a metallic melt. Scr. Mater. 2011, 65, 532–535. [Google Scholar] [CrossRef]
- McCue, I.; Gaskey, B.; Geslin, P.-A.; Karma, A.; Erlebacher, J. Kinetics and morphological evolution of liquid metal dealloying. Acta Mater. 2016, 115, 10–23. [Google Scholar] [CrossRef]
- Geslin, P.-A.; McCue, I.; Gaskey, B.; Erlebacher, J.; Karma, A. Topology-generating interfacial pattern formation during liquid metal dealloying. Nat. Commun. 2015, 6, 8887. [Google Scholar] [CrossRef]
- McCue, I.; Karma, A.; Erlebacher, J. Pattern formation during electrochemical and liquid metal dealloying. MRS Bull. 2018, 43, 27–34. [Google Scholar] [CrossRef]
- Kim, J.W.; Wada, T.; Kim, S.G.; Kato, H. Sub-micron porous niobium solid electrolytic capacitor prepared by dealloying in a metallic melt. Mater. Lett. 2014, 116, 223–226. [Google Scholar] [CrossRef]
- Kim, J.W.; Tsuda, M.; Wada, T.; Yubuta, K.; Kim, S.G.; Kato, H. Optimizing niobium dealloying with metallic melt to fabricate porous structure for electrolytic capacitors. Acta Mater. 2015, 84, 497–505. [Google Scholar] [CrossRef]
- Kim, J.W.; Wada, T.; Kim, S.G.; Kato, H. Enlarging the surface area of an electrolytic capacitor of porous niobium by Mg Ce eutectic liquid dealloying. Scr. Mater. 2016, 122, 68–71. [Google Scholar] [CrossRef]
- Chen-Wiegart, Y.-C.K.; Wada, T.; Butakov, N.; Xiao, X.; De Carlo, F.; Kato, H.; Wang, J.; Dunand, D.C.; Maire, É. 3D morphological evolution of porous titanium by x-ray micro- and nano-tomography. J. Mater. Res. 2013, 28, 2444–2452. [Google Scholar] [CrossRef]
- Yu, S.-G.; Yubuta, K.; Wada, T.; Kato, H. Three-dimensional bicontinuous porous graphite generated in low temperature metallic liquid. Carbon 2016, 96, 403–410. [Google Scholar] [CrossRef]
- Wada, T.; Kato, H. Three-dimensional open-cell macroporous iron, chromium and ferritic stainless steel. Scr. Mater. 2013, 68, 723–726. [Google Scholar] [CrossRef]
- Wei, D.; Koizumi, Y.; Chiba, A. Porous surface structures in biomedical Co-Cr-Mo alloy prepared by local dealloying in a metallic melt. Mater. Lett. 2018, 219, 256–259. [Google Scholar] [CrossRef]
- Zeng, L.; You, C.; Cai, X.; Wang, C.; Zhang, X.; Liang, T. Preparation of nanoporous CoCr alloy by dealloying CrCoNi medium entropy alloys. J. Mater. Res. Technol. 2020, 9, 6909–6915. [Google Scholar] [CrossRef]
- Wada, T.; Kato, H. Preparation of Nanoporous Si by Dealloying in Metallic Melt and Its Application for Negative Electrode of Lithium Ion Battery. Mater. Today: Proc. 2017, 4, 11465–11469. [Google Scholar] [CrossRef]
- Wada, T.; Yubuta, K.; Kato, H. Evolution of a bicontinuous nanostructure via a solid-state interfacial dealloying reaction. Scr. Mater. 2016, 118, 33–36. [Google Scholar] [CrossRef]
- Qian, L.H.; Chen, M. Ultrafine nanoporous gold by low-temperature dealloying and kinetics of nanopore formation. Appl. Phys. Lett. 2007, 91, 83105. [Google Scholar] [CrossRef]
- McCue, I.; Demkowicz, M.J. Alloy Design Criteria for Solid Metal Dealloying of Thin Films. JOM 2017, 69, 2199–2205. [Google Scholar] [CrossRef]
- Zhao, C.; Kisslinger, K.; Huang, X.; Lu, M.; Camino, F.; Lin, C.-H.; Yan, H.; Nazaretski, E.; Chu, Y.; Ravel, B.; et al. Bi-continuous pattern formation in thin films via solid-state interfacial dealloying studied by multimodal characterization. Mater. Horiz. 2019, 6, 1991–2002. [Google Scholar] [CrossRef]
- Han, J.; Liu, P.; Lu, Z.; Wang, H.; Wang, Z.; Watanabe, K.; Chen, M. Vapor phase dealloying: A versatile approach for fabricating 3D porous materials. Acta Mater. 2019, 163, 161–172. [Google Scholar] [CrossRef]
- Balluffi, R.W.; Alexander, B.H. Development of Porosity during Diffusion in Substitutional Solid Solutions. J. Appl. Phys. 1952, 23, 1237. [Google Scholar] [CrossRef]
- Sun, Y.; Ren, Y. New preparation method of porous copper powder through vacuum dealloying. Vacuum 2015, 122, 215–217. [Google Scholar] [CrossRef]
- Lu, Z.; Li, C.; Han, J.; Zhang, F.; Liu, P.; Wang, H.; Wang, Z.; Cheng, C.; Chen, L.; Hirata, A.T.; et al. Three-dimensional bicontunous nanoporous materials by vapor phase dealloying. Nat. Commun. 2018, 9, 1–7. [Google Scholar] [CrossRef]
- Ding, Y. and Erlebacher, J. Metallic mesoporous nanocomposites for electrocatalysts. J. Am. Chem. Soc. 2004, 126, 6876–6877. [Google Scholar] [CrossRef]
- Zielasek, V.; Jürgens, B.; Schulz, C.; Biener, J.; Biener, M.M.; Hamza, A.V.; Bäumer, M.; Bäumer, M. Gold Catalysts: Nanoporous Gold Foams. Angew. Chem. Int. Ed. 2006, 45, 8241–8244. [Google Scholar] [CrossRef]
- Wang, R.; Xu, C.; Bi, X.; Ding, Y. Nanoporous surface alloys as highly active and durable oxygen reduction reaction electrocatalysts. Energy Environ. Sci. 2012, 5, 5281–5286. [Google Scholar] [CrossRef]
- Xu, C.; Su, J.; Xu, X.; Liu, P.; Zhao, H.; Tian, A.F.; Ding, Y. Low Temperature CO Oxidation over Unsupported Nanoporous Gold. J. Am. Chem. Soc. 2007, 129, 42–43. [Google Scholar] [CrossRef] [PubMed]
- Zeis, R.; Mathur, A.; Fritz, G.; Lee, J.; Erlebacher, J. Platinum-plated nanoporous gold: An efficient, low Pt loading electrocatalyst for PEM fuel cells. J. Power Sources 2007, 165, 65–72. [Google Scholar] [CrossRef]
- Erlebacher, J.; Snyder, J. Dealloyed Nanoporous Metals for PEM Fuel Cell Catalysis. ECS Meet. Abstr. 2009, 603–612. [Google Scholar] [CrossRef]
- McCue, I.; Ryan, S.; Hemker, K.; Xu, X.; Li, N.; Chen, M.; Erlebacher, J. Size Effects in the Mechanical Properties of Bulk Bicontinuous Ta/Cu Nanocomposites Made by Liquid Metal Dealloying. Adv. Eng. Mater. 2015, 18, 46–50. [Google Scholar] [CrossRef]
- Sumner, D.R.; Galante, J.O. Determinants of Stress Shielding. Clin. Orthop. Relat. Res. 1992, 271, 202–212. [Google Scholar] [CrossRef]
- Sumner, D.R.; Turner, T.; Igloria, R.; Urban, R.; Galante, J. Functional adaptation and ingrowth of bone vary as a function of hip implant stiffness. J. Biomech. 1998, 31, 909–917. [Google Scholar] [CrossRef]
- Geetha, M.; Singh, A.K.; Asokamani, R.; Gogia, A.K. Ti based biomaterials, the ultimate choice for orthopaedic implants—A review. Prog. Mater. Sci. 2009, 54, 397–425. [Google Scholar] [CrossRef]
- Long, M.; Rack, H. Titanium alloys in total joint replacement—A materials science perspective. Biomaterials 1998, 19, 1621–1639. [Google Scholar] [CrossRef]
- Niinomi, M.; Nakai, M.; Hieda, J. Development of new metallic alloys for biomedical applications. Acta Biomater. 2012, 8, 3888–3903. [Google Scholar] [CrossRef]
- Okulov, I.; Volegov, A.; Markmann, J.; Okulov, A. Tuning microstructure and mechanical properties of open porous TiNb and TiFe alloys by optimization of dealloying parameters. Scr. Mater. 2018, 154, 68–72. [Google Scholar] [CrossRef]
- Okulov, I.; Soldatov, I.; Luthringer, B.; Willumeit-Römer, R.; Wada, T.; Kato, H.; Weissmüller, J.; Markmann, J.; Okulov, A. Open porous dealloying-based biomaterials as a novel biomaterial platform. Mater. Sci. Eng. C 2018, 88, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Okulov, A.; Volegov, A.; Weissmüller, J.; Markmann, J.; Okulov, I. Dealloying-based metal-polymer composites for biomedical applications. Scr. Mater. 2018, 146, 290–294. [Google Scholar] [CrossRef]
- Okulov, I.; Weissmüller, J.; Markmann, J. Dealloying-based interpenetrating-phase nanocomposites matching the elastic behavior of human bone. Sci. Rep. 2017, 7, 20. [Google Scholar] [CrossRef]
- Heiden, M.; Huang, S.; Nauman, E.; Stanciu, L.; Johnson, D. Nanoporous metals for biodegradable implants: Initial bone mesenchymal stem cell adhesion and degradation behavior. J. Biomed. Mater. Res. Part. A 2016, 104, 1747–1758. [Google Scholar] [CrossRef]
- Murr, L.; Gaytan, S.M.; Medina, F.; Lopez, H.; Martinez, E.; Machado, B.I.; Hernandez, D.H.; Lopez, M.I.; Wicker, R.B.; Bracke, J. Next-generation biomedical implants using additive manufacturing of complex, cellular and functional mesh arrays. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2010, 368, 1999–2032. [Google Scholar] [CrossRef]
- Revilla-León, M.; Meyer, M.J.; Özcan, M. Metal additive manufacturing technologies: Literature review of current status and prosthodontic applications. Int. J. Comput. Dent. 2019, 22, 55–67. [Google Scholar]
- Janeczek, M.; Szymczyk, P.; Dobrzynski, M.; Parulska, O.; Szymonowicz, M.; Kuropka, P.; Rybak, Z.; Zywicka, B.; Ziolkowski, G.; Marycz, K.; et al. Influence of surface modifications of a nanostructured implant on osseointegration capacity–preliminary in vivo study. RSC Adv. 2018, 8, 15533–15546. [Google Scholar] [CrossRef]
- Zhou, Z.; Buchanan, F.J.; Mitchell, C.A.; Dunne, N. Printability of calcium phosphate: Calcium sulfate powders for the application of tissue engineered bone scaffolds using the 3D printing technique. Mater. Sci. Eng. C 2014, 38, 1–10. [Google Scholar] [CrossRef]
- Liang, X.; Liao, W.; Cai, H.; Jiang, S.; Chen, S. 3D-Printed Artificial Teeth: Accuracy and Application in Root Canal Therapy. J. Biomed. Nanotechnol. 2018, 14, 1477–1485. [Google Scholar] [CrossRef]
- Conner, B.; Manogharan, G.P.; Martof, A.N.; Rodomsky, L.M.; Rodomsky, C.M.; Jordan, D.C.; Limperos, J.W. Making sense of 3-D printing: Creating a map of additive manufacturing products and services. Addit. Manuf. 2014, 1–4, 64–76. [Google Scholar] [CrossRef]
- Frazier, W.E. Metal Additive Manufacturing: A. Review. J. Mater. Eng. Perform. 2014, 23, 1917–1928. [Google Scholar] [CrossRef]
- Wieneke, H.; Dirsch, O.; Sawitowski, T.; Gu, Y.L.; Brauer, H.; Dahmen, U.; Fischer, A.; Wnendt, S.; Erbel, R. Synergistic effects of a novel nanoporous stent coating and tacrolimus on intima proliferation in rabbits. Catheter. Cardiovasc. Interv. 2003, 60, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Varga, Z.; Filipcsei, G.; Zrínyi, M. Smart composites with controlled anisotropy. Polymer 2005, 46, 7779–7787. [Google Scholar] [CrossRef]
- Standard Terminology for Additive Manufacturing. F2792-12a, ASTM Standard Terminology for Additive Manufacturing Technologies; ASTM International: West Conshohocken, PA, USA, 2015. [Google Scholar]
- Zhang, Y.; Jarosinski, W.; Jung, Y.-G.; Zhang, J. Additive manufacturing processes and equipment. In Additive Manufacturing; Elsevier BV: Amsterdam, The Netherlands, 2018; pp. 39–51. [Google Scholar]
- Lewis, J.A.; Smay, J.E.; Stuecker, J.; Cesarano, J. Direct Ink Writing of Three-Dimensional Cermaic Structures. J. Am. Ceram. Soc. 2006, 89, 3599–3609. [Google Scholar] [CrossRef]
- Jakus, A.E.; Taylor, S.; Geisendorfer, N.R.; Dunand, D.C.; Shah, R.N. Metallic Architectures from 3D-Printed Powder-Based Liquid Inks. Adv. Funct. Mater. 2015, 25, 6985–6995. [Google Scholar] [CrossRef]
- Lewis, J.A. Direct Ink Writing of 3D Functional Materials. Adv. Funct. Mater. 2006, 16, 2193–2204. [Google Scholar] [CrossRef]
- Xu, C.; Quinn, B.; Lebel, L.L.; Therriault, D.; L’Espérance, G. Multi-Material Direct Ink Writing (DIW) for Complex 3D Metallic Structures with Removable Supports. ACS Appl. Mater. Interfaces 2019, 11, 8499–8506. [Google Scholar] [CrossRef]
- Yap, C.Y.; Chua, C.K.; Dong, Z.; Liu, Z.H.; Zhang, D.Q.; Loh, L.E.; Sing, S.L. Review of selective laser melting: Materials and applications. Appl. Phys. Rev. 2015, 2, 041101. [Google Scholar] [CrossRef]
- Thijs, L.; Kempen, K.; Kruth, J.-P.; Van Humbeeck, J. Fine-structured aluminium products with controllable texture by selective laser melting of pre-alloyed AlSi10Mg powder. Acta Mater. 2013, 61, 1809–1819. [Google Scholar] [CrossRef]
- Murr, L.; Martinez, E.; Hernandez, J.; Collins, S.; Amato, K.N.; Gaytan, S.M.; Shindo, P.W. Microstructures and Properties of 17-4 PH Stainless Steel Fabricated by Selective Laser Melting. J. Mater. Res. Technol. 2012, 1, 167–177. [Google Scholar] [CrossRef]
- Thijs, L.; Verhaeghe, F.; Craeghs, T.; Van Humbeeck, J.; Kruth, J.-P. A study of the microstructural evolution during selective laser melting of Ti–6Al–4V. Acta Mater. 2010, 58, 3303–3312. [Google Scholar] [CrossRef]
- Attar, H.; Prashanth, K.G.; Zhang, L.; Calin, M.; Okulov, I.; Scudino, S.; Yang, C.; Eckert, J. Effect of Powder Particle Shape on the Properties of In Situ Ti–TiB Composite Materials Produced by Selective Laser Melting. J. Mater. Sci. Technol. 2015, 31, 1001–1005. [Google Scholar] [CrossRef]
- Shi, Q.; Gu, D.; Xia, M.; Cao, S.; Rong, T. Effects of laser processing parameters on thermal behavior and melting/solidification mechanism during selective laser melting of TiC/Inconel 718 composites. Opt. Laser Technol. 2016, 84, 9–22. [Google Scholar] [CrossRef]
- Prashanth, K.; Kolla, S.; Eckert, J. Additive Manufacturing Processes: Selective Laser Melting, Electron Beam Melting and Binder Jetting—Selection Guidelines. Materials 2017, 10, 672. [Google Scholar] [CrossRef]
- Ding, Y.; Erlebacher, J. Nanoporous Metals with Controlled Multimodal Pore Size Distribution. J. Am. Chem. Soc. 2003, 125, 7772–7773. [Google Scholar] [CrossRef] [PubMed]
- Juarez, T.; Biener, J.; Weissmüller, J.; Hodge, A.M. Nanoporous Metals with Structural Hierarchy: A Review. Adv. Eng. Mater. 2017, 19, 1700389. [Google Scholar] [CrossRef]
- Nyce, G.W.; Hayes, J.R.; Hamza, A.V.; Satcher, J.H. Synthesis and Characterization of Hierarchical Porous Gold Materials. Chem. Mater. 2007, 19, 344–346. [Google Scholar] [CrossRef]
- Liu, H.; Lu, X.; Xiao, D.; Zhou, M.; Xu, D.; Sun, L.; Song, Y. Hierarchical Cu–Co–Ni nanostructures electrodeposited on carbon nanofiber modified glassy carbon electrode: Application to glucose detection. Anal. Methods 2013, 5, 6360. [Google Scholar] [CrossRef]
- Qi, Z.; Weissmüller, J. Hierarchical Nested-Network Nanostructure by Dealloying. ACS Nano 2013, 7, 5948–5954. [Google Scholar] [CrossRef]
- Wada, T.; Geslin, P.-A.; Kato, H. Preparation of hierarchical porous metals by two-step liquid metal dealloying. Scr. Mater. 2018, 142, 101–105. [Google Scholar] [CrossRef]
- Song, T.; Yan, M.; Qian, M. The enabling role of dealloying in the creation of specific hierarchical porous metal structures—A review. Corros. Sci. 2018, 134, 78–98. [Google Scholar] [CrossRef]
- Vaezi, M.; Seitz, H.; Yang, S. A review on 3D micro-additive manufacturing technologies. Int. J. Adv. Manuf. Technol. 2012, 67, 1721–1754. [Google Scholar] [CrossRef]
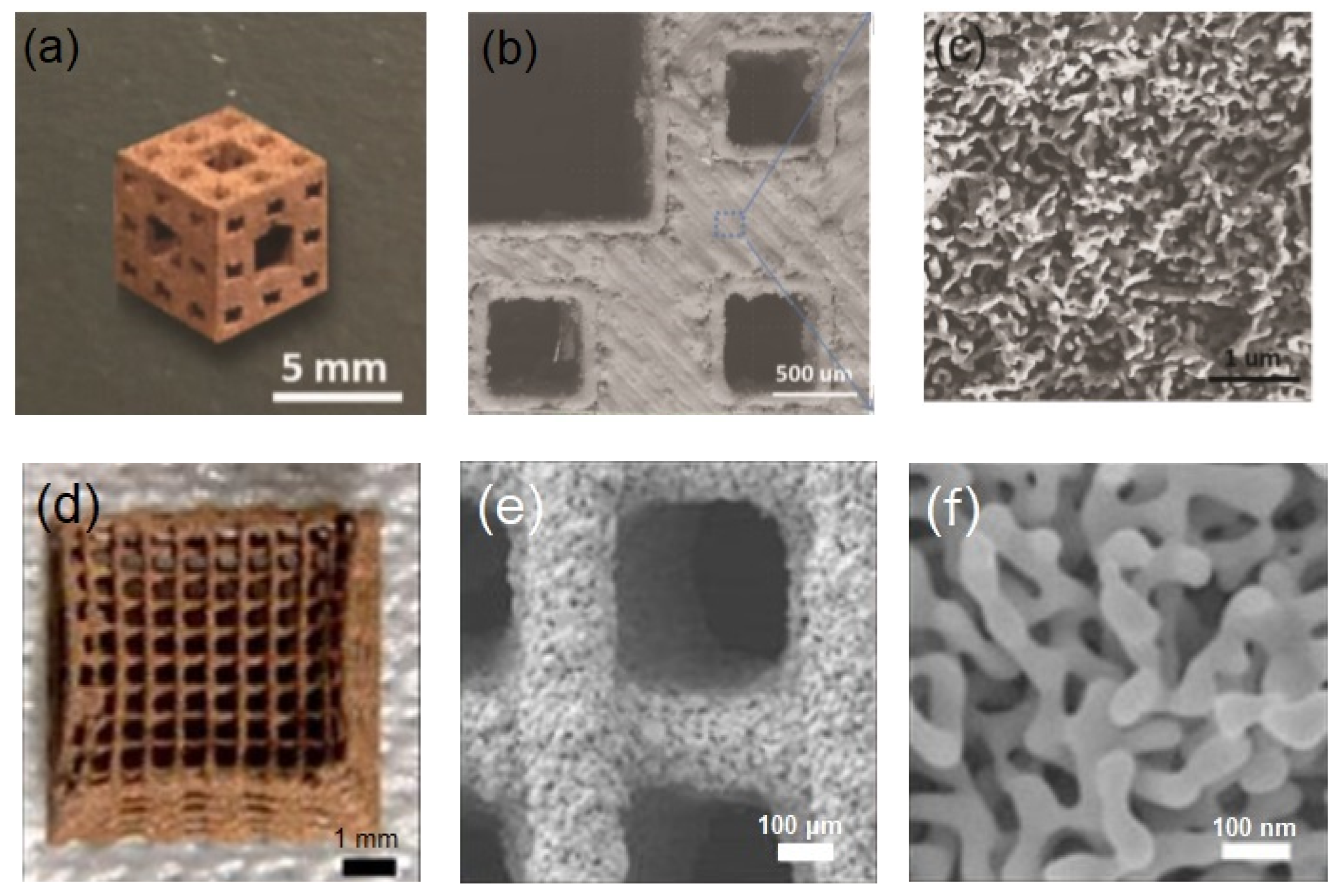
- Zhu, C.; Qi, Z.; Beck, V.A.; Luneau, M.; Lattimer, J.; Chen, W.; Worsley, M.A.; Ye, J.; Duoss, E.B.; Spadaccini, C.M.; et al. Toward digitally controlled catalyst architectures: Hierarchical nanoporous gold via 3D printing. Sci. Adv. 2018, 4, eaas9459. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, X.; Nomura, N.; Fujita, T. Hierarchical Nanoporous Copper Architectures via 3D Printing Technique for Highly Efficient Catalysts. Small 2019, 15, e1805432. [Google Scholar] [CrossRef]
- Chen, Q.; Sieradzki, K. Mechanisms and Morphology Evolution in Dealloying. J. Electrochem. Soc. 2013, 160, C226–C231. [Google Scholar] [CrossRef]
- Tan, H.W.; An, J.; Chua, C.K.; Tran, T. Metallic Nanoparticle Inks for 3D Printing of Electronics. Adv. Electron. Mater. 2019, 5, 1800831. [Google Scholar] [CrossRef]
- Tao, Y.; Zeng, G.; Xiao, C.; Liu, Y.; Qian, Y.; Feng, J. Porosity controlled synthesis of nanoporous silicon by chemical dealloying as anode for high energy lithium-ion batteries. J. Colloid Interface Sci. 2019, 554, 674–681. [Google Scholar] [CrossRef]
- Ullrich, K.S.; Frick, T.; Schmidt, M. New Developments of Laser Processing Aluminum Alloys via Additive Manufacturing Technique. Phys. Procedia 2011, 12, 393–401. [Google Scholar]
- Greenidge, G.; Erlebacher, J. Porous graphite fabricated by liquid metal dealloying of silicon carbide. Carbon 2020, 165, 45–54. [Google Scholar] [CrossRef]
- Mott, M.; Evans, J.R.G. Solid Freeforming of Silicon Carbide by Inkjet Printing Using a Polymeric Precursor. J. Am. Ceram. Soc. 2004, 84, 307-13. [Google Scholar] [CrossRef]
- Girardin, E.; Barucca, G.; Mengucci, P.; Fiori, F.; Bassoli, E.; Gatto, A.; Iuliano, L.; Rutkowski, B. Biomedical Co-Cr-Mo Components Produced by Direct Metal Laser Sintering1. Mater. Today Proc. 2016, 3, 889–897. [Google Scholar] [CrossRef]
- Li, W.; Chen, X.; Yan, L.; Zhang, J.; Zhang, X.; Liou, F. Additive manufacturing of a new Fe-Cr-Ni alloy with gradually changing compositions with elemental powder mixes and thermodynamic calculation. Int. J. Adv. Manuf. Technol. 2017, 95, 1013–1023. [Google Scholar] [CrossRef]
- Zhuravleva, K.; Bönisch, M.; Prashanth, K.; Hempel, U.; Helth, A.; Gemming, T.; Calin, M.; Scudino, S.; Schultz, L.; Eckert, J.; et al. Production of Porous β-Type Ti–40Nb Alloy for Biomedical Applications: Comparison of Selective Laser Melting and Hot Pressing. Materials 2013, 6, 5700–5712. [Google Scholar] [CrossRef] [PubMed]
- Mikler, C.V.; Chaudhary, V.; Borkar, T.; Soni, V.; Jaeger, D.; Chen, X.; Contieri, R.; Ramanujan, R.; Banerjee, R. Laser Additive Manufacturing of Magnetic Materials. JOM 2017, 69, 532–543. [Google Scholar] [CrossRef]
- Yang, C.; Zhao, Y.; Kang, L.; Li, D.; Zhang, W.; Zhang, L. High-strength silicon brass manufactured by selective laser melting. Mater. Lett. 2018, 210, 169–172. [Google Scholar] [CrossRef]
- Volkert, C.A.; Lilleodden, E.; Kramer, D.; Weissmüller, J. Approaching the theoretical strength in nanopoous Au. Appl. Phys. Lett. 2006, 89, 061920. [Google Scholar] [CrossRef]
- Wang, K.; Weissmüller, J. Composites of Nanoporous Gold and Polymer. Adv. Mater. 2013, 25, 1280–1284. [Google Scholar] [CrossRef]
- Wang, K.; Kobler, A.; Kübel, C.; Jelitto, H.; Schneider, G.; Weissmüller, J.; Kübel, C. Nanoporous-gold-based composites: Toward tensile ductility. NPG Asia Mater. 2015, 7, e187. [Google Scholar] [CrossRef]
- Li, J.; Zhao, F.; Shih, W.-C. Direct-write patterning of nanoporous gold microstructures by in situ laser-assisted dealloying. Opt. Express 2016, 24, 23610. [Google Scholar] [CrossRef]
- Zou, L.; Ge, M.; Zhao, C.; Meng, Q.; Wang, H.; Liu, X.; Lin, C.-H.; Xiao, X.; Lee, W.-K.; Shen, Q.; et al. Designing Multiscale Porous Metal by Simple Dealloying with 3D Morphological Evolution Mechanism Revealed via X-ray Nano-tomography. ACS Appl. Mater. Interfaces 2019, 12, 2793–2804. [Google Scholar] [CrossRef] [PubMed]
- Song, T.; Tang, H.P.; Li, Y.; Qian, M. Liquid metal dealloying of titanium-tantalum (Ti-Ta) alloy to fabricate ultrafine Ta ligament structures: A comparative study in molten copper (Cu) and Cu-based alloys. Corros. Sci. 2020, 169, 108600. [Google Scholar] [CrossRef]
- Manfredi, D.; Calignano, F.; Krishnan, M.; Canali, R.; Paola, E.; Biamino, S.; Ugues, D.; Pavese, M.; Fino, M.P.A.P. Additive Manufacturing of Al Alloys and Aluminium Matrix Composites (AMCs). In Light Metal Alloys Applications; IntechOpen: London, UK, 2014. [Google Scholar]
- Vrancken, B.; Thijs, L.; Kruth, J.-P.; Van Humbeeck, J. Microstructure and mechanical properties of a novel β titanium metallic composite by selective laser melting. Acta Mater. 2014, 68, 150–158. [Google Scholar] [CrossRef]
- Zheng, B.; Zhou, Y.; Smugeresky, J.; Schoenung, J.; Lavernia, E. Thermal Behavior and Microstructure Evolution during Laser Deposition with Laser-Engineered Net Shaping: Part II. Experimental Investigation and Discussion. Met. Mater. Trans. A 2008, 39, 2237–2245. [Google Scholar] [CrossRef]
- Van Petegem, S.; Brandstetter, S.; Maass, R.; Hodge, A.M.; El-Dasher, B.S.; Biener, J.; Schmitt, B.; Borca, C.; Van Swygenhoven, H. On the Microstructure of Nanoporous Gold: An X-ray Diffraction Study. Nano Lett. 2009, 9, 1158–1163. [Google Scholar] [CrossRef]
- Tammas-Williams, S.; Withers, P.J.; Todd, I.; Prangnell, P.B. Porosity regrowth during heat treatment of hot isostatically pressed additively manufactured titanium components. Scr. Mater. 2016, 122, 72–76. [Google Scholar] [CrossRef]
- Wang, S.; Liu, L. Fabrication of novel nanoporous copper powder catalyst by dealloying of ZrCuNiAl amorphous powders for the application of wastewater treatments. J. Hazard. Mater. 2017, 340, 445–453. [Google Scholar] [CrossRef]
- Wang, D.J.; Li, Z.H.; Rahman, A.; Shen, J. Nanosized Metal Oxide and Nanobelts Prepared by Selective Dealloying of Ti-Based Amorphous Powders. Langmuir 2013, 29, 8108–8115. [Google Scholar] [CrossRef]
- Balaji, T.; Govindaiah, R.; Sharma, M.; Purushotham, Y.; Kumar, A.; Prakash, T. Sintering and electrical properties of tantalum anodes for capacitor applications. Mater. Lett. 2002, 56, 560–563. [Google Scholar] [CrossRef]
- Gaskey, B.; McCue, I.; Chuang, A.; Erlebacher, J. Self-assembled porous metal-intermetallic nanocomposites via liquid metal dealloying. Acta Mater. 2019, 164, 293–300. [Google Scholar] [CrossRef]

| Compatibility of Precursor Alloys with Dealloying and AM Methods | |||
|---|---|---|---|
| Additive Manufacturing Methods | |||
| Direct Ink Writing | Selective Laser Melting/Sintering | ||
| Dealloying methods | Electrochemical dealloying | Au-Ag [86], Ni-Cu [14,89] | Cu-Mn [87], Al-Si [90,91] |
| Liquid metal dealloying | SiC [92,93] | Co-Cr-Mo [28,94], Fe-Cr-Ni [27,95], Nb-Ti [54,96] | |
| Solid-state dealloying | – | Fe-Ni [33,97] | |
| Vapor phase dealloying | – | Brass [37,98] | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chuang, A.; Erlebacher, J. Challenges and Opportunities for Integrating Dealloying Methods into Additive Manufacturing. Materials 2020, 13, 3706. https://doi.org/10.3390/ma13173706
Chuang A, Erlebacher J. Challenges and Opportunities for Integrating Dealloying Methods into Additive Manufacturing. Materials. 2020; 13(17):3706. https://doi.org/10.3390/ma13173706
Chicago/Turabian StyleChuang, A., and J. Erlebacher. 2020. "Challenges and Opportunities for Integrating Dealloying Methods into Additive Manufacturing" Materials 13, no. 17: 3706. https://doi.org/10.3390/ma13173706
APA StyleChuang, A., & Erlebacher, J. (2020). Challenges and Opportunities for Integrating Dealloying Methods into Additive Manufacturing. Materials, 13(17), 3706. https://doi.org/10.3390/ma13173706




