Collagen-Based Materials Modified by Phenolic Acids—A Review
Abstract
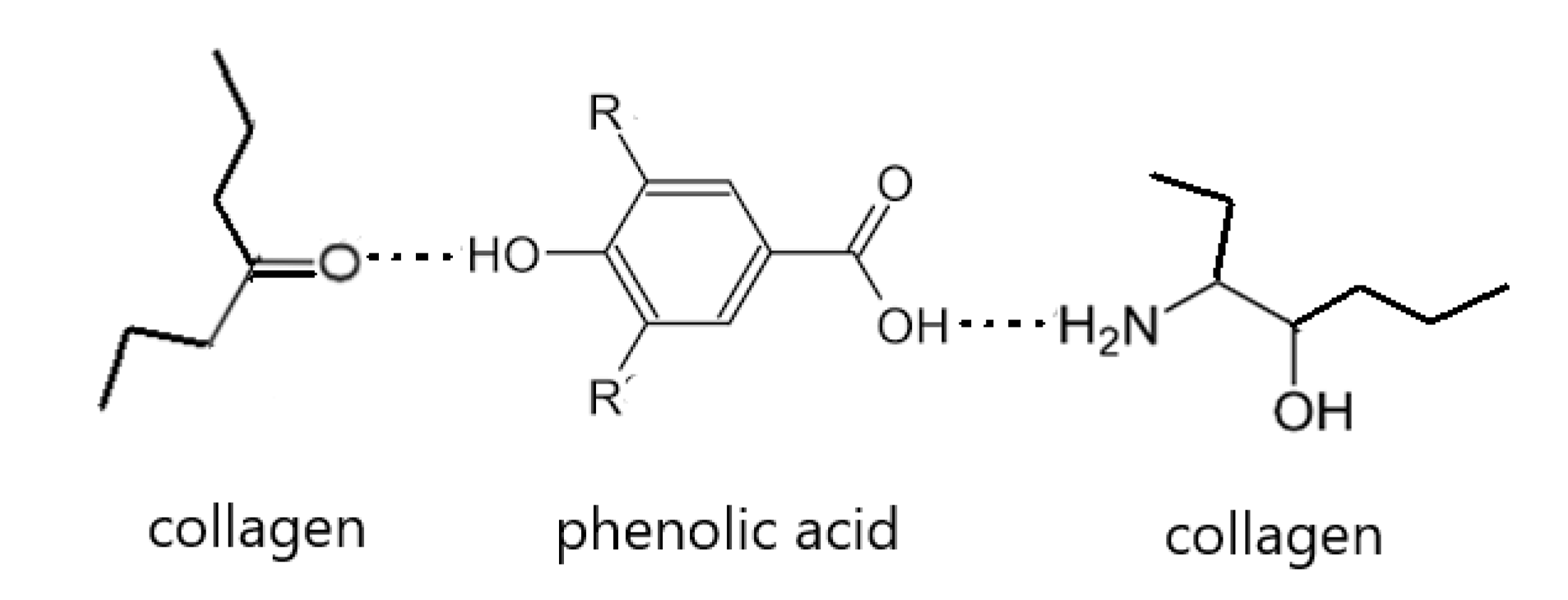
1. Introduction
2. Collagen
2.1. Types of Collagen
2.2. Collagen Application
2.3. Biomedical Aspect
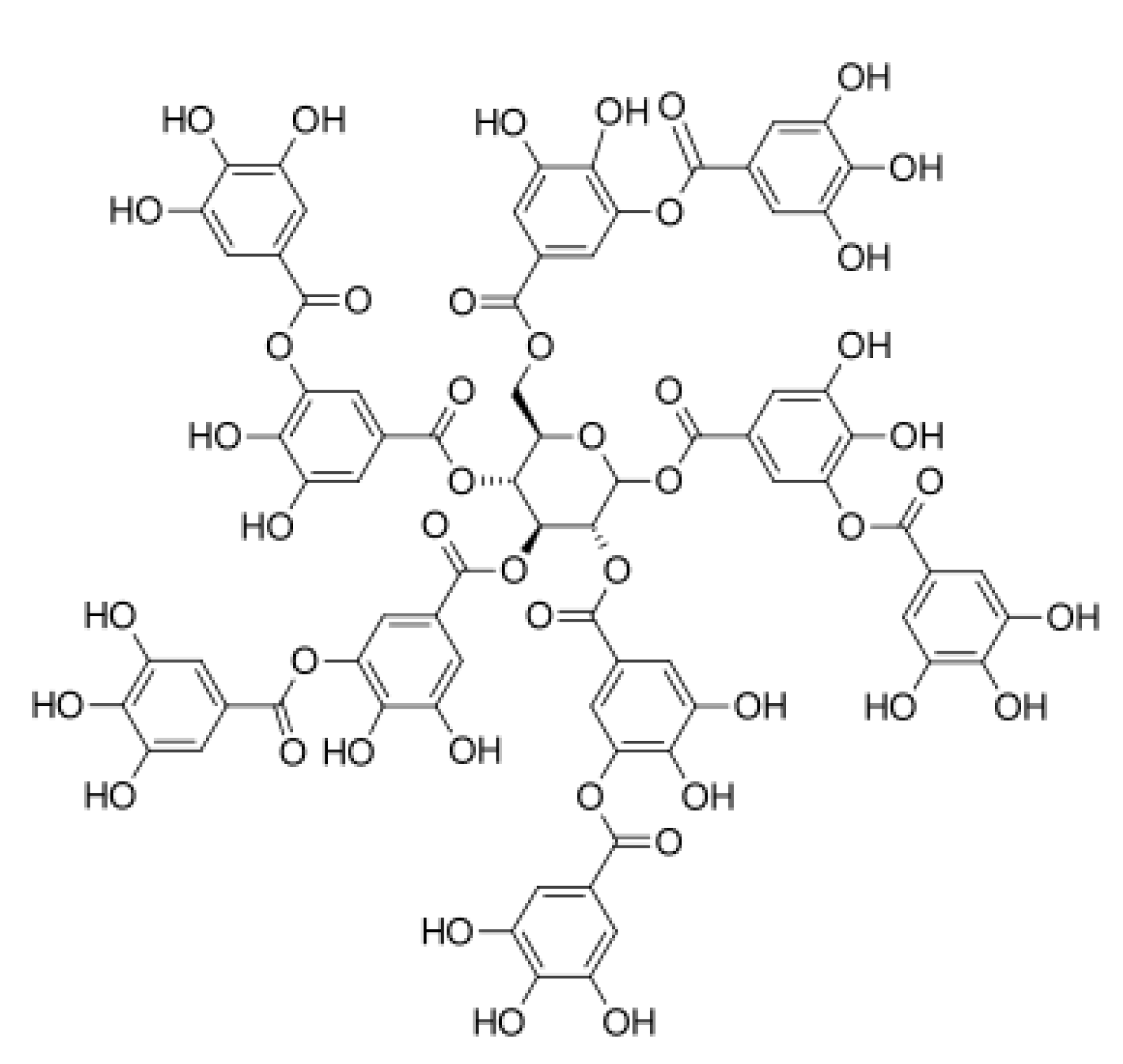
2.4. Collagen–Tannic Acid
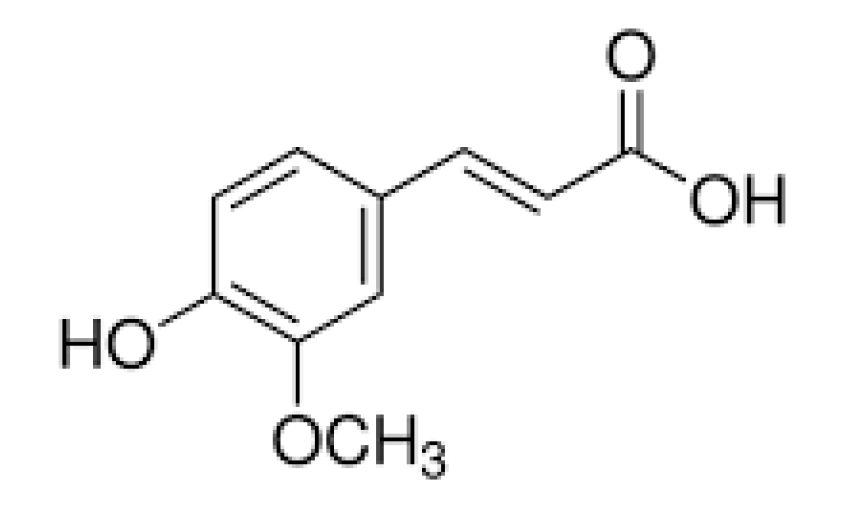
2.5. Collagen–Ferulic Acid
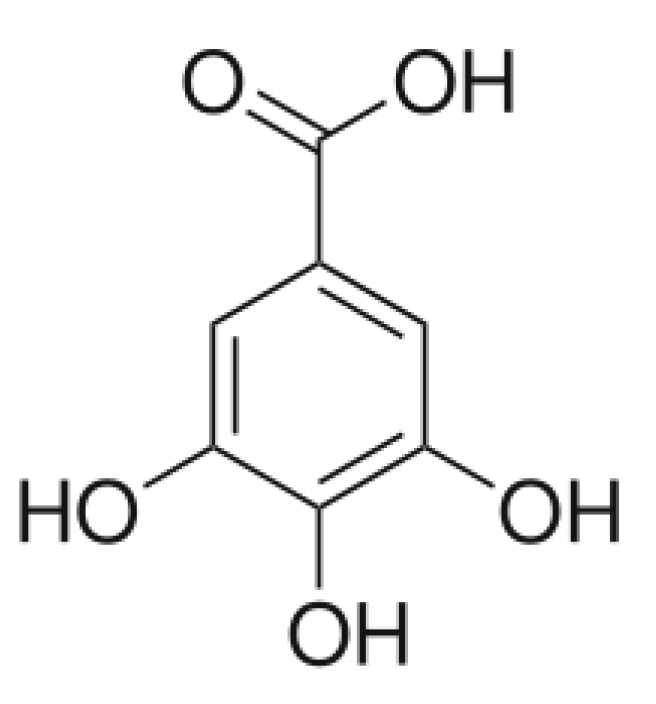
2.6. Collagen–Gallic Acid
2.7. Collagen with Other Phenolic Acids
2.8. Phenolic Acids with Other Natural Polymers
3. Conclusions
Funding
Conflicts of Interest
References
- Qiu, T.; Hanna, E.; Dabbous, M.; Borislav, B.; Toumi, M. Regenerative Medicine Regulatory Policies: A Systematic Review and International Comparison. Health Policy 2020, 124, 701–713. [Google Scholar] [CrossRef] [PubMed]
- Itaba, N.; Shiota, G. A report of the 18th congress of the Japanese Society for Regenerative Medicine. Regen. Ther. 2020, 14, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Han, D.; Wu, J.C. Molecular Imaging of Cardiac Regenerative Medicine. Curr. Opin. Biomed. Eng. 2019, 9, 66–73. [Google Scholar] [CrossRef]
- Yuan, A.R.; Bian, Q.; Gao, J.Q. Current advances in stem cell-based therapies for hair regeneration. Eur. J. Pharmacol. 2020, 881, 173197. [Google Scholar] [CrossRef] [PubMed]
- Luinenburg, D.G.; de Haan, G. MicroRNAs in Hematopoietic Stem Cell Aging. Mech. Ageing. Dev. 2020, 189, 111281. [Google Scholar] [CrossRef]
- Earthman, J.C.; Sheets, C.G.; Paquette, J.M.; Kaminishi, R.M.; Nordland, W.P.; Keim, R.G.; Wu, J.C. Tissue engineering in dentistry. Clin. Plast. Surg. 2003, 30, 621–639. [Google Scholar] [CrossRef]
- Chen, F.M.; Zhang, J.; Zhang, M.; An, Y.; Chen, F.; Wu, Z.F. A review on endogenous regenerative technology in periodontal regenerative medicine. Biomaterials 2010, 31, 7892–7927. [Google Scholar] [CrossRef]
- Nishigaki, F.; Ezoe, S.; Kitajima, H.; Hata, K. Human resource development contributes to the creation of outstanding regenerative medicine products. Regen. Ther. 2017, 7, 17–23. [Google Scholar] [CrossRef]
- Peng, J.; Zhao, H.; Tu, C.; Xu, Z.; Ye, L.; Zhao, L.; Zongheng, G.; Zhao, D.; Jie, Z.; Feng, Z. In situ hydrogel dressing loaded with heparin and basic fibroblast growth factor for accelerating wound healing in rat. Mater. Sci. Eng. C 2020, 116, 111169. [Google Scholar] [CrossRef]
- Taşın, S.; Turp, I.; Bozdağ, E.; Sünbüloğlu, E.; Üşümez, A. Evaluation of strain distribution on an edentulous mandible generated by cobalt-chromium metal alloy fixed complete dentures fabricated with different techniques: An in vitro study. J. Prosthet. Dent. 2019, 122, 47–53. [Google Scholar] [CrossRef]
- Arshad, M.; Zubair, M.; Rahman, S.S.; Ullah, A. Polymers for advanced applications. Polym. Sci. Nanotechnol. 2020, 325–340. [Google Scholar] [CrossRef]
- Samadian, H.; Maleki, H.; Allahyari, Z.; Jaymand, M. Natural polymers-based light-induced hydrogels: Promising biomaterials for biomedical applications. Coord. Chem. Rev. 2020, 420, 213432. [Google Scholar] [CrossRef]
- Rani, S.; Kadam, V.; Rose, N.M.; Jose, S.; Yadav, S.; Shakyawar, D.B. Wheat starch, gum arabic and chitosan biopolymer treatment of wool fabric for improved shrink resistance finishing. Int. J. Biol. Macromol. 2020, 163, 1044–1052. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Jeong, D.; Kanmani, P. Study on physical and mechanical properties of the biopolymer/silver based active nanocomposite films with antimicrobial activity. Carbohydr. Polym. 2019, 224, 115159. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.F.M.; Pogacnik, L. Polyphenols from food and natural products: Neuroprotection and safety. Antioxidants 2020, 10, 61. [Google Scholar] [CrossRef]
- Abbas, M.; Saeed, F.; Anjum, F.M.; Afzaal, M.; Tufail, T.; Bashir, M.S. Natural polyphenols: An overview. Int. J. Food Prop. 2020, 20, 1689–1699. [Google Scholar] [CrossRef]
- Scalbert, A.; Johnson, I.T.; Saltmarsh, M. Polyphenols: Antioxidants and beyond. Am. J. Clin. Nutr. 2005, 81, 215S–217S. [Google Scholar] [CrossRef]
- D’Archivio, M.; Filesi, C.; di Benedetto, R.; Gargiulo, R.; Giovannini, C.; Masella, R. Polyphenols, dietary sources and bioavailability. Ann. Ist. Super Sanita 2007, 43, 348. [Google Scholar]
- Bailey, A.; Paul, R. Collagen-is not so simple protein. J. Soc. Leather Technol. Chem. 1998, 82, 104–108. [Google Scholar]
- Myllyharju, J.; Kivirikko, K. Collagens, modifying enzymes and their mutations in humans, flies and worms. Trends Genet. 2004, 20, 33–43. [Google Scholar] [CrossRef]
- Mitura, S.; Sionkowska, A.; Jaiswal, A. Biopolymers for hydrogels in cosmetics: Review. J. Mater. Sci. Mater. Med. 2020, 31. [Google Scholar] [CrossRef] [PubMed]
- Rusaouen, M.; Pujol, J.P.; Bocquet, J.; Veillard, A.; Borel, J.P. Evidence of collagen in the egg capsule of the dogfish, scyliorhinus canicular. Comp. Biochem. Phys. B 1976, 53, 539–543. [Google Scholar] [CrossRef]
- Luong, T.T.; Boutillon, M.M.; Garrone, R.; Knight, D.P. Characterization of Selachian Egg Case Collagen. Biochem. Biophys. Res. Commun. 1998, 250, 657–663. [Google Scholar] [CrossRef] [PubMed]
- Iconomidou, V.A.; Georgaka, M.E.; Chryssikos, G.D.; Gionis, V.; Megalofonou, P.; Hamodrakas, S.J. Dogfish egg case structural studies by ATR FT-IR and FT-Raman spectroscopy. Int. J. Biol. Macromol. 2007, 41, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Heinemann, S.; Ehrlich, H.; Douglas, T.; Heinemann, C.; Worch, H.; Schatton, W.; Hanke, T. Ultrastructural studies on the collagen of the marine sponge Chondrosia reniformis Nardo. Biomacromolecules 2007, 8, 3452–3457. [Google Scholar] [CrossRef]
- Hoyer, B.; Bernhardt, A.; Lode, A.; Heinemann, S.; Sewing, J.; Klinger, M.; Notbohm, H.; Gelinsky, M. Jellyfish collagen scaffolds for cartilage tissue engineering. Acta Biomater. 2014, 10, 883–892. [Google Scholar] [CrossRef]
- Ehrlich, H. Chitin and collagen as universal and alternative templates in biomineralization. Int. Geol. Rev. 2010, 52, 661–699. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, W.; Li, G.; Shi, B.; Miao, Y.; Wu, X. Isolation and partial characterization of pepsin-soluble collagen from the skin of grass carp (Ctenopharyngodon idella). Food Chem. 2007, 103, 906–912. [Google Scholar] [CrossRef]
- Sionkowska, A.; Skrzyński, S.; Śmiechowski, K.; Kołodziejczak, A. The review of versatile application of collagen. Polym. Adv. Technol. 2016, 28, 4–9. [Google Scholar] [CrossRef]
- Szatkowski, T.; Kopczyński, K.; Motylenko, M.; Borrmann, H.; Mania, B.; Graś, M.; Lota, G.; Bazhenov, V.; Rafaja, D.; Roth, F.; et al. Extreme biomimetics: A carbonized 3D spongin scaffold as a novel support for nanostructured manganese oxide(IV) and its electrochemical applications. Nano Res. 2019, 11, 4199–4214. [Google Scholar] [CrossRef]
- Petrenko, I.; Summers, A.P.; Simon, P.; Żółtowska-Aksamitowska, S.; Motylenko, M.; Schimpf, C.; Rafaja, D.; Roth, F.; Kummer, K.; Brendler, E.; et al. Extreme biomimetics: Preservation of molecular detail in centimeter-scale samples of biological meshes laid down by sponges. Mater. Sci. 2019, 5. [Google Scholar] [CrossRef] [PubMed]
- Kadler, K.E.; Baldock, C.; Bella, J.; Boot-Handford, R.P. Collagens at a glance. J. Cell Sci. 2001, 120, 1955–1958. [Google Scholar] [CrossRef]
- Koob, T.J.; Cox, D.L. Stabilization and sclerotization ofRaja erinacea egg capsule proteins. Environ. Biol. Fishes 1993, 38, 151–157. [Google Scholar] [CrossRef]
- Ehrlich, H.; Wysokowski, M.; Żółtowska-Aksamitowska, S.; Petrenko, I.; Jesionowski, T. Collagens of Poriferan Origin. Mar. Durgs 2018, 16, 79. [Google Scholar] [CrossRef] [PubMed]
- Fathima, N.N.; Baias, M.; Blumich, B.; Ramasami, T. Structure and dynamics of water in native and tanned collagen fibers: Effect of crosslinking. Int. J. Biol. Macromol. 2010, 1, 590–596. [Google Scholar] [CrossRef] [PubMed]
- Jesionowski, T.; Norman, M.; Żółtowska-Aksamitowska, S.; Petrenko, I.; Joseph, Y.; Ehrlich, H. Marine spongin: Naturally prefabricated 3D scaffold–based biomaterial. Mar. Drugs 2018, 16, 88. [Google Scholar] [CrossRef]
- Ehrlich, H.; Hanke, T.; Simon, P.; Born, R.; Fisher, C.; Frolov, A.; Langrock, T.; Hoffmann, R.; Schwarzenbolz, U.; Henle, T.; et al. Carboxymethylation of the fibrillar collagen with respect to formation of hydroxyapatite. J. Biomed. Mater. Res. B. 2010, 92, 542–551. [Google Scholar] [CrossRef]
- Funt, D.; Pavicic, T. Dermal fillers in aesthetics: An overview of adverse events and treatment approaches. Clin. Cosmet. Investig. Dermatol. 2013, 6, 295–316. [Google Scholar] [CrossRef]
- Heneke, E. Managing Complications of Fillers: Rare and Not-So-Rare. J. Cutan. Aesthet. Surg. 2015, 8, 198–210. [Google Scholar] [CrossRef]
- Heinemann, S.; Ehrlich, H.; Knieb, C.; Hanke, T. Biomimetically inspired hybrid materials based on silicified collagen. Int. J. Mater. Res. 2007, 98, 603–608. [Google Scholar] [CrossRef]
- Subhan, F.; Hussain, Z.; Tauseef, I.; Shehzad, A.; Wahid, F. A review on recent advances and applications of fish collagen. Crit. Rev. Food Sci. Nutr. 2020, 29, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Heinemann, S.; Heinemann, C.; Ehrlich, H.; Meyer, M.; Baltzer, H.; Worch, H.; Hanke, T. A Novel Biomimetic Hybrid Material Made of Silicified Collagen: Perspectives for Bone Replacement. Adv. Eng. Mater. 2007, 9, 1061–1068. [Google Scholar] [CrossRef]
- Murugesan, S.M.; Aaron, K.P.; Muralidharan, C.; Rose, C. Highly biocompatible novel polyphenol cross-linked collagen scaffold for potential tissue engineering applications. React. Funct. Polym. 2020, 104630. [Google Scholar]
- Garcia, Y.; Collighan, R.; Griffin, M.; Pandit, A. Assessment of cell viability in a three-dimensional enzymatically cross-linked collagen scaffold. J. Mater. Sci. Mater. Med. 2007, 18, 1991–2001. [Google Scholar] [CrossRef] [PubMed]
- Nomura, Y.; Toki, S.; Ishii, Y.; Shirai, K. Effect of transglutaminase on reconstruction and physicochemical properties of collagen gel from shark type I collagen. Biomacromolecules 2001, 2, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Oh, H.; Uemura, T.; Yamaguchi, I.; Ikoma, T.; Tanaka, J. Effect of enzymatically cross-linked tilapia scale collagen for osteoblastic differentiation of human mesenchymal stem cells. J. Bioact. Compat. Polym. 2016, 31, 31–41. [Google Scholar] [CrossRef]
- Garcia, Y.; Hemantkumar, N.; Collighan, R.; Griffin, M.; Rodriguez-Cabello, J.; Pandit, A. In vitro characterization of a collagen scaffold enzymatically cross-linked with a tailored elastin-like polymer. Tissue Eng. A 2009, 15, 887–899. [Google Scholar] [CrossRef]
- Arakawa, C.; Ng, R.; Tan, S.; Kim, S.; Wu, B.; Lee, M. Photopolymerizable chitosan-collagen hydrogels for bone tissue engineering. Artif. Cells Blood Substit. Immobil. Biotechnol. 2014, 34, 27–39. [Google Scholar] [CrossRef]
- Yan, J.; Li, X.; Liu, L.; Wang, F.; Tai, W.; Zhang, Q. Potential use of collagen-chitosan-hyaluronan tri-copolymer scaffold for cartilage tissue engineering. Artif. Cells Blood Substit. Immobil. Biotechnol. 2006, 34, 27–39. [Google Scholar] [CrossRef]
- Yan, L.P.; Wang, Y.J.; Ren, L.; Wu, G.; Caridade, S.G.; Fan, J.B.; Wang, L.Y.; Ji, P.H.; Oliveira, J.M.; Oliveira, J.T.; et al. Genipin-cross-linked collagen/chitosan biomimetic scaffolds for articular cartilage tissue engineering applications. J. Biomed. Mater. Res. A 2010, 95, 465–475. [Google Scholar] [CrossRef]
- Haugh, M.; Jaasma, M.; O’Brien, F. The effect of dehydrothermal treatment on the mechanical and structural properties of collagen—GAG scaffolds. J. Biomed. Mater. Res. A 2009, 89, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Yannas, I.; Tobolsky, A. Cross-linking of gelatine by dehydration. Nature 1967, 215, 509–510. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Venugopal, J.; Huang, Z.; Lim, C.; Ramakrishna, S. Crosslinking of the electrospun gelatin nanofibers. Polymer 2006, 47, 2911–2917. [Google Scholar] [CrossRef]
- Lutolf, M.; Hubbell, J. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat. Biotechnol. 2005, 23, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Drexler, J.W.; Powell, H.M. Dehydrothermal crosslinking of electrospun collagen. Tissue Eng. C 2011, 17, 9–17. [Google Scholar] [CrossRef]
- Bellincampi, L.; Dunn, M. Effect of crosslinking method on collagen fiber-fibroblast interactions. J. Appl. Polym. Sci. 1997, 63, 1493–1498. [Google Scholar] [CrossRef]
- Cooper, D.; Davidson, R.J. The effect of ultraviolet irradiation on soluble collagen. Biochem. J. 1965, 97, 139–143. [Google Scholar] [CrossRef]
- Davidenko, N.; Bax, D.; Schuster, C.; Farndale, R.; Hamaia, S.; Best, S.; Cameron, R. Optimisation of UV irradiation as a binding site conserving method for crosslinking collagen-based scaffolds. J. Mater. Sci. Mater. Med. 2016, 27, 14. [Google Scholar] [CrossRef]
- Parenteau-Bareil, R.; Gauvin, R.; Berthod, F. Collagen-based biomaterials for tissue engineering applications. Materials 2010, 3, 1863–1887. [Google Scholar] [CrossRef]
- Korn, A.; Feairheller, S.; Filachione, E. Glutaraldehyde: Nature of the reagent. J. Mol. Biol. 1972, 65, 525–529. [Google Scholar] [CrossRef]
- Jastrzębska, M.; Wrzalik, R.; Kocot, A.; Zalewska-Rejdak, J.; Cywilna, B. Raman spectroscopic study of glutaraldehyde-stabilized collagen and pericardium issue. J. Biomater. Sci. Polym. Ed. 2003, 14, 185–197. [Google Scholar] [CrossRef] [PubMed]
- Nimni, M.; Cheung, D.; Strates, B.; Kodama, M.; Sheikh, K. Chemically modified collagen: A natural biomaterial for tissue replacement. J. Biomed. Mater. Res. 1987, 21, 741–771. [Google Scholar] [CrossRef] [PubMed]
- Migneault, I.; Dartiguenave, C.; Bertrand, J.; Waldron, K. Glutaraldehyde: Behavior in aqueous solution, reaction with proteins, and application to enzyme crosslinking. Biotechniques 2004, 37, 790–802. [Google Scholar] [CrossRef] [PubMed]
- Cheung, D.; Nimni, M. Mechanism of crosslinking proteins by glutaraldehyde. Cell Tissue Res. 1982, 10, 187–199. [Google Scholar] [CrossRef]
- Huang-Lee, L.; Cheung, D.; Nimni, M. Biochemical changes and cytotoxicity associated with the degradation of polymeric glutaraldehyde derived crosslinks. J. Biomed. Mater. Res. 1990, 24, 1185–1201. [Google Scholar] [CrossRef]
- Hardy, P.; Nicholls, A.; Rydon, H. The nature of glutaraldehyde in aqeous solution. J. Chem. Soc. D 1969, 1, 565–566. [Google Scholar] [CrossRef]
- Okuda, K.; Urabe, I.; Yamada, Y.; Okada, H. Reaction of glutaraldehyde with amino and thiol compounds. J. Ferment. Bioeng. 1991, 72, 100–105. [Google Scholar] [CrossRef]
- Woodroof, E.A. Use of glutaraldehyde and formaldehyde to process tissue heart valves. J. Bioeng. 1978, 2, 1–9. [Google Scholar]
- Johnson, T. Glutaraldehyde fixation chemistry: Oxygen-consuming reactions. Eur. J. Cell Biol. 1987, 45, 160–169. [Google Scholar]
- Manickam, B.; Sreedharan, R.; Elumalai, M. ‘Genipin’-the natural water soluble cross-linking agent and its importance in the modified drug delivery systems: An overview. Curr. Drug Deliv. 2014, 11, 139–145. [Google Scholar] [CrossRef]
- Chang, Y.; Lee, M.; Liang, H.; Hsu, C.; Sung, H. Acellularbovine pericardia with distinct porous structures fixed with genipin as an extracellular matrix. Tissue Eng. 2004, 10, 881–892. [Google Scholar] [CrossRef] [PubMed]
- Butler, M.F.; Ng, Y.F.; Pudney, P.D. Mechanism and kinetics of the crosslinking reaction between biopolymers containing primary amine groups and genipin. J. Polym. Sci. Pol. Chem. 2003, 41, 3941–3953. [Google Scholar] [CrossRef]
- Mu, C.; Zhang, K.; Lin, W.; Li, D. Ring-opening polymerization of genipin and its long-range crosslinking effect on collagen hydrogel. J. Biomed. Mater. Res. A 2013, 101, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Sundararaghavan, H.G.; Monteiro, G.A.; Lapin, N.A.; Chabal, Y.J.; Miksan, J.R.; Shreiber, D.I. Genipin. Induced changes in collagen gels: Correlation of mechanical properties to fluorescence. J. Biomed. Mater. Res. A 2008, 87, 308–320. [Google Scholar] [CrossRef]
- Almog, J.; Cohen, Y.; Azoury, M.; Hahn, T. Genipin-A novel fingerprint reagent with colorimetric and fluorogenic activity. J. Forensic Sci. 2004, 49, 255–257. [Google Scholar] [CrossRef]
- Macaya, D.; Ng, K.; Spector, M. Injectable collagen-genipin gel for the treatment of spinal cord injury: In vitro studies. Adv. Funct. Mater. 2011, 21, 4788–4797. [Google Scholar] [CrossRef]
- Avila, M.; Navia, J. Effect of genipin collagen crosslinking on porcine corneas. J. Cataract. Refract. Surg. 2010, 36, 659–664. [Google Scholar] [CrossRef]
- Gaudio, C.; Baiguera, S.; Boieri, M. Induction of angiogenesis using VEGF releasing genipin-crosslinked electrospun gelatin mats, Genipin, a cross-linking agent, promotes odontogenic differentiation of human dental pulp cells. Biomaterials 2013, 34, 7754–7765. [Google Scholar] [CrossRef]
- Kwon, Y.; Lim, E.; Kim, H.; Hwang, Y.; Lee, K.; Min, K. Genipin, a cross-linking agent, promotes odontogenic differentation of human dental pulp cells. J. Endod. 2015, 41, 501–507. [Google Scholar] [CrossRef]
- Narita, T.; Yunoki, S.; Ohyabu, Y.; Yahagi, N.; Uraoka, T. In situ gelation properties of a collagen–genipin sol with a potential for the treatment of gastrointestinal ulcers. Med. Devices 2016, 9, 429–439. [Google Scholar] [CrossRef]
- Zhao, Y.; Sun, Z. Effects of gelatin-polyphenol and gelatin–genipin cross-linking on the structure of gelatin hydrogels. International journal of food properties. Int. J. Food Prop. 2017, 20, 2822–2832. [Google Scholar] [CrossRef]
- Yang, C. Enhaced physicochemical properties of collagen by using EDC/NHS–crosslinking. Bull. Mater. Sci. 2012, 35, 913–918. [Google Scholar] [CrossRef]
- Goodarzi, H.; Jadidi, K.; Pourmotabed, S.; Sharifi, E.; Aghamollaei, H. Preparation and in vitro characterization of cross-linked collagen-gelatin hydrogel using EDC/NHS for corneal tissue engineering applications. Int. J. Biol. Macromol. 2019, 126, 620–632. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarek, B.; Sionkowska, A.; Kozlowska, J.; Osyczka, A.M. New composite materials prepared by calcium phosphateprecipitation in chitosan/collagen/hyaluronic acid spongecross-linked by EDC/NHS. Int. J. Biol. Macromol. 2018, 107, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Pfeifer, V.; Sohns, V.; Conway, H.; Lancaster, E.; Dabic, S.; Griffin, E. Two-stage process for dialdehyde starch using electrolytic regeneration of periodic acid. Ind. Eng. Chem. 1960, 52, 201–206. [Google Scholar] [CrossRef]
- Mu, C.; Liu, F.; Cheng, Q.; Li, H.; Wu, B.; Zhang, G.; Lin, W. Collagen cryogel cross-linked by dialdehyde starch. Macromol. Mater. Eng. 2010, 295, 100–107. [Google Scholar] [CrossRef]
- Langmaier, F.; Mladek, M.; Mokrejs, P.; Kolomaznik, K. Biodegradable packing materials based on waste collagen hydrolysate cured with dialdehyde starch. J. Therm. Anal. Calorim. 2008, 93, 547–552. [Google Scholar] [CrossRef]
- Martucci, J.; Ruseckaite, R. Tensile properties, barrierproperties, and biodegradation in soil of compression: Molded gelatin-dialdehyde starch films. J. Appl. Polym. Sci. 2009, 112, 2166–2178. [Google Scholar] [CrossRef]
- Langmaier, F.; Mládek, M.; Mokrejš, P. Hydrogels of collagen hydrolysate cross-linked with dialdehyde starch. J. Therm. Anal. Calorim. 2009, 98, 807–812. [Google Scholar] [CrossRef]
- Liu, Y.; Acharya, G.; Lee, C. Hydrogels of collagen hydrolysate cross-linked with dialdehyde starch. J. Biomed. Mater. Res. A 2011, 99, 485–492. [Google Scholar] [CrossRef]
- Sionkowska, A.; Michalska-Sionkowska, M.; Walczak, M. Preparation and characterization of collagen/hyaluronic acid/chitosan film crosslinked with dialdehyde starch. Int. J. Biol. Macromol. 2020, 149, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Grabska-Zielińska, S.; Sionkowska, A.; Reczyńska, K.; Pamuła, E. Physico-chemical characterization and biological tests of collagen/silk fibroin/chitosan scaffolds cross-linked by dialdehyde starch. Polymers 2020, 12, 372. [Google Scholar]
- Kaczmarek, B.; Sionkowska, A. Chitosan/collagen blends with inorganic and organic additive—A review. Adv. Polym. Technol. 2018, 37, 2367–2376. [Google Scholar] [CrossRef]
- Sionkowska, A.; Wisniewski, M.; Skopinska, J.; Kennedy, C.; Wess, T. Molecularinter-actions in collagen and chitosan blends. Biomaterials 2004, 25, 795–801. [Google Scholar] [CrossRef]
- Claudio-Rizo, J.A.; González-Lara, I.A.; Flores-Guía, T.E.; Cano-Salazar, L.F.; Cabrera-Munguía, D.A.; Becerra-Rodríguez, J.J. Study of the polyacrylate interpenetration in a collagen-polyurethane matrix to prepare novel hydrogels for biomedical applications. Int. J. Biol. Macromol. 2020, 156, 27–39. [Google Scholar] [CrossRef]
- Tsurkan, D.; Wysokowski, M.; Petrenko, I.; Voronkina, A.; Khrunyk, Y.; Fursov, A.; Ehrlich, H. Modern scaffolding strategies based on naturally pre-fabricated 3D biomaterials of poriferan origin. App. Phys. A 2020, 126, 382. [Google Scholar] [CrossRef]
- Wu, L.; Shao, H.; Fang, Z.; Zhao, Y.; Cao, C.Y.; Li, Q. Mechanism and effects of polyphenol derivatives for modifying collagen. ACS Biomater. Sci. Eng. 2019, 5, 4272–4284. [Google Scholar] [CrossRef]
- Violeta Ghica, M.; Georgiana Albu, M.; Popa, L.; Leca, M.; Brǎzdaru, L.; Cotruţ, C.; Trandafir, V. Drug delivery systems based on collagen-tannic acid matrices. Rev. Roum. Chim. 2009, 54, 1103–1110. [Google Scholar]
- Kaczmarek, B.; Sionkowska, A.; Osyczka, A.M. Scaffolds based on chitosan and collagen with glycosaminoglycans cross-linked by tannic acid. Polym. Test. 2018, 65, 163–168. [Google Scholar] [CrossRef]
- Albu, M.G.; Deselnicu, V.; Ioannidis, I.; Deselnicu, D.; Chelaru, C. Chemical functionalization and stabilization of type I collagen with organic tanning agents. Korean J. Chem. Eng. 2014, 32, 354–361. [Google Scholar] [CrossRef]
- Kaczmarek, B.; Sionkowska, A.; Osyczka, A.M. The comparison of physic-chemical properties of chitosan/collagen/hyaluronic acid composites with nano-hydroxyapatite cross-linked by dialdehyde starch and tannic acid. Polym. Test. 2017, 62, 171–176. [Google Scholar] [CrossRef]
- Iqbal, M.H.; Schroder, A.; Kerdjoudj, H.; Njel, C.; Senger, B.; Ball, V.; Meyer, F.; Boulmedais, F. Effect of the buffer on the buildup and stability of tannic acid/collagen multilayer films applied as antibacterial coatings. ACS App. Mater. Interfaces 2020, 12, 22601–22612. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarek, B. Tannic acid with antiviral and antibacterial activity as a promising component of biomaterials—A minireview. Materials 2020, 13, 3224. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhao, L.; Guo, S.; Xia, Y.; Zhou, P. Modification of fish skin collagen film and absorption property of tannic acid. J. Food Sci. Technol. 2014, 51, 1102–1109. [Google Scholar] [CrossRef] [PubMed]
- Velmurugan, P.; Singam, E.R.A.; Jonnalagadda, R.R.; Subramanian, V. Investigation on interaction of tannic acid with type i collagen and its effect on thermal, enzymatic, and conformational stability for tissue engineering applications. Biopolymers 2014, 101, 471–483. [Google Scholar] [CrossRef]
- Wu, J.; Liao, W.; Zhang, J.; Chen, W. Thermal behavior of collagen crosslinked with tannic acid under microwave heating. J. Therm. Anal. Calorim. 2019, 135, 2329–2335. [Google Scholar] [CrossRef]
- Cass, C.A.P.; Burg, K.J.L. Tannic acid cross-linked collagen scaffolds and their anti-cancer potential in a tissue engineered breast implant. J. Biomed. Sci. Polym. Ed. 2012, 23, 281–298. [Google Scholar] [CrossRef]
- Baldwin, A.; Uy, L.; Frank-Kamenetskii, A.; Strizzi, L.; Booth, B.W. The in vivo biocompatibility of novel tannic acid-collagen type I injectable bead scaffold material for breast reconstruction post-lumpectomy. J. Biomater. App. 2020, 34, 1315–1329. [Google Scholar] [CrossRef]
- Kaczmarek, B.; Sionkowska, A.; Otrocka-Domagała, I.; Polkowska, I. In vivo studies of novel scaffolds with tannic acid addition. Polym. Deg. Stab. 2018, 158, 26–30. [Google Scholar] [CrossRef]
- Natarajan, V.; Krithica, N.; Madhan, B.; Sehgal, P.K. Preparation and properties of tannic acid cross-linked collagen scaffold and its application in wound healing. J. Biomed. Mater. Res. B Appl. Biomater. 2013, 101, 560–567. [Google Scholar] [CrossRef]
- Bridgeman, C.J.; Nguyen, T.-U.; Kishore, V. Anticancer efficacy of tannic acid is dependent on the stiffness of the underlying matrix. J. Biomater. Sci. Polym. Ed. 2018, 29, 412–427. [Google Scholar] [CrossRef] [PubMed]
- Zduńska, K.; Dana, A.; Kołodziejczak, A.; Rotsztejn, H. Antioxidant properties of ferulic acid and its possible application. Skin Pharmacol. Phys. 2018, 31, 332–336. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.-H.; Park, J.-K.; Kim, K.-M.; Lee, H.-J.; Kim, S. In vitro and in vivo antithrombotic and cytotoxicity effects of ferulic acid. J. Biochem. Mol. Toxicol. 2018, 32, e22004. [Google Scholar] [CrossRef] [PubMed]
- Kumar, C.S.; Soloman, A.M.; Thangam, R.; Perumal, R.K.; Gopinath, A.; Madhan, B. Ferulic acid-loaded collagen hydrolysate and polycaprolactone nanofibres for tissue engineering applications. IET Nanobiotechnol. 2020, 14, 202–209. [Google Scholar] [CrossRef]
- Kaczmarek, B.; Lewandowska, K.; Sionkowska, A. Modification of collagen properties with ferulic acid. Materials 2020, 13, 3419. [Google Scholar] [CrossRef]
- Altan, A.; Yuce, H.; Karatas, O.; Taskan, M.; Gevrek, F.; Colak, S.; Akbulut, N. Free and liposome form of gallic acid improves calvarial bone wound healing in Wistar rats. Asian Pac. J. Trop. Biomed. 2020, 10, 156–163. [Google Scholar] [CrossRef]
- Sahiner, M.; Alpaslan, D.; Bitlisli, B.O. Collagen-based hydrogel films as drug-delivery devices with antimicrobial properties. Polym. Bull. 2014, 71, 3017–3033. [Google Scholar] [CrossRef]
- Zhao, Q.; Yu, X.; Zhou, C.; Yagoub, A.E.A.; Ma, H. Effects of collagen and casein with phenolic compounds interactions on protein in vitro digestion and antioxidation. LWT 2020, 124, 109192. [Google Scholar] [CrossRef]
- Krishnamoorthy, G.; Selvakumar, R.; Sastry, T.P.; Sadulla, S.; Mandal, A.B.; Doble, M. Experimental and theoretical studies on Gallic acid assisted EDC/NHS initiated crosslinked collagen scaffolds. Mater. Sci. Eng. C 2014, 43, 164–171. [Google Scholar] [CrossRef]
- Shaik, M.M.; Kowshik, M. Ellagic acid containing collagen-chitosan scaffolds as potential antioxidative bio-materials for tissue engineering applications. Int. J. Polym. Mater. Polym. Biomater. 2019, 68, 208–215. [Google Scholar] [CrossRef]
- Petrisor, G.; Ion, R.M.; Brachais, C.-H.; Boni, G.; Plasseraud, L.; Couvercelle, J.-P.; Chambin, O. In vitro release of local anaesthetic and anti-inflammatory drugs from crosslinked collagen based device. J. Macromol. Sci. A 2012, 49, 699–705. [Google Scholar] [CrossRef]
- Song, H.S.; Park, T.W.; Sohn, U.D.; Shin, Y.K.; Choi, B.C.; Kim, C.J.; Sim, S.S. The effect of caffeic acid on wound healing in skin-incised mice. Korean J. Physiol. Pharmacol. 2008, 12, 343–347. [Google Scholar] [CrossRef] [PubMed]
- Magnani, C.; Isaac, V.L.B.; Correa, M.A.; Salgado, H.R.N. Caffeic acid: A review of its potential use in medications and cosmetics. Anal. Methods 2014, 6, 3203–3210. [Google Scholar] [CrossRef]
- Thongchai, K.; Chuysinuan, P.; Thanyacharoen, T.; Techasakul, S.; Ummartyotin, S. Characterization, release, and antioxidant activity of caffeic acid-loaded collagen and chitosan hydrogel composites. J. Mater. Res. Technol. 2020. [Google Scholar] [CrossRef]
- Wang, Y.; Du, H.; Xie, M.; Ma, G.; Yang, W.; Hu, Q.; Pei, F. Characterization of the physical properties and biological activity of chitosan films grafted with gallic acid and caffeic acid: A comparison study. Food Packag. Shelf Life 2019, 22, 100401. [Google Scholar] [CrossRef]
- Rubentheren, V.; Ward, T.A.; Chee, C.Y.; Tang, C.A. Processing and analysis of chitosan nanocomposites reinforced with chitin whiskers and tannic acid as a crosslinker. Carbohyd. Polym. 2015, 115, 379–387. [Google Scholar] [CrossRef]
- Kaczmarek, B.; Wekwejt, M.; Nadolna, K.; Owczarek, K.; Mazur, O.; Pałubicka, A. The mechanical properties and bactericidal degradation effectiveness of tannic acid-based thin films for wound care. J. Mech. Beh. Biomed. Mater. 2020, 110, 103916. [Google Scholar] [CrossRef]
- Ninan, N.; Foget, A.; Shastri, V.P.; Voelcker, N.H.; Blencowe, A. Antibacterial and anti-inflammatory ph-responsive tannic acid-carboxylated agarose composite hydrogels for wound healing. ACS Appl. Mater. Interfaces 2016, 8, 28511. [Google Scholar] [CrossRef]
- Zhu, F. Interactions between starch and phenolic compound. Trends Food Sci. Technol. 2015, 43, 129. [Google Scholar] [CrossRef]
- Gao, X.; Dai, Q.; Yao, L.; Dong, H.; Li, Q.; Cao, X. A medical adhesive used in a wet environment by blending tannic acid and silk fibroin. Biomater. Sci. 2020, 8, 2694–2701. [Google Scholar] [CrossRef]
- Lee, H.Y.; Hwang, C.H.; Kim, H.E.; Jeong, S.H. Enhancement of bio-stability and mechanical properties of hyaluronic acid hydrogels by tannic acid treatment. Carbohydr. Polym. 2018, 186, 290. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.Y.; Sun, Y.; Zheng, Y.D.; He, W.; Yang, Y.Y.; Xie, Y.J.; Feng, Z.X.; Qiao, K. A biocompatible bacterial cellulose/tannic acid composite with antibacterial and anti-biofilm activities for biomedical applications. Mater. Sci. Eng. C 2020, 106, 110249. [Google Scholar] [CrossRef] [PubMed]
- Bam, P.; Bhatta, A.; Krishnamoorthy, G. Design of biostable scaffold based on collagen crosslinked by dialdehyde chitosan with presence of gallic acid. Int. J. Biol. Macromol. 2019, 130, 836. [Google Scholar] [CrossRef] [PubMed]
- Wary, R.; Sivaray, S.; Gurukarthikeyan, K.R.; Suray, S.; Sadararaju, G.; Kannayiram, G. Chitosan gallic acid microsphere incorporated collagen matrix for chronic wounds: Biophysical and biochemical characterization. Int. J. Pharm. Pharm. Sci. 2014, 6, 94–100. [Google Scholar]
- Lam, R.K.K.; Fung, Y.K.; Han, W.; Yu, K.N. Rescue effects: Irradiated cells helped by unirradiated bystander cells. Int. J. Mol. Sci. 2015, 16, 2591–2609. [Google Scholar] [CrossRef]
- Shao, Y.; Luo, W.; Guo, Q.; Li, X.; Zhang, Q.; Li, J. In vitro and in vivo effect of hyaluronic acid modified, doxorubicin and gallic acid co-delivered lipid-polymeric hybrid nano-system for leukemia therapy. Drug Des. Devel. Ther. 2019, 13, 2043. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, L.; Xu, H.; Liang, Y.; Zheng, B. Understanding the digestibility of rice starch-gallic acid complexes formed by high pressure homogenization. Int. J. Biol. Macromol. 2019, 134, 856–863. [Google Scholar] [CrossRef]
- Wutticharoenmongkol, P.; Hannirojram, P.; Nuthong, P. Gallic acid-loaded electrospun cellulose acetate nanofibers as potential wound dressing materials. Polym. Adv. Technol. 2019, 30, 1135–1147. [Google Scholar] [CrossRef]
- Jayamani, J.; Naisini, A.; Madhan, B.; Shanmugam, G. Ferulic acid, a natural phenolic compound, as a potential inhibitor for collagen fibril formation and its propagation. Int. J. Biol. Macromol. 2018, 113, 277–284. [Google Scholar] [CrossRef]
- Anwar, M.; Nisa, K.; Indirayati, N. Acid-base evaluation of chitosan-ferulic acid conjugate by a free radical grafting method. IOP Conf. Ser. Earth Environ. Sci. 2019, 251, 012023. [Google Scholar] [CrossRef]
- Li, K.; Zhu, J.; Guan, G.; Wu, H. Preparation of chitosan-sodium alginate films through layer-by-layer assembly and ferulic acid crosslinking: Film properties, characterization, and formation mechanism. Int. J. Biol. Macromol. 2019, 122, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Naseri, A.; Shekarchizadeh, H.; Kadivar, M. Octenylsuccination of sago starch and investigation of the effect of calcium chloride and ferulic acid on physicochemical and functional properties of the modified starch film. J. Food Process. Pres. 2019, 43, e13898. [Google Scholar] [CrossRef]
- Trombino, S.; Cassano, R.; Bloise, E.; Muzzalupo, R.; Tavano, L.; Picci, N. Synthesis and antioxidant activity evaluation of a novel cellulose hydrogel containing trans-ferulic acid. Carbohyd. Polym. 2009, 75, 184–188. [Google Scholar] [CrossRef]
- Hafezi, R.; Dadfarnia, S.; Shabani, A.; Amraei, R.; Moghaddam, Z. Doxycycline drug delivery using hydrogels of o-carboxymethyl chitosan conjugated with caffeic acid and its composite with polyacrylamide synthesized by electron beam irradiation. Int. J. Biol. Macromol. 2020, 154, 962–973. [Google Scholar] [CrossRef]
- Zheng, Y.; Tian, J.; Kong, X.; Yang, W.; Yin, X.; Xu, E.; Chen, S.; Liu, D.; Ye, X. Physicochemical and digestibility characterisation of maize starch–caffeic acid complexes. LWT 2020, 121, 108857. [Google Scholar] [CrossRef]
- Ding, C.; Bi, H.; Wang, D.; Kang, M.; Tian, Z.; Zhnag, Y.; Wang, H.; Zhu, T.; Ma, J. Preparation of chitosan/alginate-ellagic acid sustained-release microspheres and their inhibition of preadipocyte adipogenic differentiation. Curr. Pharm. Biotechnol. 2019, 20, 1213–1222. [Google Scholar] [CrossRef]
- Tirado-Gallegos, J.M.; Zamudio-Flores, P.B.; Ornelas-Paz, J.; Rios-Velasco, C.; Orozco, G.I.O.; Espino-Díaz, M.; Baeza-Jiménez, R.; Buenrostro-Figueroa, J.J.; Aguilar-González, M.A.; Lardizábal-Gutierrez, D.; et al. Elaboration and characterization of active apple starch films incorporated with ellagic acid. Coatings 2018, 8, 384. [Google Scholar] [CrossRef]
- Barnaby, S.N.; Nakatsuka, N.; Frayne, S.H.; Fath, K.R.; Banerjee, I.A. Formation of hyaluronic acid–ellagic acid microfiber hybrid hydrogels and their applications. Coll. Polym. Sci. 2013, 291, 515. [Google Scholar] [CrossRef]
- Ferrochio, L.; Cendoya, E.; Farnochi, M.C.; Massad, W.; Ramírez, M.L. Evaluation of ability of ferulic acid to control growth and fumonisin production of Fusarium verticillioides and Fusarium proliferatum on maize based media. Int. J. Food Microbiol. 2013, 167, 215–220. [Google Scholar] [CrossRef]
- Coma, V.; Portes, E.; Gardrat, C.; Richard-Forget, F.; Castellan, A. In vitro inhibitory effect of tetrahydrocurcuminoids on Fusarium proliferatum growth and fumonisin B1 biosynthesis. Food Addit. Contam. 2011, 2, 218–225. [Google Scholar] [CrossRef]
- Boutigny, A.L.; Atanasova-Pénichon, V.; Benet, M.; Barreau, C.; Richard-Forget, F. Natural phenolic acids from wheat bran inhibit Fusarium culmorum trichothecene biosynthesis in vitro by repressing Tri gene expression. Eur. J. Plant Pathol. 2010, 127, 275–286. [Google Scholar] [CrossRef]
- Dambolena, J.S.; Zygadlo, J.A.; Rubinstein, H.R. Antifumonisin activity of natural phenolic compounds. A structure-property-activity relationship study. Int. J. Food Microbiol. 2011, 145, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.; Huang, J.; Li, K. A new formaldehyde-free wood adhesive from renewable materials. Int. J. Adhes. Adhes. 2011, 31, 754–759. [Google Scholar] [CrossRef]
- Li, J.; Luo, J.; Li, X.; Zhang, Y.; Gao, Q.; Li, J. Soybean meal-based wood adhesive enhanced by ethylene glycol diglycidyl ether and diethylenetriamine. Ind. Crops Prod. 2015, 74, 613–618. [Google Scholar] [CrossRef]
- Wu, L.Q.; Embree, H.D.; Balgley, B.M.; Smith, P.J.; Payne, G.F. Utilizing renewable resources to create functional polymers: Chitosan-based associative thickener environmental. Environ. Sci. Technol. 2002, 36, 3446. [Google Scholar] [CrossRef]
- Kamari, A.; Ngah, W.S.W.; Liew, L.K. Chitosan and chemically modified chitosan beads for acid dyes sorption. J. Environ. Sci. 2009, 21, 296–302. [Google Scholar]
- Kristiansen, K.A.; Potthast, A.; Christensen, B.E. Periodate oxidation of polysaccharides for modification of chemical and physical properties. Carbohydr. Res. 2010, 345, 1264–1271. [Google Scholar] [CrossRef]
- Sing, K.S.W. Adsorption methods for the characterization of porous materials. Adv. Colloid Interface Sci. 1998, 76, 3–11. [Google Scholar] [CrossRef]
- Silva, G.S.; Oliveira, P.C.; Giordani, D.S.; De Castro, H.F. Chitosan/Siloxane Hybrid Polymer: Synthesis, Characterization and Performance as a Support for Immobilizing Enzyme. J. Braz. Chem. Soc. 2011, 22, 1407–1417. [Google Scholar] [CrossRef]
- Qu, B.J. Recent developments in photo-initiated crosslinking of polyethylene and its industrial applications. Chin. J. Polym. Sci. 2001, 19, 189–207. [Google Scholar]
- Qu, B.J.; Xu, Y.H.; Shi, W.F.; Rånby, B. Photoinitiated crosslinking of low-density polyethylene. 6. Spin-trapping ESR studies on radical intermediates. Macromolecules 1992, 25, 5215–5219. [Google Scholar] [CrossRef]
- Kempe, K.; Becer, C.R.; Schubert, U.S. Microwave-assisted polymerizations: Recent status and future perspectives. Macromolecules 2011, 44, 5825–5842. [Google Scholar] [CrossRef]
- Mol, J.C. Application of olefin metathesis in oleochemistry: An example of green chemistry. Green Chem. 2002, 4, 5–13. [Google Scholar] [CrossRef]
- Kuijpers, A.J.; Engbers, G.H.; Krijgsveld, J.; Zaat, S.A.; Dankert, J.; Feijen, J. Cross-linking and characterization of gelatin matrices for biomedical applications. J. Biomater. Sci. Polym. Ed. 2012, 11, 225–243. [Google Scholar] [CrossRef] [PubMed]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaczmarek, B.; Mazur, O. Collagen-Based Materials Modified by Phenolic Acids—A Review. Materials 2020, 13, 3641. https://doi.org/10.3390/ma13163641
Kaczmarek B, Mazur O. Collagen-Based Materials Modified by Phenolic Acids—A Review. Materials. 2020; 13(16):3641. https://doi.org/10.3390/ma13163641
Chicago/Turabian StyleKaczmarek, Beata, and Olha Mazur. 2020. "Collagen-Based Materials Modified by Phenolic Acids—A Review" Materials 13, no. 16: 3641. https://doi.org/10.3390/ma13163641
APA StyleKaczmarek, B., & Mazur, O. (2020). Collagen-Based Materials Modified by Phenolic Acids—A Review. Materials, 13(16), 3641. https://doi.org/10.3390/ma13163641





