Biomaterials with Potential Use in Bone Tissue Regeneration—Collagen/Chitosan/Silk Fibroin Scaffolds Cross-Linked by EDC/NHS
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Silk Fibroin
2.3. Preparation of Collagen
2.4. Preparation of Three-Component Scaffolds
2.5. Cross-Linking of Scaffolds
2.6. Chemical Structure Studies—Fourier Transform Infrared Spectroscopy (FTIR)
2.7. Swelling Properties, Liquid Uptake and Moisture Content
2.8. Microstructure Studies—Scanning Electron Microscopy, Porosity, Density
2.9. Mechanical Properties Studies
2.10. Biological Studies—In Vitro Cytocompatibility Assay—Metabolic Activity
2.11. Statistical Analysis
3. Results and Discussion
3.1. Chemical Structure Studies—Fourier Transform Infrared Spectroscopy (FTIR)
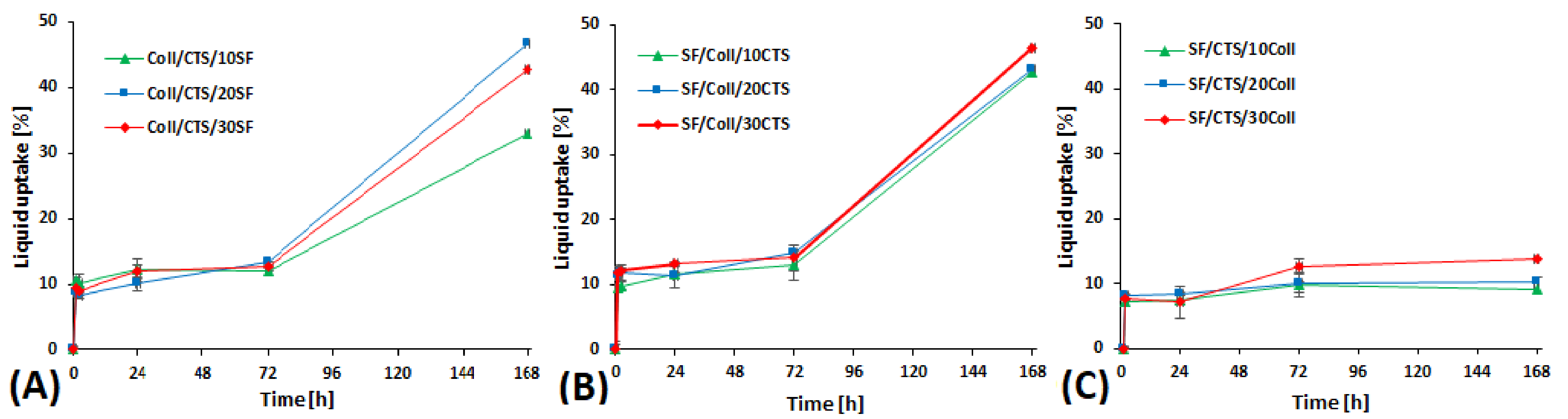
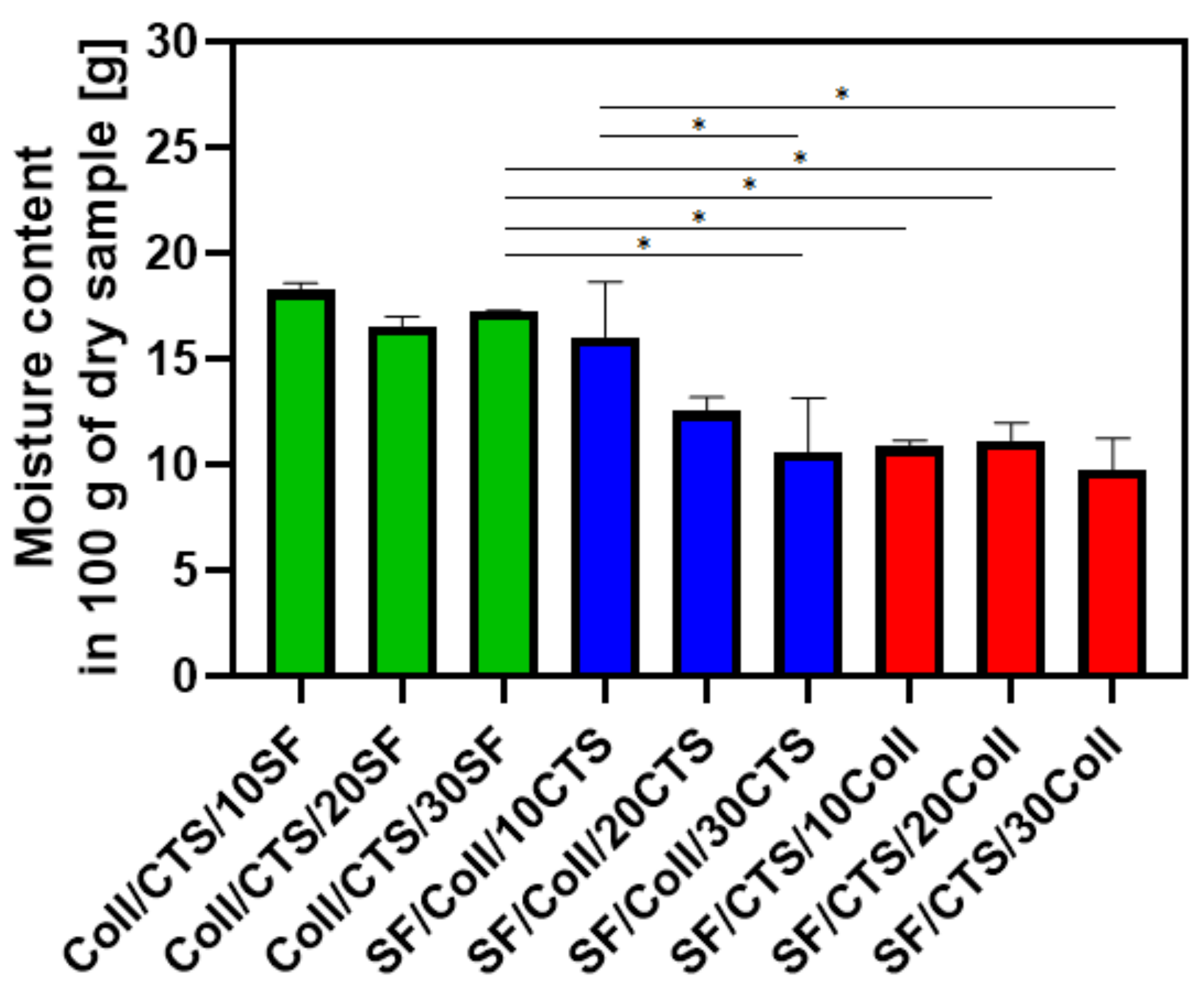
3.2. Swelling Properties, Liquid Uptake and Moisture Content
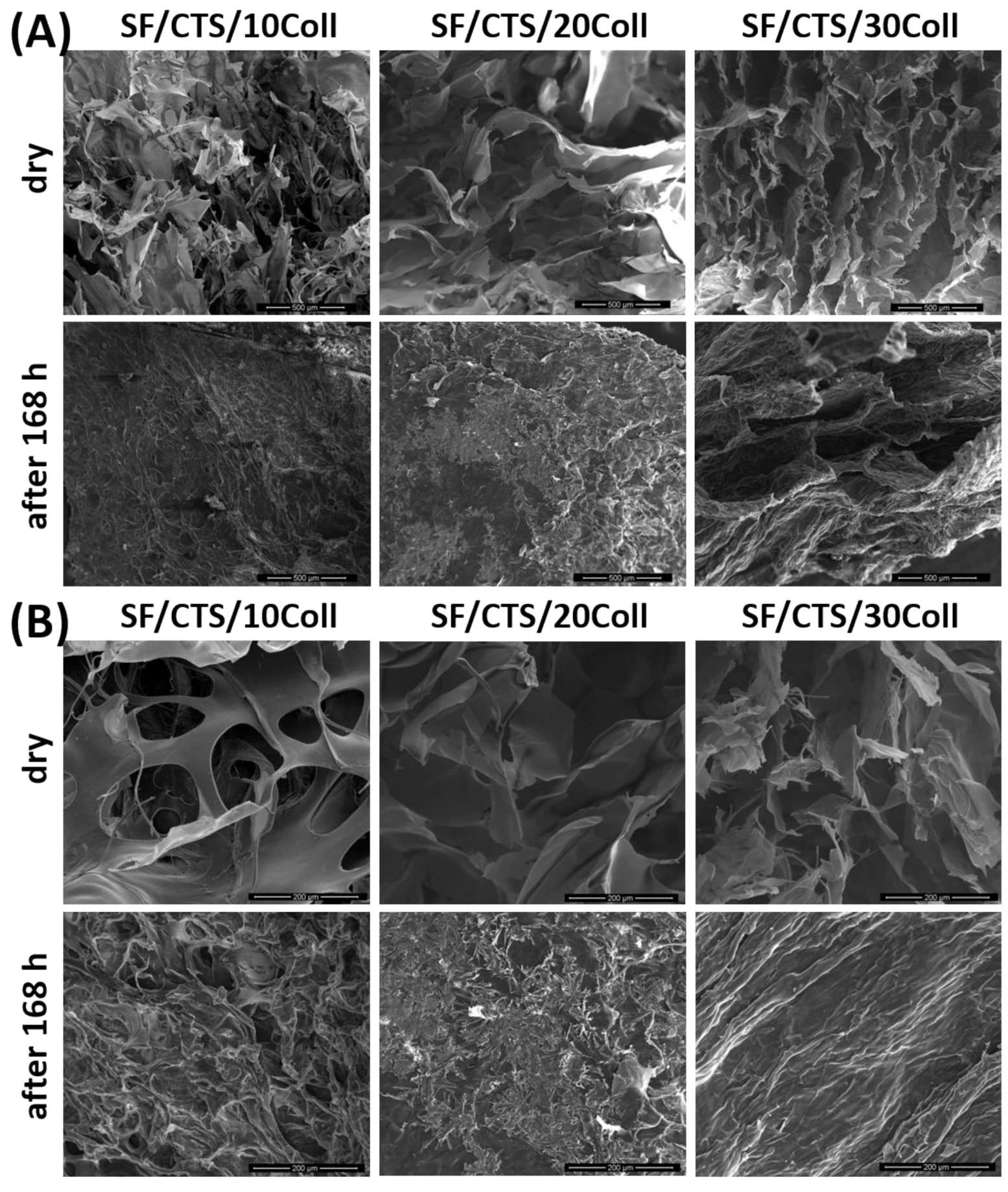
3.3. Microstructure Studies—Scanning Electron Microscopy, Porosity, Density
3.4. Mechanical Properties Studies
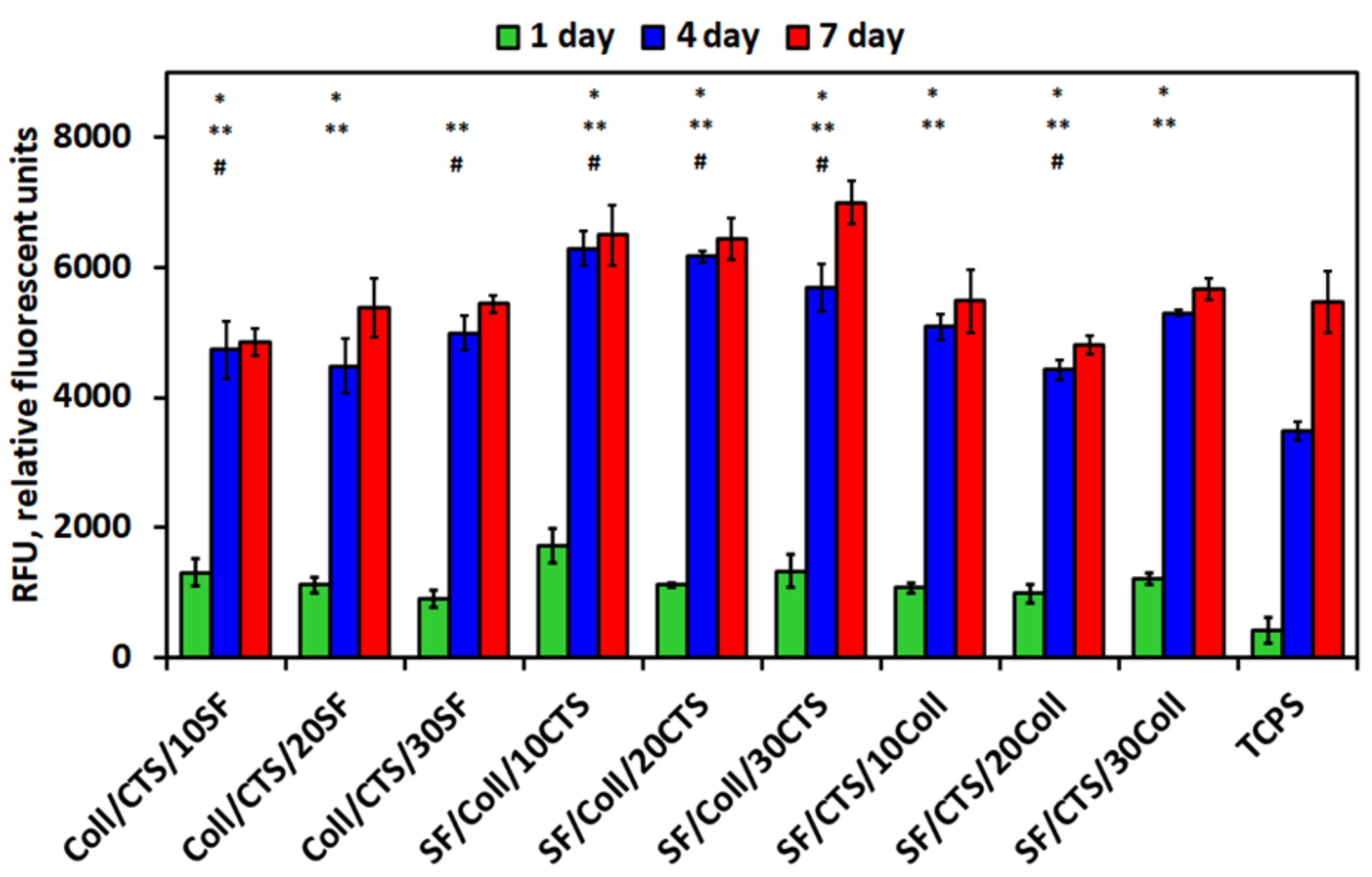
3.5. Biological Studies—In Vitro Cytocompatibility Assay—Metabolic Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baldwin, A.D.; Kiick, K.L. Polysaccharide-Modified Synthetic Polymeric Biomaterials. Biopolymers 2010, 94, 128–140. [Google Scholar] [CrossRef]
- Reddy, N.; Reddy, R.; Jiang, Q. Crosslinking Biopolymers for Biomedical Applications. Trends Biotechnol. 2015, 33, 362–369. [Google Scholar] [CrossRef] [PubMed]
- Sionkowska, A. Current Research on the Blends of Natural and Synthetic Polymers as New Biomaterials: Review. Prog. Polym. Sci. 2011, 36, 1254–1276. [Google Scholar] [CrossRef]
- Cicciù, M.; Fiorillo, L.; Cervino, G.; Habal, M.B. BMP Application as Grafting Materials for Bone Regeneration in the Craniofacial Surgery: Current Application and Future Directions by an RCT Analysis. J. Craniofac. Surg. 2020. [Google Scholar] [CrossRef] [PubMed]
- Cicciù, M.; Cervino, G.; Herford, A.S.; Famà, F.; Bramanti, E.; Fiorillo, L.; Lauritano, F.; Sambataro, S.; Troiano, G.; Laino, L. Facial Bone Reconstruction Using Both Marine or Non-Marine Bone Substitutes: Evaluation of Current Outcomes in a Systematic Literature Review. Mar. Drugs 2018, 16. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarek, B.; Mazur, O. Collagen-Based Materials Modified by Phenolic Acids—A Review. Materials 2020, 13. [Google Scholar] [CrossRef] [PubMed]
- Hoyer, B.; Bernhardt, A.; Lode, A.; Heinemann, S.; Sewing, J.; Klinger, M.; Notbohm, H.; Gelinsky, M. Jellyfish Collagen Scaffolds for Cartilage Tissue Engineering. Acta Biomater. 2014, 10, 883–892. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.M.; Gentile, P.; Chiono, V.; Ciardelli, G. Collagen for Bone Tissue Regeneration. Acta Biomater. 2012, 8, 3191–3200. [Google Scholar] [CrossRef] [PubMed]
- Stoop, R. Smart Biomaterials for Tissue Engineering of Cartilage. Injury 2008, 39, S77–S87. [Google Scholar] [CrossRef] [PubMed]
- Levengood, S.K.L.; Zhang, M. Chitosan-Based Scaffolds for Bone Tissue Engineering. J. Mater. Chem. B 2014, 2, 3161–3184. [Google Scholar] [CrossRef] [PubMed]
- Melke, J.; Midha, S.; Ghosh, S.; Ito, K.; Hofmann, S. Silk fibroin as biomaterial for bone tissue engineering. Acta Biomater. 2016, 31, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Yunus Basha, R.; Sampath Kumar, T.S.; Doble, M. Design of Biocomposite Materials for Bone Tissue Regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 57, 452–463. [Google Scholar] [CrossRef]
- Li, X.; Ma, X.; Fan, D.; Zhu, C. New Suitable for Tissue Reconstruction Injectable Chitosan/Collagen-Based Hydrogels. Soft Matter 2012, 8, 3781–3790. [Google Scholar] [CrossRef]
- Deng, Y.; Ren, J.; Chen, G.; Li, G.; Wu, X.; Wang, G.; Gu, G.; Li, J. Injectable in Situ Cross-Linking Chitosan-Hyaluronic Acid Based Hydrogels for Abdominal Tissue Regeneration. Sci. Rep. 2017, 7, 2699. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Ahn, S.-H.; Kim, G.H. Three-Dimensional Collagen/Alginate Hybrid Scaffolds Functionalized with a Drug Delivery System (DDS) for Bone Tissue Regeneration. Chem. Mater. 2012, 24, 881–891. [Google Scholar] [CrossRef]
- Gilarska, A.; Lewandowska-Łańcucka, J.; Horak, W.; Nowakowska, M. Collagen/Chitosan/Hyaluronic Acid—Based Injectable Hydrogels for Tissue Engineering Applications—Design, Physicochemical and Biological Characterization. Colloids Surf. B Biointerfaces 2018, 170, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, J.; Jayakumar, R.; Anil, S.; Chalisserry, E.P.; Pallela, R.; Kim, S.-K. Development of Alginate-Chitosan-Collagen Based Hydrogels for Tissue Engineering. J. Biomater. Tissue Eng. 2015, 5, 458–464. [Google Scholar] [CrossRef]
- Li, J.; Yang, B.; Qian, Y.; Wang, Q.; Han, R.; Hao, T.; Shu, Y.; Zhang, Y.; Yao, F.; Wang, C. Iota-Carrageenan/Chitosan/Gelatin Scaffold for the Osteogenic Differentiation of Adipose-Derived MSCs in Vitro. J. Biomed. Mater. Res. B Appl. Biomater. 2015, 103, 1498–1510. [Google Scholar] [CrossRef] [PubMed]
- Thakur, G.; Rodrigues, F.C.; Singh, K. Crosslinking Biopolymers for Advanced Drug Delivery and Tissue Engineering Applications. Adv. Exp. Med. Biol. 2018, 1078, 213–231. [Google Scholar] [CrossRef]
- Chronopoulou, L.; Toumia, Y.; Cerroni, B.; Pandolfi, D.; Paradossi, G.; Palocci, C. Biofabrication of Genipin-Crosslinked Peptide Hydrogels and Their Use in the Controlled Delivery of Naproxen. New Biotechnol. 2017, 37, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Jin, R.; Moreira Teixeira, L.S.; Dijkstra, P.J.; Zhong, Z.; van Blitterswijk, C.A.; Karperien, M.; Feijen, J. Enzymatically Crosslinked Dextran-Tyramine Hydrogels as Injectable Scaffolds for Cartilage Tissue Engineering. Tissue Eng. Part A 2010, 16, 2429–2440. [Google Scholar] [CrossRef]
- Korytkowska-Wałach, A.; Śmiga-Matuszowicz, M.; Łukaszczyk, J. Polymeric in situ forming systems for biomedical applications. Part II. Injectable hydrogel systems. Polimery 2015, 60. [Google Scholar] [CrossRef]
- Ostrowska-Czubenko, J.; Pieróg, M.; Gierszewska, M. Modification of chitosan—A concise overview. Wiad. Chem. 2016, 70, 9–10. [Google Scholar]
- Dmour, I.; Taha, M. Natural and semisynthetic polymers in pharmaceutical nanotechnology. In Organic Materials as Smart Nanocarriers for Drug Delivery; Elsevier Inc.: Cambridge, MA, USA, 2018; ISBN 978-0-12-813663-8. [Google Scholar]
- Chiono, V.; Pulieri, E.; Vozzi, G.; Ciardelli, G.; Ahluwalia, A.; Giusti, P. Genipin-Crosslinked Chitosan/Gelatin Blends for Biomedical Applications. J. Mater. Sci. Mater. Med. 2008, 19, 889–898. [Google Scholar] [CrossRef]
- Kaczmarek, B.; Sionkowska, A.; Monteiro, F.J.; Carvalho, A.; Lukowicz, K.; Osyczka, A.M. Characterization of Gelatin and Chitosan Scaffolds Cross-Linked by Addition of Dialdehyde Starch. Biomed. Mater. 2017, 13, 015016. [Google Scholar] [CrossRef] [PubMed]
- Salgado, C.L.; Grenho, L.; Fernandes, M.H.; Colaço, B.J.; Monteiro, F.J. Biodegradation, Biocompatibility, and Osteoconduction Evaluation of Collagen-Nanohydroxyapatite Cryogels for Bone Tissue Regeneration. J. Biomed. Mater. Res. A 2016, 104, 57–70. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Tuffin, J.; Lei, I.M.; Ruggeri, F.S.; Lewis, N.S.; Gill, E.L.; Savin, T.; Huleihel, L.; Badylak, S.F.; Knowles, T.; et al. Solution Fibre Spinning Technique for the Fabrication of Tuneable Decellularised Matrix-Laden Fibres and Fibrous Micromembranes. Acta Biomater. 2018, 78, 111–122. [Google Scholar] [CrossRef]
- Ma, G.; Lin, W.; Yuan, Z.; Wu, J.; Qian, H.; Xu, L.; Chen, S. Development of Ionic Strength/PH/Enzyme Triple-Responsive Zwitterionic Hydrogel of the Mixed l-Glutamic Acid and l-Lysine Polypeptide for Site-Specific Drug Delivery. J. Mater. Chem. B 2017, 5, 935–943. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zhang, X.; Luo, Q.; Hou, C.; Xu, J.; Liu, J. Engineering Nonmechanical Protein-Based Hydrogels with Highly Mechanical Properties: Comparison with Natural Muscles. Biomacromolecules 2020, 21, 4212–4219. [Google Scholar] [CrossRef] [PubMed]
- Beauchamp, R.O.; St Clair, M.B.; Fennell, T.R.; Clarke, D.O.; Morgan, K.T.; Kari, F.W. A Critical Review of the Toxicology of Glutaraldehyde. Crit. Rev. Toxicol. 1992, 22, 143–174. [Google Scholar] [CrossRef] [PubMed]
- Kashirina, A.; Yao, Y.; Liu, Y.; Leng, J. Biopolymers as Bone Substitutes: A Review. Biomater. Sci. 2019, 7, 3961–3983. [Google Scholar] [CrossRef] [PubMed]
- Sathiyavimal, S.; Vasantharaj, S.; LewisOscar, F.; Selvaraj, R.; Brindhadevi, K.; Pugazhendhi, A. Natural Organic and Inorganic–Hydroxyapatite Biopolymer Composite for Biomedical Applications. Prog. Org. Coat. 2020, 147, 105858. [Google Scholar] [CrossRef]
- Bose, S.; Koski, C.; Vu, A.A. Additive Manufacturing of Natural Biopolymers and Composites for Bone Tissue Engineering. Mater. Horiz. 2020, 7, 2011–2027. [Google Scholar] [CrossRef]
- Nosrati, H.; Pourmotabed, S.; Sharifi, E. A Review on Some Natural Biopolymers and Their Applications in Angiogenesis and Tissue Engineering. J. Appl. Biotechnol. Rep. 2018, 5, 81–91. [Google Scholar] [CrossRef][Green Version]
- Apinun, J.; Honsawek, S.; Kuptniratsaikul, S.; Jamkratoke, J.; Kanokpanont, S. Osteogenic Differentiation of Rat Bone Marrow-Derived Mesenchymal Stem Cells Encapsulated in Thai Silk Fibroin/Collagen Hydrogel: A Pilot Study in Vitro. Asian Biomed. 2018, 12, 273–279. [Google Scholar] [CrossRef]
- Zeng, S.; Liu, L.; Shi, Y.; Qiu, J.; Fang, W.; Rong, M.; Guo, Z.; Gao, W. Characterization of Silk Fibroin/Chitosan 3D Porous Scaffold and In Vitro Cytology. PLoS ONE 2015, 10, e0128658. [Google Scholar] [CrossRef]
- Sionkowska, A.; Płanecka, A. Preparation and Characterization of Silk Fibroin/Chitosan Composite Sponges for Tissue Engineering. J. Mol. Liq. 2013, 178, 5–14. [Google Scholar] [CrossRef]
- Qiang, L.; Chuan-Bao, C.; Ying, Z.; He-Sun, Z. Preparation and Properties of Insoluble Fibroin Films by Novel Method. Chem. J. Chin. Univ. 2004, 25, 1752–1755. [Google Scholar]
- Sionkowska, A.; Kozłowska, J. Properties and Modification of Porous 3-D Collagen/Hydroxyapatite Composites. Int. J. Biol. Macromol. 2013, 52, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarek, B.; Sionkowska, A.; Kozlowska, J.; Osyczka, A.M. New Composite Materials Prepared by Calcium Phosphate Precipitation in Chitosan/Collagen/Hyaluronic Acid Sponge Cross-Linked by EDC/NHS. Int. J. Biol. Macromol. 2018, 107, 247–253. [Google Scholar] [CrossRef]
- Sionkowska, A.; Grabska, S. Preparation and Characterization of 3D Collagen Materials with Magnetic Properties. Polym. Test. 2017, 62, 382–391. [Google Scholar] [CrossRef]
- Kaczmarek, B.; Sionkowska, A.; Osyczka, A.M. The Comparison of Physic-Chemical Properties of Chitosan/Collagen/Hyaluronic Acid Composites with Nano-Hydroxyapatite Cross-Linked by Dialdehyde Starch and Tannic Acid. Polym. Test. 2017, 62, 171–176. [Google Scholar] [CrossRef]
- Grabska-Zielińska, S.; Sionkowska, A.; Reczyńska, K.; Pamuła, E. Physico-Chemical Characterization and Biological Tests of Collagen/Silk Fibroin/Chitosan Scaffolds Cross-Linked by Dialdehyde Starch. Polymers 2020, 12. [Google Scholar] [CrossRef] [PubMed]
- Sionkowska, A.; Michalska, M.; Walczak, M.; Śmiechowski, K.; Grabska, S. Preparation and Characterization of Silk Fibroin/Collagen Sponge Modified by Chemical Cross-Linking. Mol. Cryst. Liq. Cryst. 2016, 640, 180–190. [Google Scholar] [CrossRef]
- Jawad, A.H.; Norrahma, S.S.A.; Hameed, B.H.; Ismail, K. Chitosan-Glyoxal Film as a Superior Adsorbent for Two Structurally Different Reactive and Acid Dyes: Adsorption and Mechanism Study. Int. J. Biol. Macromol. 2019, 135, 569–581. [Google Scholar] [CrossRef]
- Sionkowska, A.; Michalska-Sionkowska, M.; Walczak, M. Preparation and Characterization of Collagen/Hyaluronic Acid/Chitosan Film Crosslinked with Dialdehyde Starch. Int. J. Biol. Macromol. 2020, 149, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Panjapheree, K.; Kamonmattayakul, S.; Meesane, J. Biphasic Scaffolds of Silk Fibroin Film Affixed to Silk Fibroin/Chitosan Sponge Based on Surgical Design for Cartilage Defect in Osteoarthritis. Mater. Des. 2018, 141, 323–332. [Google Scholar] [CrossRef]
- Grabska, S.; Sionkowska, A.; Kaczmarek, B. The Physicochemical Properties of 3D Materials Based on Hyaluronic Acid Modified by Tannic Acid Addition. Mol. Cryst. Liq. Cryst. 2018, 670, 90–96. [Google Scholar] [CrossRef]
- Suwantong, O.; Pavasant, P.; Supaphol, P. Electrospun Zein Fibrous Membranes Using Glyoxal as Cross-Linking Agent: Preparation, Characterization and Potential for Use in Biomedical Applications. Chiang Mai J. Sci. 2010, 38, 56–70. [Google Scholar]
- Spanneberg, R.; Schymanski, D.; Stechmann, H.; Figura, L.; Glomb, M.A. Glyoxal Modification of Gelatin Leads to Change in Properties of Solutions and Resulting Films. Soft Matter 2012, 8, 2222–2229. [Google Scholar] [CrossRef]
- Grabska-Zielińska, S.; Sionkowska, A.; Coelho, C.C.; Monteiro, F.J. Silk Fibroin/Collagen/Chitosan Scaffolds Cross-Linked by a Glyoxal Solution as Biomaterials toward Bone Tissue Regeneration. Materials 2020, 13, 3433. [Google Scholar] [CrossRef]
- Lin, X.-L.; Gao, L.-L.; Li, R.; Cheng, W.; Zhang, C.-Q.; Zhang, X. Mechanical Property and Biocompatibility of Silk Fibroin–Collagen Type II Composite Membrane. Mater. Sci. Eng. C 2019, 105, 110018. [Google Scholar] [CrossRef] [PubMed]
- Polo-Corrales, L.; Latorre-Esteves, M.; Ramirez-Vick, J.E. Scaffold Design for Bone Regeneration. J. Nanosci. Nanotechnol. 2014, 14, 15–56. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarek, B.; Sionkowska, A.; Gołyńska, M.; Polkowska, I.; Szponder, T.; Nehrbass, D.; Osyczka, A.M. In Vivo Study on Scaffolds Based on Chitosan, Collagen, and Hyaluronic Acid with Hydroxyapatite. Int. J. Biol. Macromol. 2018, 118, 938–944. [Google Scholar] [CrossRef]
- Wang, J.; Sun, X.; Zhang, Z.; Wang, Y.; Huang, C.; Yang, C.; Liu, L.; Zhang, Q. Silk Fibroin/Collagen/Hyaluronic Acid Scaffold Incorporating Pilose Antler Polypeptides Microspheres for Cartilage Tissue Engineering. Mater. Sci. Eng. C-Mater. Biol. Appl. 2019, 94, 35–44. [Google Scholar] [CrossRef]
- Zhang, X.; Jia, C.; Qiao, X.; Liu, T.; Sun, K. Silk Fibroin Microfibers and Chitosan Modified Poly (Glycerol Sebacate) Composite Scaffolds for Skin Tissue Engineering. Polym. Test. 2017, 62, 88–95. [Google Scholar] [CrossRef]
- Asadpour, S.; Kargozar, S.; Moradi, L.; Ai, A.; Nosrati, H.; Ai, J. Natural Biomacromolecule Based Composite Scaffolds from Silk Fibroin, Gelatin and Chitosan toward Tissue Engineering Applications. Int. J. Biol. Macromol. 2020, 154, 1285–1294. [Google Scholar] [CrossRef] [PubMed]
- Sionkowska, A.; Kaczmarek, B.; Lewandowska, K.; Grabska, S.; Pokrywczyńska, M.; Kloskowski, T.; Drewa, T. 3D Composites Based on the Blends of Chitosan and Collagen with the Addition of Hyaluronic Acid. Int. J. Biol. Macromol. 2016, 89, 442–448. [Google Scholar] [CrossRef]
- Karageorgiou, V.; Kaplan, D. Porosity of 3D Biomaterial Scaffolds and Osteogenesis. Biomaterials 2005, 26, 5474–5491. [Google Scholar] [CrossRef] [PubMed]
- Sionkowska, A.; Kaczmarek, B. Preparation and Characterization of Composites Based on the Blends of Collagen, Chitosan and Hyaluronic Acid with Nano-Hydroxyapatite. Int. J. Biol. Macromol. 2017, 102, 658–666. [Google Scholar] [CrossRef]
- Hulbert, S.F.; Young, F.A.; Mathews, R.S.; Klawitter, J.J.; Talbert, C.D.; Stelling, F.H. Potential of Ceramic Materials as Permanently Implantable Skeletal Prostheses. J. Biomed. Mater. Res. 1970, 4, 433–456. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarek, B.; Sionkowska, A.; Osyczka, A.M. The Application of Chitosan/Collagen/Hyaluronic Acid Sponge Cross-Linked by Dialdehyde Starch Addition as a Matrix for Calcium Phosphate in Situ Precipitation. Int. J. Biol. Macromol. 2018, 107, 470–477. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarek, B.; Nadolna, K.; Owczarek, A. The physical and chemical properties of hydrogels based on natural polymers. In Hydrogels Based on Natural Polymers; Chen, Y., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 151–172. ISBN 978-0-12-816421-1. [Google Scholar]
- Li, J.; Wang, Q.; Gu, Y.; Zhu, Y.; Chen, L.; Chen, Y. Production of Composite Scaffold Containing Silk Fibroin, Chitosan, and Gelatin for 3D Cell Culture and Bone Tissue Regeneration. Med. Sci. Monit. 2017, 23, 5311–5320. [Google Scholar] [CrossRef] [PubMed]
- Carey, S.P.; Kraning-Rush, C.M.; Williams, R.M.; Reinhart-King, C.A. Biophysical Control of Invasive Tumor Cell Behavior by Extracellular Matrix Microarchitecture. Biomaterials 2012, 33, 4157–4165. [Google Scholar] [CrossRef]
- Sionkowska, A.; Grabska, S.; Lewandowska, K.; Andrzejczyk, A. Polymer Films Based on Silk Fibroin and Collagen—The Physico-Chemical Properties. Mol. Cryst. Liq. Cryst. 2016, 640, 13–20. [Google Scholar] [CrossRef]
- Sun, K.; Li, H.; Li, R.; Nian, Z.; Li, D.; Xu, C. Silk Fibroin/Collagen and Silk Fibroin/Chitosan Blended Three-Dimensional Scaffolds for Tissue Engineering. Eur. J. Orthop. Surg. Traumatol. 2015, 25, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Cao, C.; Ma, X.; Li, Y. Optimization of Macroporous 3-D Silk Fibroin Scaffolds by Salt-Leaching Procedure in Organic Solvent-Free Conditions. J. Mater. Sci. Mater. Med. 2012, 23, 315–324. [Google Scholar] [CrossRef]
- Ribeiro, M.; de Moraes, M.A.; Beppu, M.M.; Garcia, M.P.; Fernandes, M.H.; Monteiro, F.J.; Ferraz, M.P. Development of Silk Fibroin/Nanohydroxyapatite Composite Hydrogels for Bone Tissue Engineering. Eur. Polym. J. 2015, 67, 66–77. [Google Scholar] [CrossRef]
- Coelho, C.C.; Grenho, L.; Gomes, P.S.; Quadros, P.A.; Fernandes, M.H. Nano-Hydroxyapatite in Oral Care Cosmetics: Characterization and Cytotoxicity Assessment. Sci. Rep. 2019, 9. [Google Scholar] [CrossRef] [PubMed]
- Parekh, N.; Hushye, C.; Warunkar, S.; Gupta, S.S.; Nisal, A. In Vitro Study of Novel Microparticle Based Silk Fibroin Scaffold with Osteoblast-like Cells for Load-Bearing Osteo-Regenerative Applications. RSC Adv. 2017, 7, 26551–26558. [Google Scholar] [CrossRef]
- Barros, J.A.R.; de Melo, L.D.R.; da Silva, R.A.R.; Ferraz, M.P.; de Rodrigues Azeredo, J.C.V.; de Carvalho Pinheiro, V.M.; Colaço, B.J.A.; Fernandes, M.H.R.; de Sousa Gomes, P.; Monteiro, F.J. Encapsulated Bacteriophages in Alginate-Nanohydroxyapatite Hydrogel as a Novel Delivery System to Prevent Orthopedic Implant-Associated Infections. Nanomed. Nanotechnol. Biol. Med. 2020, 24, 102145. [Google Scholar] [CrossRef] [PubMed]









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grabska-Zielińska, S.; Sionkowska, A.; Carvalho, Â.; Monteiro, F.J. Biomaterials with Potential Use in Bone Tissue Regeneration—Collagen/Chitosan/Silk Fibroin Scaffolds Cross-Linked by EDC/NHS. Materials 2021, 14, 1105. https://doi.org/10.3390/ma14051105
Grabska-Zielińska S, Sionkowska A, Carvalho Â, Monteiro FJ. Biomaterials with Potential Use in Bone Tissue Regeneration—Collagen/Chitosan/Silk Fibroin Scaffolds Cross-Linked by EDC/NHS. Materials. 2021; 14(5):1105. https://doi.org/10.3390/ma14051105
Chicago/Turabian StyleGrabska-Zielińska, Sylwia, Alina Sionkowska, Ângela Carvalho, and Fernando J. Monteiro. 2021. "Biomaterials with Potential Use in Bone Tissue Regeneration—Collagen/Chitosan/Silk Fibroin Scaffolds Cross-Linked by EDC/NHS" Materials 14, no. 5: 1105. https://doi.org/10.3390/ma14051105
APA StyleGrabska-Zielińska, S., Sionkowska, A., Carvalho, Â., & Monteiro, F. J. (2021). Biomaterials with Potential Use in Bone Tissue Regeneration—Collagen/Chitosan/Silk Fibroin Scaffolds Cross-Linked by EDC/NHS. Materials, 14(5), 1105. https://doi.org/10.3390/ma14051105








