Nanostructured Polystyrene Doped with Acetylsalicylic Acid and Its Antibacterial Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Surface Exposure of Substrates
2.3. Substrate Characterization
2.4. Antibacterial Activity
3. Results
3.1. Surface Morphology, Roughness and Surface Area
3.2. Surface Wettability
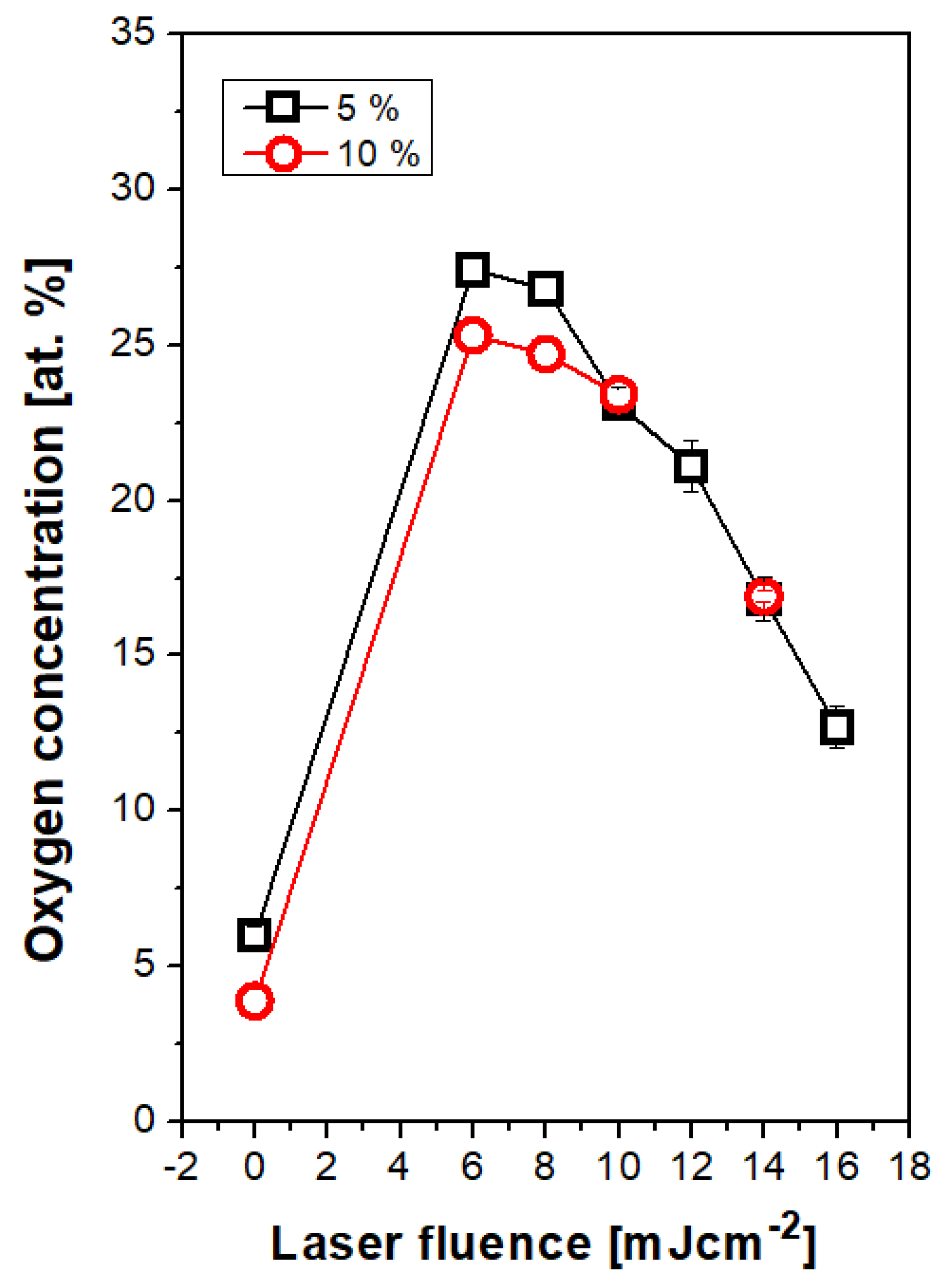
3.3. Surface Chemistry
3.4. Antibacterial Properties
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Fuzlin, A.F.A.; Misnon, I.I.; Samsudin, A.S. Conduction Properties Study on Alginate Incorporated with Glycolic Acid Based Solid Biopolymer Electrolytes. In Materials Science Forum; Trans Tech Publications Ltd.: Stafa-Zurich, Switzerland, 2020; Volume 981, pp. 34–39. [Google Scholar]
- Bonse, J.; Kirner, S.V.; Höhm, S.; Epperlein, N.; Spaltmann, D.; Rosenfeld, A.; Krüger, J. Applications of laser-induced periodic surface structures (LIPSS). In Laser-Based Micro-and Nanoprocessing XI; International Society for Optics and Photonics: San Diego, CA, USA, 2017; Volume 10092, p. 100920N. [Google Scholar]
- Bolle, M.; Lazare, S.; Le Blanc, M.; Wilmes, A. Submicron periodic structures produced on polymer surfaces with polarized excimer laser ultraviolet radiation. Appl. Phys. Lett. 1992, 60, 674–676. [Google Scholar] [CrossRef]
- Loeschner, K.; Kiesow, A.; Heilmann, A. Periodic Structure Formation in Polymer Films with Embedded Gold Nanoparticles. In Advances in Solid State Physics; Springer: Berlin/Heidelberg, Germany, 2008; pp. 73–86. [Google Scholar]
- Hendrikson, W.; Masman-Bakker, W.; van Bochove, B.; Skolski, J.; Eichstädt, J.; Koopman, B.; Rouwkema, J. Mold-Based Application of Laser-Induced Periodic Surface Structures (LIPSS) on Biomaterials for Nanoscale Patterning. Macromol. Biosci. 2016, 16, 43–49. [Google Scholar] [CrossRef]
- Rebollar, E.; Vázquez de Aldana, J.R.; Pérez-Hernández, J.A.; Ezquerra, T.A.; Moreno, P.; Castillejo, M. Ultraviolet and infrared femtosecond laser induced periodic surface structures on thin polymer films. Appl. Phys. Lett. 2012, 100, 041106. [Google Scholar] [CrossRef]
- Bonse, J.; Krüger, J.; Höhm, S.; Rosenfeld, A. Femtosecond laser-induced periodic surface structures. J. Laser Appl. 2012, 24, 042006. [Google Scholar]
- Gurevich, E.L. Mechanisms of femtosecond LIPSS formation induced by periodic surface temperature modulation. Appl. Surf. Sci. 2016, 374, 56–60. [Google Scholar] [CrossRef]
- Rebollar, E.; Ezquerra, T.A.; Nogales, A. Laser-Induced Periodic Surface Structures (LIPSS) on Polymer Surfaces. In Wrinkled Polymer Surfaces; Springer: Berlin/Heidelberg, Germany, 2019; pp. 143–155. [Google Scholar]
- Orazi, L.; Sorgato, M.; Piccolo, L.; Masato, D.; Lucchetta, G. Generation and characterization of Laser Induced Periodic Surface Structures on plastic injection molds. Lasers Manuf. Mater. Process. 2020, 7, 207–221. [Google Scholar] [CrossRef]
- Van Krevelen, D.W. Properties of Polymers; Elsevier Science B.V.: Amsterdam, The Netherlands, 1990. [Google Scholar]
- Fajstavr, D.; Michaljaničová, I.; Slepička, P.; Neděla, O.; Sajdl, P.; Kolská, Z.; Švorčík, V. Surface instability on polyethersulfone induced by dual laser treatment for husk nanostructure construction. React. Funct. Polym. 2018, 125, 20–28. [Google Scholar] [CrossRef]
- Lemoine, P.; Blau, W.; Drury, A.; Keely, C. Molecular weight effects on the 248-nm photoablation of polystyrene spun films. Polymer 1993, 34, 5020–5028. [Google Scholar] [CrossRef]
- Urech, L.; Lippert, T. Photoablation of polymer materials. Photochem. Photophys. Polym. Mater. 2010, 541–568. [Google Scholar] [CrossRef]
- Mito, T.; Masuhara, H. Laser-induced nanometer expansion and contraction dynamics of polystyrene films depending on its molecular weight. Appl. Surf. Sci. 2002, 197, 796–799. [Google Scholar] [CrossRef]
- Rebollar, E.; Oujja, M.; Castillejo, M.; Georgiou, S. Examination of photoproducts in the ablation plume of doped PMMA. Appl. Phys. A 2004, 79, 1357–1360. [Google Scholar] [CrossRef]
- Rebollar, E.; Bounos, G.; Oujja, M.; Georgiou, S.; Castillejo, M. Effect of molecular weight on the morphological modifications induced by UV laser ablation of doped polymers. J. Phys. Chem. B 2006, 110, 16452–16458. [Google Scholar] [CrossRef] [PubMed]
- Rebollar, E.; Bounos, G.; Oujja, M.; Domingo, C.; Georgiou, S.; Castillejo, M. Influence of polymer molecular weight on the chemical modifications induced by UV laser ablation. J. Phys. Chem. B 2006, 110, 14215–14220. [Google Scholar] [CrossRef] [PubMed]
- Nayak, N.C.; Lam, Y.C.; Yue, C.Y.; Sinha, A.T. CO2-laser micromachining of PMMA: The effect of polymer molecular weight. J. Micromechanics Microengineering 2008, 18, 095020. [Google Scholar] [CrossRef]
- Fajstavr, D.; Neznalová, K.; Švorčík, V.; Slepička, P. LIPSS Structures Induced on Graphene-Polystyrene Composite. Materials 2019, 12, 3460. [Google Scholar] [CrossRef]
- Fajstavr, D.; Slepička, P.; Švorčík, V. LIPSS with gold nanoclusters prepared by combination of heat treatment and KrF exposure. Appl. Surf. Sci. 2019, 465, 919–928. [Google Scholar] [CrossRef]
- Yoshida, S.; Hagiwara, K.; Hasebe, T.; Hotta, A. Surface modification of polymers by plasma treatments for the enhancement of biocompatibility and controlled drug release. Surf. Coat. Technol. 2013, 233, 99–107. [Google Scholar] [CrossRef]
- Siegel, J.; Šuláková, P.; Kaimlová, M.; Švorčík, V.; Hubáček, T. Underwater Laser Treatment of PET: Effect of Processing Parameters on Surface Morphology and Chemistry. Appl. Sci. 2018, 8, 2389. [Google Scholar] [CrossRef]
- Heitz, J.; Gumpenberger, T.; Kahr, H.; Romanin, C. Adhesion and proliferation of human vascular cells on UV-light-modified polymers. Biotechnol. Appl. Biochem. 2004, 39, 59–69. [Google Scholar] [CrossRef]
- Azuma, H.; Takeuchi, A.; Kamiya, N.; Ito, T.; Kato, M.; Shirai, S.; Narita, T.; Fukumori, K.; Tachi, K.; Matsuoka, T. New surface treatment of polymers by simultaneous exposure to vacuum ultra-violet light and nanometer-sized particles. Jpn. J. Appl. Phys. 2004, 43, L1250. [Google Scholar] [CrossRef]
- Wang, Z.; Li, H.; Chen, J.; Xue, Z.; Wu, B.; Lu, X. Acetylsalicylic acid electrochemical sensor based on PATP–AuNPs modified molecularly imprinted polymer film. Talanta 2011, 85, 1672–1679. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.M.; Lu, Q.H.; Zhu, Z.K.; Yin, J.; Wang, Z.G. The laser-induced periodic surface structure on polyimide doped with lecithin. Mater. Lett. 2003, 57, 3636–3640. [Google Scholar] [CrossRef]
- Böger, R.H.; Bode-Böger, S.M.; Gutzki, F.M.; Tsikas, D.; Weskott, H.P.; frölich, J.C. Rapid and selective inhibition of platelet aggregation and thromboxane formation by intravenous low dose aspirin in man. Clin. Sci. 1993, 84, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Attie, M.F.; Gill, J.R.; Stock, J.L.; Spiegel, A.M.; Downs, R.W.; Levine, M.A.; Marx, S.J. Urinary calcium excretion in familial hypocalciuric hypercalcemia. Persistence of relative hypocalciuria after induction of hypoparathyroidism. J. Clin. Investig. 1983, 72, 667–676. [Google Scholar] [CrossRef]
- Moore, T.J.; Joseph, M.J.; Allen, B.W.; Coury, L.A. Enzymatically amplified voltammetric sensor for microliter sample volumes of salicylate. Anal. Chem. 1995, 67, 1896–1902. [Google Scholar] [CrossRef]
- Wang, X.; Ohlin, C.H.A.; Lu, Q.; Hu, J. Cell directional migration and oriented division on three-dimensional laser-induced periodic surface structures on polystyrene. Biomaterials 2008, 29, 2049–2059. [Google Scholar] [CrossRef]
- Slepička, P.; Neznalová, K.; Fajstavr, D.; Slepičková, K.N.; Švorčík, V. Honeycomb-like pattern formation on perfluoroethylenepropylene enhanced by plasma treatment. Plasma Process. Polym. 2019, 16, 1900063. [Google Scholar] [CrossRef]
- Krajcar, R.; Siegel, J.; Slepička, P.; Fitl, P.; Švorčík, V. Silver nanowires prepared on PET structured by laser irradiation. Mater. Lett. 2014, 117, 184–187. [Google Scholar] [CrossRef]
- Slepička, P.; Neděla, O.; Sajdl, P.; Kolská, Z.; Švorčík, V. Polyethylene naphthalate as an excellent candidate for ripple nanopatterning. Appl. Surf. Sci. 2013, 285P, 885–892. [Google Scholar] [CrossRef]
- Cui, J.; Nogales, A.; Ezquerra, T.A.; Rebollar, E. Influence of substrate and film thickness on polymer LIPSS formation. Appl. Surf. Sci. 2017, 394, 125–131. [Google Scholar] [CrossRef]
- Chen, Y.; Ding, Y.; Zheng, J. A polymer nanocomposite coating with enhanced hydrophilicity, antibacterial and antibiofouling properties: Role of polymerizable emulsifier/anionic ligand. Chem. Eng. J. 2020, 379, 122268. [Google Scholar] [CrossRef]
- Slepicka, P.; Siegel, J.; Lyutakov, O.; Slepickova Kasalkova, N.; Kolska, Z.; Bacakova, L.; Svorcik, V. Polymer nanostructures for bioapplications induced by laser treatment. Biotechnol. Adv. 2018, 36, 839–855. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Liu, X.; Liu, N.; Guo, Z.; Singh, P.K.; Fu, S. Effect of surface wettability on the antibacterial activity of nanocellulose-based material with quaternary ammonium groups. Colloids Surf. A Physicochem. Eng. Asp. 2018, 554, 122–128. [Google Scholar] [CrossRef]
- Recek, N. Biocompatibility of Plasma-Treated Polymeric Implants. Materials 2019, 12, 240. [Google Scholar] [CrossRef] [PubMed]
- Kubiak, K.J.; Wilson, M.C.T.; Mathia, T.G.; Carval, P.H. Wettability versus roughness of engineering surfaces. Wear 2011, 271, 523–528. [Google Scholar] [CrossRef]
- Řezníčková, A.; Chaloupka, A.; Heitz, J.; Kolská, Z.; Švorčík, V. Surface properties of polymers treated with F2 laser. Surf. Interface Anal. 2011, 44, 296–300. [Google Scholar] [CrossRef]







© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fajstavr, D.; Neznalová, K.; Slepičková Kasálková, N.; Rimpelová, S.; Kubičíková, K.; Švorčík, V.; Slepička, P. Nanostructured Polystyrene Doped with Acetylsalicylic Acid and Its Antibacterial Properties. Materials 2020, 13, 3609. https://doi.org/10.3390/ma13163609
Fajstavr D, Neznalová K, Slepičková Kasálková N, Rimpelová S, Kubičíková K, Švorčík V, Slepička P. Nanostructured Polystyrene Doped with Acetylsalicylic Acid and Its Antibacterial Properties. Materials. 2020; 13(16):3609. https://doi.org/10.3390/ma13163609
Chicago/Turabian StyleFajstavr, Dominik, Klára Neznalová, Nikola Slepičková Kasálková, Silvie Rimpelová, Kateřina Kubičíková, Václav Švorčík, and Petr Slepička. 2020. "Nanostructured Polystyrene Doped with Acetylsalicylic Acid and Its Antibacterial Properties" Materials 13, no. 16: 3609. https://doi.org/10.3390/ma13163609
APA StyleFajstavr, D., Neznalová, K., Slepičková Kasálková, N., Rimpelová, S., Kubičíková, K., Švorčík, V., & Slepička, P. (2020). Nanostructured Polystyrene Doped with Acetylsalicylic Acid and Its Antibacterial Properties. Materials, 13(16), 3609. https://doi.org/10.3390/ma13163609





