Asbestos-Based Pottery from Corsica: The First Fiber-Reinforced Ceramic Matrix Composite
Abstract
1. Introduction
2. Materials and Methods
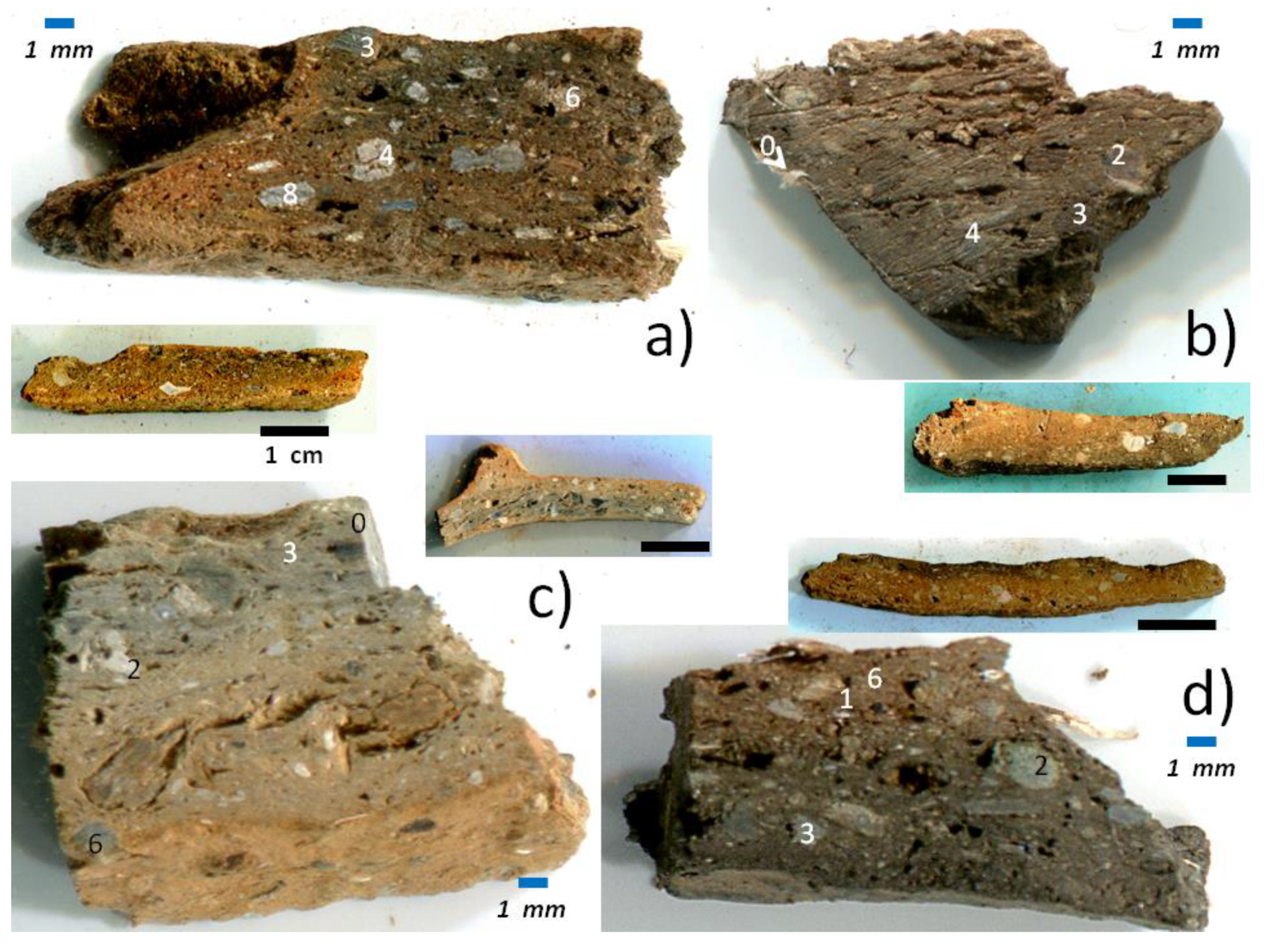
2.1. Pottery Shards
2.2. Methods
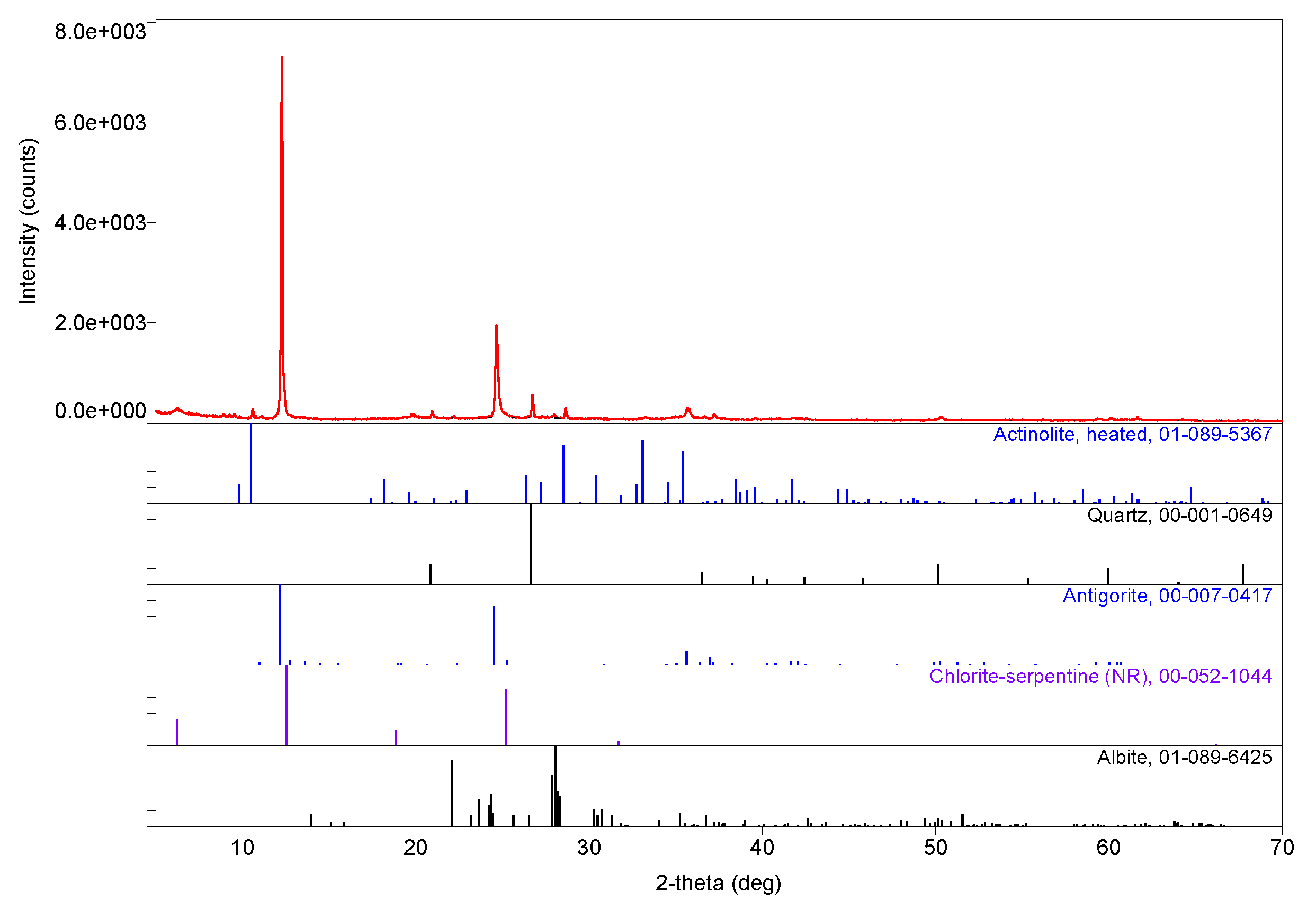
3. Results
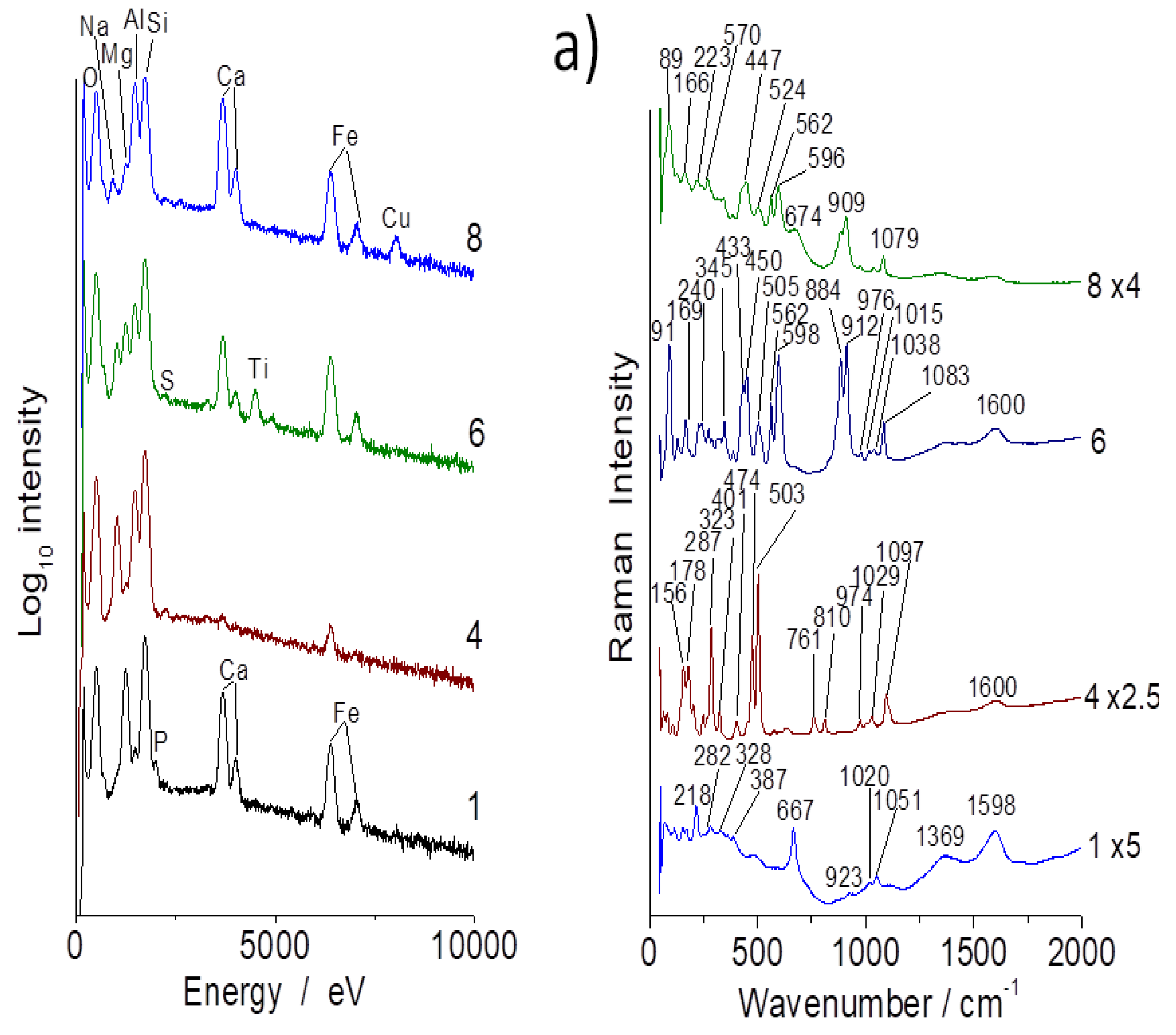
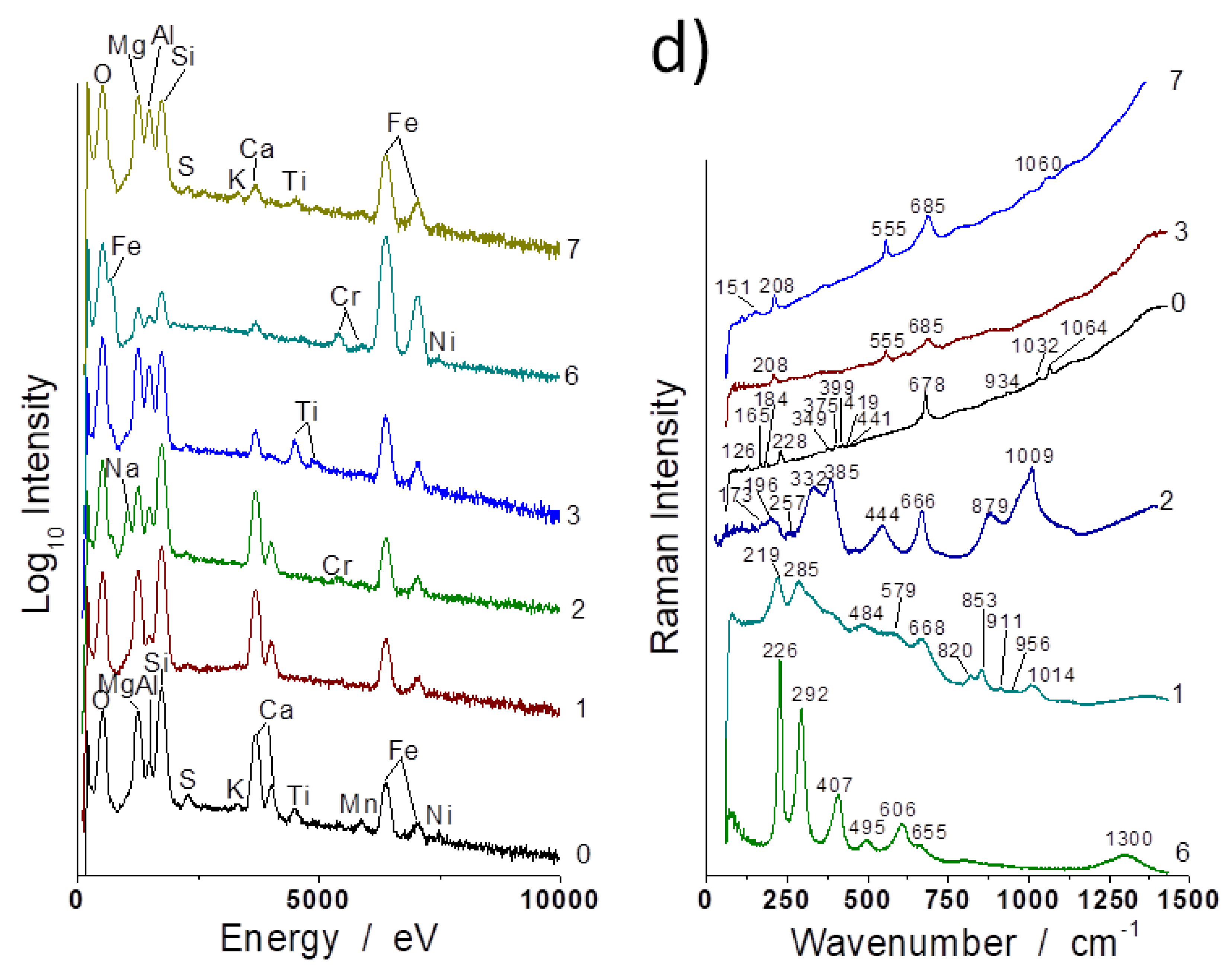
3.1. Compositions
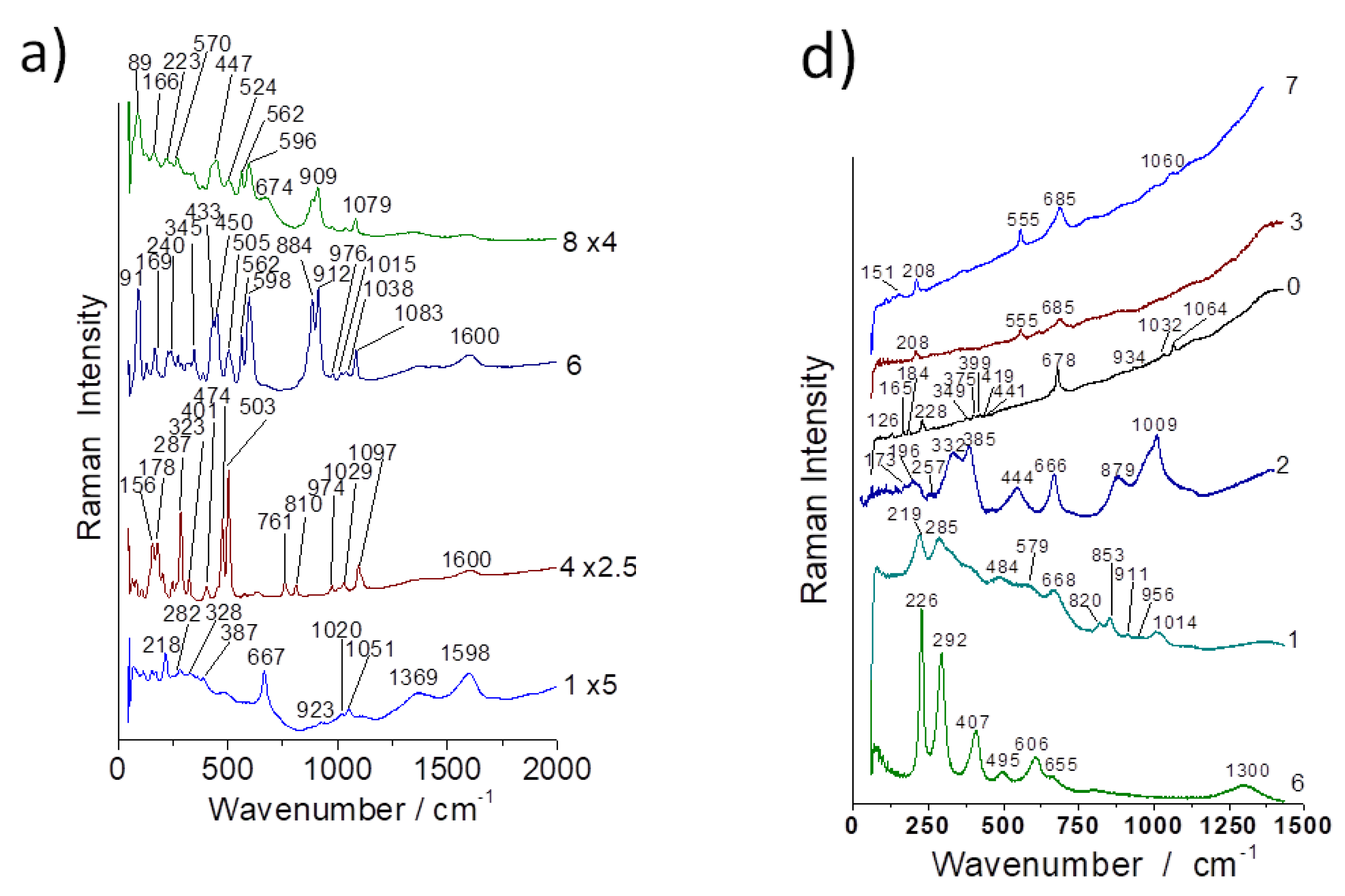
3.2. Evidence of Asbestos Fibers
3.3. Tentative Identification of Minerals
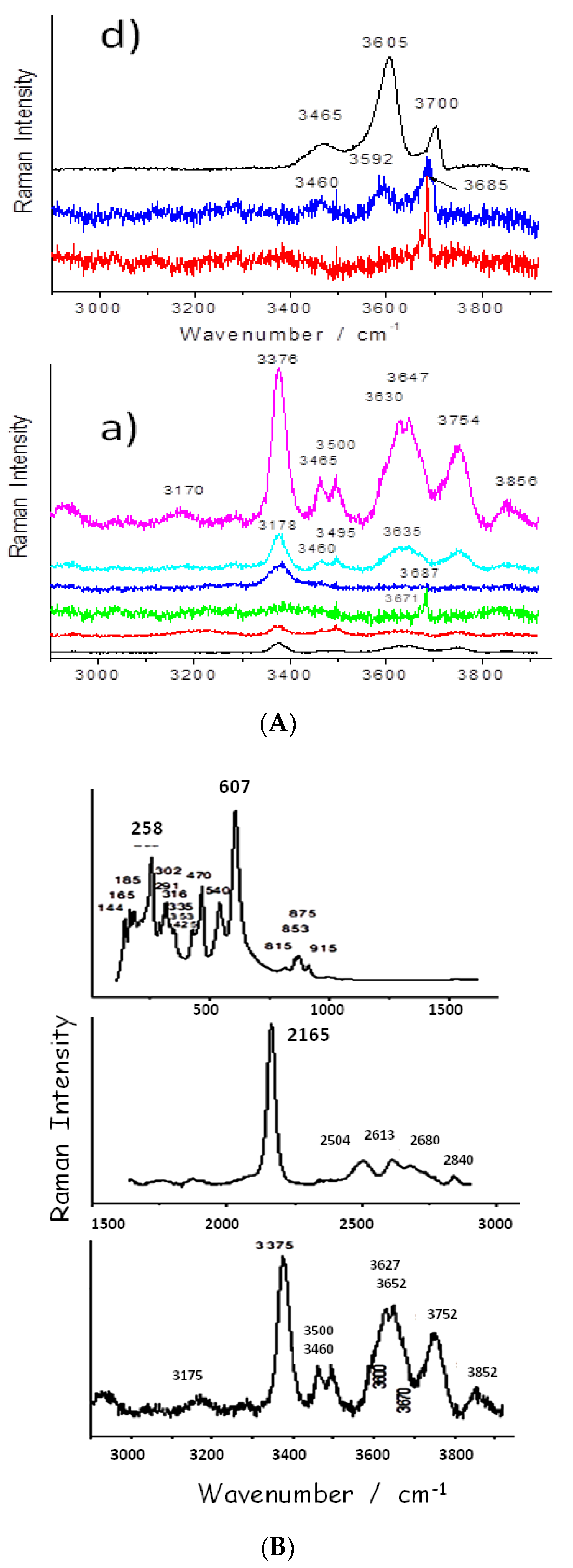
3.4. OH Groups, Water and Clays
3.5. Heating-Induced Effects and Remarks on Preparation Procedure
3.6. Evidence of Residues: Gold Ore Processing
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A

References
- Chawla, K.K. Composites Materials, Sciences and Engineering, 2nd ed.; Springer: New York, NY, USA, 1998. [Google Scholar]
- Evans, A.G.J. Perspectives on the development of high-toughness ceramics. J. Am. Ceram. Soc. 1990, 73, 187–206. [Google Scholar] [CrossRef]
- Mah, T.I.; Mendiratta, M.G.; Katz, A.P.; Mazdiyasni, K.S. Recent developments in fiber-reinforced high temperature ceramic composites. Am. Ceram. Soc. Bull. 1987, 66, 304–308. [Google Scholar]
- Mouchon, E.; Colomban, P. Oxide ceramic matrix/oxide fibre wowen fabric composites exhibiting dissipative fracture behaviour. Composites 1995, 26, 175–182. [Google Scholar] [CrossRef]
- Chiva, I.; Ojalvo, D. La poterie corse à l’amiante. Rev. Arts Tradit. Pop. 1959, 7, 203–227. [Google Scholar]
- Mezzadri, B. La Corse—Poterie traditionnelle à l’amiante. Rev. Céramique Verre 1986, 26, 6–9. [Google Scholar]
- Istria, D. L’utilisation de l’amiante en corse du XIVe au XIXe siècle. Médiévales 2007, 53, 39–50. [Google Scholar] [CrossRef][Green Version]
- Colomban, P.; Gouadec, G. The ideal Ceramic fiber/oxide matrix composite: How to conciliate antagonist physical and chemical requirements. Ann. Chim. Sci. Matériaux 2005, 30, 673–688. [Google Scholar] [CrossRef]
- Hulthén, B. On ceramic ware in northern scandinavia during the neolithic, bronze, and early iron Age: A ceramic-ecological study. In Archaeology and Environment Series (e-book), 8th ed.; University of Umea: Umea, Sweden, 1991. [Google Scholar]
- Kakoulli, I.; Prikhodko, S.V.; King, A.; Fischer, C. Earliest evidence for asbestos composites linked to Byzantine wall paintings production. J. Archaeolog. Sci. 2014, 44, 148–153. [Google Scholar] [CrossRef]
- Colomban, P.; Ambrosi, F.; Ngo, A.-T.; Lu, T.-A.; Feng, X.-L.; Chen, S.; Choi, C.-L. Comparative analysis of wucai Chinese porcelains using mobile and fixed Raman microspectrometers. Ceram. Int. 2017, 43, 14244–14256. [Google Scholar] [CrossRef]
- PDXL 2. Integrated X-Ray Powder Diffraction Software, Version 2.8.3.0; Rigaku Corporation: Tokyo, Japan, 2018. [Google Scholar]
- Levin, E.M.; Robin, C.R.; McMurdie, H.F. Phase Diagrams for Ceramists; The American Ceramic Society: Columbus, OH, USA, 1964. [Google Scholar]
- Andreozzi, G.B.; Ballirano, P.; Gianfagna, A.; Mazziotti-Tagliani, S.; Pacella, A. Structural and spectroscopic characterization of a suite of fibrous amphiboles with high environmental and health relevance from Biancavilla (Sicily, Italy). Am. Mineral. 2009, 94, 1333–1340. [Google Scholar] [CrossRef]
- Giacobbe, C.; Gualteri, A.F.; Quartieri, S.; Rinaudo, C.; Allegrina, M.; Andreozzi, G.B. Spectroscopic study of thermal transformation of chrysotile-asbestos containing materials (ACM). Eur. J. Mineral. 2010, 22, 535–546. [Google Scholar] [CrossRef]
- Ristic, M.; Czako-Nagy, I.; Music, S.; Vértes, A. Spectroscopic characterization of chrysotile asbestos from different regions. J. Mol. Struct. 2011, 993, 120–126. [Google Scholar] [CrossRef]
- Trittschack, R.; Groberty, B.M. Koch-Muller, In-situ high-temperature Raman and FTIR spectroscopy of the phase transformation of lizardite. Am. Mineral. 2012, 97, 1965–1976. [Google Scholar] [CrossRef]
- Trittschack, R.; Groberty, B. Dehydroxylation kinetics of lizardite. Eur. J. Mineral. 2012, 24, 47–57. [Google Scholar] [CrossRef]
- Trittschack, R.; Groberty, B. The dehydroxylation of crhrysotile: A combined in situ micro-Raman and micro-FTIR study. Am. Mineral. 2013, 98, 1133–1145. [Google Scholar] [CrossRef][Green Version]
- Ventura, G.; Vigliaturo, R.; Gieré, R.; Pollastri, S.; Gualtieri, A.; Iezzi, G. FTIR Spectroscopy of the regulated Asbestos Amphiboles. Minerals 2018, 8, 413. [Google Scholar] [CrossRef]
- Yang, H.-X.; Evans, B.W. X-ray structure refinements of tremolite at 140 and 295 K: Crystal chemistry and petrologic implications. Am. Mineral. 1996, 81, 1117–1125. [Google Scholar] [CrossRef]
- Gatta, D.G.; Merlini, M.; Valdrè, G.; Liermann, H.-P.; Nénert, G.; Rothkirch, A.; Kahlenberg, V.; Pavese, A. On the crystal structure and compressional behavior of talc: A mineral of interest in petrology and material science. Phys. Chem. Miner. 2013, 40, 145–156. [Google Scholar] [CrossRef]
- Hanawalt, J.D.; Rinn, H.W.; Frevel, L.K. Identification of Crystalline Materials. Ind. Eng. Anal. Chem. 1938, 10, 457–513. [Google Scholar] [CrossRef]
- Gatta, G.D.; Rinaldi, R.; Knight, K.S.; Molin, G.; Artioli, G. High temperature structural and thermoelastic behaviour of mantle orthopyroxene: An in situ neutron powder diffraction study. Phys. Chem. Miner. 2007, 34, 185–200. [Google Scholar] [CrossRef]
- National Bureau of Standards (USA). Standard x-ray diffraction powder patterns. Monographs 1967, 255, 17. [Google Scholar]
- Reynolds, R., Jr.; DiStefano, M.; Lahann, R. Randomly interstratified serpentine/chlorite: Its detection and quantification by powder X-ray diffraction methods. Clays Clay Miner. 1992, 40, 262–267. [Google Scholar] [CrossRef]
- Hess, H.; Smith, R.; Dengo, G. Antigorite from the vicinity of Caracas, Venezuela. Am. Mineral. J. Earth Planet. Mater. 1952, 37, 68–75. [Google Scholar]
- Meneghinello, E.; Alberti, A.; Cruciani, G. Order-disorder process in the tetrahedral sites of albite. Am. Mineral. 1999, 84, 1144–1151. [Google Scholar] [CrossRef]
- Evans, B.W.; Yang, H.-X. Fe-Mg order-disorder in tremolite-actinolite-ferro-actinolite at ambient and high temperature. Am. Mineral. 1998, 83, 458–475. [Google Scholar] [CrossRef]
- Dichicco, M.C.; de Bonis, A.; Mongelli, G.; Rizzo, G.; Sinisi, R. μ-Raman spectroscopy and X-ray diffraction of asbestos’ minerals for geo-environmental monitoring: The case of the southern Apennines natural sources. Appl. Clay Sci. 2017, 141, 292–299. [Google Scholar] [CrossRef]
- Jeong, H.; Moon, W.; Roh, Y. Characterization of mineralogical changes of chrysotile and its thermal decomposition by heat treatment. Econ. Environ. Geol. 2016, 49, 77–88. [Google Scholar] [CrossRef]
- Rooney, J.; Tarling, M.; Smith, S.; Gordon, K. Sub-micron raman spectroscopy mapping of serpentinite fault rocks. J. Raman Spectrosc. 2018, 49, 279–286. [Google Scholar] [CrossRef]
- Dodony, I.; Buseck, P.R. Serpentines close-up and intimate: An hrtem view. Int. Geol. Rev. 2004, 46, 507–527. [Google Scholar] [CrossRef]
- Viti, C.; Giacobbe, C.; Galtieri, A.F. Quantitative determination of chrysotile in massive serpentinites using DTA: Implications for asbestos determinations. Am. Mineral. 2011, 96, 1003–1011. [Google Scholar] [CrossRef]
- Groppo, C.; Rinaudo, C.; Cairo, S.; Gastaldi, D.; Compagnoni, R. Micro-Raman spectroscopy for a quick and reliable identification of serpentine minerals from ultramarics. Eur. J. Mineral. 2008, 18, 319–329. [Google Scholar] [CrossRef]
- Petriglieri, J.R.; Salvioli-Mariani, E.; Mantovani, L.; Tribaudino, M.; Lottici, P.P.; Laporte-Magoni, C.; Bersani, D. Micro-Raman mapping of the polymorphs of of serpentine. J. Raman Spectrosc. 2015, 46, 953–958. [Google Scholar] [CrossRef]
- Fornero, E.; Allegrina, M.; Rinaudo, C.; Mazziotti-Tagliani, S.; Gianfana, A. Micro-Raman spectroscopy applied on oriented crystals of fluoro-edenite amphibole. Per. Mineral. 2008, 77, 5–14. [Google Scholar]
- Galtieri, A.F.; Giacobbe, C.; Viti, C. The dehydroxylation of serpentine group minerals. Am. Mineral. 2012, 97, 666–680. [Google Scholar] [CrossRef]
- Kloprogge, J.T.; Wharton, D.; Hickey, L.; Frost, R.L. Infrared and Raman study of interlayer anions CO32–, NO3−, SO42− and ClO4− in Mg/Al-hydrotalcite. Am. Mineral. 2002, 87, 623–629. [Google Scholar] [CrossRef]
- Frost, R.L.; Palmer, S.J. Infrared and infrared emission spectroscopy of nesquehonite Mg(OH)(HCO3) 2H2O: Implications for the formula of nesquehonite. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2011, 78, 1255–1260. [Google Scholar] [CrossRef]
- Type of Asbestos. Available online: https://www.asbestos.com/asbestos/types/ (accessed on 4 July 2020).
- Froment, F.; Tournié, A.; Colomban, P. Raman identification of natural red to yellow pigments: Ochre and iron-containing ores. J. Raman Spectrosc. 2008, 39, 560–568. [Google Scholar] [CrossRef]
- Kouketsu, Y.; Hattori, K.; Guillot, S.; Rayner, N. Eocene to Oligocene retrogression and recrystallization of the Stak eclogite in northwest Himalaya. Lithos 2015, 240. [Google Scholar] [CrossRef]
- Afify, A.S.; Hassan, M.; Piumetti, M.; Peter, I.; Bonelli, B.; Tulliani, J.-M. Elaboration and characterization of modified sepiolites and their humidity sensing features for environmental monitoring. Appl. Clay Sci. 2015, 115, 165–173. [Google Scholar] [CrossRef]
- Asquier, M.; Colomban, P.; Milande, V. Raman and infrared analysis of glues used for pottery conservation treatments. J. Raman Spectrosc. 2009, 40, 1641–1644. [Google Scholar] [CrossRef]
- Steele, C.J. Gold Adsorption on Active Carbon. Ph.D. Thesis, University of Newcastle upon Tyne, London, UK, June 1997. Available online: https://www.researchgate.net/publication/36012399_Gold_adsorption_on_active_carbon/link/5b0e4fcd0f7e9b1ed701619c/download (accessed on 12 July 2020).
- Chadwick, B.M.; Frankiss, M.G. Vibrational spectra and structures of polycrystalline KAg(CN)2, NaAg(CN)2 and TlAg(CN)2. J. Mol. Struct. 1968, 2, 281–285. [Google Scholar] [CrossRef]
- Jia, Y.F.; Steele, C.J.; Hayward, I.P.; Thomas, M. Mechanisms of adsorption of gold and silver species on activated carbons. Carbon 1998, 36, 1299–1308. [Google Scholar] [CrossRef]
- Silver Cyanide. Available online: https://en.wikipedia.org/wiki/Silver_cyanide (accessed on 12 July 2020).
- Potassium Dicyanoaurate. Available online: https://en.wikipedia.org/wiki/Potassium_dicyanoaurate (accessed on 12 July 2020).
- Corsica Gold Extraction. Available online: https://books.google.fr/books?id=RQdHAQAAIAAJ&pg=PA211&lpg=PA211&dq=Corsica+gold+extraction&source=bl&ots=P-dxNx74tA&sig=ACfU3U1qpKUHSVka-7Q0MwdQcUc9F1xtAQ&hl=fr&sa=X&ved=2ahUKEwian5GYn8fqAhVOTBoKHaV1BYI4ChDoATAAegQIChAB#v=onepage&q=Corsica%20gold%20extraction&f=false (accessed on 12 July 2020).
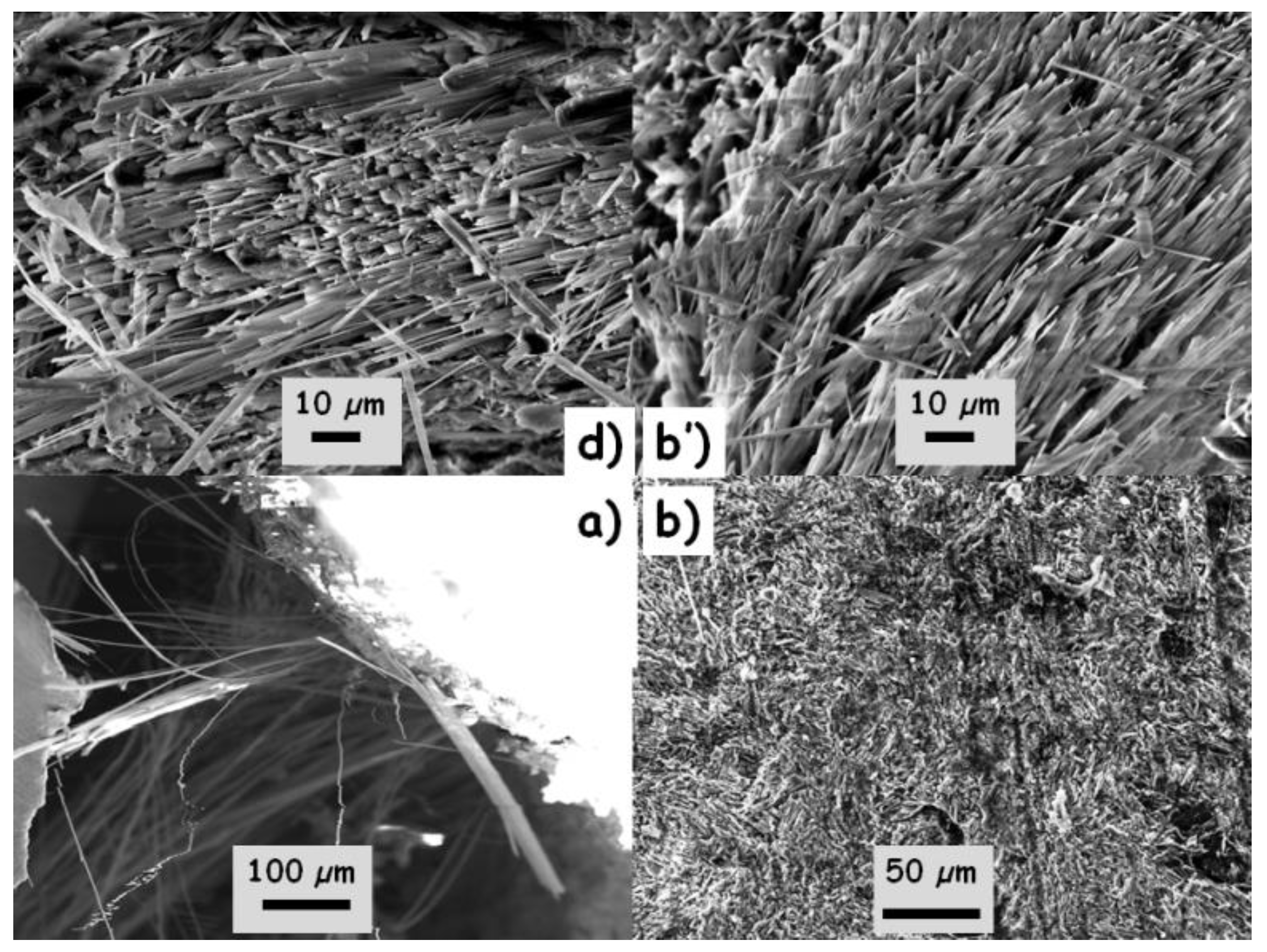


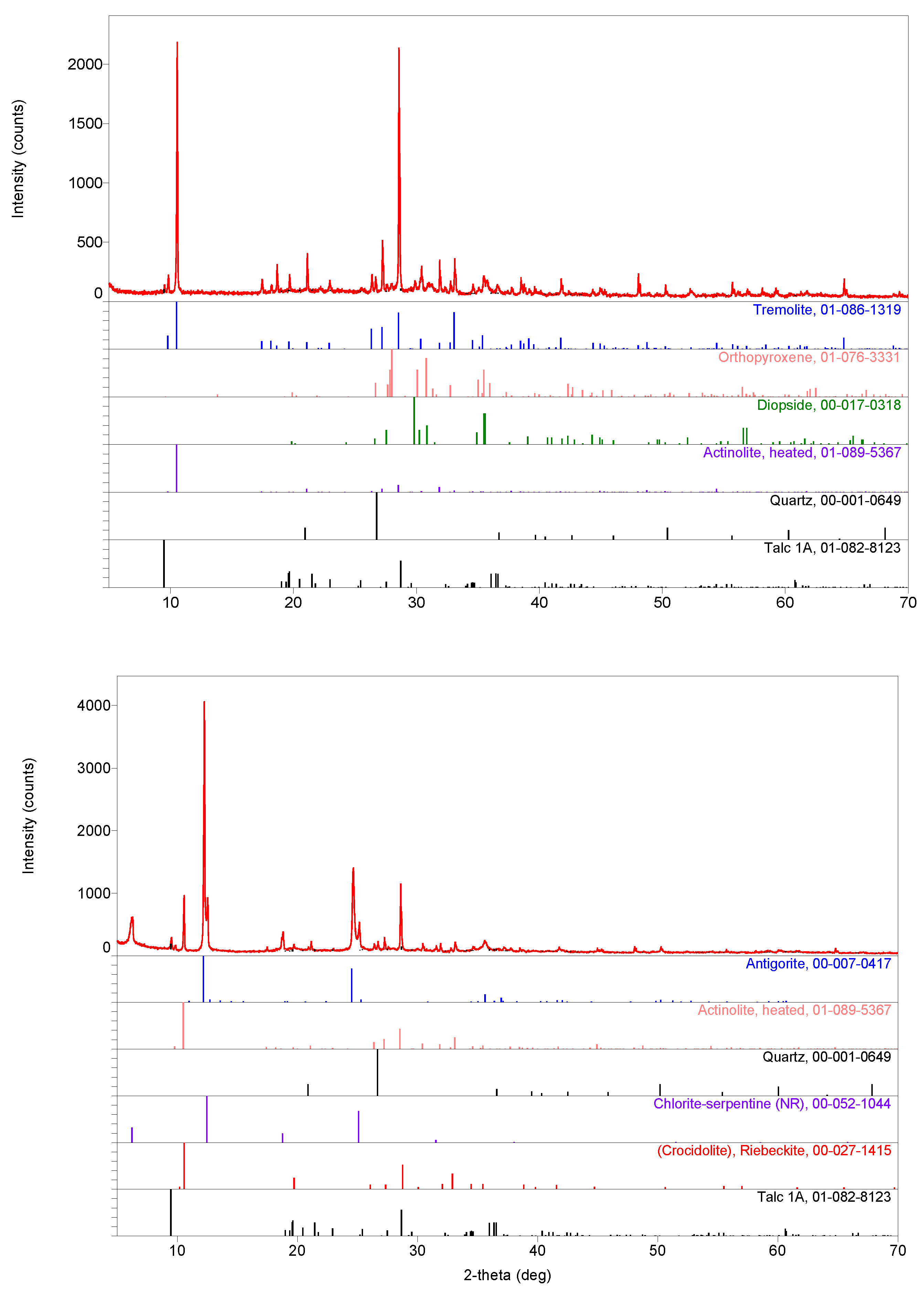
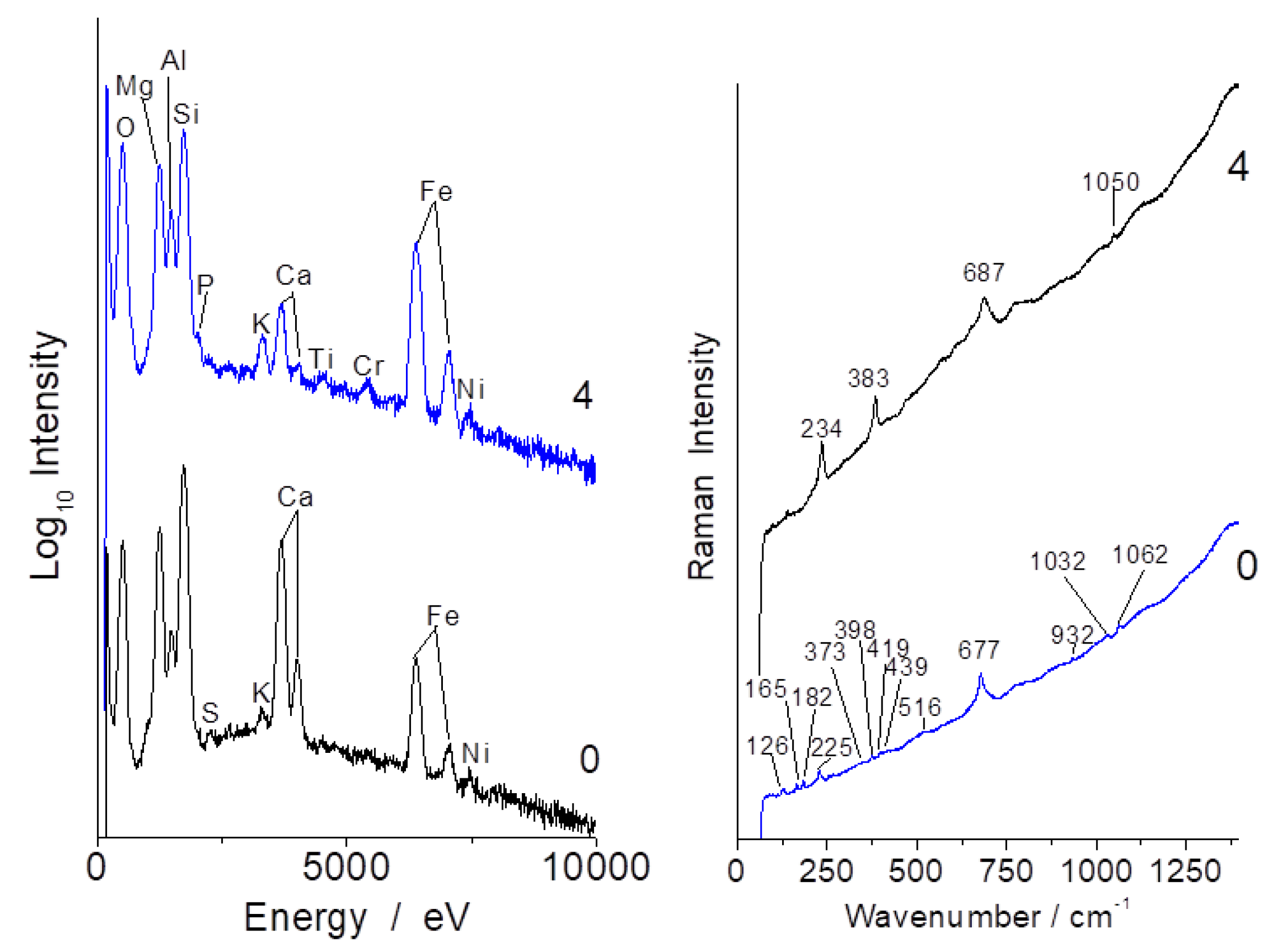
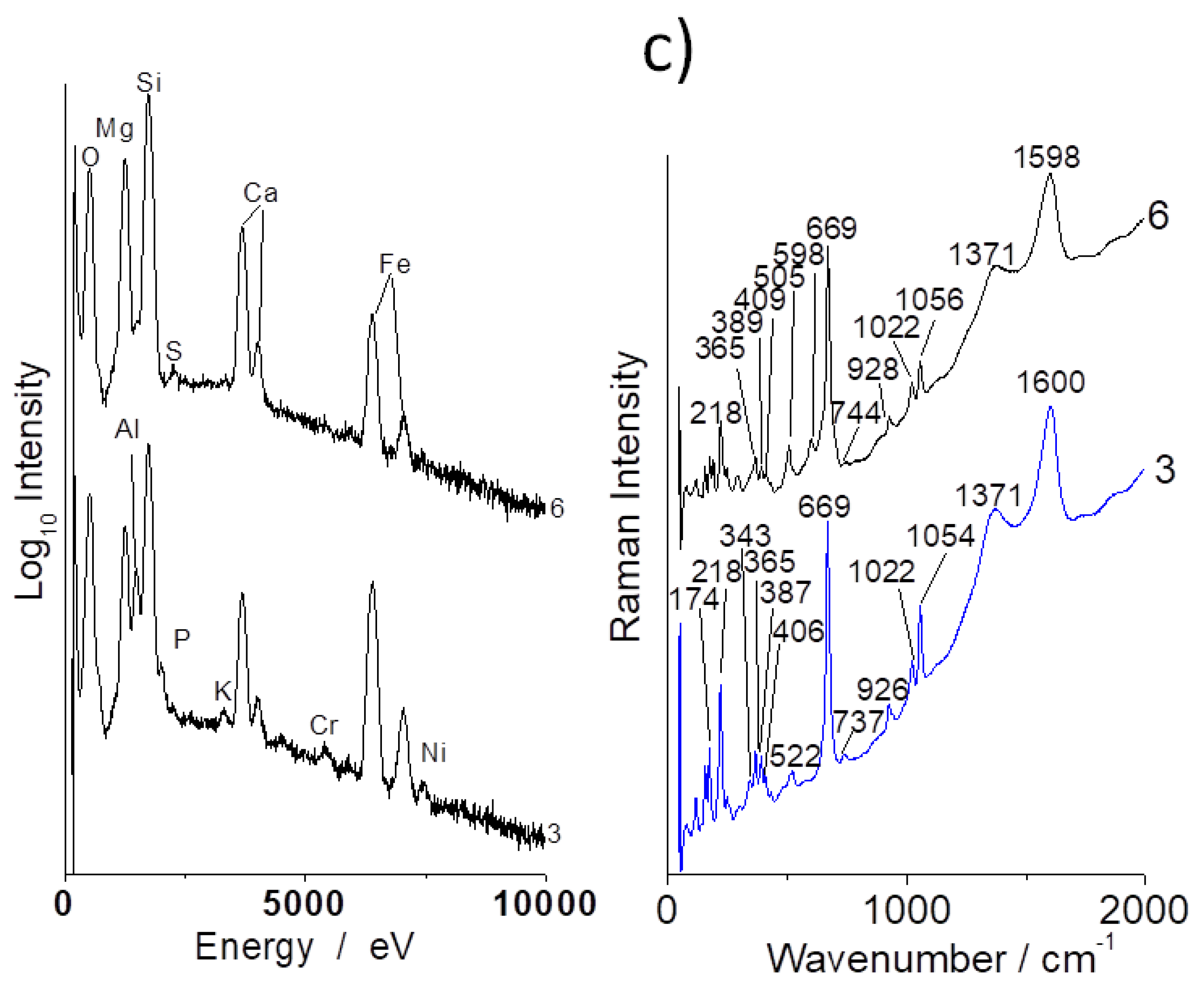
| Oxide | Sample b | Sample c | Sample a | Sample d | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| «0» | «4» | «3» | «6» | «1» | «6» | «8» | «0» | «1» | «2» | «6» | |
| SiO2 | 50.73 | 42.79 | 50.41 | 56.78 | 54.91 | 55.68 | 42.21 | 55.52 | 55.39 | 61.24 | 4.68 |
| Al2O3 | 3.47 | 11.01 | 7.63 | 1.88 | 1.63 | 14.13 | 28.29 | 3.19 | 1.92 | 4.88 | 1.26 |
| CaO | 17.87 | 2.43 | 6.44 | 9.74 | 12.81 | 6.34 | 20.08 | 11.47 | 13.06 | 11.57 | 0.82 |
| MgO | 19.74 | 23.23 | 15.84 | 23.53 | 21.99 | 8.02 | 1.93 | 22.54 | 20.03 | 11.72 | 3.45 |
| K2O | 0.35 | 0.97 | 0.29 | 0.15 | 0.18 | 0.23 | 0.09 | 0.20 | 0.13 | 0.05 | 0.09 |
| Na2O | 0.36 | 0.31 | 0.12 | 1.10 | 0.76 | 4.82 | 0.24 | 0.46 | 0.76 | 5.11 | 0.03 |
| Fe2O3 | 6.44 | 17.75 | 18.12 | 6.14 | 7.18 | 8.78 | 6.45 | 4.54 | 5.03 | 4.81 | 88.20 |
| TiO2 | 0.13 | 0.40 | 0.18 | 0.07 | 0.10 | 1.52 | 0.15 | 0.63 | 0.08 | 0.04 | 0.15 |
| NiO | 0.41 | 0.52 | 0.44 | 0.14 | 0.09 | 0.04 | 0.09 | 0.27 | 0.12 | 0.06 | 0.30 |
| MnO2 | 0.14 | 0.09 | 0.17 | 0.15 | 0.18 | 0.24 | 0.16 | 0.37 | 0.08 | 0.09 | 0.19 |
| SO3 | 0.14 | 0.05 | 0.03 | 0.22 | 0.05 | 0.16 | 0.18 | 0.70 | 0.26 | 0.20 | 0.06 |
| Cr2O3 | 0.23 | 0.48 | 0.34 | 0.11 | 0.13 | 0.05 | 0.14 | 0.12 | 0.15 | 0.24 | 0.79 |
| (a) | ||||||||||
| Samples/ Phases | Actinolite (Heated) | Antigorite | Crocidolite (Blue Asbestos) | Tremolite | Chlorite–Serpentine | Quartz | Albite | Diopside | Ortho-pyroxene | Talc |
| a | ++ | ++++ | ++ | ++ | + | |||||
| c | ++ | ++++ | + | + | ++ | + | ||||
| d | +++ | ++++ | ++ | ++ | + | + | ||||
| b | ++++ | +++ | ++ | + | ++ | ++ | + | + | + | |
| (b) | ||||||||||
| Samples/ Phases | Antigorite Chrysotile Serpentine | Crocidolite Amphibole (Blue Asbestos) | Amosite Amphibole | Quartz | Feldspar | Diopside | Pyroxene | Talc | Forsterite | |
| 690 | ~664 | 658 | 460 | 503 | 668 1015 | 680 1007 | 820–850 | |||
| a | ++ | + | + | + | ||||||
| b | +++ | +++ | ||||||||
| c | +++ | + | + | |||||||
| d | +++ | ++ | + | ++ | ||||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Colomban, P.; Kremenović, A. Asbestos-Based Pottery from Corsica: The First Fiber-Reinforced Ceramic Matrix Composite. Materials 2020, 13, 3597. https://doi.org/10.3390/ma13163597
Colomban P, Kremenović A. Asbestos-Based Pottery from Corsica: The First Fiber-Reinforced Ceramic Matrix Composite. Materials. 2020; 13(16):3597. https://doi.org/10.3390/ma13163597
Chicago/Turabian StyleColomban, Philippe, and Aleksandar Kremenović. 2020. "Asbestos-Based Pottery from Corsica: The First Fiber-Reinforced Ceramic Matrix Composite" Materials 13, no. 16: 3597. https://doi.org/10.3390/ma13163597
APA StyleColomban, P., & Kremenović, A. (2020). Asbestos-Based Pottery from Corsica: The First Fiber-Reinforced Ceramic Matrix Composite. Materials, 13(16), 3597. https://doi.org/10.3390/ma13163597







