Characteristics of 10-Methacryloyloxidecyl Dihydrogen Phosphate Monomer in Self-Etching Two-Bottled Dental Adhesive System: Comparison with Commercial Products
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Physical and Mechanical Properties
2.3. In Vitro Cytotoxicity Test
2.4. Statistical Analysis
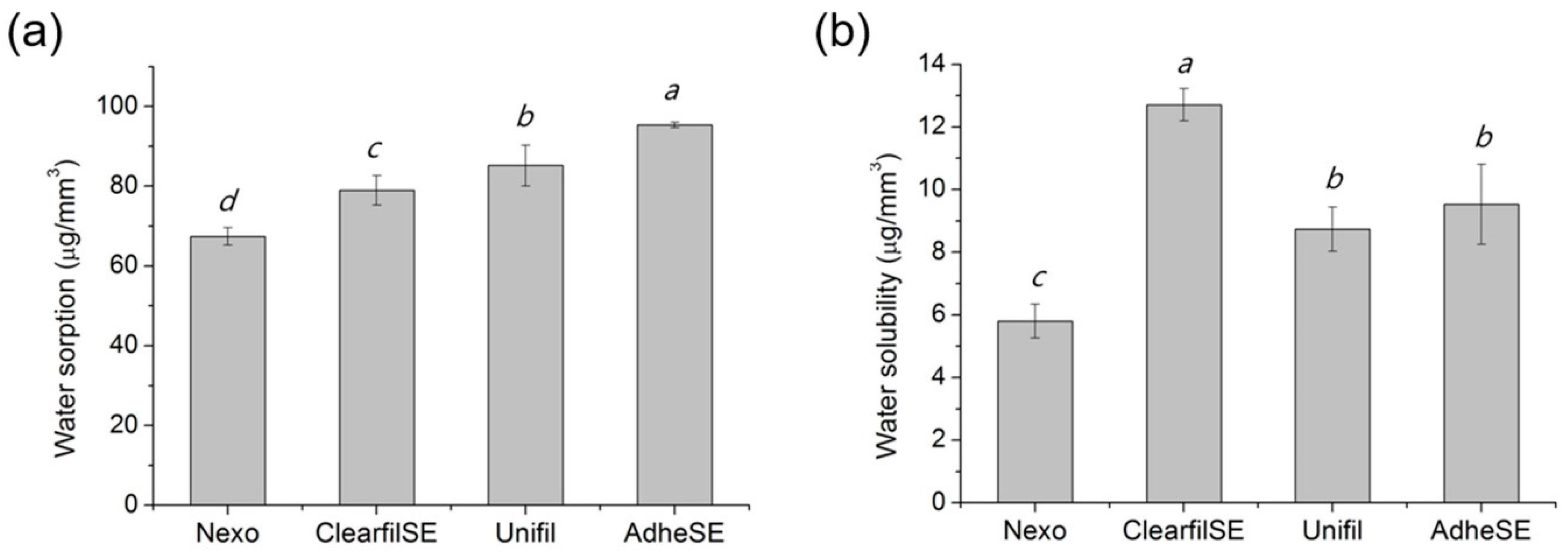
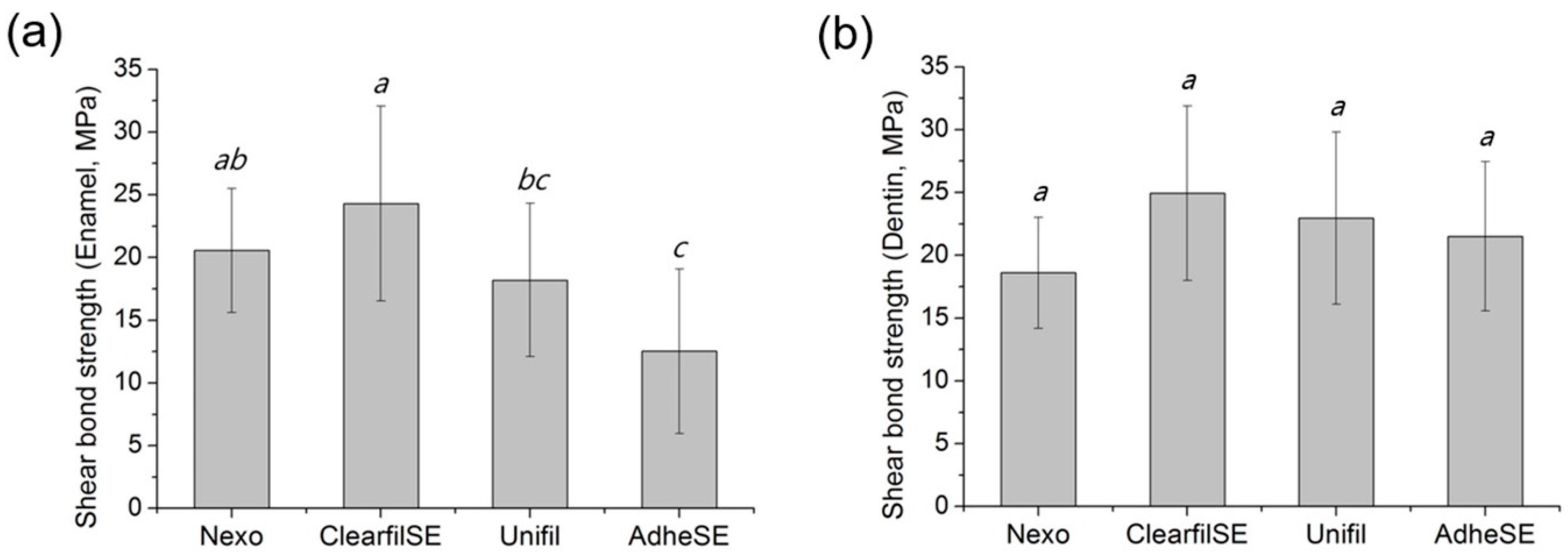
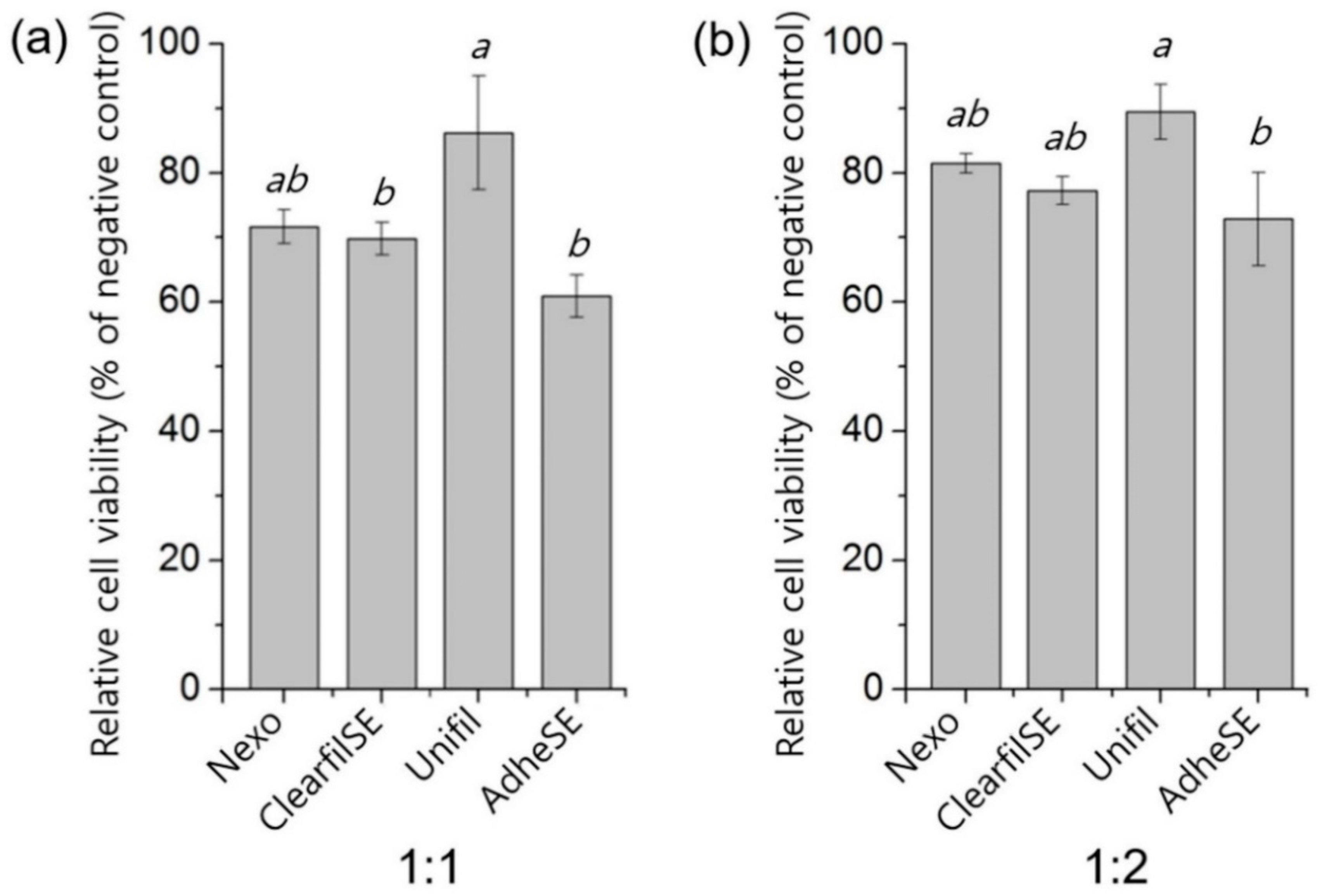
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nakabayashi, N.; Kojima, K.; Masuhara, E. The Promotion of Adhesion by the Infiltration of Monomers into Tooth Substrates. J. Biomed. Mater. Res. 1982, 16, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Perdigao, J.; Frankenberger, R.; Rosa, B.T.; Breschi, L. New trends in dentin/enamel adhesion. Am. J. Dent. 2000, 13, 25d–30d. [Google Scholar] [PubMed]
- Feitosa, V.P.; Pomacondor-Hernandez, C.; Ogliari, F.A.; Leal, F.; Correr, A.B.; Sauro, S. Chemical interaction of 10-MDP (methacryloyloxi-decyl-dihydrogen-phosphate) in zinc-doped self-etch adhesives. J. Dent. 2014, 42, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Omura, I. Dental Composition. U.S. Patent No. 5,091,441, 25 February 1992. [Google Scholar]
- Swift, E.J., Jr. Dentin/enamel adhesives: Review of the literature. Pediatr. Dent. 2002, 24, 456–461. [Google Scholar]
- Kijsamanmith, K.; Timpawat, S.; Harnirattisai, C.; Messer, H.H. Micro-tensile bond strengths of bonding agents to pulpal floor dentine. Int. Endod. J. 2002, 35, 833–839. [Google Scholar] [CrossRef]
- Araujo, M.S.; Souza, L.C.; Apolonio, F.M.; Barros, L.O.; Reis, A.; Loguercio, A.D.; Saboia, V.P. Two-year clinical evaluation of chlorhexidine incorporation in two-step self-etch adhesive. J. Dent. 2015, 43, 140–148. [Google Scholar] [CrossRef]
- International Organization for Standardization. ISO 4049: Dentistry-Polymer-Based Restorative Materials; ISO: Geneva, Switzerland, 2009. [Google Scholar]
- International Organization for Standardization. ISO 29022: Dentistry, Adhesion, Notched-Edge Shear Bond Strength Test; ISO: Geneva, Switzerland, 2013. [Google Scholar]
- Scribante, A.; Gallo, S.; Turcato, B.; Trovati, F.; Gandini, P.; Sfondrini, M.F. Fear of the Relapse: Effect of Composite Type on Adhesion Efficacy of Upper and Lower Orthodontic Fixed Retainers: In Vitro Investigation and Randomized Clinical Trial. Polymers 2020, 12, 963. [Google Scholar] [CrossRef] [Green Version]
- International Organization for Standardization. ISO 10993-5: Biological Evaluation of Medical Devices. Part 5, Tests for In Vitro Cytotoxicity; ISO: Geneva, Switzerland, 2009. [Google Scholar]
- International Organization for Standardization. ISO 10993-12: Biological Evaluation of Medical Devices. Part 12, Sample Preparation and Reference Materials; ISO: Geneva, Switzerland, 2012. [Google Scholar]
- Summitt, J.B. Fundamentals of Operative Dentistry: A Contemporary Approach; Quintessence Pub.: Chicago, IL, USA, 2006. [Google Scholar]
- Carrilho, E.; Cardoso, M.; Marques Ferreira, M.; Marto, C.M.; Paula, A.; Coelho, A.S. 10-MDP Based Dental Adhesives: Adhesive Interface Characterization and Adhesive Stability-A Systematic Review. Materials 2019, 12, 790. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, Y.; Yoshihara, K.; Nagaoka, N.; Hayakawa, S.; Torii, Y.; Ogawa, T.; Osaka, A.; Van Meerbeek, B. Self-assembled Nano-layering at the Adhesive Interface. J. Dent. Res. 2012, 91, 376–381. [Google Scholar] [CrossRef]
- Derbanne, M.A.; Besse, V.; Le Goff, S.; Sadoun, M.; Pham, T.N. The effect of functional monomer chain spacer length on the bond strength of an experimental dental adhesive. Int. J. Adhes. Adhes. 2014, 55, 95–105. [Google Scholar] [CrossRef]
- Nishiyama, N.; Tay, F.R.; Fujita, K.; Pashley, D.H.; Ikemura, K.; Hiraishi, N.; King, N.M. Hydrolysis of functional monomers in a single-bottle self-etching primer—Correlation of C-13 NMR and TEM findings. J. Dent. Res. 2006, 85, 422–426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malacarne, J.; Carvalho, R.M.; de Goes, M.F.; Svizero, N.; Pashley, D.H.; Tay, F.R.; Yiu, C.K.; Carrilho, M.R.D. Water sorption/solubility of dental adhesive resins. Dent. Mater. 2006, 22, 973–980. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, Y.; Yoshihara, K.; Hayakawa, S.; Nagaoka, N.; Okihara, T.; Matsumoto, T.; Minagi, S.; Osaka, A.; Van Landuyt, K.; Van Meerbeek, B. HEMA Inhibits Interfacial Nano-layering of the Functional Monomer MDP. J. Dent. Res. 2012, 91, 1060–1065. [Google Scholar] [CrossRef] [PubMed]
- Poggio, C.; Scribante, A.; Della Zoppa, F.; Colombo, M.; Beltrami, R.; Chiesa, M. Shear bond strength of one-step self-etch adhesives to enamel: Effect of acid pretreatment. Dent. Traumatol. 2014, 30, 43–48. [Google Scholar] [CrossRef]
- Beltrami, R.; Chiesa, M.; Scribante, A.; Allegretti, J.; Poggio, C. Comparison of shear bond strength of universal adhesives on etched and nonetched enamel. J. Appl. Biomater. Funct. Mater. 2016, 14, e78–e83. [Google Scholar] [CrossRef] [Green Version]
- Qin, W.; Lei, L.; Huang, Q.T.; Wang, L.; Lin, Z.M. Clinical effectiveness of self-etching adhesives with or without selective enamel etching in noncarious cervical lesions: A systematic review. J. Dent. Sci. 2014, 9, 303–312. [Google Scholar] [CrossRef] [Green Version]
- Marimoto, A.K.; Cunha, L.A.; Yui, K.C.K.; Huhtala, M.F.R.L.; Barcellos, D.; Prakki, A.; Goncalves, S.E.P. Influence of Nd:YAG Laser on the Bond Strength of Self-etching and Conventional Adhesive Systems to Dental Hard Tissues. Oper. Dent. 2013, 38, 447–455. [Google Scholar] [CrossRef]
- Sadr, A.; Shimada, Y.; Tagami, J. Effects of solvent drying time on micro-shear bond strength and mechanical properties of two self-etching adhesive systems. Dent. Mater. 2007, 23, 1114–1119. [Google Scholar] [CrossRef]
- Oliveira, S.S.A.; Pugach, M.K.; Hilton, J.F.; Watanabe, L.G.; Marshall, S.J.; Marshall, G.W. The influence of the dentin smear layer on adhesion: A self-etching primer vs. a total-etch system. Dent. Mater. 2003, 19, 758–767. [Google Scholar] [CrossRef]
- International Organization for Standardization. ISO 7405: Dentistry—Evaluation of Biocompatibility of Medical Devices Used in Dentistry; ISO: Geneva, Switzerland, 2008. [Google Scholar]
- Schmalz, G.; Schuster, U.; Nuetzel, K.; Schweikl, H. An in vitro pulp chamber with three-dimensional cell cultures. J. Endod. 1999, 25, 24–29. [Google Scholar] [CrossRef]
- Ulker, H.E.; Sengun, A. Cytotoxicity Evaluation of Self Adhesive Composite Resin Cements by Dentin Barrier Test on 3D Pulp Cells. Eur. J. Dent. 2009, 3, 120–126. [Google Scholar] [PubMed]
- Schmalz, G.; Schuster, U.; Thonemann, B.; Barth, M.; Esterbauer, S. Dentin barrier test with transfected bovine pulp-derived cells. J. Endod. 2001, 27, 96–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costa, C.A.; Hebling, J.; Hanks, C.T. Current status of pulp capping with dentin adhesive systems: A review. Dent. Mater. 2000, 16, 188–197. [Google Scholar] [CrossRef]


| Compound Name | 10-Methacryloyloxydecyl Dihydrogen Phosphate |
|---|---|
| SMILE | C=C(C)C(=O)OCCCCCCCCCCOP(=O)(O)O |
| CAS number | 85590-00-7 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roh, J.; Shin, H.; Hong, M.-H. Characteristics of 10-Methacryloyloxidecyl Dihydrogen Phosphate Monomer in Self-Etching Two-Bottled Dental Adhesive System: Comparison with Commercial Products. Materials 2020, 13, 3553. https://doi.org/10.3390/ma13163553
Roh J, Shin H, Hong M-H. Characteristics of 10-Methacryloyloxidecyl Dihydrogen Phosphate Monomer in Self-Etching Two-Bottled Dental Adhesive System: Comparison with Commercial Products. Materials. 2020; 13(16):3553. https://doi.org/10.3390/ma13163553
Chicago/Turabian StyleRoh, Jiyeon, Hyunjung Shin, and Min-Ho Hong. 2020. "Characteristics of 10-Methacryloyloxidecyl Dihydrogen Phosphate Monomer in Self-Etching Two-Bottled Dental Adhesive System: Comparison with Commercial Products" Materials 13, no. 16: 3553. https://doi.org/10.3390/ma13163553
APA StyleRoh, J., Shin, H., & Hong, M.-H. (2020). Characteristics of 10-Methacryloyloxidecyl Dihydrogen Phosphate Monomer in Self-Etching Two-Bottled Dental Adhesive System: Comparison with Commercial Products. Materials, 13(16), 3553. https://doi.org/10.3390/ma13163553






