Study of the Air-Entraining Behavior Based on the Interactions between Cement Particles and Selected Cationic, Anionic and Nonionic Surfactants
Abstract
1. Introduction
2. Materials and Methods
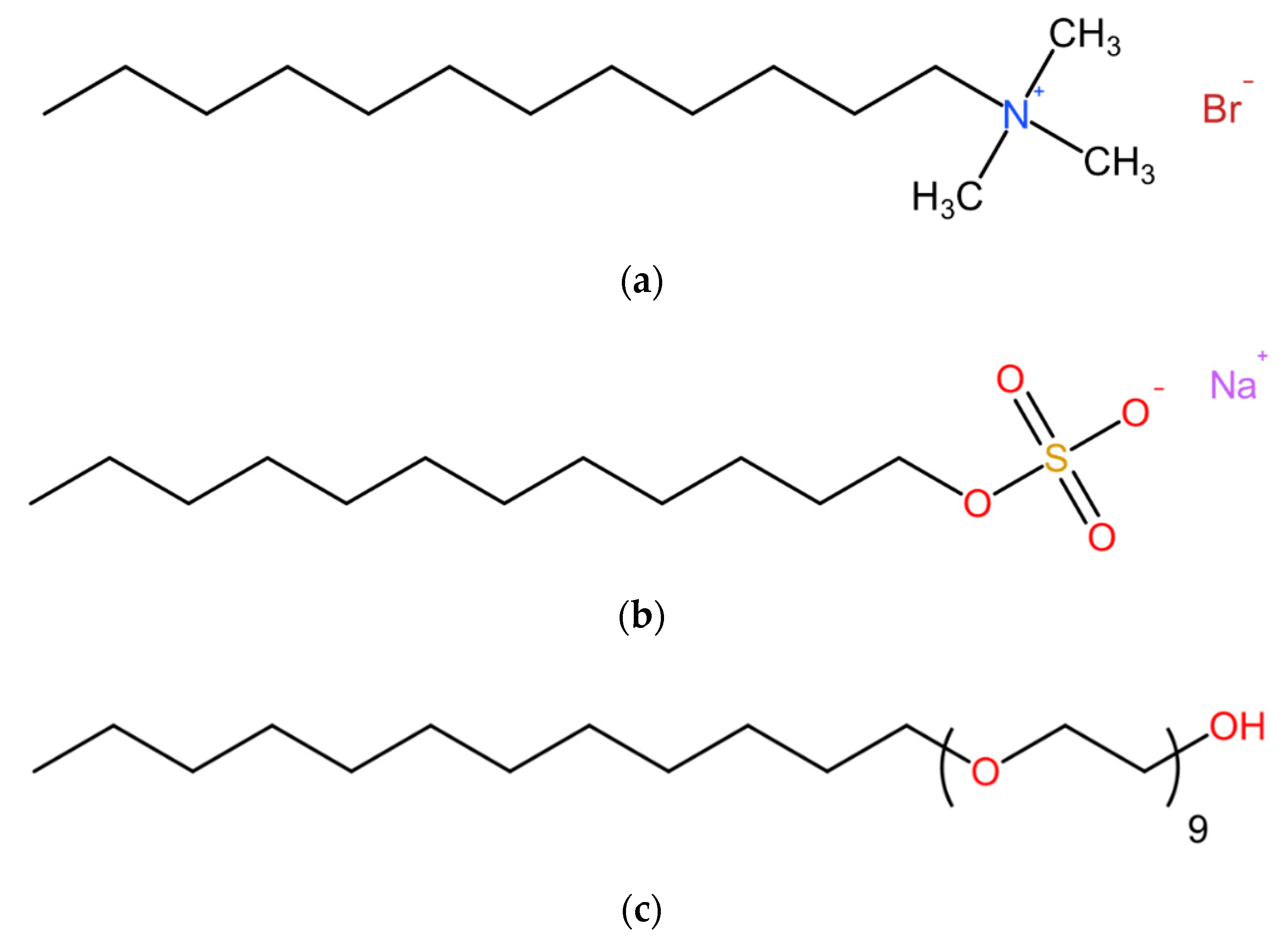
2.1. Air-Entrained Mortar Mixtures
2.2. Air Void Parameters of Hardened Mortar at 28 d
2.3. Foam Index Test
2.4. Zeta Potential Measurements
2.5. In Situ Attenuated Total Reflection-Fourier Transform Infrared Spectroscopy
3. Results and Discussion
3.1. Air Void Structure of Hardened Mortars
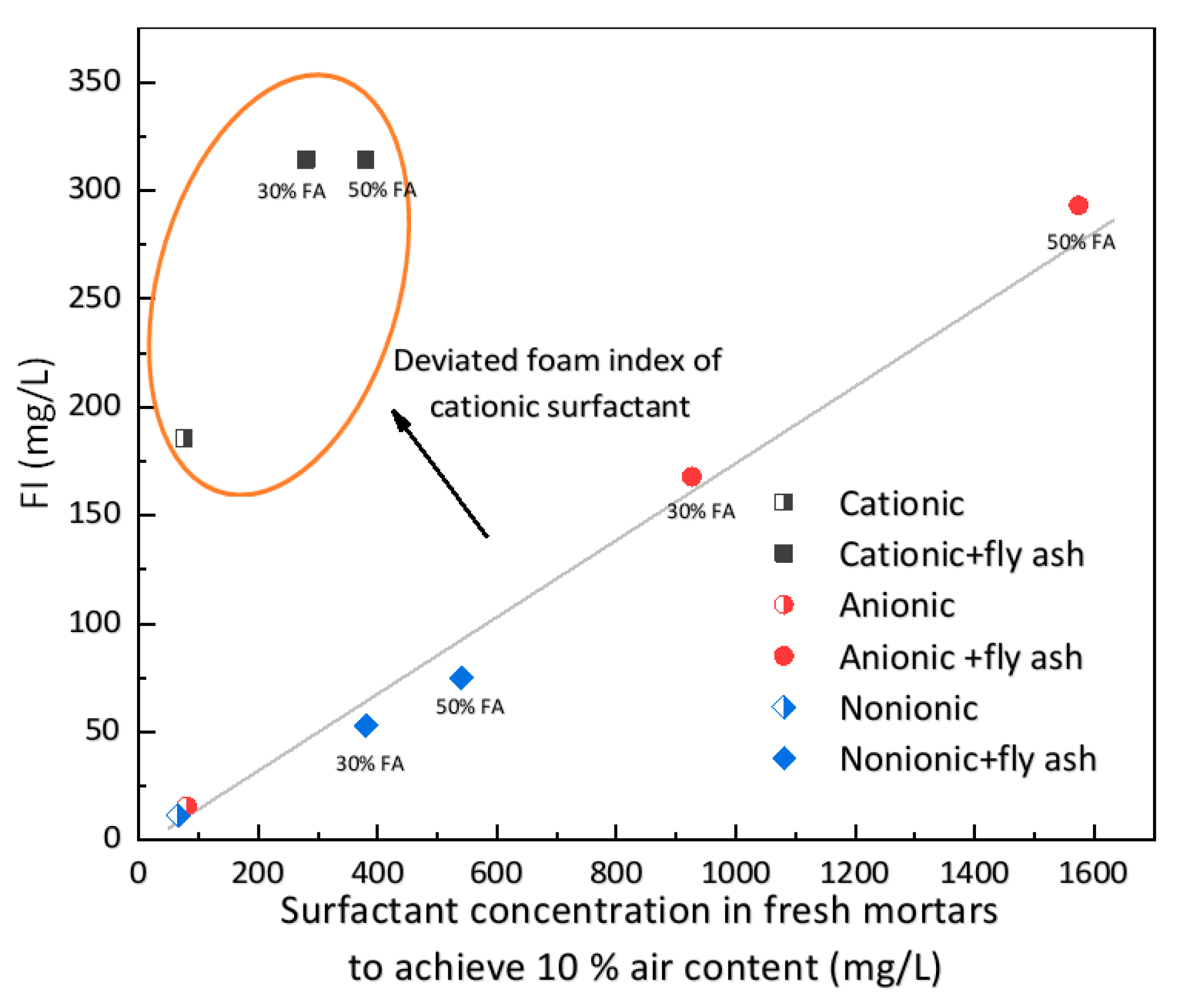
3.2. Foam Index Performance
3.3. Interaction between Cement Particles and Surfactants
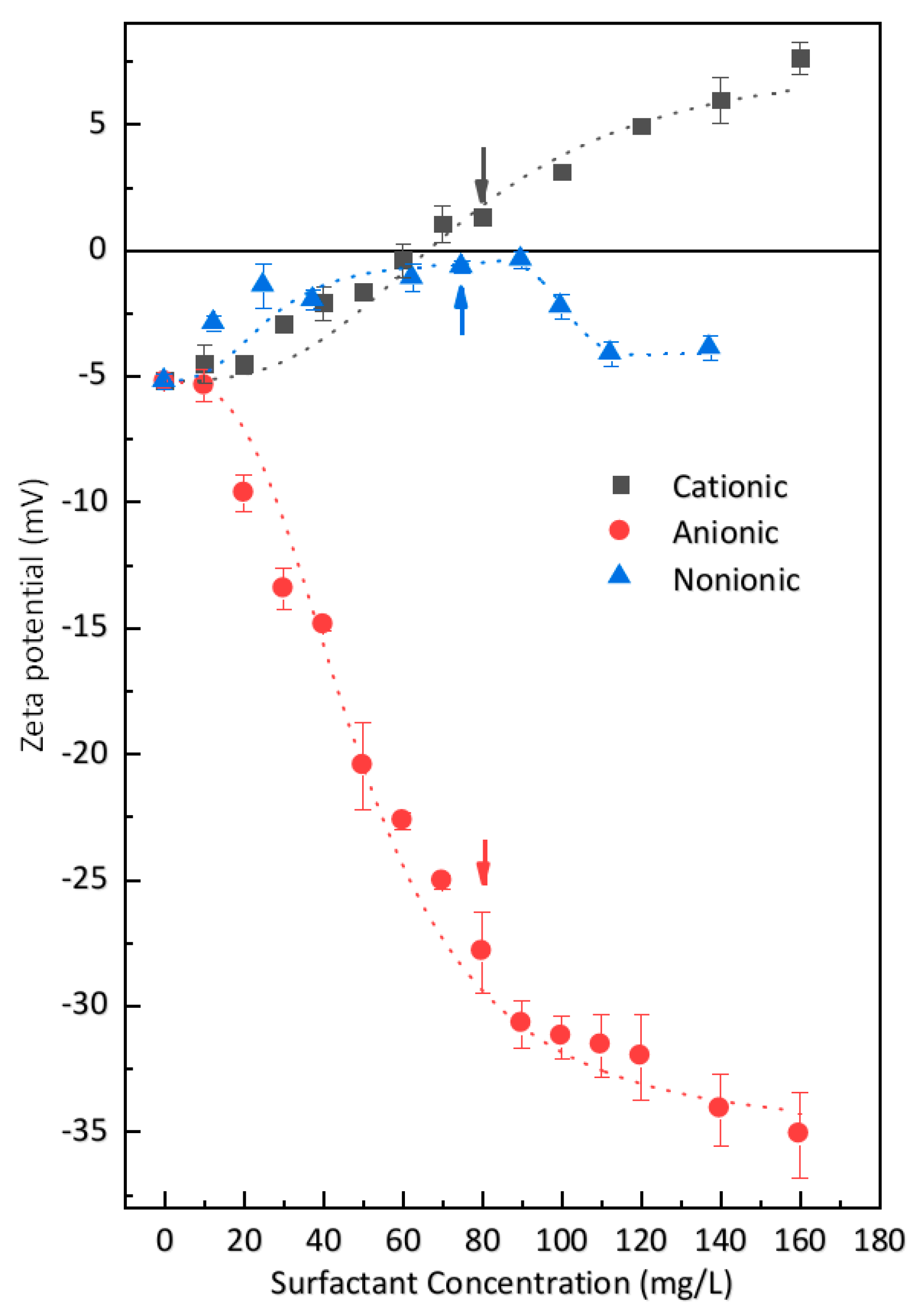
3.3.1. Zeta Potential of Cement Particles in Model Solutions
3.3.2. Zeta Potential of Cement Particles in Surfactant Solutions
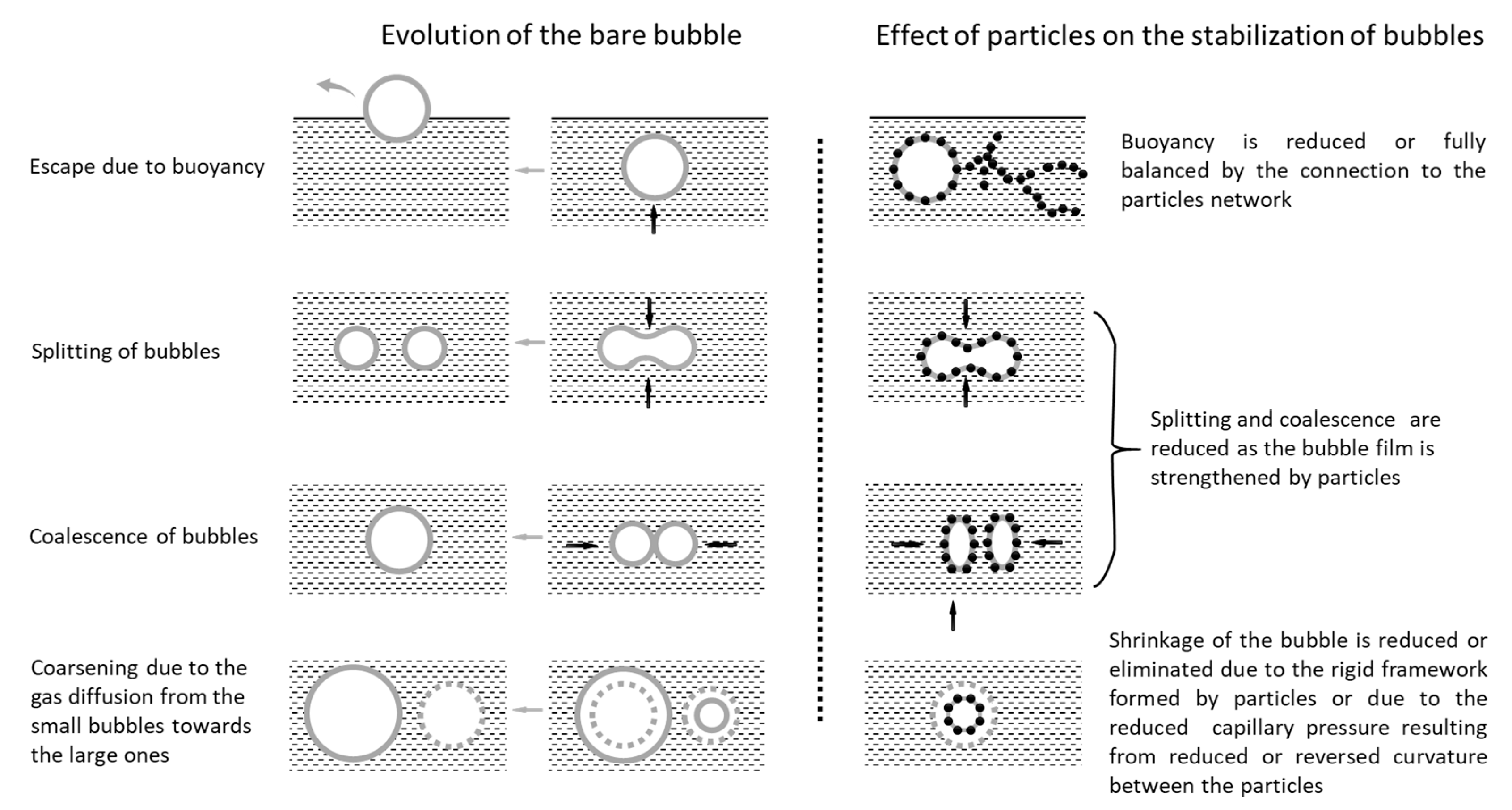
3.4. Interaction between Cement Particles and Air Bubbles
3.4.1. Model of the Interaction between Cement Particles and Air Bubbles
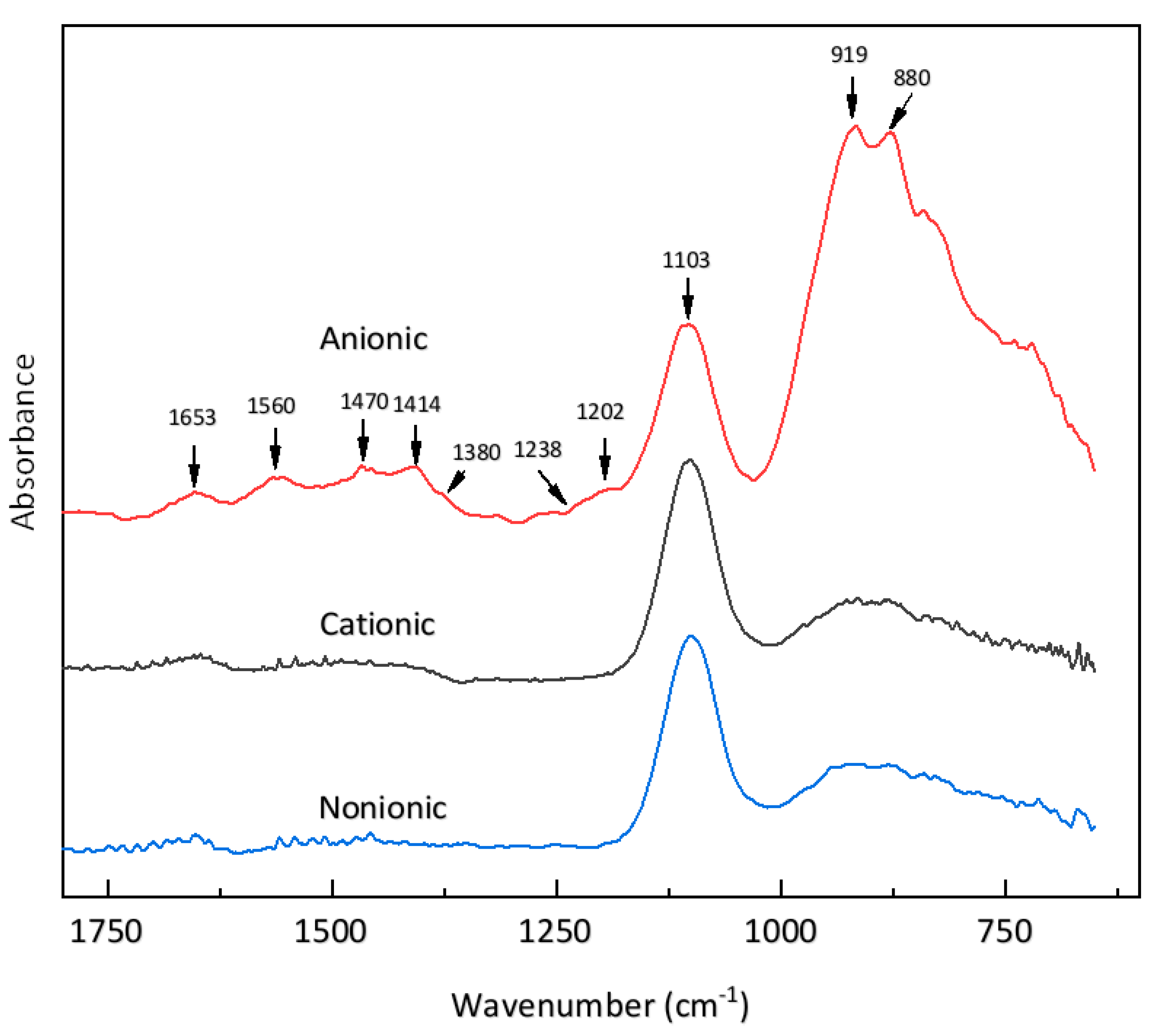
3.4.2. ATR Difference Spectra of Cement Particles in Surfactant Solutions
3.5. Summary
4. Conclusions
- (1)
- The selected cationic (DTAB), anionic (SDS), and nonionic (AEO-9) surfactants in this research could all entrain an adequate air content in cement mortars. However, for mortars with about 10% air content, air voids entrained by the cationic surfactant were much coarser, as more than 25% of the entrained air voids were larger than 2000 μm, while the anionic surfactant (SDS) and the nonionic surfactant (AEO-9) entrained mid-sized and smaller air voids, respectively.
- (2)
- Overestimated surfactant dosage in foam index tests is an indication of improper use as an air-entraining admixture in cementitious materials. When the cationic surfactant (DTAB) was used as the air-entraining admixture, it had an overestimated foam index and entrained excessive coarse air voids.
- (3)
- Zeta potential is a method to reflect the interaction between surfactant and cement particles. Due to the overall negative charge of cement particles, the cationic (DTAB), anionic (SDS), and nonionic (AEO-9) surfactants interacted with cement particles differently, according to their electrostatic interactions. Results indicate that the adsorption of the cationic surfactant (DTAB) arises from electrostatic interactions with the negative charge sites of cement particles. Correspondingly, the anionic surfactant (SDS) interacts with the positive charge sites of cement particles. Both surfactant molecules orient their tail to the aqueous phase. The nonionic surfactant (AEO-9) interacted moderately with cement particles. The degree of interaction between cement particles and the selected surfactants followed the order: nonionic (AEO-9) < anionic (SDS) < cationic (DTAB).
- (4)
- Under the condition of monolayer adsorption of surfactants on cement particles orienting their hydrophobic tails towards the aqueous phase, the stronger interaction between surfactant molecules and cement particles results in a stronger interaction between air bubbles and cement particles, which further influences the air void size distribution of hardened mortars. The degree of interaction between cement particles and air bubbles formed by selected surfactants in this study followed the order: nonionic (AEO-9) < anionic (SDS)< cationic (DTAB). The stronger the interaction between air bubbles and the cement particles, the larger the air voids entrained.
Author Contributions
Funding
Conflicts of Interest
References
- Du, L.; Folliard, K.J. Mechanisms of air entrainment in concrete. Cem. Concr. Res. 2005, 35, 1463–1471. [Google Scholar] [CrossRef]
- Liu, G.; Chen, L. Development of a new type of green switch air entraining agent for wet-mix shotcrete and its engineering application. Adv. Mater. Sci. Eng. 2016, 2016, 6981091. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, H.; Gao, H.; He, Y.; Jiang, M. Effect of bubble feature parameters on rheological properties of fresh concrete. Constr. Build. Mater. 2019, 196, 245–255. [Google Scholar] [CrossRef]
- Barfield, M.; Ghafoori, N. Air-entrained self-consolidating concrete: A study of admixture sources. Constr. Build. Mater. 2012, 26, 490–496. [Google Scholar] [CrossRef]
- Puthipad, N.; Ouchi, M.; Rath, S.; Attachaiyawuth, A. Enhancement in self-compactability and stability in volume of entrained air in self-compacting concrete with high volume fly ash. Constr. Build. Mater. 2016, 128, 349–360. [Google Scholar] [CrossRef]
- Łaźniewska-Piekarczyk, B. The influence of selected new generation admixtures on the workability, air-voids parameters and frost-resistance of self compacting concrete. Constr. Build. Mater. 2012, 31, 310–319. [Google Scholar] [CrossRef]
- Łaźniewska-Piekarczyk, B. The type of air-entraining and viscosity modifying admixtures and porosity and frost durability of high performance self-compacting concrete. Constr. Build. Mater. 2013, 40, 659–671. [Google Scholar] [CrossRef]
- Zhang, J.; Gao, X.; Yu, L. Improvement of viscosity-modifying agents on air-void system of vibrated concrete. Constr. Build. Mater. 2020, 239, 117843. [Google Scholar] [CrossRef]
- Sypek, M.; Latawiec, R.; Pichór, W. Gypsum dehydration in cement and its impact on air-void structure in air-entrained concrete. Constr. Build. Mater. 2019, 220, 396–402. [Google Scholar] [CrossRef]
- Rehman, A.; Usman, M.; Bokhari, T.H.; Rahman, H.M.A.U.; Mansha, A.; Siddiq, M.; Rasheed, A.; Nisa, M.U. Effects of nonionic surfactant (TX-100) on solubilizing power of cationic surfactants (CTAB and CPC) for Direct Red 13. Colloids Surfaces A Physicochem. Eng. Asp. 2020, 586, 124241. [Google Scholar] [CrossRef]
- Rehman, A.; Usman, M.; Bokhari, T.H.; Haq, A.U.; Saeed, M.; Rahman, H.M.A.U.; Siddiq, M.; Rasheed, A.; Nisa, M.U. The application of cationic-nonionic mixed micellar media for enhanced solubilization of Direct Brown 2 dye. J. Mol. Liq. 2020, 301, 112408. [Google Scholar] [CrossRef]
- Xu, J.; Feng, W.; Jiang, L.; Xu, Y.; Song, Y.; Cao, Y.; Tan, Q. Influence of surfactants on chloride binding in cement paste. Constr. Build. Mater. 2016, 125, 369–374. [Google Scholar] [CrossRef]
- Ramachandran, V.S. Concrete Admixtures Handbook: Properties, Science and Technology. Noyes Publications: Park Ridge, NJ, USA, 1995. [Google Scholar]
- Dodson, V.H. Concrete Admixtures; Springer Science & Business Media: New York, NY, USA, 2013. [Google Scholar]
- Chen, J.; Qiao, M.; Gao, N.; Ran, Q.; Wu, J.; Shan, G.; Qi, S.; Wu, S. Cationic oligomeric surfactants as novel air entraining agents for concrete. Colloids Surfaces A Physicochem. Eng. Asp. 2018, 538, 686–693. [Google Scholar] [CrossRef]
- Dean, S.W.; Harris, N.J.; Hover, K.C.; Folliard, K.J.; Ley, M.T. The use of the foam index test to predict AEA dosage in concrete containing fly ash: Part I—Evaluation of the state of practice. J. ASTM Int. 2008, 5, 101601. [Google Scholar] [CrossRef]
- Mendes, J.C.; Moro, T.K.; Figueiredo, A.S.; Silva, K.D.D.C.; Silva, G.C.; Silva, G.J.B.; Peixoto, R.A.F. Mechanical, rheological and morphological analysis of cement-based composites with a new LAS-based air entraining agent. Constr. Build. Mater. 2017, 145, 648–661. [Google Scholar] [CrossRef]
- Ley, M.T.; Folliard, K.J.; Hover, K.C. Observations of air-bubbles escaped from fresh cement paste. Cem. Concr. Res. 2009, 39, 409–416. [Google Scholar] [CrossRef]
- Zhang, S.; Lan, Q.; Liu, Q.; Xu, J.; Sun, D. Aqueous foams stabilized by Laponite and CTAB. Colloids Surfaces A Physicochem. Eng. Asp. 2008, 317, 406–413. [Google Scholar] [CrossRef]
- Petit, P.; Javierre, I.; Jezequel, P.H.; Biance, A.L. Generation and stability of bubbles in a cement based slurry. Cem. Concr. Res. 2014, 60, 37–44. [Google Scholar] [CrossRef]
- Kam, S.I.; Rossen, W.R. Anomalous capillary pressure, stress, and stability of solids-coated bubbles. J. Colloid Interface Sci. 1999, 213, 329–339. [Google Scholar] [CrossRef]
- Abkarian, M.; Subramaniam, A.B.; Kim, S.H.; Larsen, R.J.; Yang, S.M.; Stone, H.A. Dissolution arrest and stability of particle-covered bubbles. Phys. Rev. Lett. 2007, 99, 188301. [Google Scholar] [CrossRef]
- Martinez, A.C.; Rio, E.; Delon, G.; Saint-Jalmes, A.; Langevin, D.; Binks, B.P. On the origin of the remarkable stability of aqueous foams stabilised by nanoparticles: Link with microscopic surface properties. Soft Matter 2008, 4, 1531. [Google Scholar] [CrossRef]
- Qiao, M.; Shan, G.; Chen, J.; Wu, S.; Gao, N.; Ran, Q.; Liu, J. Effects of salts and adsorption on the performance of air entraining agent with different charge type in solution and cement mortar. Constr. Build. Mater. 2020, 242, 118188. [Google Scholar] [CrossRef]
- Tunstall, L.E.; Scherer, G.W.; Prud’Homme, R.K. Studying AEA interaction in cement systems using tensiometry. Cem. Concr. Res. 2017, 92, 29–36. [Google Scholar] [CrossRef]
- Şahin, Y.; Akkaya, Y.; Boylu, F.; Taşdemir, M. Characterization of air entraining admixtures in concrete using surface tension measurements. Cem. Concr. Compos. 2017, 82, 95–104. [Google Scholar] [CrossRef]
- Andersen, P. The effect of superplasticizers and air-entraining agents on the Zeta potential of cement particles. Cem. Concr. Res. 1986, 16, 931–940. [Google Scholar] [CrossRef]
- Plank, J.; Hirsch, C. Impact of zeta potential of early cement hydration phases on superplasticizer adsorption. Cem. Concr. Res. 2007, 37, 537–542. [Google Scholar] [CrossRef]
- Mollah, M.Y.A.; Adams, W.J.; Schennach, R.; Cocke, D.L. A review of cement–superplasticizer interactions and their models. Adv. Cem. Res. 2000, 12, 153–161. [Google Scholar] [CrossRef]
- Zhang, T.; Shang, S.; Yin, F.; Aishah, A.; Salmiah, A.; Ooi, T. Adsorptive behavior of surfactants on surface of Portland cement. Cem. Concr. Res. 2001, 31, 1009–1015. [Google Scholar] [CrossRef]
- Lummer, N.R.; Plank, J. Combination of lignosulfonate and AMPS®-co-NNDMA water retention agent—An example for dual synergistic interaction between admixtures in cement. Cem. Concr. Res. 2012, 42, 728–735. [Google Scholar] [CrossRef]
- Katsioti, M.; Tsakiridis, P.; Giannatos, P.; Tsibouki, Z.; Marinos, J. Characterization of various cement grinding aids and their impact on grindability and cement performance. Constr. Build. Mater. 2009, 23, 1954–1959. [Google Scholar] [CrossRef]
- Jiao, L.; Jiao, L.; Chen, D.; Chen, D.; Feng, D.; Feng, D.; Wang, X.; Wang, X.; Zhang, J.; Zhang, J. Potential for significantly improving performances of oil-well cement by soap-free Emulsions. Mater. Struct. 2016, 49, 279–288. [Google Scholar] [CrossRef]
- Ran, Q.; Song, F.; Wang, T.; Fan, S.; Ma, J.; Yang, Y.; Liu, J. Effect of the different hydrophobic groups of polycarboxylate superplasticizers on the properties in cement mortars. Polym. Compos. 2015, 38, 1783–1791. [Google Scholar] [CrossRef]
- Chinese National Standard. Common Portland Cement; Chinese National Standard: Beijing, China, 2007; GB175-2007. [Google Scholar]
- Fonseca, P.C.; Scherer, G.W. An image analysis procedure to quantify the air void system of mortar and concrete. Mater. Struct. 2014, 48, 3087–3098. [Google Scholar] [CrossRef]
- Ataie, F.F.; Riding, K. Influence of agricultural residue ash on early cement hydration and chemical admixtures adsorption. Constr. Build. Mater. 2016, 106, 274–281. [Google Scholar] [CrossRef]
- Liu, Q.; Yang, Y.; Chen, Z.; Gao, J. Development of a foam index test method by investigating the effect of test parameters. Measurment 2020, 152, 107307. [Google Scholar] [CrossRef]
- Robert, J.; Hunter, R.; Ottewill, H.; Rowell, R.L. Zeta Potential in Colloid Science: Principles and Applications; Academic Press: London, UK, 2013; Volume 2. [Google Scholar]
- Hind, A.R.; Bhargava, S.K.; Grocott, S.C. Adsorption of quaternary ammonium compounds on the surface of sodium oxalate: FTIR/ATR investigation under high-ionic-strength, highly alkaline conditions. Langmuir 1997, 13, 6255–6259. [Google Scholar] [CrossRef]
- Hind, A.R.; Bhargava, S.K.; McKinnon, A. At the solid/liquid interface: FTIR/ATR—The tool of choice. Adv. Colloid Interface Sci. 2001, 93, 91–114. [Google Scholar] [CrossRef]
- Hind, A.R.; Bhargava, S.K.; Grocott, S.C. Attenuated total reflection fourier transform infrared spectroscopic investigation of the solid/aqueous interface of low surface area, water-soluble solids in high ionic strength, highly alkaline, aqueous media. Langmuir 1997, 13, 3483–3487. [Google Scholar] [CrossRef]
- Liu, Z.; Hansen, W.; Wang, F.; Zhang, W. Simulation of air-void system in hardened concrete using a geometrical model. Mag. Concr. Res. 2019, 71, 680–689. [Google Scholar] [CrossRef]
- Rath, S.; Puthipad, N.; Attachaiyawuth, A.; Ouchi, M. Critical size of entrained air to stability of air volume in mortar of self-compacting concrete at fresh Stage. J. Adv. Concr. Technol. 2017, 15, 29–37. [Google Scholar] [CrossRef]
- Backstrom, J.E. Orgin, evolution, and effects of the air void system in concrete. Part 3—Influence of water-cement ratio and compaction. Aci Struct. J. 1958, 55, 359–375. [Google Scholar]
- Backstrom, J.E. Orgin, evolution, and effects of the air void system in concrete. Part 2—Air-entraining agent. Aci Struct. J. 1958, 55, 261–272. [Google Scholar]
- Moradian, M.; Hu, Q.; Aboustait, M.; Ley, M.T.; Hanan, J.C.; Xiao, X.; Scherer, G.W.; Zhang, Z. Direct observation of void evolution during cement hydration. Mater. Des. 2017, 136, 137–149. [Google Scholar] [CrossRef]
- Ley, M.T.; Chancey, R.; Juenger, M.C.; Folliard, K.J. The physical and chemical characteristics of the shell of air-entrained bubbles in cement paste. Cem. Concr. Res. 2009, 39, 417–425. [Google Scholar] [CrossRef]
- Nachbaur, L.; Nkinamubanzi, P.C.; Nonat, A.; Mutin, J.C. Electrokinetic properties which control the coagulation of silicate cement suspensions during early age hydration. J. Colloid Interface Sci. 1998, 202, 261–268. [Google Scholar] [CrossRef]
- Ouyang, X.; Koleva, D.; Ye, G.; Van Breugel, K. Understanding the adhesion mechanisms between C S H and fillers. Cem. Concr. Res. 2017, 100, 275–283. [Google Scholar] [CrossRef]
- Ouyang, X. Filler-Hydrates Adhesion Properties in Cement Paste System: Development of Sustainable Building Materials; Delft University of Technology: Delft, The Netherlands, 2017. [Google Scholar] [CrossRef]
- Lu, Z.; Kong, X.; Zhang, C.; Xing, F.; Zhang, Y. Effect of colloidal polymers with different surface properties on the rheological property of fresh cement pastes. Colloids Surfaces A Physicochem. Eng. Asp. 2017, 520, 154–165. [Google Scholar] [CrossRef]
- Kong, X.; Pakusch, J.; Jansen, D.; Emmerling, S.; Neubauer, J.; Goetz-Neuhoeffer, F.; Goetz-Neunhoeffer, F. Effect of polymer latexes with cleaned serum on the phase development of hydrating cement pastes. Cem. Concr. Res. 2016, 84, 30–40. [Google Scholar] [CrossRef]
- Zhang, Y.; Kong, X.M.; Lu, Z.B.; Lu, Z.C.; Hou, S.-S. Effects of the charge characteristics of polycarboxylate superplasticizers on the adsorption and the retardation in cement pastes. Cem. Concr. Res. 2015, 67, 184–196. [Google Scholar] [CrossRef]
- Çelik, M. Adsorption of ethoxylated sulfonate and nonionic homologs on coal. J. Colloid Interface Sci. 1989, 129, 428–440. [Google Scholar] [CrossRef]
- Möbius, D.; Miller, R.; Fainerman, V.B. Surfactants: Chemistry, Interfacial Properties, Applications; Elsevier: Amsterdam, The Netherlands, 2001; Volume 13. [Google Scholar]
- Shen, Y.H. Sorption of non-ionic surfactants to soil: The role of soil mineral composition. Chemosphere 2000, 41, 711–716. [Google Scholar] [CrossRef]
- Tkachenko, N.H.; Yaremko, Z.; Bellmann, C.; Soltys, M.M. The influence of ionic and nonionic surfactants on aggregative stability and electrical surface properties of aqueous suspensions of titanium dioxide. J. Colloid Interface Sci. 2006, 299, 686–695. [Google Scholar] [CrossRef] [PubMed]
- Hughes, T.L.; Methven, C.M.; Jones, T.G.J.; Pelham, S.E.; Fletcher, P.; Hall, C. Determining cement composition by fourier transform infrared spectroscopy. Adv. Cem. Based Mater. 1995, 2, 91–104. [Google Scholar] [CrossRef]
- Mollah, M.; Kesmez, M.; Cocke, D. An X-ray diffraction (XRD) and Fourier transform infrared spectroscopic (FT-IR) investigation of the long-term effect on the solidification/stabilization (S/S) of arsenic(V) in Portland cement type-V. Sci. Total. Environ. 2004, 325, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.; Lee, M.; Choe, E.K. Characterization of cotton fabric scouring by FT-IR ATR spectroscopy. Carbohydr. Polym. 2004, 58, 417–420. [Google Scholar] [CrossRef]
- Dobson, K.D.; McQuillan, A.J. In situ infrared spectroscopic analysis of the adsorption of aromatic carboxylic acids to TiO2, ZrO2, Al2O3, and Ta2O5 from aqueous solutions. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2000, 56, 557–565. [Google Scholar] [CrossRef]
- Scheuing, D.R. Fourier Transform Infrared Spectroscopy in Colloid and Interface Science; American Chemical Society: Washington, DC, USA, 1991. [Google Scholar]
- Li, H.; Tripp, C.P. Spectroscopic identification and dynamics of adsorbed cetyltrimethylammonium bromide structures on TiO2Surfaces. Langmuir 2002, 18, 9441–9446. [Google Scholar] [CrossRef]
- Bai, B.; Hankins, N.P.; Hey, M.J.; Kingman, S. In situ mechanistic study of SDS adsorption on hematite for optimized froth flotation. Ind. Eng. Chem. Res. 2004, 43, 5326–5338. [Google Scholar] [CrossRef]
- Kawai, T.; Umemura, J.; Takenaka, T. Fourier transform infrared study on the phase transitions of a sodium dodecyl sulfate-water system. Bull. Inst. Chem. Res. 1983, 61, 314–323. [Google Scholar]
- Umemura, J.; Cameron, D.G.; Mantsch, H.H. A fourier transform infrared spectroscopic study of the molecular interaction of cholesterol with 1,2-dipalmitoyl-sn-glycero-3-phosphocholine. Biochim. Biophys. Acta 1980, 602, 32–44. [Google Scholar] [CrossRef]
- Cameron, D.G.; Casal, H.L.; Mantsch, H.H. Characterization of the pretransition in 1,2-dipalmitoyl-sn-glycero-3-phosphocholine by Fourier transform infrared spectroscopy. Biochemistry 1980, 19, 3665–3672. [Google Scholar] [CrossRef] [PubMed]
- Lanzón, M.; Garrido, A.; Garcia-Ruiz, P.A. Stabilization of sodium oleate as calcium oleate in cement-based mortars made with limestone fillers. Constr. Build. Mater. 2011, 25, 1001–1008. [Google Scholar] [CrossRef]
- Kirwan, L.J.; Fawell, P.D.; Van Bronswijk, W. In situ FTIR-ATR examination of poly(acrylic acid) adsorbed onto hematite at low pH. Langmuir 2003, 19, 5802–5807. [Google Scholar] [CrossRef]
- Mielczarski, J.A.; Mielczarski, E. Determination of molecular orientation and thickness of self-assembled monolayers of oleate on apatite by FTIR reflection spectroscopy. J. Phys. Chem. 1995, 99, 3206–3217. [Google Scholar] [CrossRef]
- De Ninno, A.; Castellano, A.C. Deprotonation of glutamic acid induced by weak magnetic field: An FTIR-ATR study. Bioelectromagnetics 2010, 32, 218–225. [Google Scholar] [CrossRef]
- Gao, X.; Chorover, J. Adsorption of sodium dodecyl sulfate (SDS) at ZnSe and α-Fe2O3 surfaces: Combining infrared spectroscopy and batch uptake studies. J. Colloid Interface Sci. 2010, 348, 167–176. [Google Scholar] [CrossRef]
- Peak, D.; Ford, R.G.; Sparks, D.L. An in situ ATR-FTIR investigation of sulfate bonding mechanisms on goethite. J. Colloid Interface Sci. 1999, 218, 289–299. [Google Scholar] [CrossRef]
- Sperline, R.P.; Song, Y.; Freiser, H. Fourier transform infrared attenuated total reflection spectroscopy linear dichroism study of sodium dodecyl sulfate adsorption at the alumina/water interface using alumina-coated optics. Langmuir 1992, 8, 2183–2191. [Google Scholar] [CrossRef]
- Li, H.; Tripp, C.P. Use of infrared bands of the surfactant headgroup to identify mixed surfactant structures adsorbed on titania. J. Phys. Chem. B 2004, 108, 18318–18326. [Google Scholar] [CrossRef]
- Scheuing, D.R.; Weers, J.G. A Fourier transform infrared spectroscopic study of dodecyltrimethylammonium chloride/sodium dodecyl sulfate surfactant mixtures. Langmuir 1990, 6, 665–671. [Google Scholar] [CrossRef]
- Hug, S.J. In situ fourier transform infrared measurements of sulfate adsorption on hematite in aqueous solutions. J. Colloid Interf. Sci. 1997, 188, 415–422. [Google Scholar] [CrossRef]
- Eggleston, C.M.; Hug, S.; Stumm, W.; Sulzberger, B.; Afonso, M.D.S. Surface complexation of sulfate by hematite surfaces: FTIR and STM observations. Geochim. Cosmochim. Acta 1998, 62, 585–593. [Google Scholar] [CrossRef]
- Viallis-Terrisse, H.; Nonat, A.; Petit, J. GOLDBECK zeta-potential study of calcium silicate hydrates interacting with alkaline cations. J. Colloid Interf. Sci. 2001, 244, 58–65. [Google Scholar] [CrossRef]
- Zingg, A.; Winnefeld, F.; Holzer, L.; Pakusch, J.; Becker, S.; Gauckler, L. Adsorption of polyelectrolytes and its influence on the rheology, zeta potential, and microstructure of various cement and hydrate phases. J. Colloid Interface Sci. 2008, 323, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Holzer, L.; Gasser, P.; Kaech, A.; Wegmann, M.; Zingg, A.; Wepf, R.; Muench, B. Cryo-FIB-nanotomography for quantitative analysis of particle structures in cement suspensions. J. Microsc. 2007, 227 Pt 3, 216–228. [Google Scholar] [CrossRef]
- Schönlein, M.; Plank, J. Influence of PCE kind and dosage on ettringite crystallization performed under terrestrial and microgravity conditions. J. Am. Ceram. Soc. 2018, 101, 3575–3584. [Google Scholar] [CrossRef]
- Berodier, E.; Scrivener, K. Understanding the filler effect on the nucleation and growth of C-S-H. J. Am. Ceram. Soc. 2014, 97, 3764–3773. [Google Scholar] [CrossRef]
- Hewlett, P.; Liska, M. Lea’s Chemistry of Cement and Concrete; Butterworth-Heinemann: Cambridge, MA, USA, 2019; p. 30. [Google Scholar]

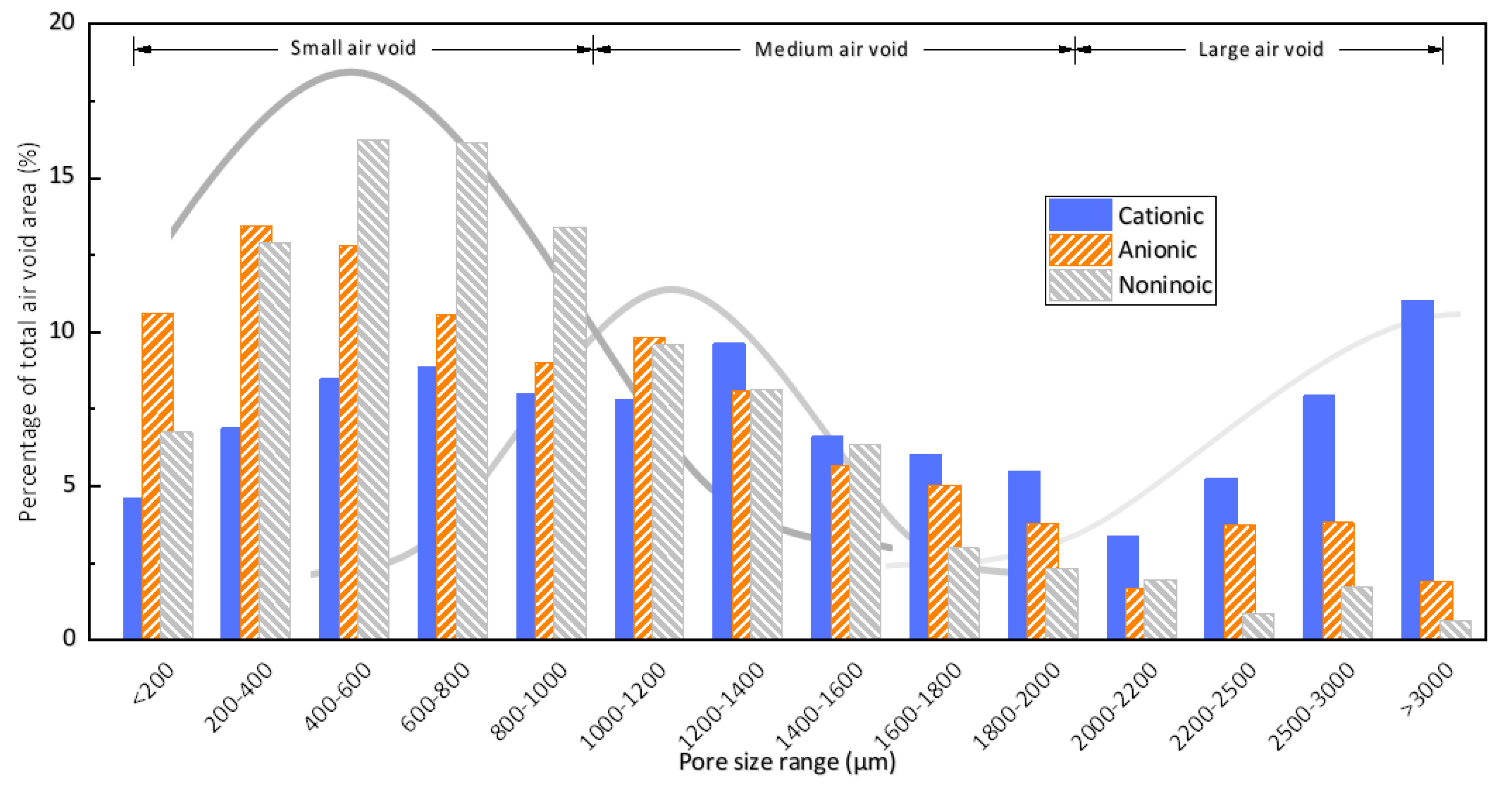
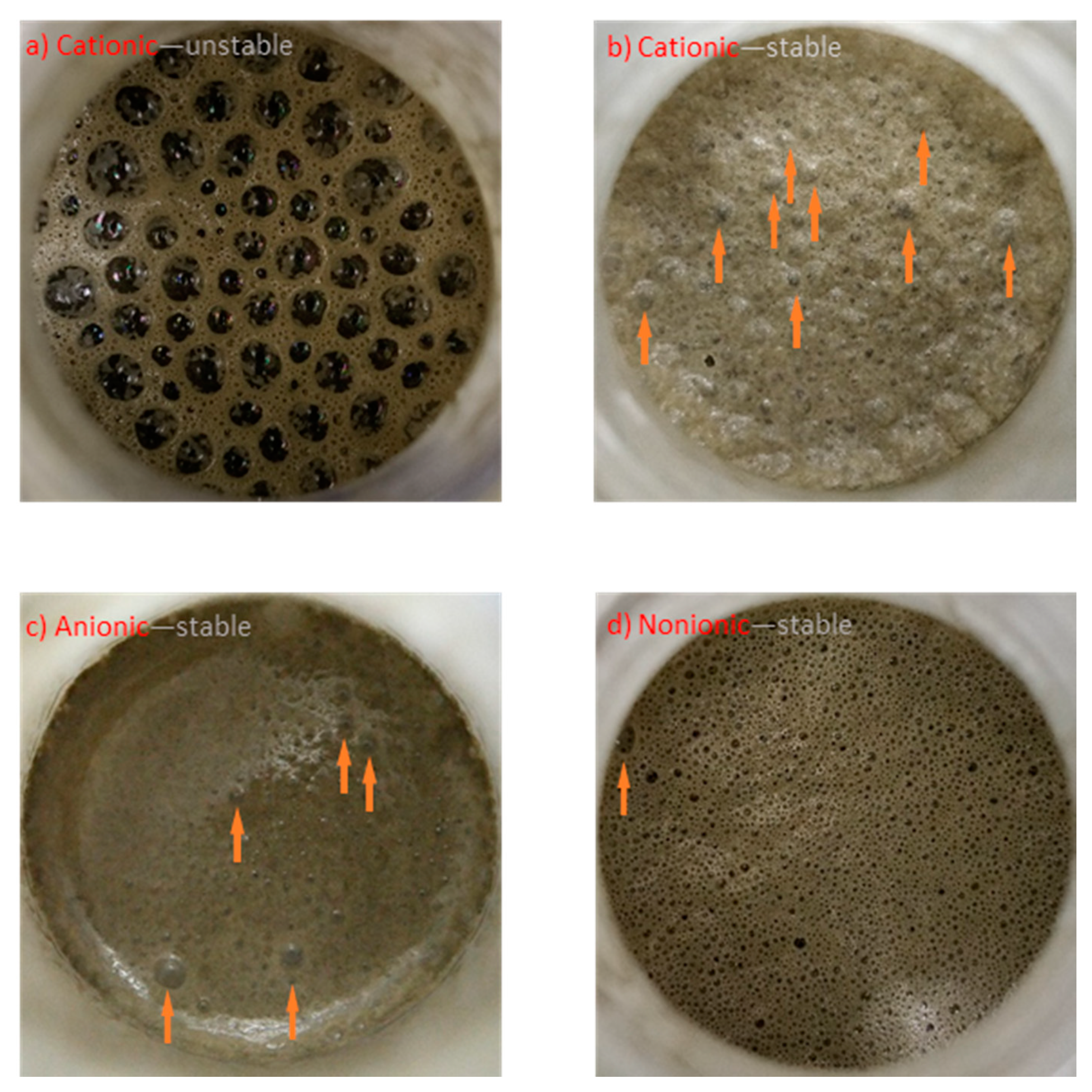

| Type of Surfactant | Proportion of Air Voids in Each Distribution Range/% | ||
|---|---|---|---|
| Small Air Voids (0–1000 μm) | Medium Air Voids (1000–2000 μm) | Large Air Voids (2000–3000 μm) | |
| Cationic | 36.88 | 35.56 | 27.56 |
| Anionic | 56.47 | 32.41 | 11.12 |
| Nonionic | 65.43 | 29.38 | 5.19 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Q.; Chen, Z.; Yang, Y. Study of the Air-Entraining Behavior Based on the Interactions between Cement Particles and Selected Cationic, Anionic and Nonionic Surfactants. Materials 2020, 13, 3514. https://doi.org/10.3390/ma13163514
Liu Q, Chen Z, Yang Y. Study of the Air-Entraining Behavior Based on the Interactions between Cement Particles and Selected Cationic, Anionic and Nonionic Surfactants. Materials. 2020; 13(16):3514. https://doi.org/10.3390/ma13163514
Chicago/Turabian StyleLiu, Qi, Zhitao Chen, and Yingzi Yang. 2020. "Study of the Air-Entraining Behavior Based on the Interactions between Cement Particles and Selected Cationic, Anionic and Nonionic Surfactants" Materials 13, no. 16: 3514. https://doi.org/10.3390/ma13163514
APA StyleLiu, Q., Chen, Z., & Yang, Y. (2020). Study of the Air-Entraining Behavior Based on the Interactions between Cement Particles and Selected Cationic, Anionic and Nonionic Surfactants. Materials, 13(16), 3514. https://doi.org/10.3390/ma13163514





