Compressive Strength and Durability of FGD Gypsum-Based Mortars Blended with Ground Granulated Blast Furnace Slag
Abstract
1. Introduction
2. Materials and Methods
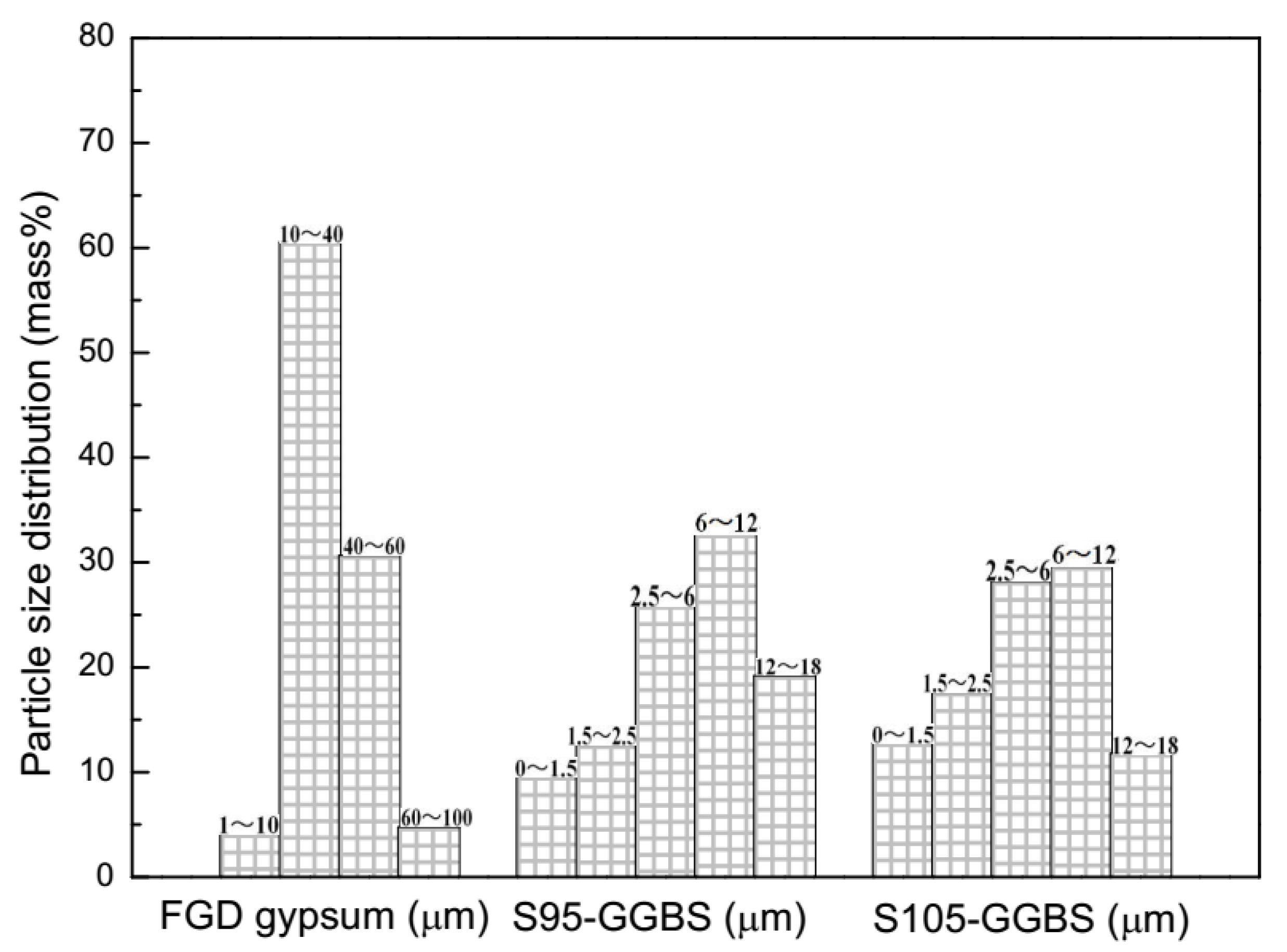
2.1. Materials
2.2. Compressive Strength
2.3. Resistance to Water
2.4. Resistance to Carbonation
2.5. Resistance to Freeze-Thaw Cycles
2.6. Drying Shrinkage
2.7. Microstructure Analysis
3. Results and Discussion
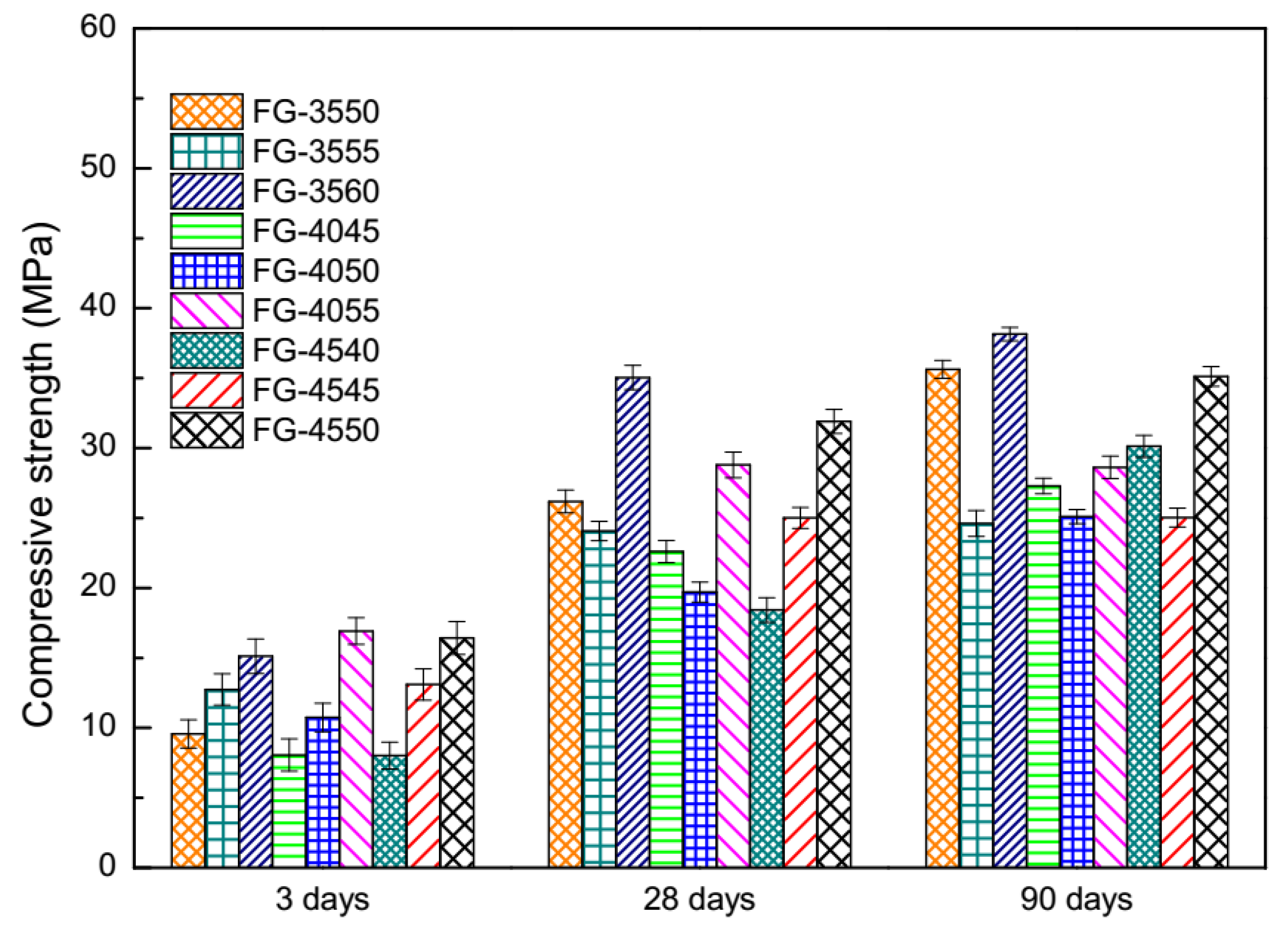
3.1. Compressive Strength under the Normal Curing Condition
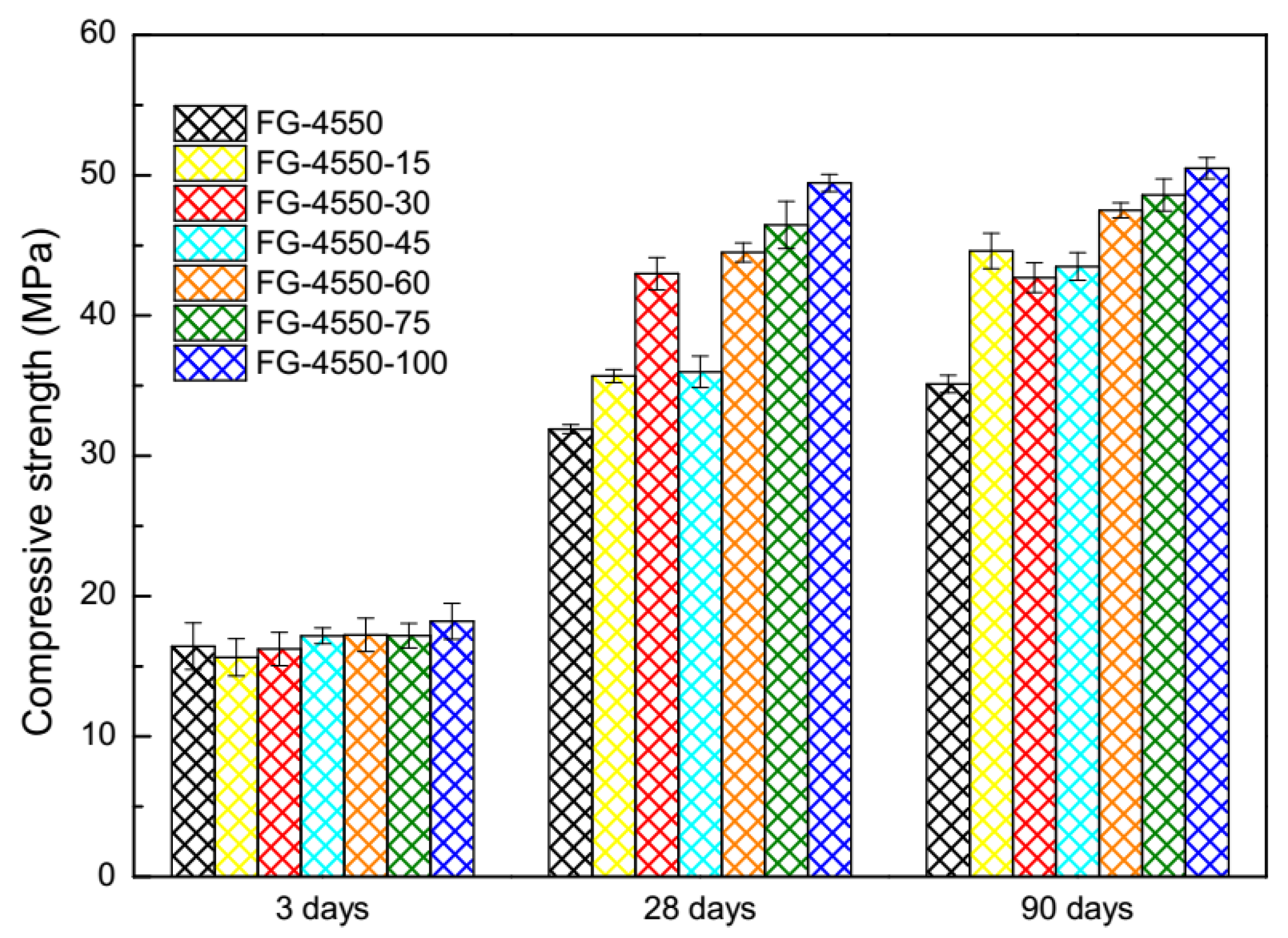
3.2. Compressive Strength of Activated FG-4550 under the Steam Curing Condition
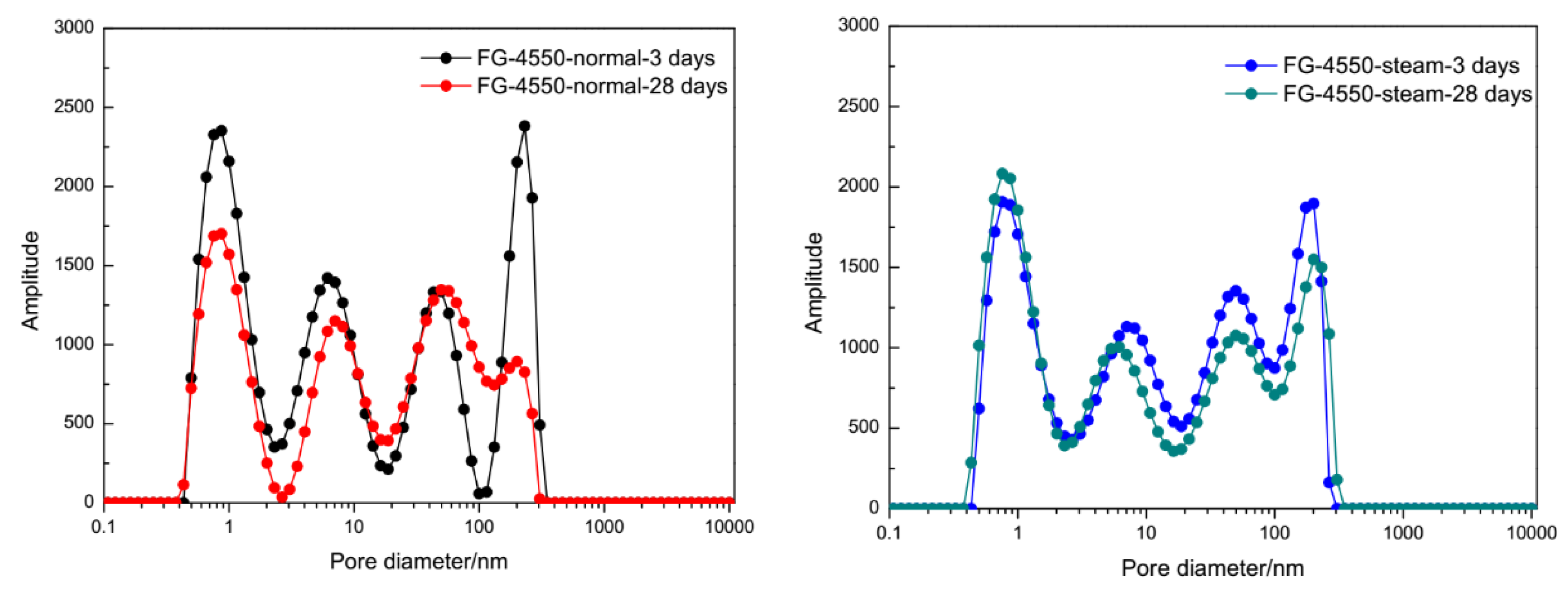
3.3. Durability of FG-4550 under the Steam Curing Condition
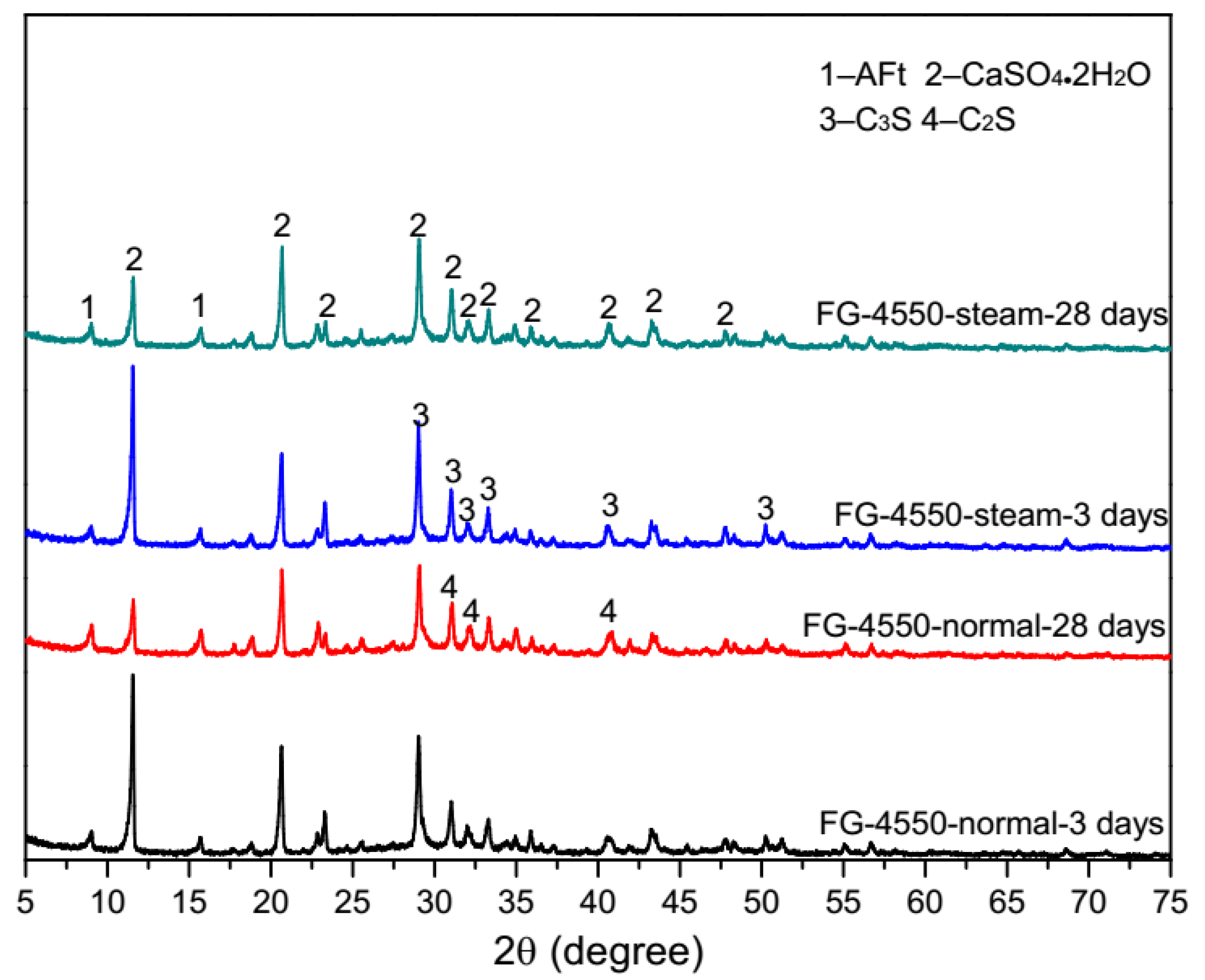
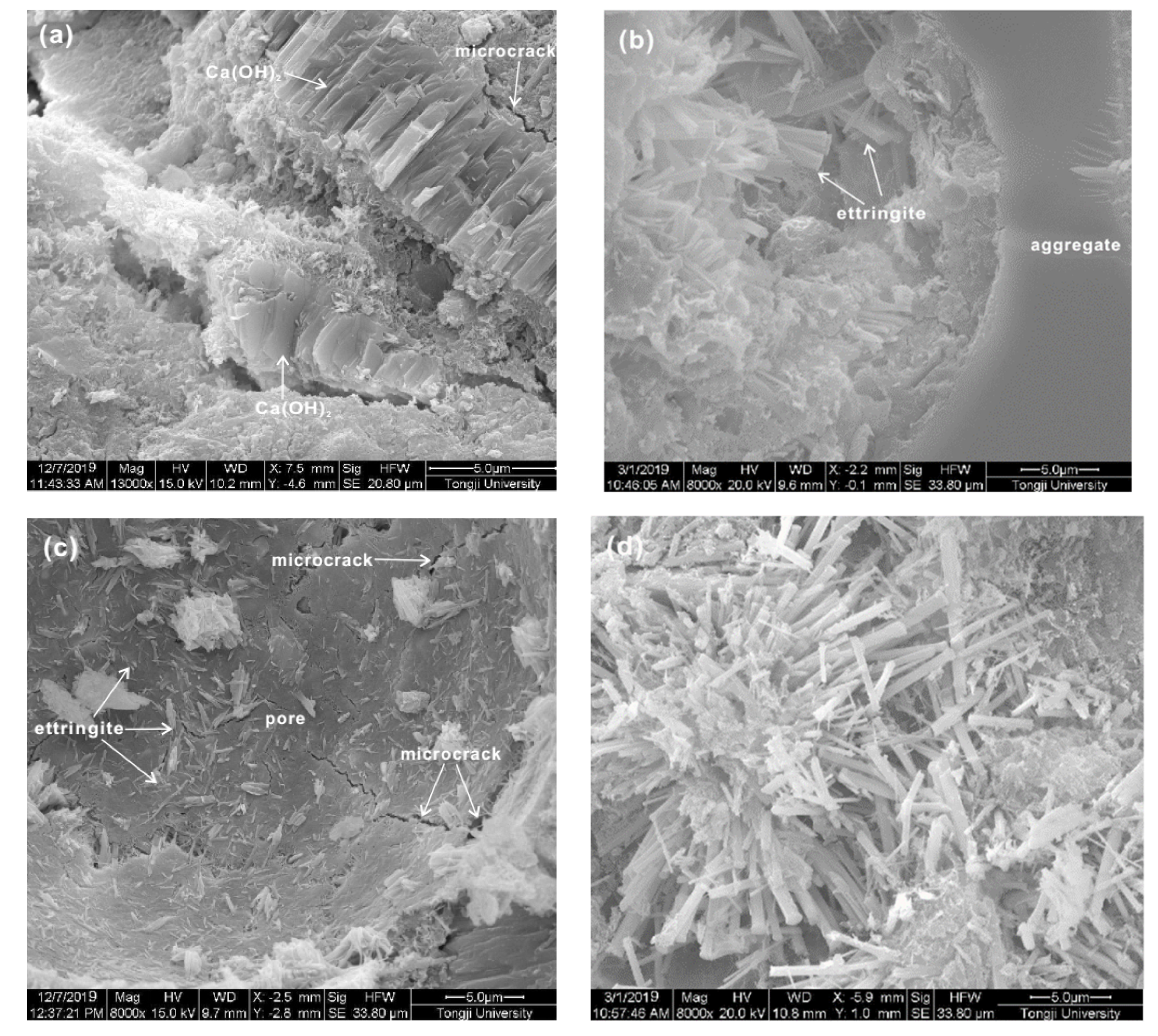
3.4. Microscopic Characteristics of FG-4550
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Charola, E.A.; Pühringer, J.; Steiger, M. Gypsum: A review of its role in the deterioration of building materials. Environ. Geol. 2007, 52, 339–352. [Google Scholar] [CrossRef]
- Chen, Z.; Sucech, S.; Faber, K.T. A hierarchical study of the mechanical properties of gypsum. J. Mater. Sci. 2010, 45, 4444–4453. [Google Scholar] [CrossRef]
- Stout, W.L.; Priddy, W.E. Use of flue gas desulfurization (FGD) by-product gypsum on alfalfa. Commun. Soil Sci. Plant Anal. 1996, 27, 2419–2432. [Google Scholar] [CrossRef]
- Yost, L.J.; Shock, S.S.; Holm, S.E.; Lowney, Y.W.; Noggle, J.J. Lack of complete exposure pathways for metals in natural and FGD gypsum. Hum. Ecol. Risk Assess. 2010, 16, 317–339. [Google Scholar] [CrossRef]
- Lei, D.Y.; Guo, L.P.; Sun, W.; Liu, J.P.; Miao, C.W. Study on properties of untreated FGD gypsum-based high-strength building materials. Constr. Build. Mater. 2017, 153, 765–773. [Google Scholar] [CrossRef]
- Goodwin, R.W. Resource recovery from flue gas desulfurization systems. J. Air Pollut. Control. Assoc. 1982, 32, 986–989. [Google Scholar] [CrossRef]
- Coppola, L.; Belz, G.; Dinelli, G.; Collepardi, M. Prefabricated building elements based on FGD gypsum and ashes from coal-fired electric generating plants. Mater. Struct. 1996, 29, 305–311. [Google Scholar] [CrossRef]
- Lee, J.Y.; Cho, K.M.; Cheng, L.; Keener, T.C.; Jegadeesan, G.; Al-Abed, S.R. Investigation of a mercury speciation technique for flue gas desulfurization materials. J. Air Waste Manag. Assoc. 2009, 59, 972–979. [Google Scholar] [CrossRef]
- Rust, D.; Rathbone, R.; Mahboub, K.C.; Robl, T. Formulating low-energy cement products. J. Mater. Civ. Eng. 2012, 24, 1125–1131. [Google Scholar] [CrossRef]
- Guan, B.H.; Lou, W.B.; Ye, Q.Q.; Fu, H.L.; Wu, Z.B. Calorimetric study of calcium aluminate cement blended with flue gas desulfurization gypsum. J. Therm. Anal. Calorim. 2009, 98, 737–742. [Google Scholar] [CrossRef]
- Tzouvalas, G.; Rantis, G.; Tsimas, S. Alternative calcium-sulfate-bearing materials as cement retarders: Part II. FGD Gypsum. Cem. Concr. Res. 2004, 34, 2119–2125. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Yu, P.; Pan, F.; He, Y. The synergistic effect of AFt enhancement and expansion in portland cement-aluminate cement-FGD gypsum composite cementitious system. Constr. Build. Mater. 2018, 190, 985–994. [Google Scholar] [CrossRef]
- Lou, W.B.; Guan, B.H.; Wu, Z.B. Calorimetric study of ternary binder of calcium aluminate cement, portland-limestone cement and FGD gypsum. J. Therm. Anal. Calorim. 2010, 101, 119–127. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Zhang, Y.H.; Guo, Y.X.; Chu, P.K.; Tu, S.C. Porous materials composed of flue gas desulfurization gypsum and textile fiber wastes. Waste Biomass Valoriz. 2017, 8, 203–207. [Google Scholar] [CrossRef]
- Wu, Q.S.; Ma, H.G.; Chen, Q.J.; Gu, B.; Li, S.P.; Zhu, H.J. Effect of silane modified styrene-acrylic emulsion on the waterproof properties of flue gas desulfurization gypsum. Constr. Build. Mater. 2019, 197, 506–512. [Google Scholar] [CrossRef]
- Guan, B.H.; Shen, Z.X.; Wu, Z.B.; Yang, L.C.; Ma, X.F. Effect of pH on the preparation of α-calcium sulfate hemihydrate from FGD gypsum with the hydrothermal method. J. Am. Ceram. Soc. 2008, 91, 3835–3840. [Google Scholar] [CrossRef]
- Shen, Z.X.; Guan, B.H.; Fu, H.L.; Yang, L.C. Effect of potassium sodium tartrate and sodium citrate on the preparation of a-calcium sulfate hemihydrate from flue gas desulfurization gypsum in a concentrated electrolyte solution. J. Am. Ceram. Soc. 2009, 92, 2894–2899. [Google Scholar] [CrossRef]
- Jinawath, S.; Thitianan, C. Production of multiphase plaster and anhydrite from Mae Moh flue-gas desulphurised gypsum. Adv. Cem. Res. 2002, 14, 121–126. [Google Scholar] [CrossRef]
- Prakaypun, W.; Jinawath, S. Comparative effect of additives on the mechanical properties of plasters made from flue-gas desulfurized and natural gypsums. Mater. Struct. 2003, 36, 51–58. [Google Scholar] [CrossRef]
- Guo, X.L.; Shi, H.S. Influence of thermally treated flue gas desulfurization (FGD) gypsum on performance of the slag powder concrete. J. Wuhan Univ. Technol.-Mater. Sci. Ed. 2013, 28, 1122–1127. [Google Scholar] [CrossRef]
- Matschei, T.; Bellmann, F.; Stark, J. Hydration behaviour of sulphate-activated slag cements. Adv. Cem. Res. 2005, 17, 167–178. [Google Scholar] [CrossRef]
- Gruskovnjak, A.; Lothenbach, B.; Winnefeld, F.; Figi, R.; Ko, S.C.; Adler, M.; Mäder, U. Hydration mechanisms of super sulphated slag cement. Cem. Concr. Res. 2008, 38, 983–992. [Google Scholar] [CrossRef]
- Masoudi, R.; Hooton, R.D. Examining the hydration mechanism of supersulfated cements made with high and low-alumina slags. Cem. Concr. Compos. 2019, 103, 193–203. [Google Scholar] [CrossRef]
- Nguyena, H.A.; Chang, T.P.; Shih, J.Y.; Chen, C.T. Influence of low calcium fly ash on compressive strength and hydration product of low energy super sulfated cement paste. Cem. Concr. Compos. 2019, 99, 40–48. [Google Scholar] [CrossRef]
- Rubert, S.; Angulski da Luz, C.; Varela, M.V.F.; Pereira Filho, J.I.; Hooton, R.D. Hydration mechanisms of supersulfated cement-The role of alkali activator and calcium sulfate content. J. Therm. Anal. Calorim. 2018, 134, 971–980. [Google Scholar] [CrossRef]
- Liu, S.H.; Wang, L.; Gao, Y.X.; Yu, B.Y.; Bai, Y. Comparing study on hydration properties of various cementitious systems. J. Therm. Anal. Calorim. 2014, 118, 1483–1492. [Google Scholar] [CrossRef]
- Ding, S.; Shui, Z.H.; Chen, W.; Lu, J.X.; Tian, S.F. Properties of supersulphated phosphogysum-slag cement (SSC) concrete. J. Wuhan Univ. Technol.-Mater. Sci. Ed. 2014, 29, 109–113. [Google Scholar] [CrossRef]
- Grounds, T.; Nowell, D.V.; Wilburn, F.W. Resistance of supersulfated cement to strongsulfate solutions. J. Therm. Anal. Calorim. 2003, 72, 181–190. [Google Scholar] [CrossRef]
- Collier, N.C.; Milestone, N.B.; Gordon, L.E.; Ko, S.C. The suitability of a supersulfated cement for nuclear waste immobilisation. J. Nucl. Mater. 2014, 452, 457–464. [Google Scholar] [CrossRef]
- Collier, N.C.; Li, X.; Bai, Y.; Milestone, N.B. The effect of sulfate activation on the early age hydration of BFS: PC composite cement. J. Nucl. Mater. 2015, 464, 128–134. [Google Scholar] [CrossRef]
- Liu, J.; Hu, L.; Tang, L.P.; Zhang, E.Q.; Ren, J. Shrinkage behaviour, early hydration and hardened properties of sodium silicate activated slag incorporated with gypsum and cement. Constr. Build. Mater. 2020, 248, 118687. [Google Scholar] [CrossRef]
- Nguyen, H.; Adesanya, E.; Ohenoja, K.; Kriskova, L.; Pontikes, Y.; Kinnunen, P.; Illikainen, M. Byproduct-based ettringite binder-a synergy between ladle slag and gypsum. Constr. Build. Mater. 2019, 197, 143–151. [Google Scholar] [CrossRef]
- Standard for Test Method of Testing-Determination of Strength (GB/T17671-1999); Chinese State Bureau of Quality Technical Supervision: Beijing, China, 1999.
- Standard for Test Method of Long-term Performance and Durability of Ordinary Concrete (GBT50082-2009); Ministry of Housing and Urban-Rural Development of the People’s Republic of China: Beijing, China, 2009.
- Muller, A.C.A.; Scrivener, K.L.; Gajewicz, A.M.; McDonald, P.J. Densification of C-S-H measured by 1H NMR relaxometry. J. Phys. Chem. C 2013, 117, 403–412. [Google Scholar] [CrossRef]
- Bortolotti, V.; Brizi, L.; Brown, R.J.S.; Fantazzini, P.; Mariani, M. Nano and sub-nano multiscale porosity formation and other features revealed by 1H NMR relaxometry during cement hydration. Langmuir 2014, 30, 10871–10877. [Google Scholar] [CrossRef] [PubMed]
- Cano-Barrita, P.F.d.J.; Balcom, B.J.; Castellanos, F. Carbonation front in cement paste detected by T2 NMR measurements using a low field unilateral magnet. Mater. Struct. 2017, 50, 150. [Google Scholar] [CrossRef]
- Tziotziou, M.; Karakosta, E.; Karatasios, I.; Diamantopoulos, G.; Sapalidis, A.; Fardis, M.; Maravelaki-Kalaitzaki, P.; Papavassiliou, G.; Kilikoglou, V. Application of 1H NMR to hydration and porosity studies of lime–pozzolan mixtures. Microporous Mesoporous Mater. 2011, 139, 16–24. [Google Scholar] [CrossRef]
- Valckenborg, R.M.E.; Pel, L.; Hazrati, K.; Kopinga, K.; Marchand, J. Pore water distribution in mortar during drying as determined by NMR. Mater. Struct. 2001, 34, 599–604. [Google Scholar] [CrossRef]
- Sun, Z.P.; Pang, M.; Yu, Y.; Yang, P.Q.; Yu, W.W. Effect of superplasticizer on transverse relaxation time curve of cement paste. J. Chin. Ceram. Soc. 2011, 39, 537–543. [Google Scholar]
- Yu, Y.; Sun, Z.P.; Pang, M.; Yang, P.Q. Probing development of microstructure of early cement paste using 1H low-field NMR. J. Wuhan Univ. Technol.-Mater. Sci. Ed. 2013, 28, 963–967. [Google Scholar] [CrossRef]
- Halperin, W.P.; Jehng, J.Y.; Song, Y.Q. Application of spin-spin relaxation to measurement of surface area and pore size distributions in a hydrating cement paste. Magn. Reson. Imaging 1994, 12, 169–173. [Google Scholar] [CrossRef]
- Riding, K.; Silva, D.A.; Scrivener, K. Early age strength enhancement of blended cement systems by CaCl2 and diethanol-isopropanolamine. Cem. Concr. Res. 2010, 40, 935–946. [Google Scholar] [CrossRef]
- Juenger, M.C.G.; Monteiro, P.J.M.; Gartner, E.M.; Denbeaux, G.P. A soft X-ray microscope investigation into the effects of calcium chloride on tricalcium silicate hydration. Cem. Concr. Res. 2005, 35, 19–25. [Google Scholar] [CrossRef]
- Aldea, C.M.; Young, F.; Wang, K.J.; Shah, S.P. Effects of curing conditions on properties of concrete using slag replacement. Cem. Concr. Res. 2000, 30, 465–472. [Google Scholar] [CrossRef]
- Liu, C.B.; Gao, J.M.; Tang, Y.B.; Chen, X.M. Preparation and characterization of gypsum-based materials used for 3D robocasting. J. Mater. Sci. 2018, 53, 16415–16422. [Google Scholar] [CrossRef]
- Filippov, A.V.; Khosina, E.V.; Khosin, V.G. Liquid self-diffusion in pores of hardened gypsum: Pulsed field gradient NMR study. J. Mater. Sci. 1996, 31, 1809–1814. [Google Scholar] [CrossRef]


| Components | SiO2 | CaO | Al2O3 | Fe2O3 | MgO | Na2O | K2O | TiO2 | SO3 | Crystal Water |
|---|---|---|---|---|---|---|---|---|---|---|
| FGD gypsum | 1.42 | 32.2 | 2.17 | 0.75 | 0.52 | 0.08 | 0.1 | 0.1 | 41.25 | 18.28 |
| Cement | 20.8 | 61.3 | 6.34 | 3.1 | 1.0 | 0.35 | 0.5 | 0.29 | 2.3 | - |
| S95-GGBS | 31.2 | 41.4 | 14.6 | 0.4 | 0.3 | 0.28 | 0.27 | 0.66 | 2.3 | - |
| Sample | FGD Gypsum | S95-GGBS | Cement |
|---|---|---|---|
| FG-3550 | 35 | 50 | 15 |
| FG-3555 | 35 | 55 | 10 |
| FG-3560 | 35 | 60 | 5 |
| FG-4045 | 40 | 45 | 15 |
| FG-4050 | 40 | 50 | 10 |
| FG-4055 | 40 | 55 | 5 |
| FG-4540 | 45 | 40 | 15 |
| FG-4545 | 45 | 45 | 10 |
| FG-4550 | 45 | 50 | 5 |
| Sample | FGD Gypsum | Cement | S95-GGBS | S105-GGBS |
|---|---|---|---|---|
| FG-4550 | 45 | 5 | 50 | 0 |
| FG-4550-15 | 45 | 5 | 42.5 | 7.5 |
| FG-4550-30 | 45 | 5 | 15 | 15 |
| FG-4550-45 | 45 | 5 | 27.5 | 22.5 |
| FG-4550-60 | 45 | 5 | 20 | 30 |
| FG-4550-75 | 45 | 5 | 12.5 | 37.5 |
| FG-4550-100 | 45 | 5 | 0 | 50 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pang, M.; Sun, Z.; Huang, H. Compressive Strength and Durability of FGD Gypsum-Based Mortars Blended with Ground Granulated Blast Furnace Slag. Materials 2020, 13, 3383. https://doi.org/10.3390/ma13153383
Pang M, Sun Z, Huang H. Compressive Strength and Durability of FGD Gypsum-Based Mortars Blended with Ground Granulated Blast Furnace Slag. Materials. 2020; 13(15):3383. https://doi.org/10.3390/ma13153383
Chicago/Turabian StylePang, Min, Zhenping Sun, and Huihao Huang. 2020. "Compressive Strength and Durability of FGD Gypsum-Based Mortars Blended with Ground Granulated Blast Furnace Slag" Materials 13, no. 15: 3383. https://doi.org/10.3390/ma13153383
APA StylePang, M., Sun, Z., & Huang, H. (2020). Compressive Strength and Durability of FGD Gypsum-Based Mortars Blended with Ground Granulated Blast Furnace Slag. Materials, 13(15), 3383. https://doi.org/10.3390/ma13153383




