Novel Etching Technique for Delineation of Prior-Austenite Grain Boundaries in Low, Medium and High Carbon Steels
Abstract
1. Introduction
1.1. Chemical Etching
1.2. Oxidation Etching
1.3. Thermal Etching
1.4. Other Methods
2. Materials and Methods
Atomic Force Microscopy
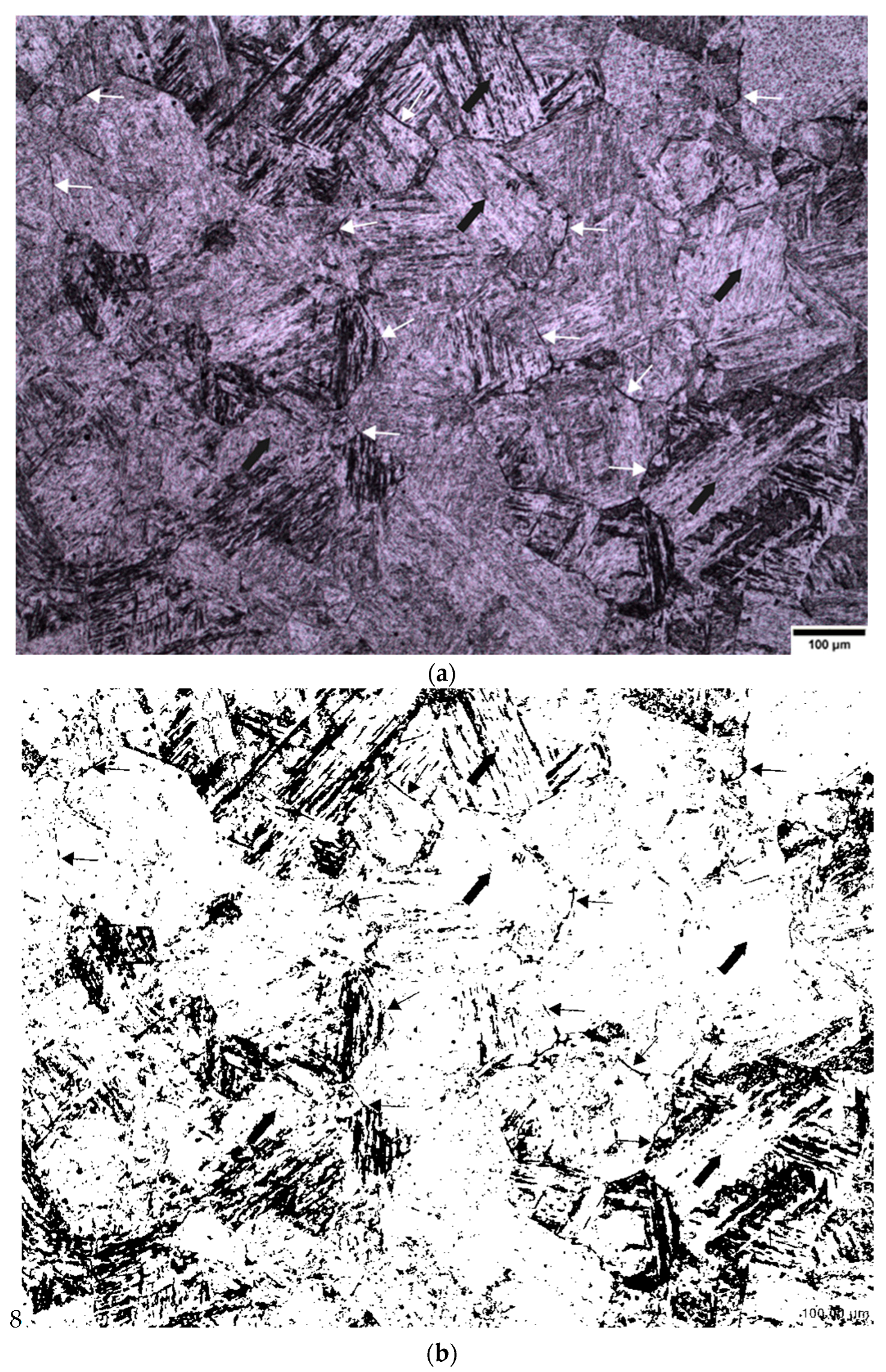
3. Results
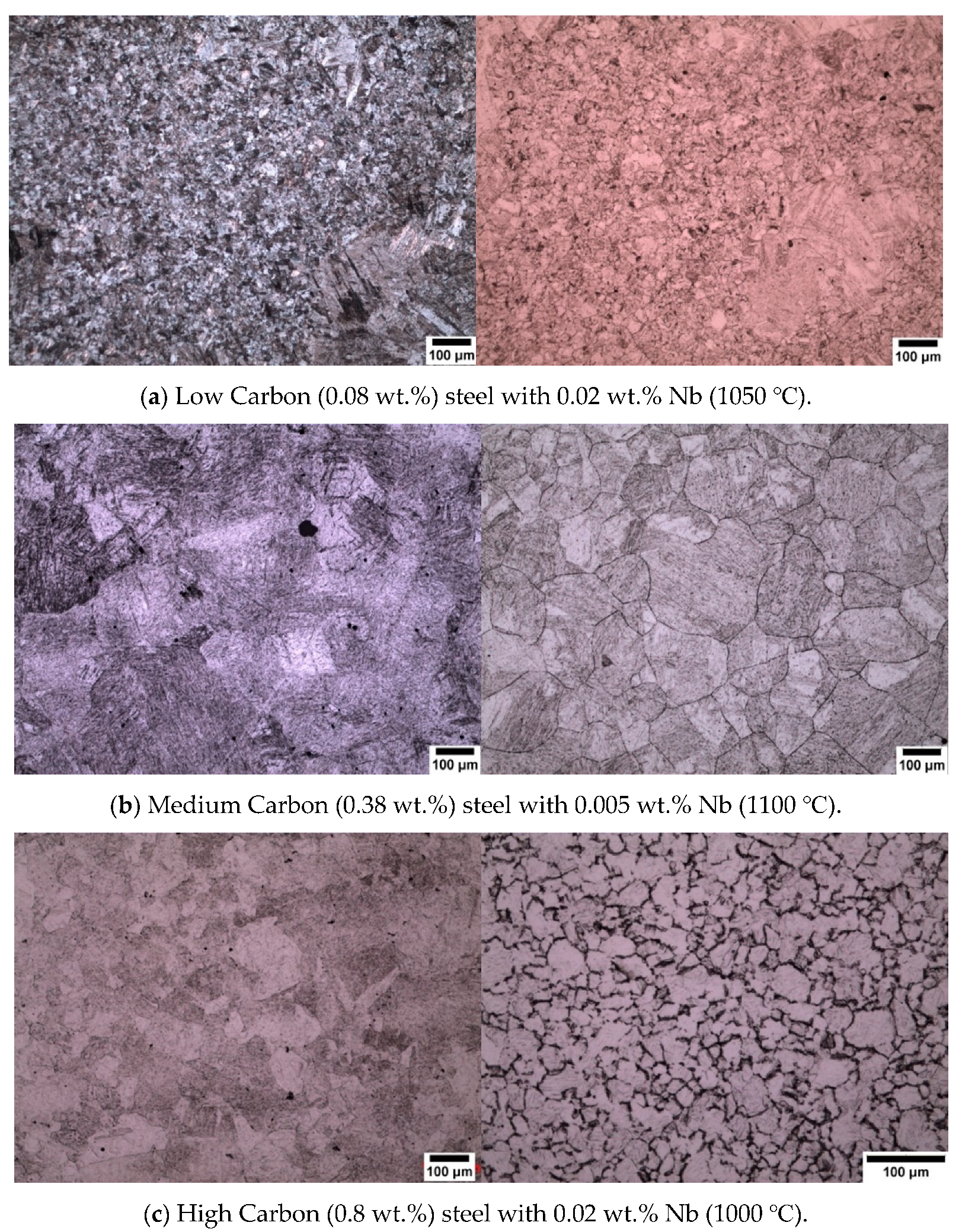
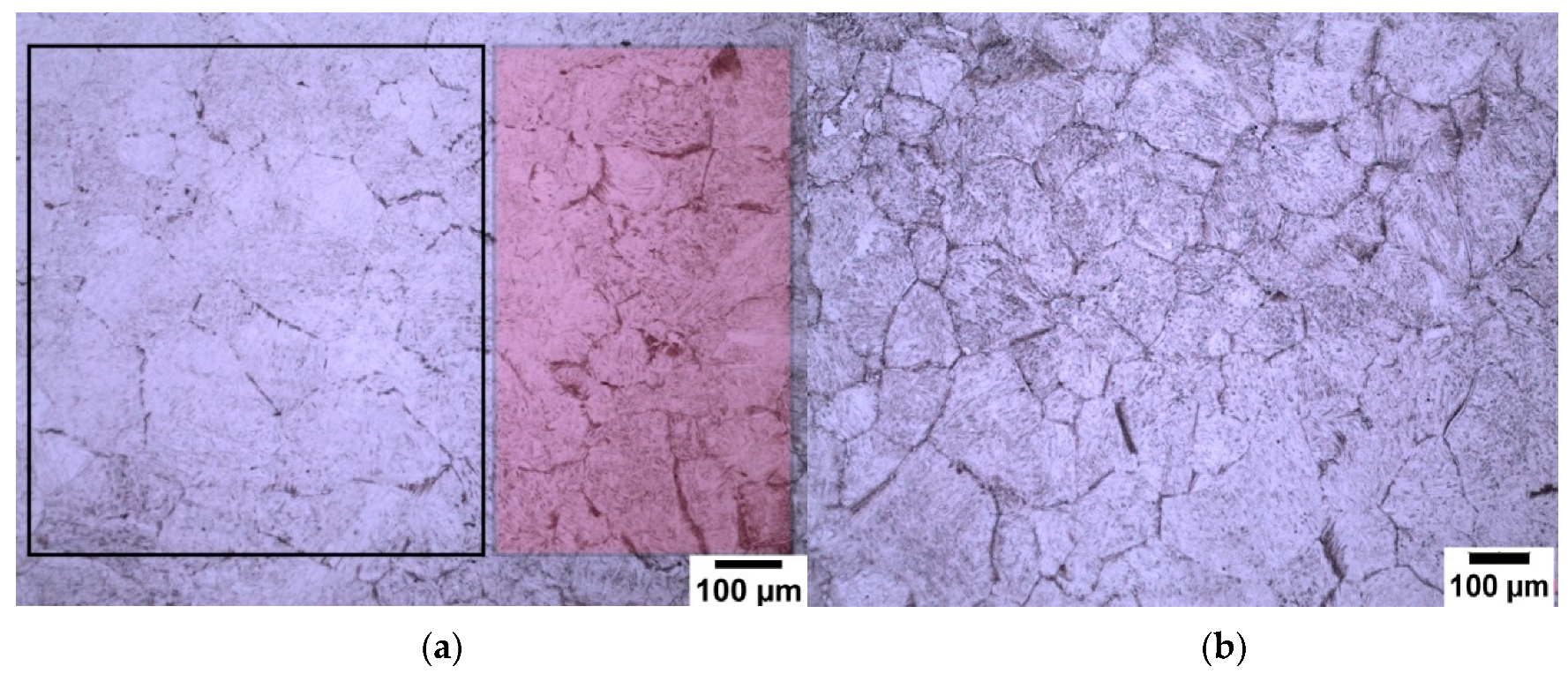
3.1. Sodium Alkylate Sulfonate (Teepol)
3.2. New Wetting Agents
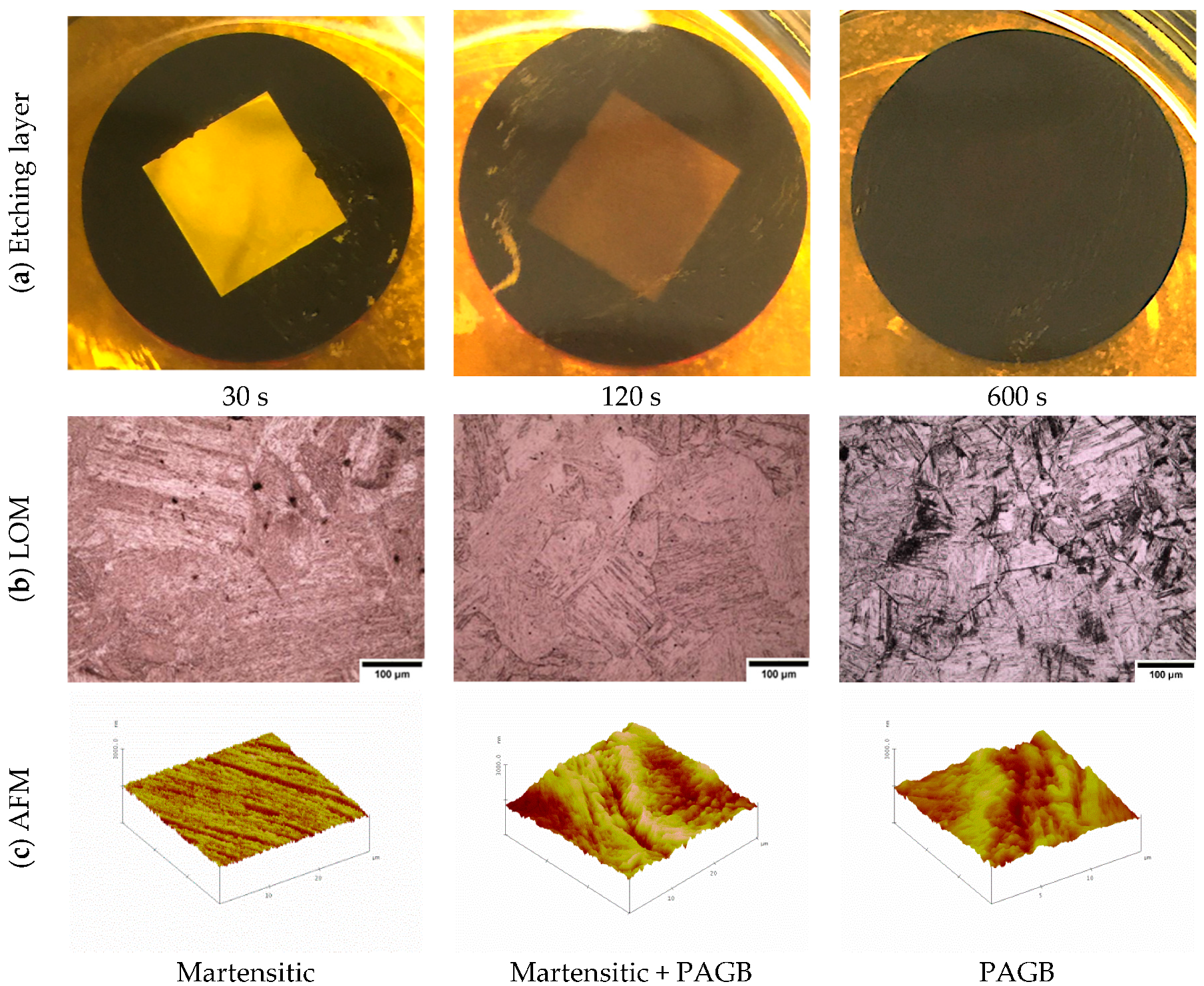
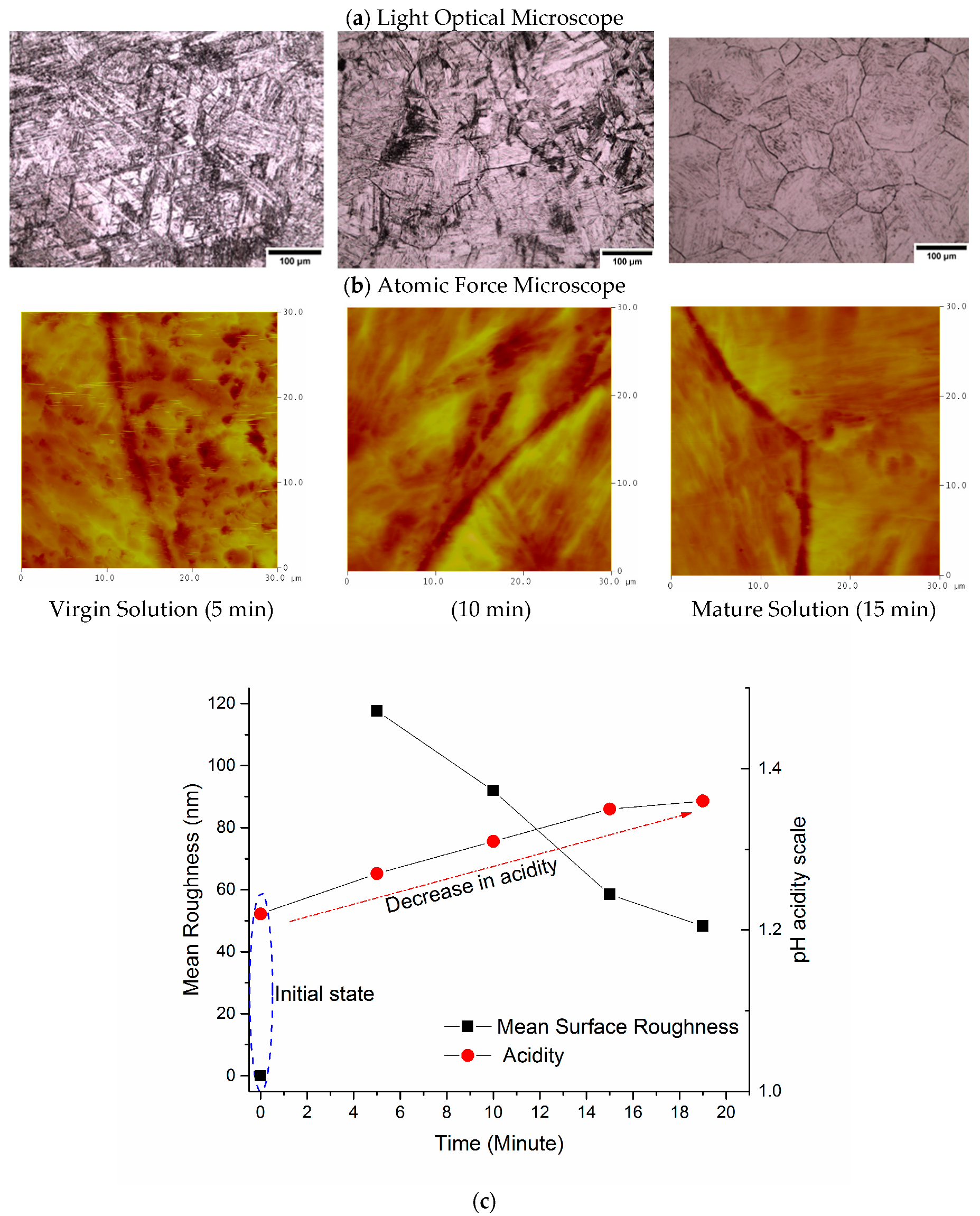
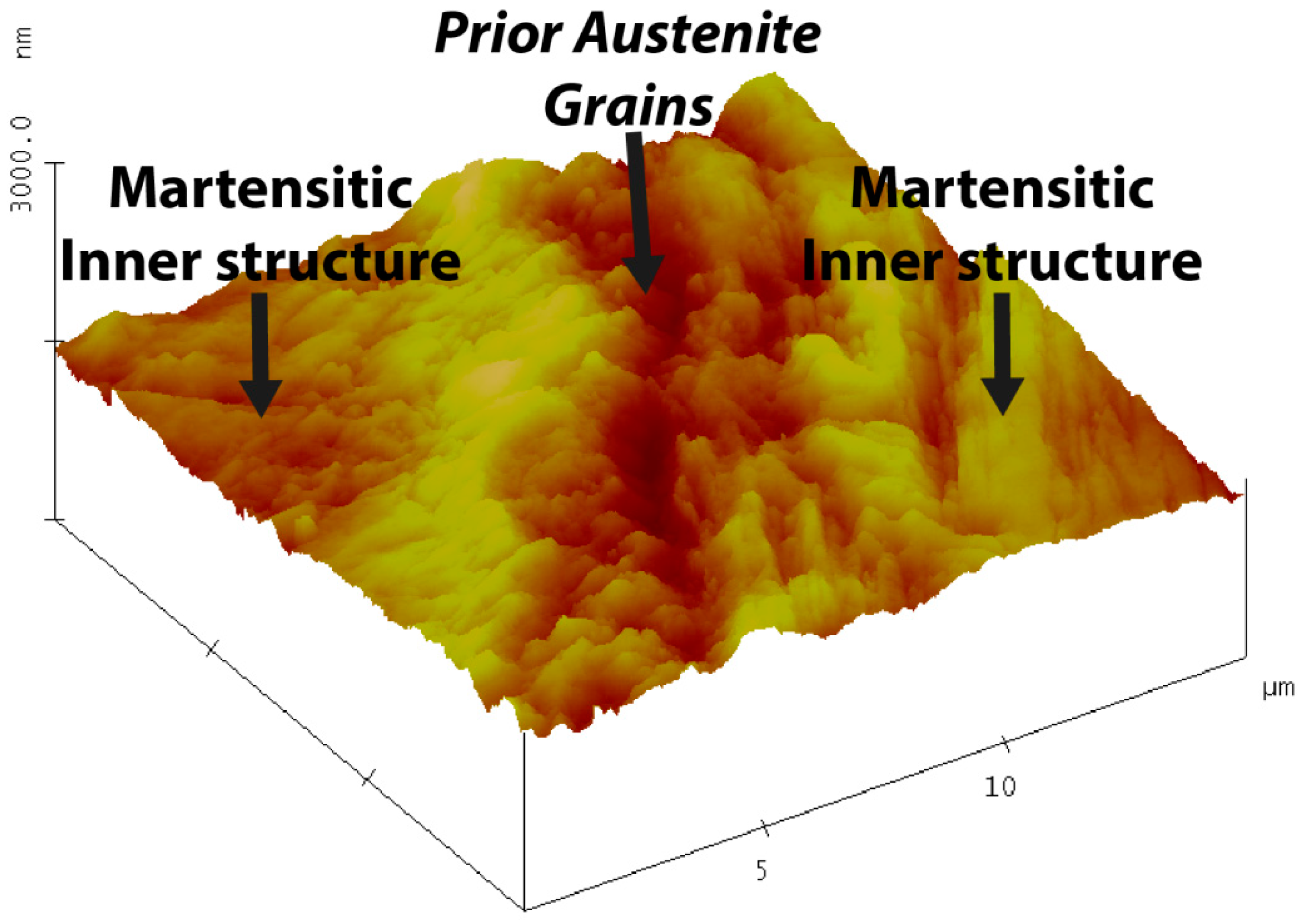
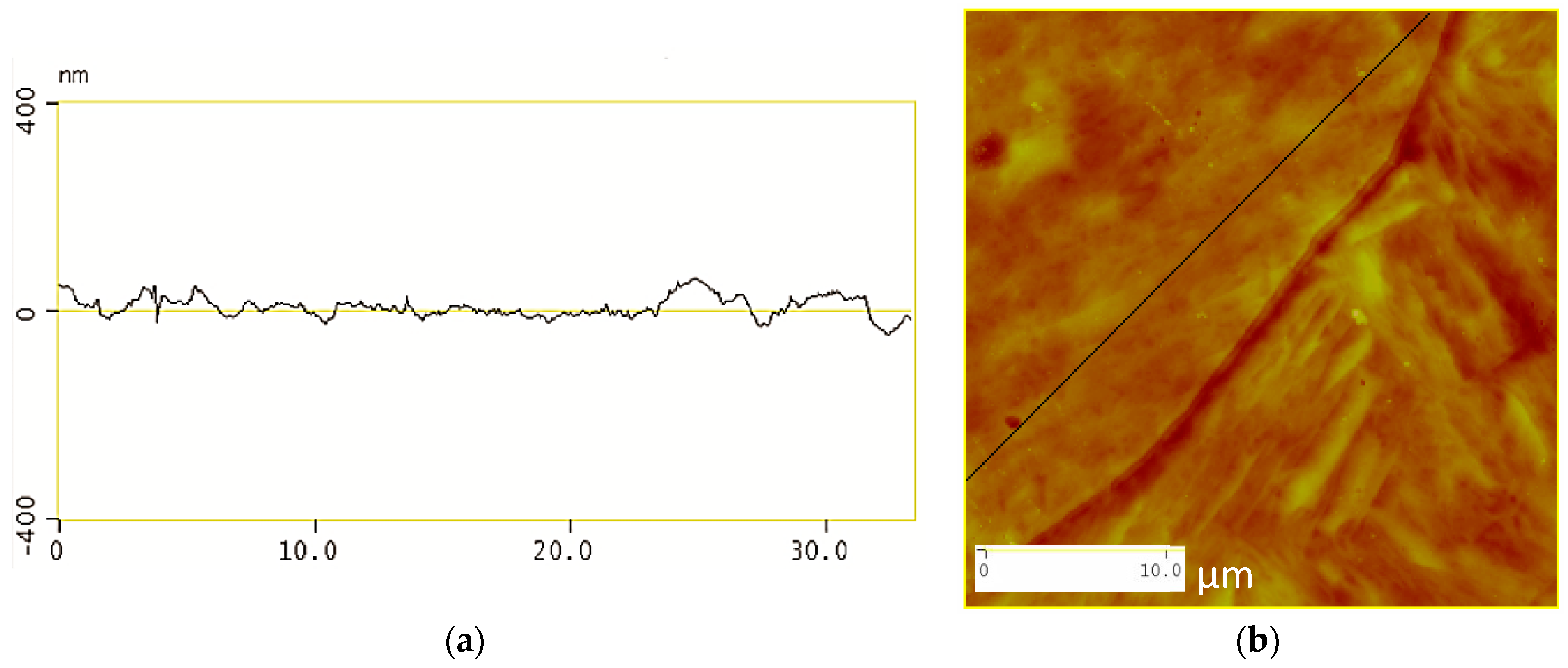
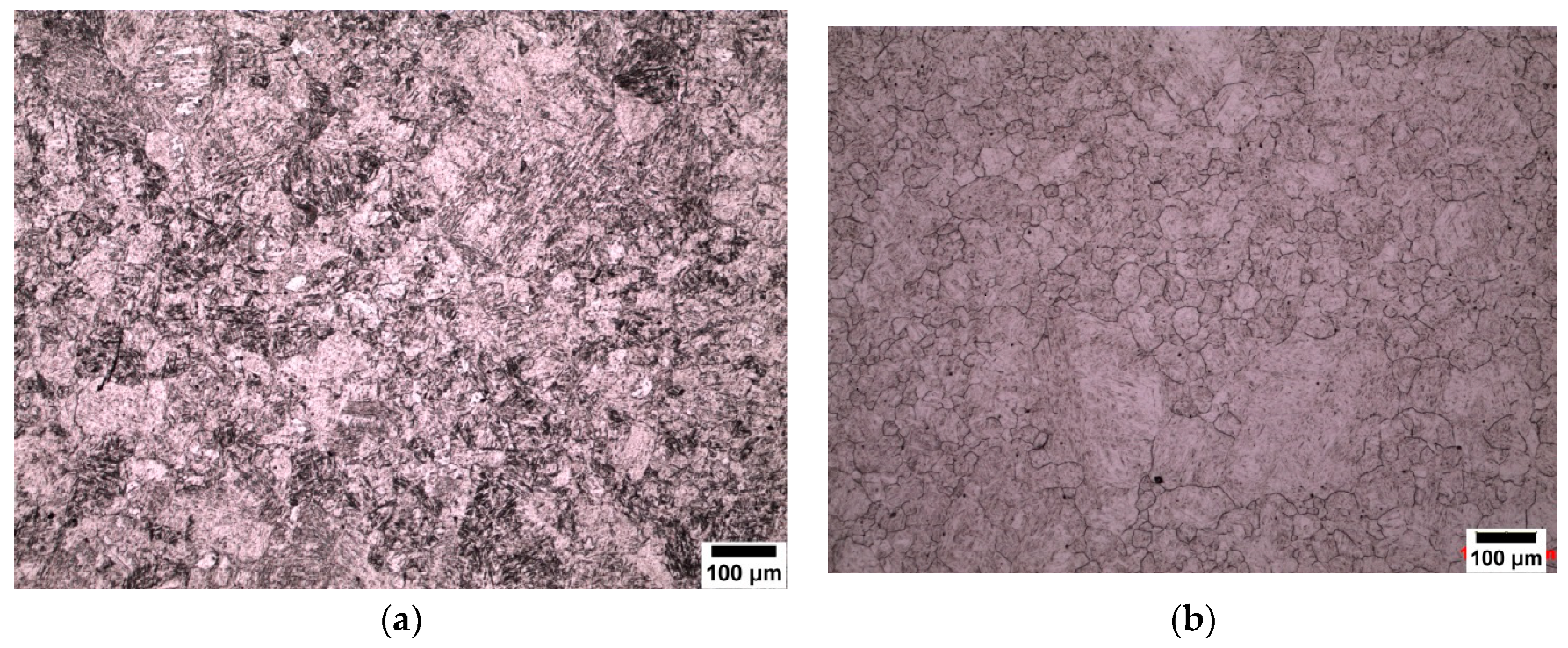
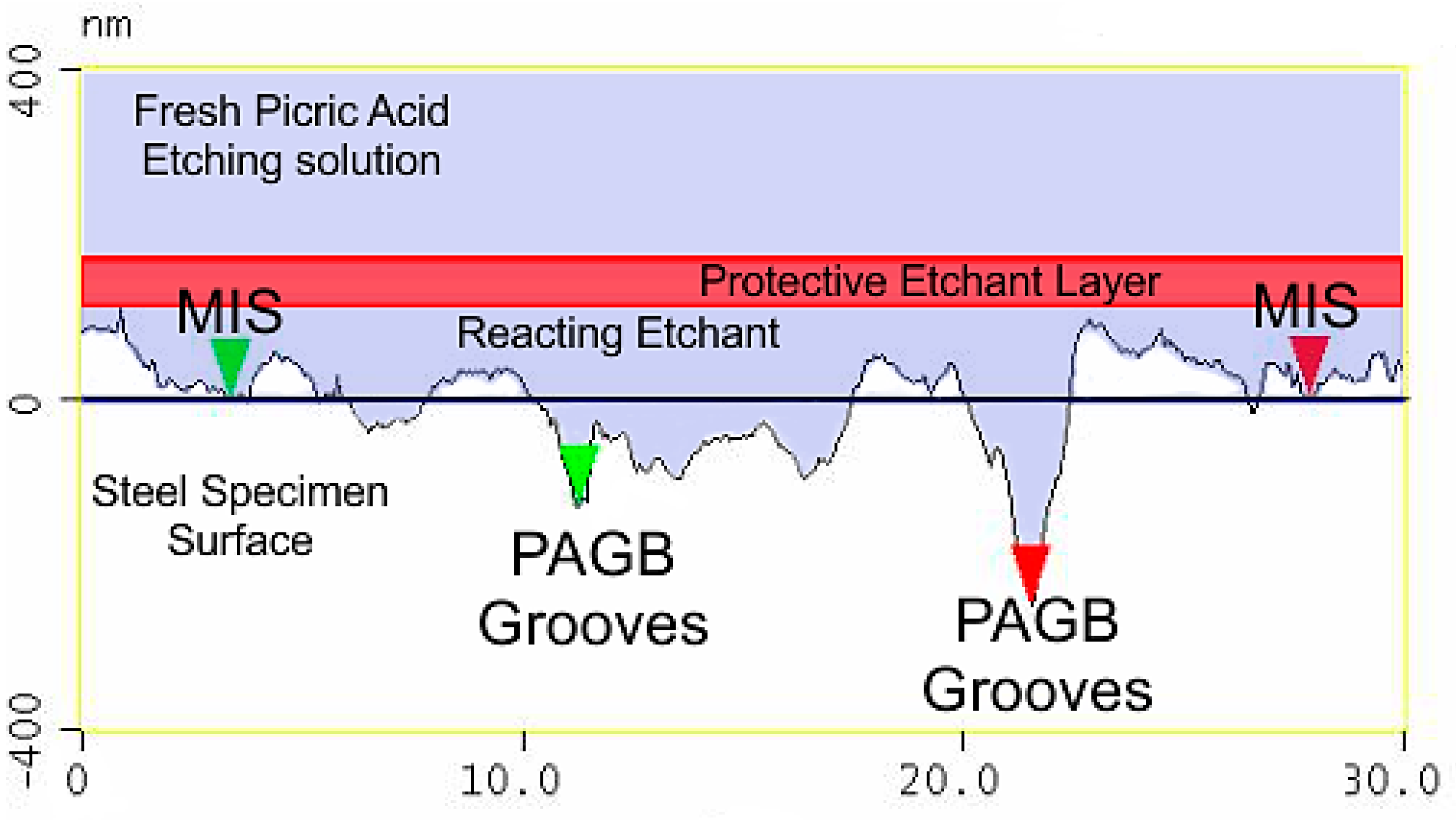
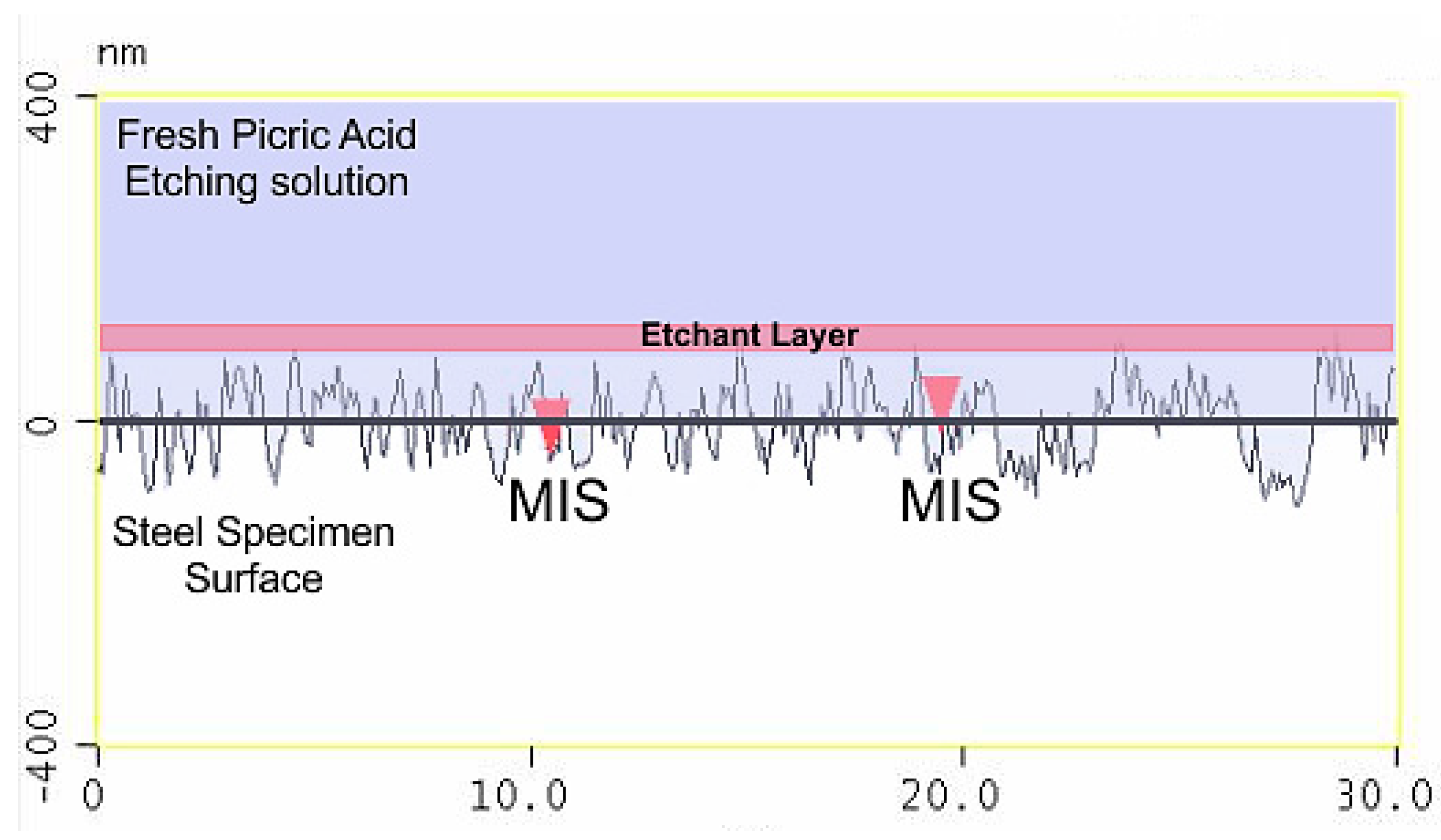
3.3. Atomic Force Microscopy (AFM)
4. Discussion
4.1. Effect of Wetting Agent
4.2. Effect of pH Concentration
4.3. Effect of the Etchant Layer
4.4. Novel Etching Mechanisms
5. Conclusions
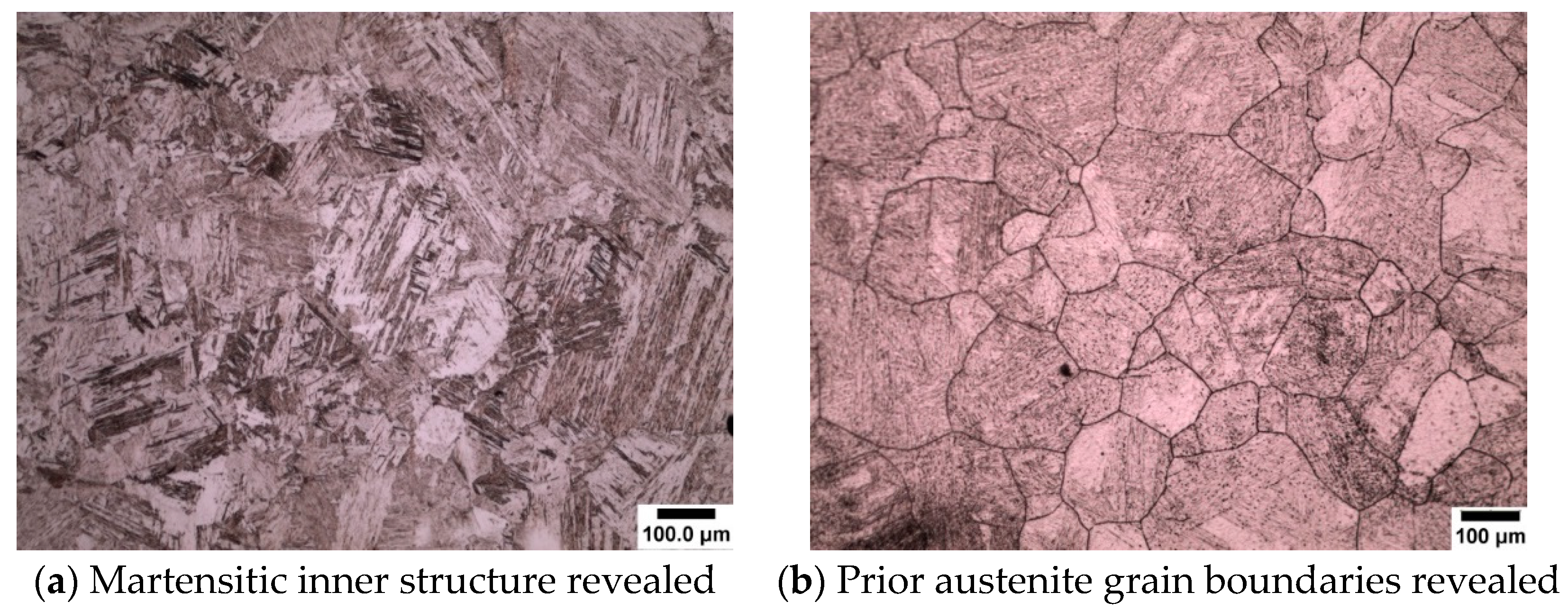
- The use of sodium dodecyl sulfate (SDS) as a wetting agent for high purity low (0.08 wt.%) and medium (0.38 wt.%) carbon microalloyed steel gives superior results for the delineation of prior austenite grain boundaries.
- The use of sodium dodecylbenzene sulfonate (SDBS) was shown to give good results in the revealing of prior austenite grain boundaries in high purity high carbon (0.8 wt.%) microalloyed steel.
- The etchant layer which forms on the specimen surface is an important part of the overall etching mechanism.
- Swabbing should not be done during etching after the etchant layer has formed on the surface.
- Using dummy specimens in the etchant solution prior to etching the real samples reduces the risk of needing to re-polish and re-etch.
- The roughness of the etched specimens decreases with a decrease in the acidity of the etching solution as indicated by the atomic force micrographs.
Author Contributions
Funding
Conflicts of Interest
References
- Voort, G.F.V. Metallography Principles and Practice; ASM International: New York, NY, USA, 1984; p. 438. [Google Scholar]
- Barraclough, D.R. Etching of Prior Austenite Grain Boundaries in Martensite. Metallography 1973, 6, 465–472. [Google Scholar] [CrossRef]
- Andres, C.G.d.; Bartolome, M.J.; Capdevila, C.; Martin, D.S.; Caballero, F.G.; Lopez, V. Metallographic techniques for the determination of the austenite grain size in medium-carbon microalloyed steels. Mater. Charact. 2001, 46, 389–398. [Google Scholar] [CrossRef]
- Andres, C.G.D.; Caballero, F.G.; Capdevila, C.; Martin, D.S. Revealing austenite grain boundaries by thermal etching: Advantages and disadvantages. Mater. Charact. 2003, 49, 121–127. [Google Scholar] [CrossRef]
- Lozinskii, M.G. High Temperature Metallography. Metal Sci. Heat Treat. 1967, 9, 405–412. [Google Scholar] [CrossRef]
- Davenport, E.S.; Bain, E.C. General Relations Between Grain-Size and Hardenability and the Normality of steels in ASM; ASM: New York, NY, USA, 1934. [Google Scholar]
- Brownrigg, A.; Curcio, P.; Boelen, R. Etching of Prior Austenite Grain Boundaries in Martensite. Metallography 1975, 8, 529–533. [Google Scholar] [CrossRef]
- Misra, R.D.K.; Balasubramanian, T.V. Effects of microstructure on grain boundary segregation processes in low alloy steels. Acta Metall. Mater. 1990, 38, 2357–2366. [Google Scholar] [CrossRef]
- Papworth, A.J.; Williams, D.B. Segregation to prior austenite grain boundaries in low-alloy steels. Scr. Mater. 2000, 42, 1107–1112. [Google Scholar] [CrossRef]
- Song, S.; Yuan, Z.; Shen, D.; Weng, L. Effects of phosphorus grain boundary segregation and hardness on the ductile-to-brittle transition for a 2.25Cr1Mo steel. J. Wuhan Univ. Technol.-Mater. Sci. Ed. 2007, 22, 1–6. [Google Scholar] [CrossRef]
- Zhang, Z.; Lin, Q.; Yu, Z. Grain boundary segregation in ultra-low carbon steel. Mater. Sci. Eng. A 2000, 291, 22–26. [Google Scholar] [CrossRef]
- Mahajan, S.W.; Venkataraman, G.; Mallikk, A.K. Grain Refinement of Steel by Cyclic Rapid Heating. Metallography 1973, 6, 337–345. [Google Scholar] [CrossRef]
- Voort, G.F.V. Wetting Agents in Metallography. Mater. Charact. 1995, 35, 35–137. [Google Scholar] [CrossRef]
- Nelson, J.A. The Use of Wetting Agents in Metallographic Etchants. Praktische Metall. 1967, 4, 192–198. [Google Scholar]
- Bodnar, R.L.; McGraw, V.E.; Brandemarte, A.V. Technique for Revealing Prior Austenite Grain Boundaries in CrMoV Turbine Rotor Steel. Metallography 1984, 17, 109–114. [Google Scholar] [CrossRef]
- Zhang, L.; Guo, D.C. A general etchant for revealing prior-austenite grain boundaries in steels. Mater. Charac. 1993, 30, 299–305. [Google Scholar] [CrossRef]
- Brockmeier, A.; Rodriguez, F.J.S.; Harrison, M.; Hilleringmann, U. Surface tension and its role for vertical wet etching of silicon. Micromech. Microeng. 2012, 22, 1–7. [Google Scholar] [CrossRef]
- Voort, G.F.V. Microstructure. In Metallography Princoples and Practice; ASM International: New York, NY, USA, 1984; p. 196. [Google Scholar]
- Petzow, G. Metallographic Etching; American Society for Metals: Cleveland, OH, USA, 1978. [Google Scholar]
- West, J.M. Basic corrosion and oxidation. In Why Corrosion Happens; John Wiley & Sons: New York, NY, USA, 1980; pp. 72–73. [Google Scholar]
- Trethewey, K.; Chamberlain, J. Differential-Aeration Corrosion. In Corrosion for Science and Engineering; Longman: London, UK, 1995; p. 169. [Google Scholar]




| C, wt.% | Mn, wt.% | Si, wt.% | S, wt.% | P, wt.% | Nb, wt.% |
|---|---|---|---|---|---|
| 0.080 | 0.98 | 0.20 | 0.003 | 0.01 | 0 |
| 0.080 | 1.00 | 0.19 | 0.003 | 0.01 | 0.005 |
| 0.080 | 0.98 | 0.17 | 0.003 | 0.01 | 0.01 |
| 0.080 | 0.98 | 0.18 | 0.003 | 0.01 | 0.02 |
| C, wt.% | Mn, wt.% | Si, wt.% | S, wt.% | P, wt.% | Nb, wt.% |
|---|---|---|---|---|---|
| 0.38 | 1.00 | 0.20 | 0.003 | 0.018 | 0 |
| 0.38 | 0.98 | 0.20 | 0.007 | 0.018 | 0.005 |
| 0.38 | 1.00 | 0.20 | 0.008 | 0.016 | 0.008 |
| 0.38 | 1.00 | 0.20 | 0.007 | 0.016 | 0.017 |
| C, wt.% | Mn, wt.% | Si, wt.% | S, wt.% | P, wt.% | Nb, wt.% |
|---|---|---|---|---|---|
| 0.80 | 0.98 | 0.21 | 0.003 | 0.01 | 0.005 |
| 0.80 | 0.99 | 0.21 | 0.003 | 0.01 | 0 |
| 0.80 | 0.96 | 0.20 | 0.003 | 0.01 | 0.01 |
| 0.80 | 0.96 | 0.20 | 0.003 | 0.01 | 0.02 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thackray, R.; Palmiere, E.J.; Khalid, O. Novel Etching Technique for Delineation of Prior-Austenite Grain Boundaries in Low, Medium and High Carbon Steels. Materials 2020, 13, 3296. https://doi.org/10.3390/ma13153296
Thackray R, Palmiere EJ, Khalid O. Novel Etching Technique for Delineation of Prior-Austenite Grain Boundaries in Low, Medium and High Carbon Steels. Materials. 2020; 13(15):3296. https://doi.org/10.3390/ma13153296
Chicago/Turabian StyleThackray, Richard, Eric J. Palmiere, and Omar Khalid. 2020. "Novel Etching Technique for Delineation of Prior-Austenite Grain Boundaries in Low, Medium and High Carbon Steels" Materials 13, no. 15: 3296. https://doi.org/10.3390/ma13153296
APA StyleThackray, R., Palmiere, E. J., & Khalid, O. (2020). Novel Etching Technique for Delineation of Prior-Austenite Grain Boundaries in Low, Medium and High Carbon Steels. Materials, 13(15), 3296. https://doi.org/10.3390/ma13153296







