Development of Novel Lightweight Metastable Metal–(Metal + Ceramic) Composites Using a New Powder Metallurgy Approach
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Methods
2.2.1. Processing
2.2.2. Microstructural Characterization
2.2.3. Thermal Characterization
2.2.4. Mechanical Testing
3. Results
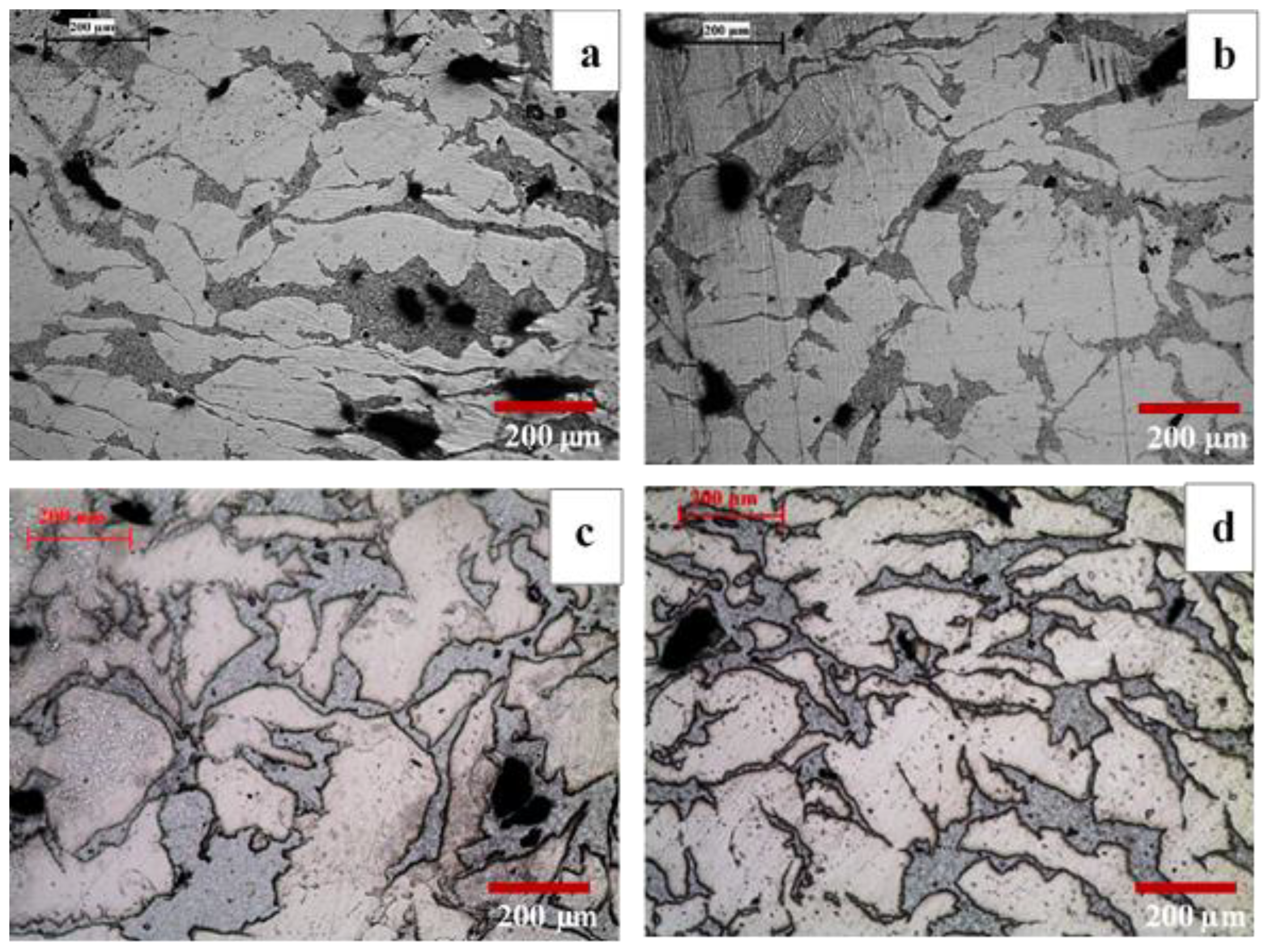
3.1. Microstructure
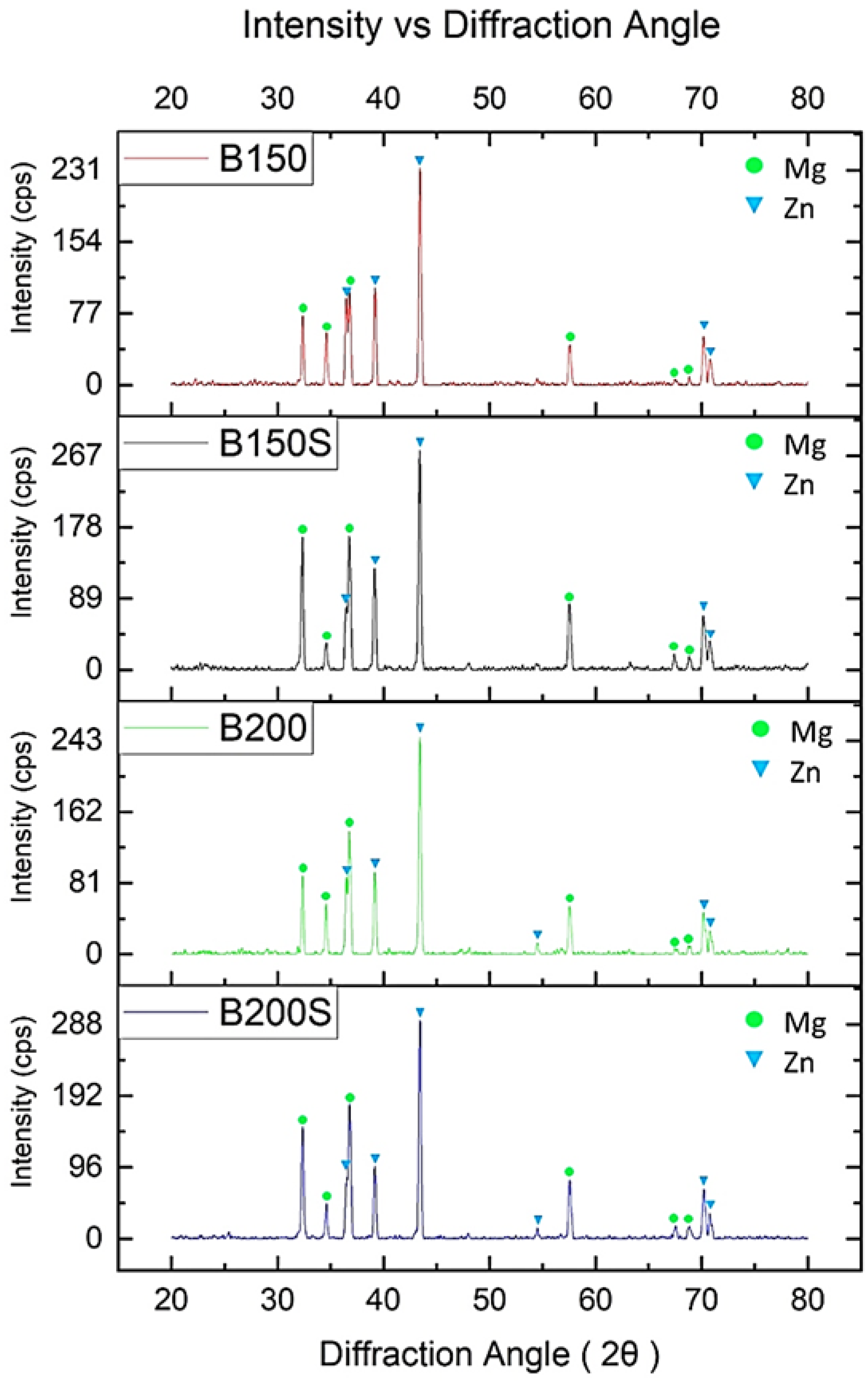
3.2. X-ray Diffraction Analysis
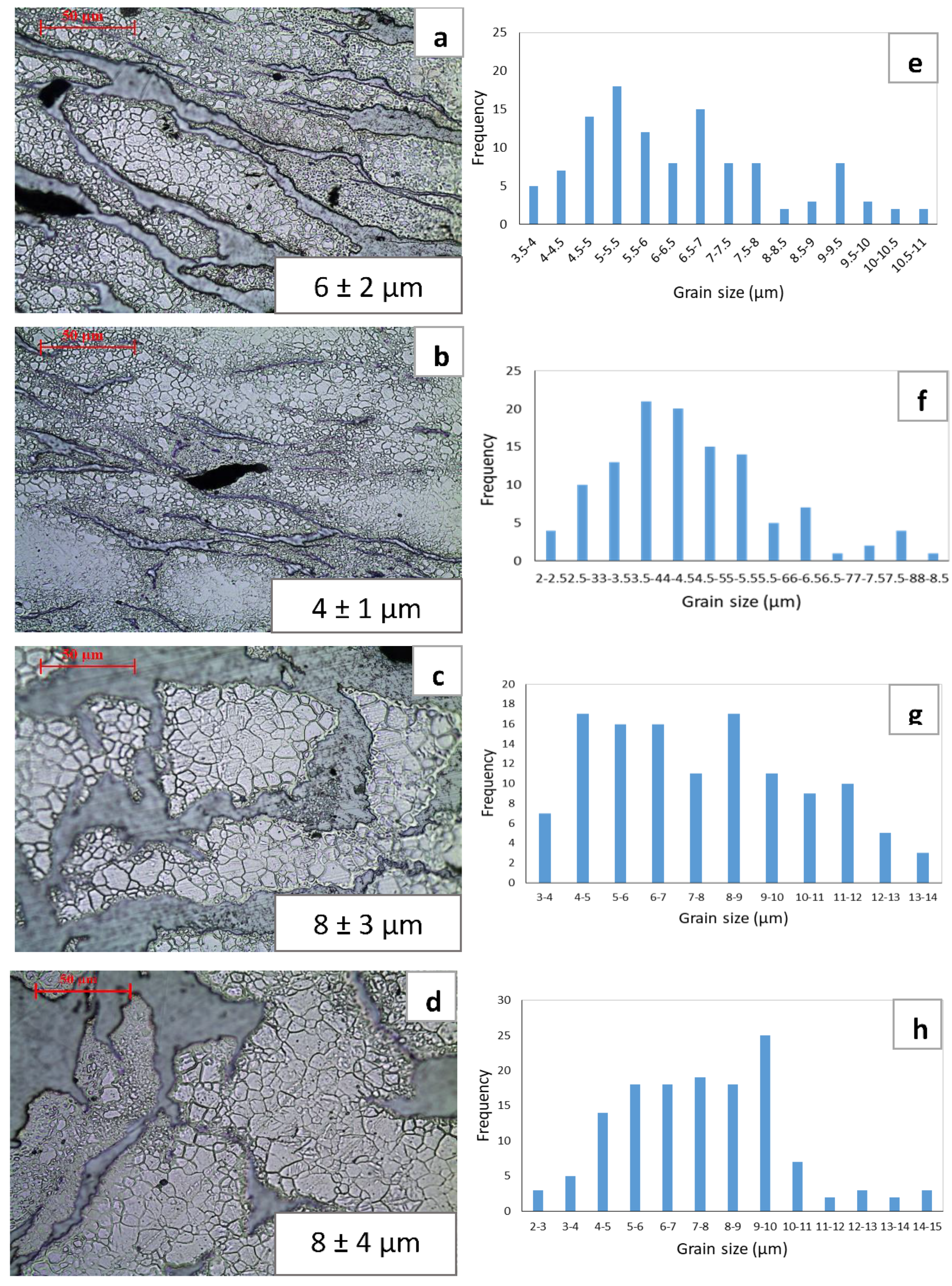
3.3. Grain Size
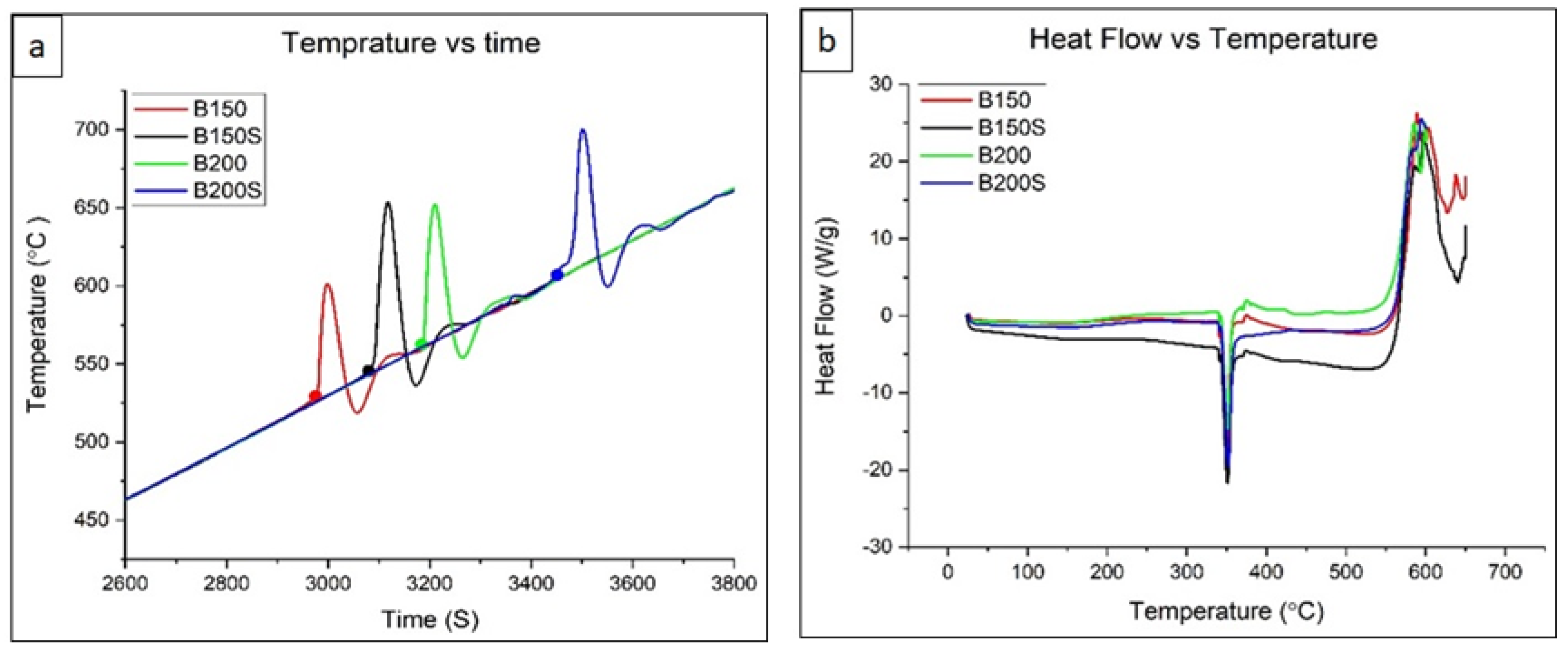
3.4. Thermal Properties
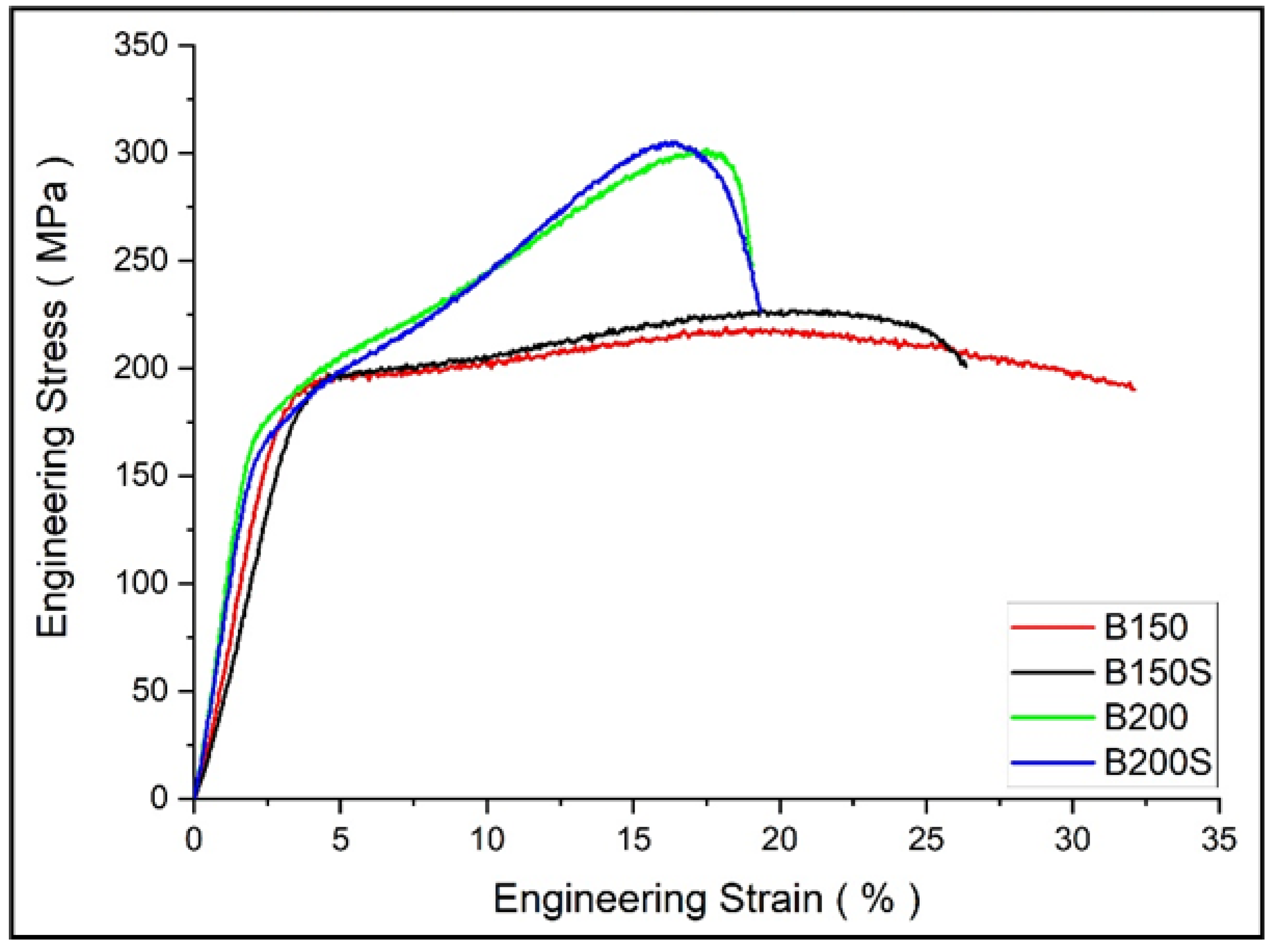
3.5. Mechanical Properties
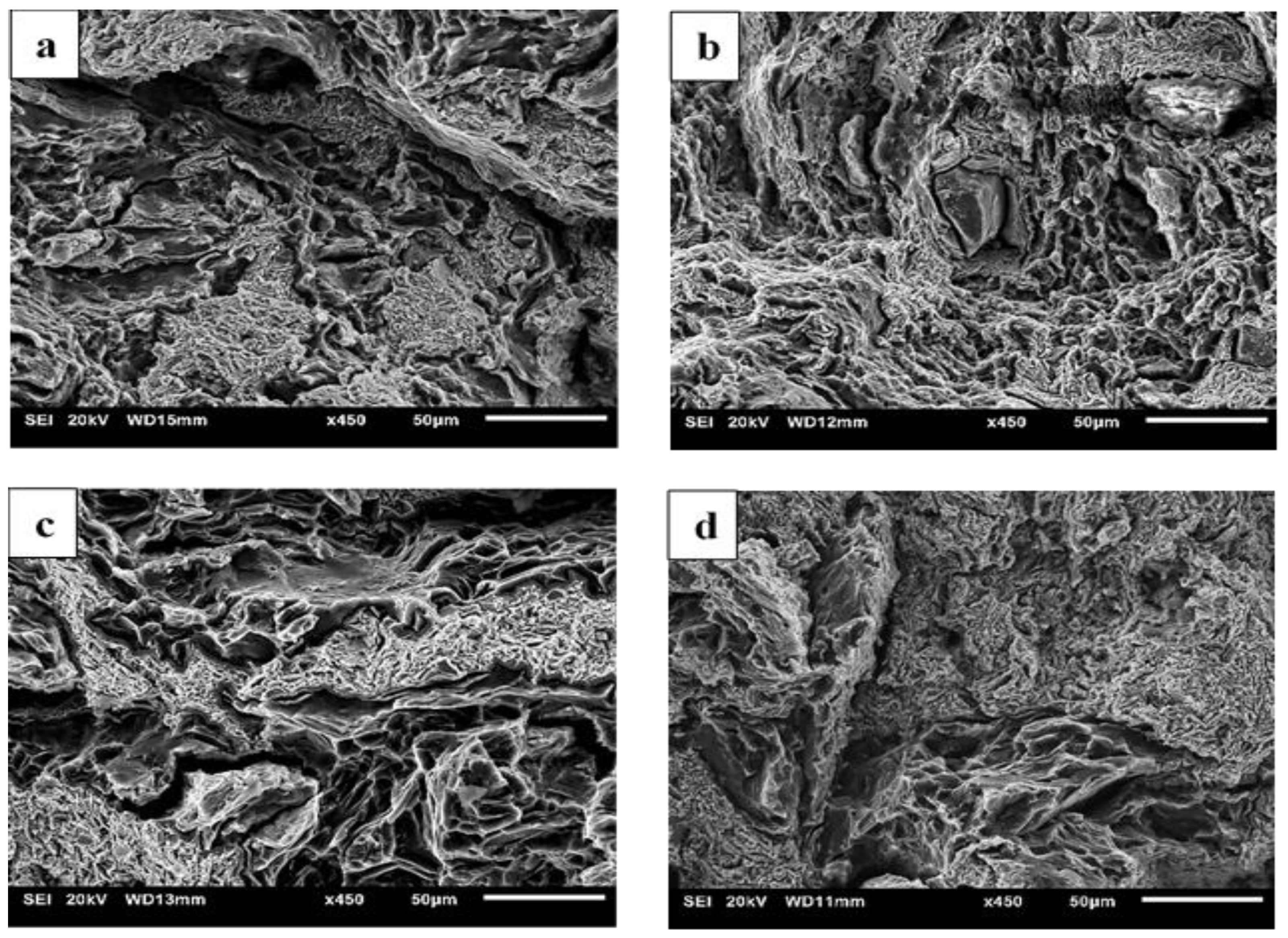
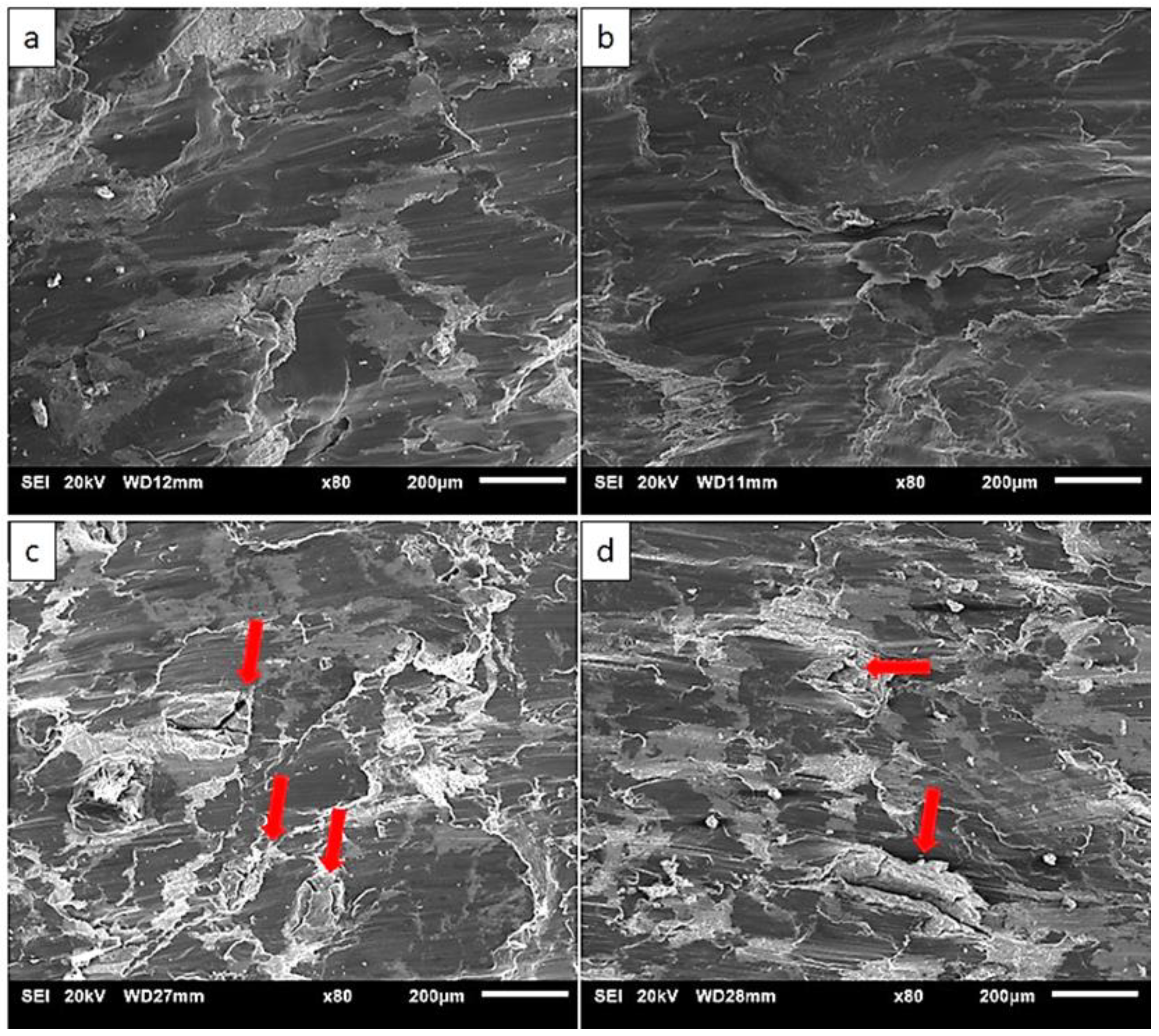
3.6. Fractography
4. Discussion
4.1. Evolution of Microstructure
4.2. Grain Size and Distribution
4.3. Effect of Soaking and Extrusion on Igniton
4.4. Mechanical Responses of Synthesized Materials
5. Conclusions
- No evidence of intermetallic formation was observed in all developed metal–(metal + ceramic) composites fulfilling the aim of current investigation. This made the currently established sinter-less PM method to be suitable for synthesis of metastable metal–(metal + ceramic) composites using either Mg or Zn as matrix material.
- With the use of low extrusion temperature (150 °C), fine recrystallized grains were abundantly found in the composites. Whereas coarsening of recrystallized grains was observed at high extrusion temperature (200 °C). Due to negligible difference in grain size between soaked and un-soaked materials it can be inferred that soaking condition (with or without soaking prior to extrusion) has minimal effect on the grain size variation and it is mainly influenced by extrusion temperature.
- Based on the grain distribution analysis, heterogeneous grain distribution was observed in the composites processed at low temperature (B150 and B150S) whereas a relatively more homogeneous grain distribution was observed in those processed at high temperature (B200 and B200S).
- Thermal analysis using TGA revealed the highest ignition temperature in B200S. The presence of relatively uniform grains in B200S facilitated the formation of a more protective oxide layer delaying the onset of ignition. Large grain size variations observed in B150 and B150S caused an adverse effect on ignition temperature.
- The bulk hardness assessed in terms of Rockwell hardness test revealed similar level of macrohardness. A small variation in average hardness follows the grain size variation while the highest hardness of 82 HRB was realized in B150S which displayed finest average grain size.
- Between B150 and B150S, despite having finer grains, B150S showed insignificant but a decrease in average tensile yield strength. A higher level of grain size heterogeneity in B150S is accountable for the yield strength reduction. Regardless of the extrusion temperature or soaking condition, all synthesized composites showed brittle nature with low tensile ductility of 2.4–3.4% under tensile loading.
- The existence of finer grains provided compressive yield strength increment in B150 and B150S when compared to B200 and B200S. However, post yielding deformation was influenced by the grains heterogeneity causing relatively linear strain hardening with a reduced compressive strength in B150 and B150S. Whereas common phenomenon of rapid strain hardening during plastic deformation with an increase compressive strength was realized in B200 and B200S.
Author Contributions
Funding
Conflicts of Interest
References
- Saris, N.-E.L.; Mervaala, E.; Karppanen, H.; Khawaja, J.A.; Lewenstam, A. Magnesium An update on physiological, clinical and analytical aspects. Clin. Chim. Acta 2000, 294, 1–26. [Google Scholar] [CrossRef]
- Radha, R.; Sreekanth, D. Insight of magnesium alloys and composites for orthopedic implant applications —A review. J. Magnes. Alloy. 2017, 5, 286–312. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, Z.; Smith, C.; Sankar, J. Recent advances on the development of magnesium alloys for biodegradable implants. Acta. Biomater. 2014, 10, 4561–4573. [Google Scholar] [CrossRef] [PubMed]
- Hermawan, H. Updates on the research and development of absorbable metals for biomedical applications. Prog. Biomater. 2018, 7, 93–110. [Google Scholar] [CrossRef]
- Dorozhkin, S. V Calcium orthophosphate coatings on magnesium and its biodegradable alloys. Acta. Biomater. 2014, 10, 2919–2934. [Google Scholar] [CrossRef]
- Staiger, M.P.; Pietak, A.M.; Huadmai, J.; Dias, G. Magnesium and its alloys as orthopedic biomaterials: A review. Biomaterials 2006, 27, 1728–1734. [Google Scholar] [CrossRef] [PubMed]
- Bommala, V.K.; Krishna, M.G.; Rao, C.T. Magnesium matrix composites for biomedical applications: A review. J. Magnes. Alloy. 2019, 7, 72–79. [Google Scholar] [CrossRef]
- Sezer, N.; Evis, Z.; Kayhan, S.M.; Tahmasebifar, A.; Koç, M. Review of magnesium-based biomaterials and their applications. J. Magnes. Alloy. 2018, 6, 23–43. [Google Scholar] [CrossRef]
- Ding, Y.; Wen, C.; Hodgson, P.; Li, Y. Effects of alloying elements on the corrosion behavior and biocompatibility of biodegradable magnesium alloys: A review. J. Mater. Chem. B 2014, 2, 1912–1933. [Google Scholar] [CrossRef]
- Zhang, B.P.; Wang, Y.; Geng, L. Research on Mg-Zn-Ca Alloy as Degradable Biomaterial. In Biomaterials-Physics and Chemistry; InTech: Rijeka, Croatia, 2011. [Google Scholar]
- Yan, Y.; Liu, H.; Fang, H.; Yu, K.; Zhang, T.; Xu, X.; Zhang, Y.; Dai, Y. Effects of the intermetallic phases on microstructure and properties of biodegradable magnesium matrix and zinc matrix prepared by powder metallurgy. Mater. Trans. 2018, 59, 1837–1844. [Google Scholar] [CrossRef]
- Rosalbino, F.; De Negri, S.; Saccone, A.; Angelini, E.; Delfino, S. Bio-corrosion characterization of Mg-Zn-X (X = Ca, Mn, Si) alloys for biomedical applications. J. Mater. Sci. Mater. Med. 2010, 21, 1091–1098. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, B.; Wang, Y.; Geng, L.; Jiao, X. Preparation and characterization of a new biomedical Mg-Zn-Ca alloy. Mater. Des. 2012, 34, 58–64. [Google Scholar] [CrossRef]
- Zhang, E.; Yin, D.; Xu, L.; Yang, L.; Yang, K. Microstructure, mechanical and corrosion properties and biocompatibility of Mg-Zn-Mn alloys for biomedical application. Mater. Sci. Eng. C 2008, 29, 987–993. [Google Scholar] [CrossRef]
- Han, Y.Y.; You, C.; Zhao, Y.; Chen, M.F.; Wang, L. Effect of Mn Element Addition on the Microstructure, Mechanical Properties, and Corrosion Properties of Mg-3Zn-0.2Ca Alloy. Front. Mater. 2019, 6, 1–10. [Google Scholar] [CrossRef]
- Kubásek, J.; Dvorský, D.; Šedý, J.; Msallamová, Š.; Levorová, J.; Foltán, R.; Vojtěch, D. The fundamental comparison of Zn-2Mg and Mg-4Y-3RE alloys as a perspective biodegradable materials. Materirals 2019, 12, 3745. [Google Scholar] [CrossRef]
- Yang, H.; Jia, B.; Zhang, Z.; Qu, X.; Li, G.; Lin, W.; Zhu, D.; Dai, K.; Zheng, Y. Alloying design of biodegradable zinc as promising bone implants for load-bearing applications. Nat. Commun. 2020, 11, 1–16. [Google Scholar] [CrossRef]
- Vojtěch, D.; Kubásek, J.; Šerák, J.; Novák, P. Mechanical and corrosion properties of newly developed biodegradable Zn-based alloys for bone fixation. Acta. Biomater. 2011, 7, 3515–3522. [Google Scholar] [CrossRef]
- Alves, M.M.; Prošek, T.; Santos, C.F.; Montemor, M.F. Evolution of the: In vitro degradation of Zn-Mg alloys under simulated physiological conditions. RSC Adv. 2017, 7, 28224–28233. [Google Scholar] [CrossRef]
- Baker, H.; Okamoto, H.; Henry, S.D.; Scott, W.W.; Uhl, R.C.; Assistance, P.E.; Wheaton, N.D.; Mills, K.; Plickert, D.S.; Starr, S.; et al. ASM Handbook Volume 3 Alloy Phase Diagrams; ASM International: Cleveland, OH, USA, 1992. [Google Scholar]
- Kazakov, N.F. Diffusion Bonding of Materials; Elsevier Science: Amsterdam, The Netherlands, 2013; ISBN 9781483150550. [Google Scholar]
- Kawasaki, M.; Ahn, B.; Lee, H.; Zhilyaev, A.P.; Langdon, T.G. Using high-pressure torsion to process an aluminum-magnesium nanocomposite through diffusion bonding. J. Mater. Res. 2016, 31, 88–99. [Google Scholar] [CrossRef]
- Gietzelt, T.; Toth, V.; Huell, A. Diffusion Bonding: Influence of Process Parameters and Material Microstructure. Join. Technol. 2016. [Google Scholar] [CrossRef]
- Tekumalla, S.; Gupta, N.; Gupta, M. Influence of turning speed on the microstructure and properties of magnesium ZK60 alloy pre-processed via turning-induced-deformation. J. Alloys Compd. 2020, 831, 154840. [Google Scholar] [CrossRef]
- Li, S.; Chen, L.; Tang, J.; Zhao, G.; Zhang, C. Microstructure and mechanical properties of hot extruded Mg-8.89Li-0.96Zn alloy. Results. Phys. 2019, 13, 102148. [Google Scholar] [CrossRef]
- Chen, B.; Li, X.; Lu, C.; Lin, D. Recrystallization and Microstructural Evolution During Hot Extrusion of Mg97Y2Zn1 Alloy. Met. Mater. Int. 2014, 20, 489–497. [Google Scholar] [CrossRef]
- Haddadi, F.; Prangnell, P. Grain structure evolution in aluminium to steel ultrasonic spot welding. Mater. Sci. Technol. Conf. Exhib. 2013, 2, 1252–1259. [Google Scholar] [CrossRef]
- Anilchandra, A.R.; Surappa, M.K. Influence of tool rake angle on the quality of pure magnesium chip-consolidated product. J. Mater. Process. Technol. 2010, 210, 423–428. [Google Scholar] [CrossRef]
- Meng, J.; Sun, L.; Zhang, Y.; Xue, F.; Chu, C.; Bai, J. Evolution of recrystallized grain and texture of cold-drawn pure Mg wire and their effect on mechanical properties. Materials 2020, 13, 427. [Google Scholar] [CrossRef]
- Tekumalla, S.; Gupta, M. An insight into ignition factors and mechanisms of magnesium based materials: A review. Mater. Des. 2017, 113, 84–98. [Google Scholar] [CrossRef]
- Fassell, W.M.; Gulbransen, L.B.; Lewis, J.R.; Hamilton, J.H. Ignition Temperatures of Magnesium and Magnesium Alloys. JOM 1951, 3, 522–528. [Google Scholar] [CrossRef]
- Tekumalla, S.; Gupta, M.; Min, K.H. Using CaO Nanoparticles to Improve Mechanical and Ignition Response of Magnesium. Curr. Nanomater. 2018, 3, 44–51. [Google Scholar] [CrossRef]
- Merson, D.; Vasiliev, E.; Markushev, M.; Vinogradov, A. On the corrosion of ZK60 magnesium alloy after severe plastic deformation. Lett. Mater. 2017, 7, 421–427. [Google Scholar] [CrossRef]
- Berbenni, S.; Favier, V.; Berveiller, M. Impact of the grain size distribution on the yield stress of heterogeneous materials. Int. J. Plast. 2007, 23, 114–142. [Google Scholar] [CrossRef]
- Chao, H.Y.; Yang, Y.; Wang, X.; Wang, E.D. Effect of grain size distribution and texture on the cold extrusion behavior and mechanical properties of AZ31 Mg alloy. Mater. Sci. Eng. A 2011, 528, 3428–3434. [Google Scholar] [CrossRef]
- Patra, S.; Hasan, S.M.; Narasaiah, N.; Chakrabarti, D. Effect of bimodal distribution in ferrite grain sizes on the tensile properties of low-carbon steels. Mater. Sci. Eng. A 2012, 538, 145–155. [Google Scholar] [CrossRef]
- Tun, K.S.; Jayaramanavar, P.; Nguyen, Q.B.; Chan, J.; Kwok, R.; Gupta, M. Investigation into tensile and compressive responses of Mg-ZnO composites. Mater. Sci. Technol. 2012, 28, 582–588. [Google Scholar] [CrossRef]
- Tun, K.S.; Zhang, Y.; Parande, G.; Manakari, V.; Gupta, M. Enhancing the hardness and compressive response of magnesium using complex composition alloy reinforcement. Metals 2018, 8, 276. [Google Scholar] [CrossRef]
- Tun, K.S.; Gupta, M. Role of microstructure and texture on compressive strength improvement of Mg/(Y2O3 + Cu) hybrid nanocomposites. J. Compos. Mater. 2010, 44, 3033–3050. [Google Scholar] [CrossRef]
- Tun, K.S.; Gupta, M. Compressive deformation behavior of Mg and Mg/(Y2O3 + Ni) nanocomposites. Mater. Sci. Eng. A 2010, 527, 5550–5556. [Google Scholar] [CrossRef]
- Nguyen, Q.B.; Gupta, M. Enhancing compressive response of AZ31B magnesium alloy using alumina nanoparticulates. Compos. Sci. Technol. 2008, 68, 2185–2192. [Google Scholar] [CrossRef]
- Anilchandra, A.R.; Basu, R.; Samajdar, I.; Surappa, M.K. Microstructure and compression behavior of chip consolidated magnesium. J. Mater. Res. 2012, 27, 709–719. [Google Scholar] [CrossRef]
- Chi, Y.Q.; Zhou, X.H.; Qiao, X.G.; Brokmeier, H.G.; Zheng, M.Y. Tension-compression asymmetry of extruded Mg-Gd-Y-Zr alloy with a bimodal microstructure studied by in-situ synchrotron diffraction. Mater. Des. 2019, 170, 107705. [Google Scholar] [CrossRef]

| Materials | Supplier | Purity (%) | Form |
|---|---|---|---|
| Magnesium | Acros Organics (Fair Lawn, NJ, USA) | >99.9 | Chips |
| Zinc | Good Fellow (Huntingdon, UK) | 98.8 | Powder |
| Manganese | Good Fellow (Huntingdon, UK) | >97.5 | Powder |
| Calcium | Merck (Singapore) | 99 | Granules |
| Sample | Soaking Temperature (°C) | Soaking Time (h) | Extrusion Temperature (°C) |
|---|---|---|---|
| B150 | - | - | 150 |
| B150S | 150 | 1 | 150 |
| B200 | - | - | 200 |
| B200S | 200 | 1 | 200 |
| Material | Ignition Temperature (°C) | Phase Transformation Temperature (°C) |
|---|---|---|
| B150 | 530 | 338.5 |
| B150S | 545 | 338.5 |
| B200 | 560 | 338.9 |
| B200S | 606 | 338.8 |
| Material | 0.2% TYS (MPa) | UTS (MPa) | Ductility (%) |
|---|---|---|---|
| B150 | 131 ± 18 | 178 ± 3 | 3.4 ± 1.5 |
| B150S | 122 ± 3 | 172 ± 17 | 3.4 ± 1.8 |
| B200 | 120 ± 2 | 142 ± 8 | 2.4 ± 0.3 |
| B200S | 118 ± 13 | 143 ± 13 | 2.5 ± 1 |
| Material | 0.2% CYS (MPa) | UCS (MPa) | Ductility (%) |
|---|---|---|---|
| B150 | 186 ± 3 | 219 ± 1 | 33 ± 5 |
| B150S | 185 ± 6 | 217 ± 9 | 26 ± 2 |
| B200 | 175 ± 3 | 308 ± 1 | 21 ± 3 |
| B200S | 169 ± 9 | 314 ± 8 | 20 ± 2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tun, K.S.; Padnuru Sripathy, A.; Tekumalla, S.; Gupta, M. Development of Novel Lightweight Metastable Metal–(Metal + Ceramic) Composites Using a New Powder Metallurgy Approach. Materials 2020, 13, 3283. https://doi.org/10.3390/ma13153283
Tun KS, Padnuru Sripathy A, Tekumalla S, Gupta M. Development of Novel Lightweight Metastable Metal–(Metal + Ceramic) Composites Using a New Powder Metallurgy Approach. Materials. 2020; 13(15):3283. https://doi.org/10.3390/ma13153283
Chicago/Turabian StyleTun, Khin Sandar, Akshay Padnuru Sripathy, Sravya Tekumalla, and Manoj Gupta. 2020. "Development of Novel Lightweight Metastable Metal–(Metal + Ceramic) Composites Using a New Powder Metallurgy Approach" Materials 13, no. 15: 3283. https://doi.org/10.3390/ma13153283
APA StyleTun, K. S., Padnuru Sripathy, A., Tekumalla, S., & Gupta, M. (2020). Development of Novel Lightweight Metastable Metal–(Metal + Ceramic) Composites Using a New Powder Metallurgy Approach. Materials, 13(15), 3283. https://doi.org/10.3390/ma13153283







