The Effectiveness of Laser-Activated Irrigation on the Apical Microleakage Qualities of MTA Repair HP and NeoMTA Plus in Simulated Immature Teeth: A Comparative Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approval
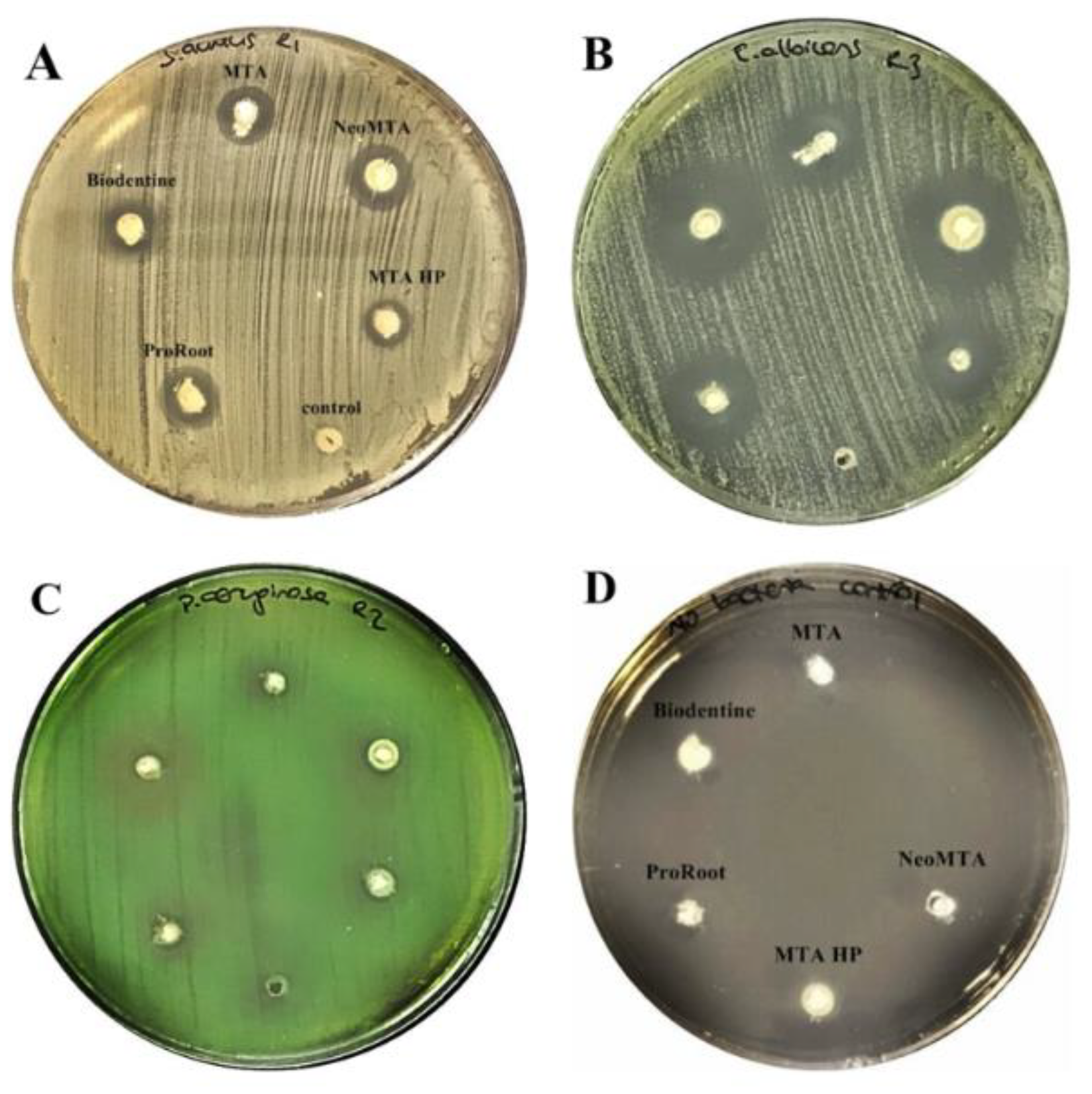
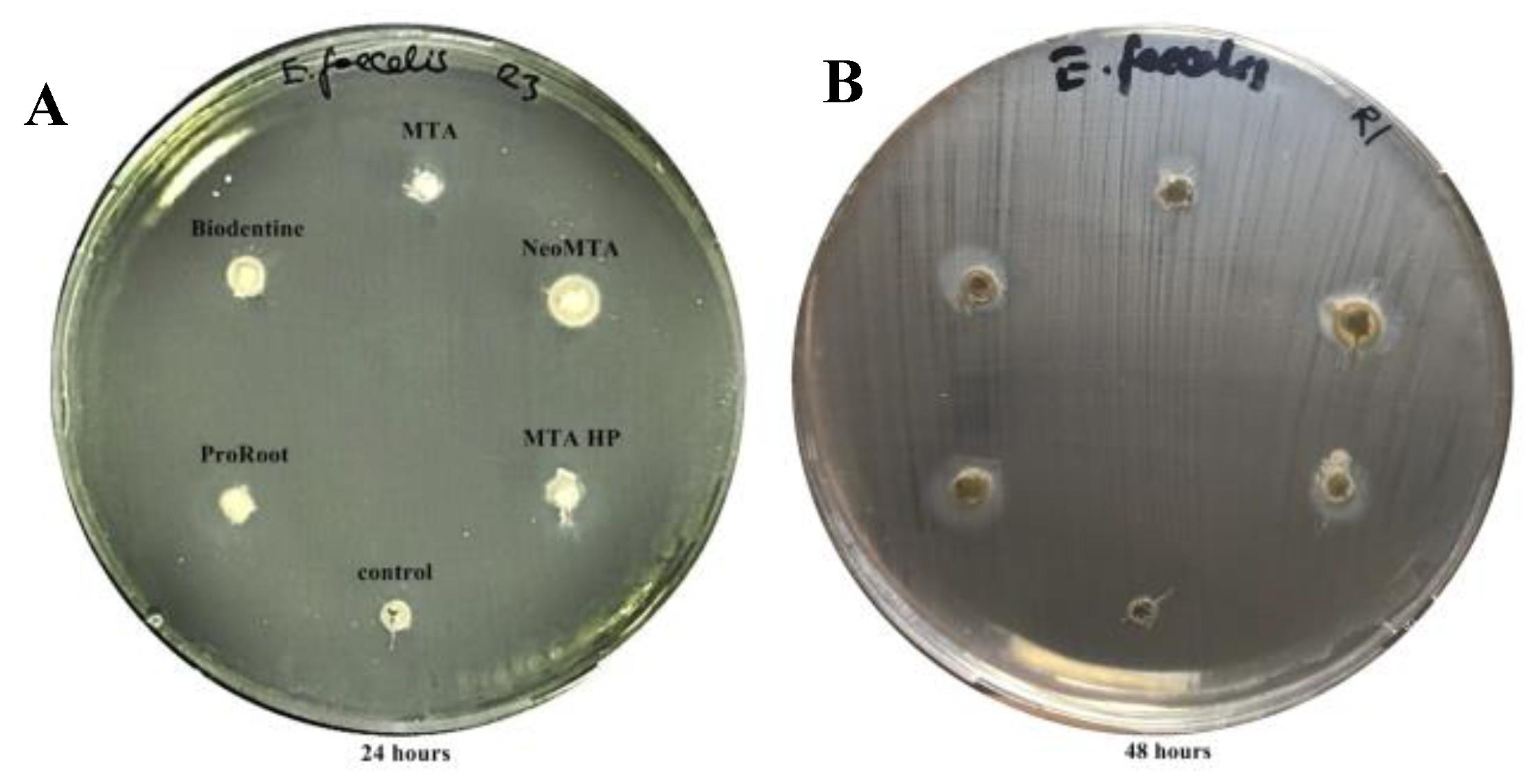
2.2. Antimicrobial Agar Diffusion Test (ADT)
2.3. Preparation of Simulated Immature Teeth Models
2.4. Determination of Groups for Microleakage Evaluation and Laser-Activated Irrigation
2.5. Glucose Leakage Model
2.6. Measurement of Microleakage
2.7. Statistical Analysis
3. Results
3.1. Antimicrobial Activity
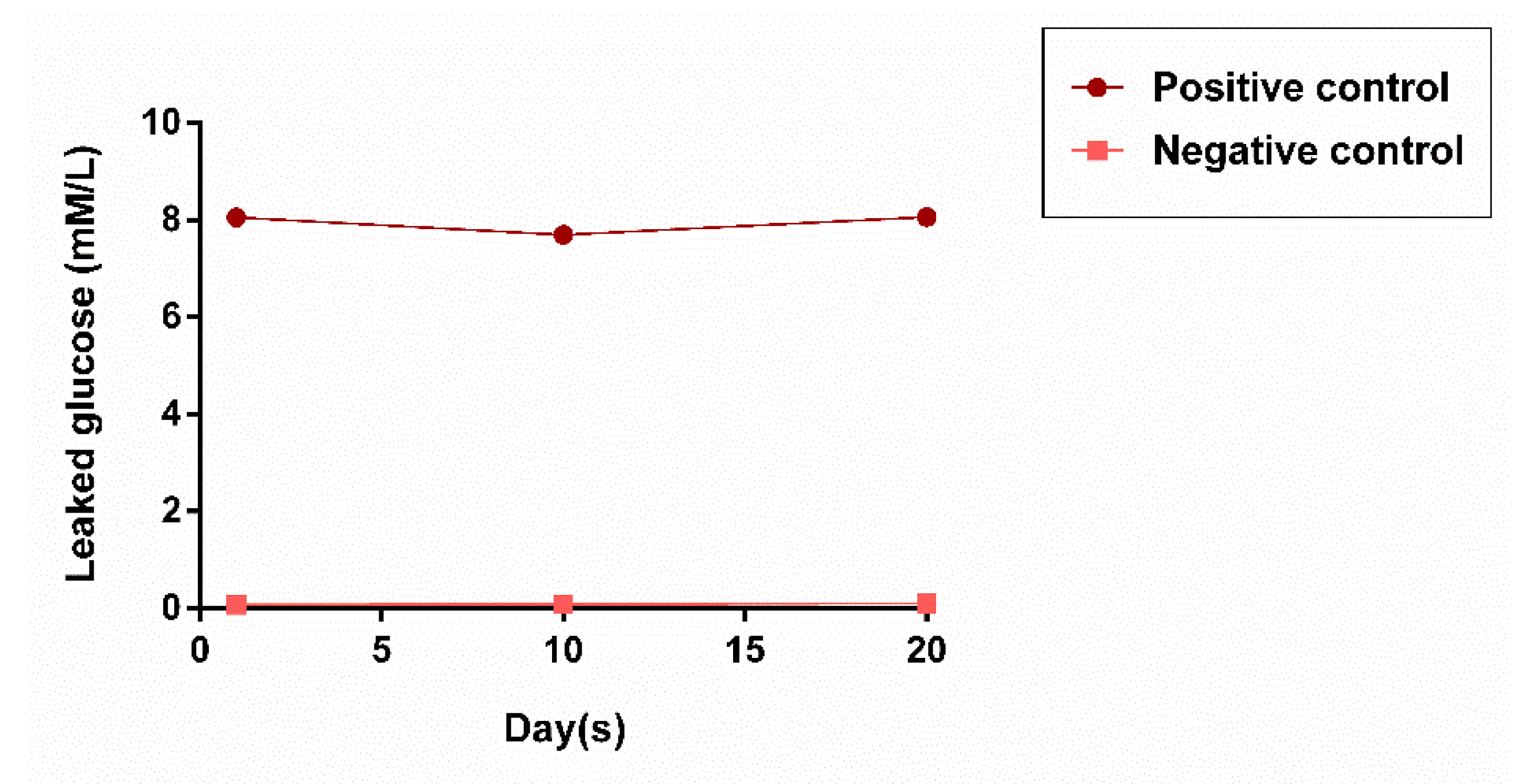
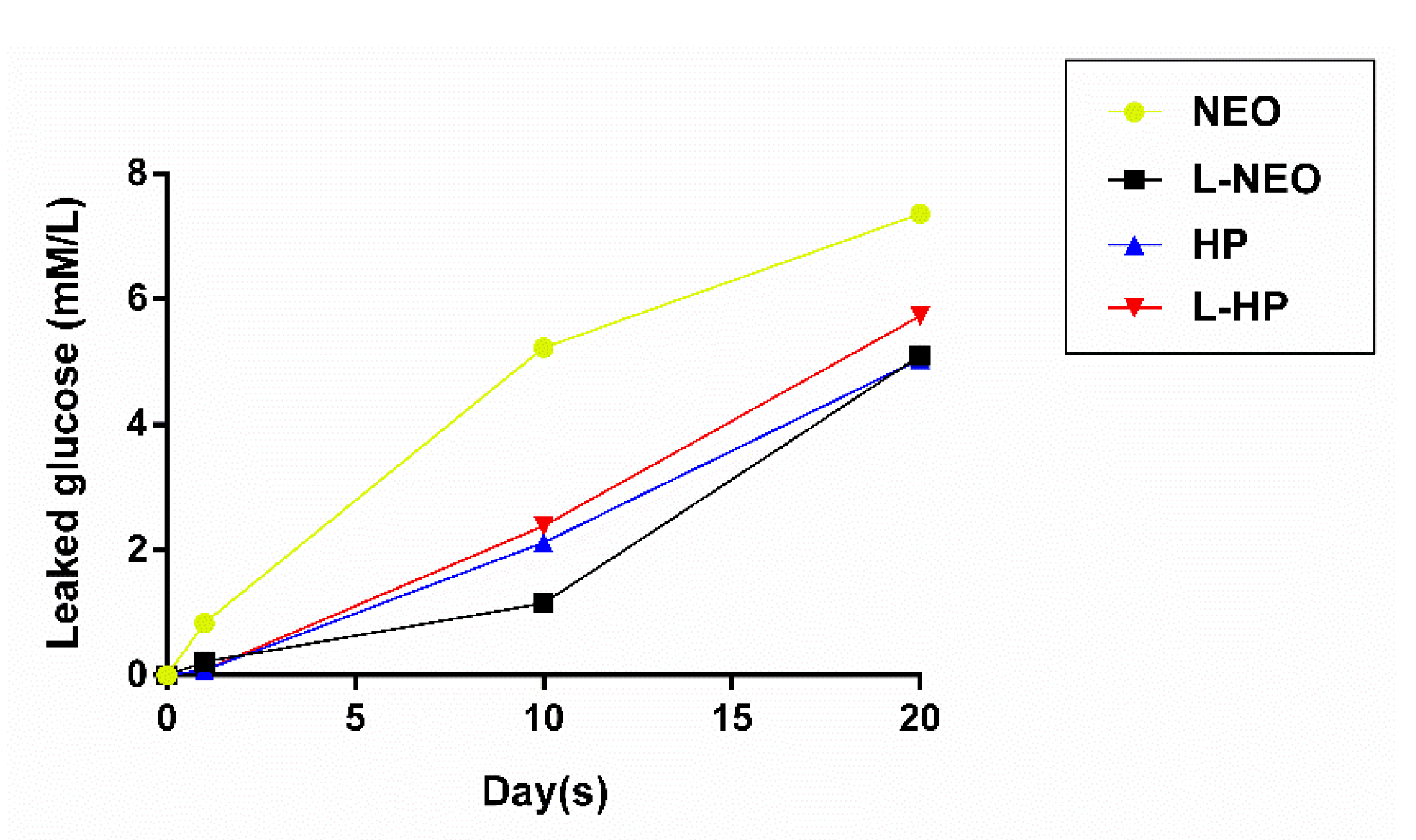
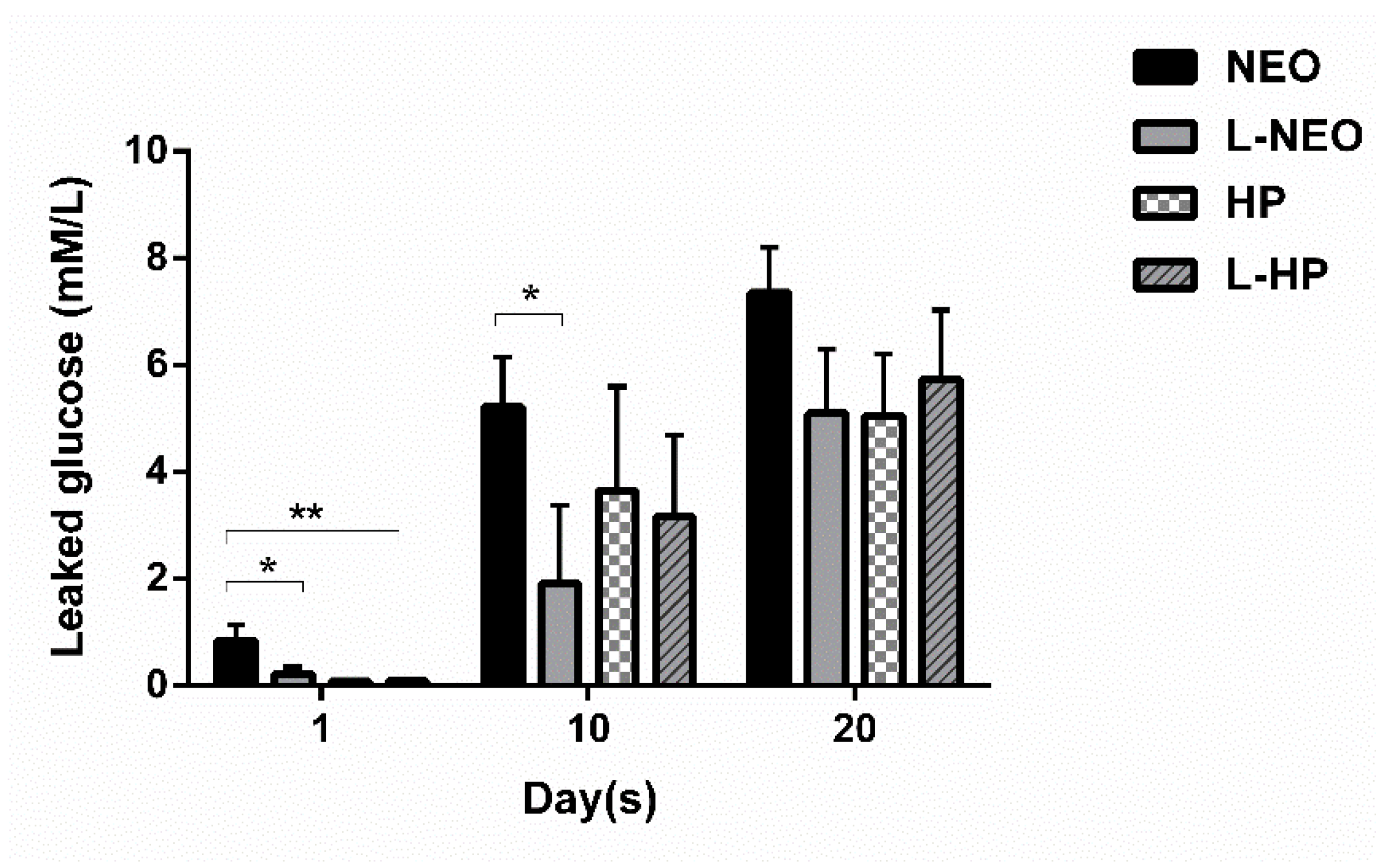
3.2. Evaluation of Microleakage
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hargreaves, K.M.; Diogenes, A.; Teixeira, F.B. Treatment options: Biological basis of regenerative endodontic procedures. J. Endod. 2013, 39, 30–43. [Google Scholar] [CrossRef]
- Kalaskar, R.R.; Kalaskar, A.R. Maturogenesis of on-vital immature permanent teeth. Contemp. Clin. Dent. 2013, 4, 268–270. [Google Scholar] [CrossRef] [PubMed]
- Shabahang, S. Treatment options: Apexogenesis and apexification. J. Endod. 2013, 39, 26–29. [Google Scholar] [CrossRef] [PubMed]
- Rafter, M. Apexification: A review. Dent. Traumatol. 2005, 21, 1–8. [Google Scholar] [CrossRef] [PubMed]
- De Sa-maria, A.B.; Nunes, E.; Cortes, M.S.G.; Silveira, F.F. A Short Time Period in the Treatment of an Open Apice Intruded Tooth: An 8-year Follow-up. Int. J. Clin. Ped. Dent. 2019, 12, 160–163. [Google Scholar]
- Songtrakul, K.; Azarpajouh, T.; Malek, M.; Sigurdsson, A.; Kahler, B.; Lin, L.M. Modified Apexification Procedure for Immature Permanent Teeth with a Necrotic Pulp/Apical Periodontitis: A Case Series. J. Endod. 2020, 46, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Goyal, A.; Nikhil, V.; Jha, A. Absorbable Suture as an Apical Matrix in Single Visit Apexification with Mineral Trioxide Aggregate. Case Rep. Dent. 2016, 2016, 4505093. [Google Scholar] [CrossRef] [PubMed]
- Coomaraswamy, K.S.; Lumley, P.J.; Hofmann, M.P. Effect of bismuth oxide radioopacifier content on the material properties of an endodontic Portlan cement-based (MTA-like) system. J. Endod. 2007, 33, 295–298. [Google Scholar] [CrossRef]
- Erdem, A.P.; Sepet, E. Mineral trioxide aggregate for obturation of maxillary central incisors with necrotic pulp and open apices. Dent. Traumatol. 2008, 24, 38–41. [Google Scholar] [CrossRef]
- Torabinejad, M.; Parirokh, M.; Dummer, P.M.H. Mineral trioxide aggregate and other bioactive endodontic cements: An updated overview-part II: Other clinical applications and complications. Int. Endod. J. 2018, 51, 284–317. [Google Scholar] [CrossRef]
- Hosoya, N.; Takigawa, T.; Horie, T.; Maeda, H.; Yamamoto, Y.; Momoi, Y.; Yamamoto, K.; Okiji, T. A review of the literature on the efficacy of mineral trioxide aggregate in conservative dentistry. Dent. Mater.J. 2019, 38, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Parirokh, M.; Torabinejad, M.; Dummer, P.M.H. Mineral trioxide aggregate and other bioactive cements: An updated overview-part I: Vital pulp therapy. Int. Endod. J. 2018, 51, 177–205. [Google Scholar] [CrossRef] [PubMed]
- Marciano, M.A.; Guimaraes, B.; Amoroso-Silva, P.; Camilleri, J.; Hungaro–Duarte, M.A. Physical and Chemical Properties and Subcutaneous Implantation of Mineral Trioxide Aggregate Mixed with Propylene Glycol. J. Endod. 2016, 42, 474–479. [Google Scholar] [CrossRef] [PubMed]
- Tomas-Catala, C.J.; Collado-Gonzalez, M.; Garcia-Bernal, D.; Onate-Sanchez, R.E.; Forner, L.; Llena, C. Comparative analysis of the biological effects of endodontic bioactive cements MTA-Angelus, MTA Repair HP and NeoMTA Plus on human dental pulp stem cells. Int. Endod. J. 2017, 50, 63–72. [Google Scholar] [CrossRef]
- Guimares, B.M.; Prati, C.; Duarte, M.A.H.; Bramante, C.M.; Gandolfi, M.G. Physicochemical properties of calcium silicate–based formulations MTA Repair HP and MTA Vitalcem. J. Appl. Oral Sci. 2018, 26, e2017115. [Google Scholar] [CrossRef]
- Fogel, H.M.; Peikoff, M.D. Microleakage of root-end filling materials. J. Endod. 2001, 27, 456–458. [Google Scholar] [CrossRef]
- Chng, H.K.; Islam, I.; Yap, A.U.; Tong, Y.W.; Koh, E.T. Properties of a new root-end filling material. J. Endod. 2005, 31, 665–668. [Google Scholar] [CrossRef]
- Namazikhah, M.S.; Nekoofar, M.H.; Sheykhrezae, M.S.; Salariyeh, S.; Hayes, S.J.; Bryant, S.T.; Mohammadi, M.M.; Dummer, P.M. The effect of pH on surface hardness and microstructure of mineral trioxide aggregate. Int. Endod.J. 2008, 41, 108–116. [Google Scholar] [CrossRef]
- Aranha, A.C.; Domıngues, F.B.; Franco, V.O.; Gutknecht, N.; Eduardo, C.P. Effects of Er:YAG and Nd:YAG lasers on dentin permeability in root surfaces: A preliminary in vitro study. Photomed. Laser Ther. 2005, 23, 504–508. [Google Scholar] [CrossRef]
- El-Ma’aita, A.M.; Qualtrough, A.J.; Watts, D.C. The effect of smear layer on the push-out bond strength of root canal calcium-silicate cements. Dent. Mater. 2013, 29, 797–803. [Google Scholar] [CrossRef]
- Gordon, W.; Atabakhsh, V.A.; Meza, F.; Doms, A.; Nissan, R.; Rizoiu, I.; Stevens, R.H. The antimicrobial efficacy of the erbium, chromium:yttrium-scandiumgallium- garnet laser with radial emitting tips on root canal dentin walls infected with Enterococcus faecalis. J. Am. Dent. Assoc. 2007, 138, 992–1002. [Google Scholar] [CrossRef] [PubMed]
- Blanken, J.W.; Verdaasdonk, R.M. Cavitation as a working mechanism of the Er,Cr:YSGG laser in endodontics: A visualization study. J. Oral. Laser Appl. 2007, 7, 97–106. [Google Scholar]
- De Moor, R.J.; Blanken, J.; Meire, M.; Verdaasdonk, R. Laser induced explosive vapor and cavitation resulting in effective irrigation of the root canal. Part 2: Evaluation of the efficacy. Lasers Surg. Med. 2009, 41, 520–523. [Google Scholar] [CrossRef] [PubMed]
- Alamoudi, R.A.; Abu Zeid, S.T. Effects of Irrigants on the Push-Out Bond Strength of Two Bioceramic Root Repair Materials. Materials 2019, 12, 1921. [Google Scholar] [CrossRef]
- Nagas, E.; Küçükkaya, S.; Eymirli, A.; Uyanık, M.O.; Çehreli, Z.C. Effect of Laser-Activated Irrigation on the Push-Out Bond Strength of ProRoot Mineral Trioxide Aggregate and Biodentine in Furcal Perforations. Photomed. Laser Surg. 2017, 35, 231–235. [Google Scholar] [CrossRef]
- Xu, Q.; Fan, M.W.; Fan, B.; Cgeung, G.S.P.; Hu, H.L. A new quantitative method using glucose for analysis of endodontic leakage. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2005, 99, 107–111. [Google Scholar] [CrossRef]
- Walsh, R.M.; Woodmansey, K.F.; He, J.; Svoboda, K.K.; Primus, C.M.; Opperman, L.A. Histology of NeoMTA Plus and Quick-Set2 in Contact with Pulp and Periradicular Tissues in a Canine Model. J. Endod. 2018, 44, 1389–1395. [Google Scholar] [CrossRef]
- Türker, S.A.; Uzunoğlu, E.; Bilgin, B. Comparative evaluation of push-out bond strength of Neo MTA Plus with Biodentine and white ProRoot MTA. J. Adhes. Sci. Technol. 2017, 5, 502–508. [Google Scholar] [CrossRef]
- Bani, M.; Ekçi, E.S.; Odabai, E. Efficacy of Biodentine as an Apical Plug in Nonvital Permanent Teeth with Open Apices: An In Vitro Study. BioMed 2015, 2015, 359275. [Google Scholar] [CrossRef]
- Çiçek, E.; Yılmaz, N.; Koçak, M.; Sağlam, B.C.; Koçak, S.; Bilgin, B. Effect of Mineral Trioxide Aggregate Apical Plug Thickness on Fracture Resistance of Immature Teeth. Basic Research—Technology. J. Endod 2017, 43, 1697–1700. [Google Scholar] [CrossRef]
- Bailon-Sanchez, M.E.; Gonzalez-Castillo, S.; Gonzalez-Rodriguez, M.P.; Poyatos-Martinez, R.; Ferrer-Luque, C.M. Intraorifice sealing ability of different materials in endodontically treated teeth. Med. Oral Patol. Oral Cir. Bucal. 2011, 16, 105–109. [Google Scholar] [CrossRef][Green Version]
- Shemesh, H.; Wu, M.K.; Wesselink, P.R. Leakage along apical root fillings with and without smear layer using two different leakage models: A two-month longitudinal ex vivo study. Int. Endod. J. 2006, 39, 968–976. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, C.M.A.; Sassone, L.M.; Gonçalves, A.S.; de Carvalho, J.J.; Tomas-Catala, C.J.; Bernal, D.G.; Onate-Sanchez, R.E.; Rodriguez-Lozano, F.J.; Leal-Silva, E.J.N. Physicochemical, cytotoxicity and in vivo biocompatibility of a high-plasticity calcium-silicate based material. Sci. Rep. 2019, 9, 3933. [Google Scholar] [CrossRef] [PubMed]
- Iménez-Sánchez, M.C.; Segura-Egea, J.J.; Díaz-Cuenca, A. Higher hydration performance and bioactive response of the new endodontic bioactive cement MTA HP repair compared with ProRoot MTA white and NeoMTA plus. J. Biomed. Mater. Res. B Part B 2019, 9999B, 1–12. [Google Scholar] [CrossRef] [PubMed]
- De Groot, S.D.; Verhaagen, B.; Versluis, M.; Wu, M.K.; Wesselink, P.R.; van der Sluis, L.W. Laser–activated irrigation within root canals: Cleaning efficacy and flow visualization. Int. Endod. J. 2009, 42, 1077–1083. [Google Scholar] [CrossRef] [PubMed]
- Montero–Miralles, P.; Torres-Lagares, D.; Segura-Egea, J.J.; Serrera-Figallo, M.A.; Gutierrez-Perez, J.L.; Castillo-Dali, G. comparative study of debris and smear layer removal with EDTA and Er,Cr:YSGG laser. J. Clin. Exp. Dent. 2018, 10, e598–e602. [Google Scholar] [CrossRef]
- Christo, J.E.; Zilm, P.S.; Sullivan, T.; Cathro, P. Efficacy of low concentrations of sodium hypochlorite and low-powered Er,Cr:YSGG activated irrigation against an Enterecoccusfaecalis biofilm. Int. Endod. J. 2016, 49, 279–286. [Google Scholar] [CrossRef]
- American Association of Endodontics. AAE Clinical Considerations for a Regenerative Procedure. Revised: 4/1/2018; American Association of Endodontics: Chicago, IL, USA, 2018. [Google Scholar]
- Betancourt, P.; Merlos, A.; Sierra, J.M.; Camps-Font, O.; Arnabat-Dominguez, J.; Vinas, M. Effectiveness of low concentration of sodium hypochlorite activated by Er,Cr:YSGG laser against Enterococcus faecalis biofilm. Lasers Med. Sci. 2019, 34, 247–254. [Google Scholar] [CrossRef]
- Eneide, C.; Castagnola, R.; Martini, C.; Grande, N.M.; Bugli, F.; Patini, R.; Cordaro, M.; Sanguinetti, M.; Olivi, G.; Isola, G.; et al. Antibiofilm Activity of Three Different Irrigation Techniques: An in Vitro Study. Antibiotics 2019, 8, 112. [Google Scholar] [CrossRef]
- Huang, Y.; Li, X.; Mandal, P.; Wu, Y.; Liu, L.; Gui, H.; Liu, J. The in vitro antimicrobial activities of four endodontic sealers. BMC Oral Health 2019, 19, 118. [Google Scholar] [CrossRef]
- Stuart, C.H.; Schwartz, A.S.; Beeson, T.J.; Owatz, C.B. Enterecoccusfaecalis: Its role in root canal treatment failure and current concepts in retreatment. J. Endod. 2006, 32, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Torabinejad, M.; Hong, C.U.; McDonald, F.; Pitt-Ford, T.R. Physical and chemical properties of a new root-end filling material. J. Endod. 1995, 21, 349–353. [Google Scholar] [CrossRef]
- McHugh, C.P.; Zhang, P.; Michalek, S.; Eleazer, P.D. pH required to kill Enterococcus faecalis in vitro. J. Endod. 2004, 30, 218–219. [Google Scholar] [CrossRef] [PubMed]
- Oliver, J.D. The viable but non culturablestate in bacteria. J. Microbiol. 2005, 43, 93–100. [Google Scholar] [PubMed]


| Material | Chemical Compositions | Manufacturer |
|---|---|---|
| Biodentine | Powder: Tricalcium silicate (3CaO.SiO2), dicalcium silicate (2CaO.SiO2), calcium carbonate (CaCO3), calcium oxide (CaO), and zirconium oxide (ZrO2). Liquid: Water, calcium chloride (CaCI2), Hydrosoluble polymer | Septodont Saint-Maur-des-Fosses, CEDEX, France |
| ProRoot MTA | Powder: Tricalciumsilicate (Ca3SiO5), dicalcium silicate (CaSiO4), tricalciumaluminate (3CaO.Al2O3), tetracalciumaluminoferrite (4CaO.Al2O3Fe2O3), free calcium oxide (CaO), bismuth oxide (Bi2O3). Liquid: Water (H2O) | Dentsply, Tulsa Dental Specialities, Germany |
| NeoMTA Plus | Powder: Tricalcium silicate (Ca3SiO5), Dicalcium silicate (Ca2SiO4) and Tantalum oxide (Ta2O5). Liquid: Water (H2O) and proprietary polymers | NuSmile, Houston TX USA |
| MTA Repair HP | Powder: Tricalciumsilicate (Ca3SiO5), dicalcium silicate (Ca2SiO4), tricalciumaluminate (3CaO.Al2O3), calcium oxide (CaO), and calcium tungstate (CaWC4). Liquid: Water and polymer plasticizer | Angelus, Londrina, Brasil |
| MTA Angelus | Tricalcium silicate, dicalcium silicate, tricalcium aluminate, tetracalciumaluminoferrite, bismuth oxide (MSDS) | Angelus, Londrina, Brasil |
| Time (Days) | NeoMTA Plus | L-NeoMTA Plus | MTA Repair HP | L-MTA Repair HP |
|---|---|---|---|---|
| 1 | 0.835 ± 0.973 | 0.219 ± 0.398 | 0.083 ± 0.005 | 0.087 ± 0.019 |
| 10 | 5.220 ± 2.939 | 1.908 ± 4.665 | 3.644 ± 6.164 | 3.153 ± 4.836 |
| 20 | 7.356 ± 2.660 | 5.099 ± 3.752 | 5.043 ± 3.663 | 5.729 ± 4.114 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Çırakoğlu, S.; Baddal, B.; İslam, A. The Effectiveness of Laser-Activated Irrigation on the Apical Microleakage Qualities of MTA Repair HP and NeoMTA Plus in Simulated Immature Teeth: A Comparative Study. Materials 2020, 13, 3287. https://doi.org/10.3390/ma13153287
Çırakoğlu S, Baddal B, İslam A. The Effectiveness of Laser-Activated Irrigation on the Apical Microleakage Qualities of MTA Repair HP and NeoMTA Plus in Simulated Immature Teeth: A Comparative Study. Materials. 2020; 13(15):3287. https://doi.org/10.3390/ma13153287
Chicago/Turabian StyleÇırakoğlu, Serenad, Buket Baddal, and Aylin İslam. 2020. "The Effectiveness of Laser-Activated Irrigation on the Apical Microleakage Qualities of MTA Repair HP and NeoMTA Plus in Simulated Immature Teeth: A Comparative Study" Materials 13, no. 15: 3287. https://doi.org/10.3390/ma13153287
APA StyleÇırakoğlu, S., Baddal, B., & İslam, A. (2020). The Effectiveness of Laser-Activated Irrigation on the Apical Microleakage Qualities of MTA Repair HP and NeoMTA Plus in Simulated Immature Teeth: A Comparative Study. Materials, 13(15), 3287. https://doi.org/10.3390/ma13153287






