Synthesis, Spectroscopic, Thermal and Catalytic Properties of Four New Metal (II) Complexes with Selected N- and O-Donor Ligands
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials, Synthesis and Analysis
2.2. Methods and Instruments
3. Results
3.1. Elemental Analysis
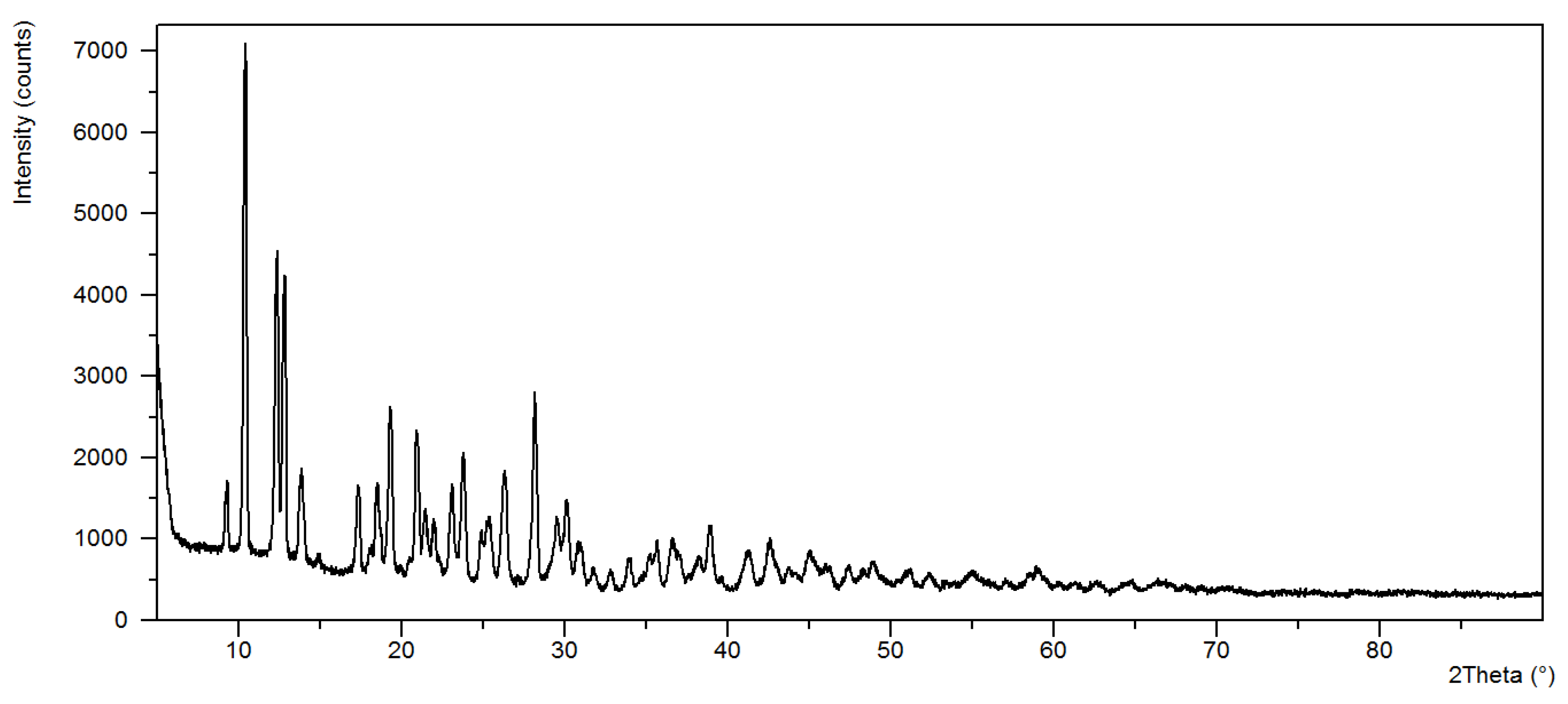
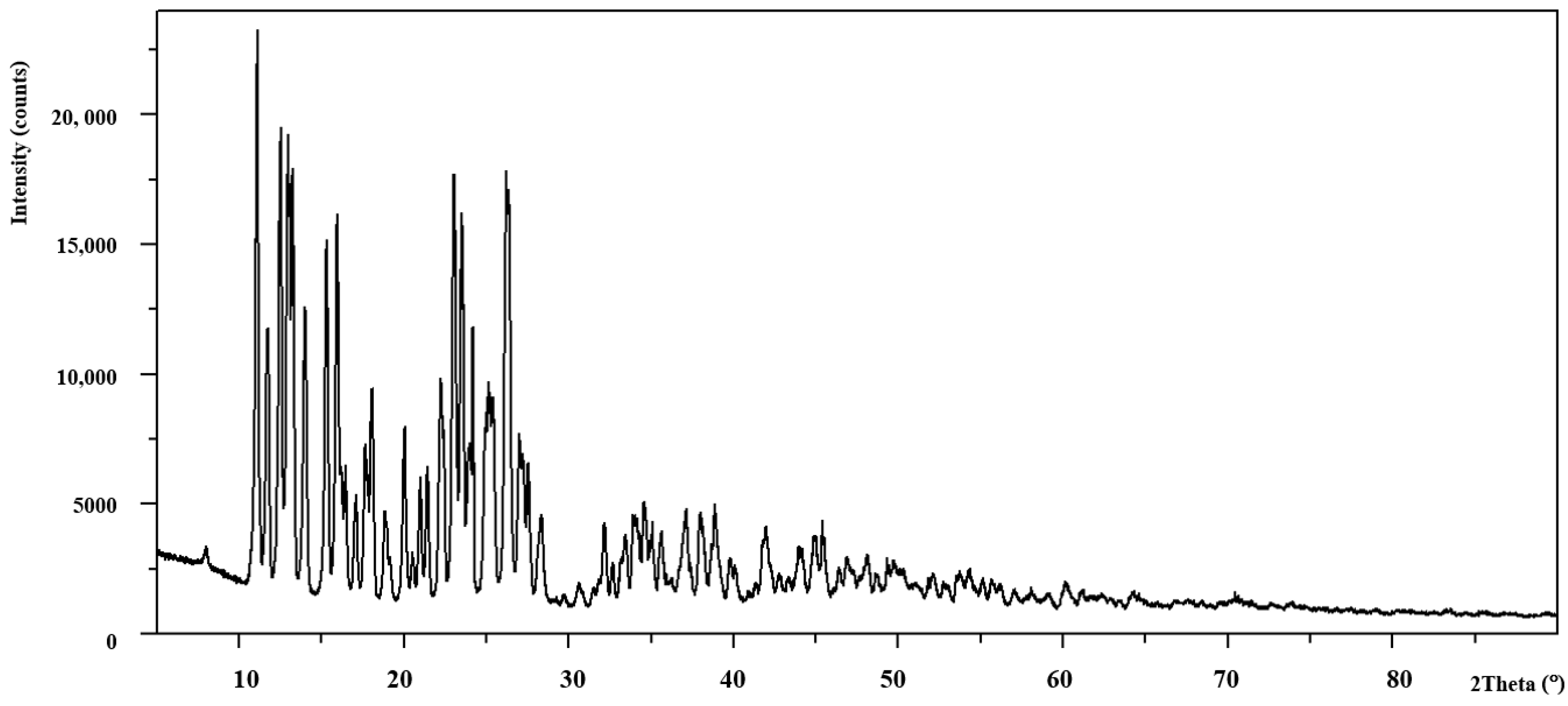
3.2. X-ray Diffraction Data
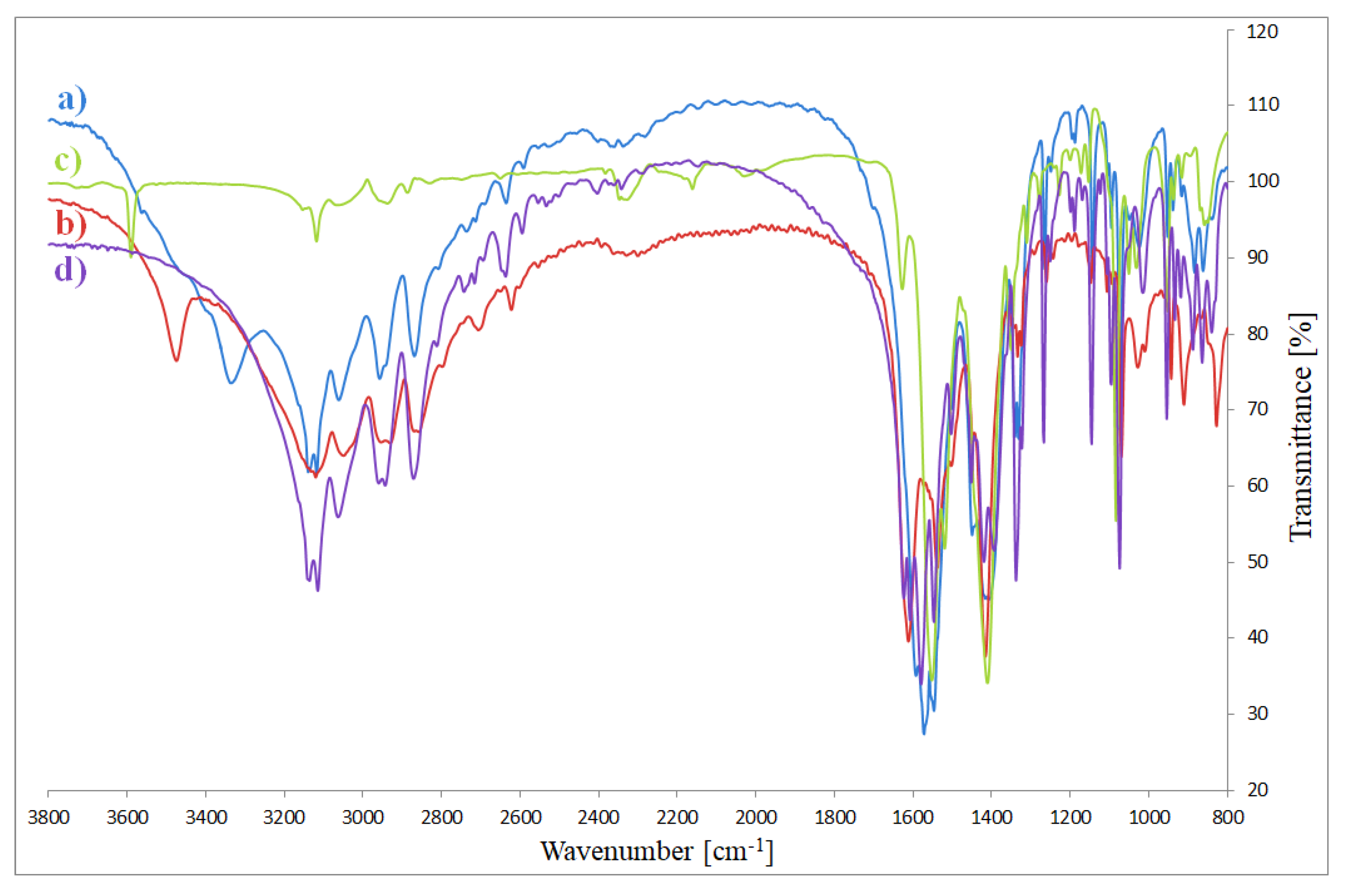
3.3. FTIR Spectra
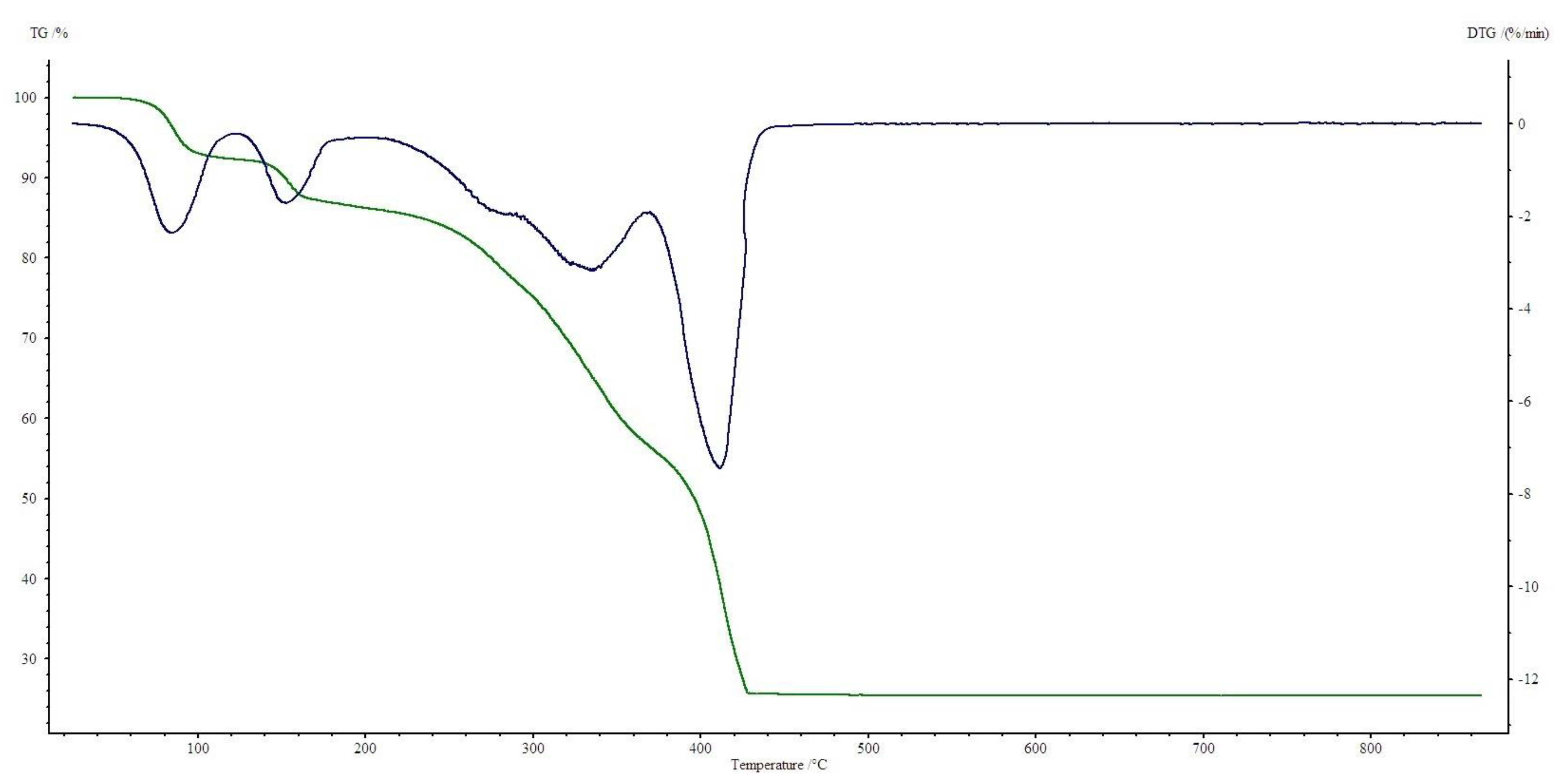
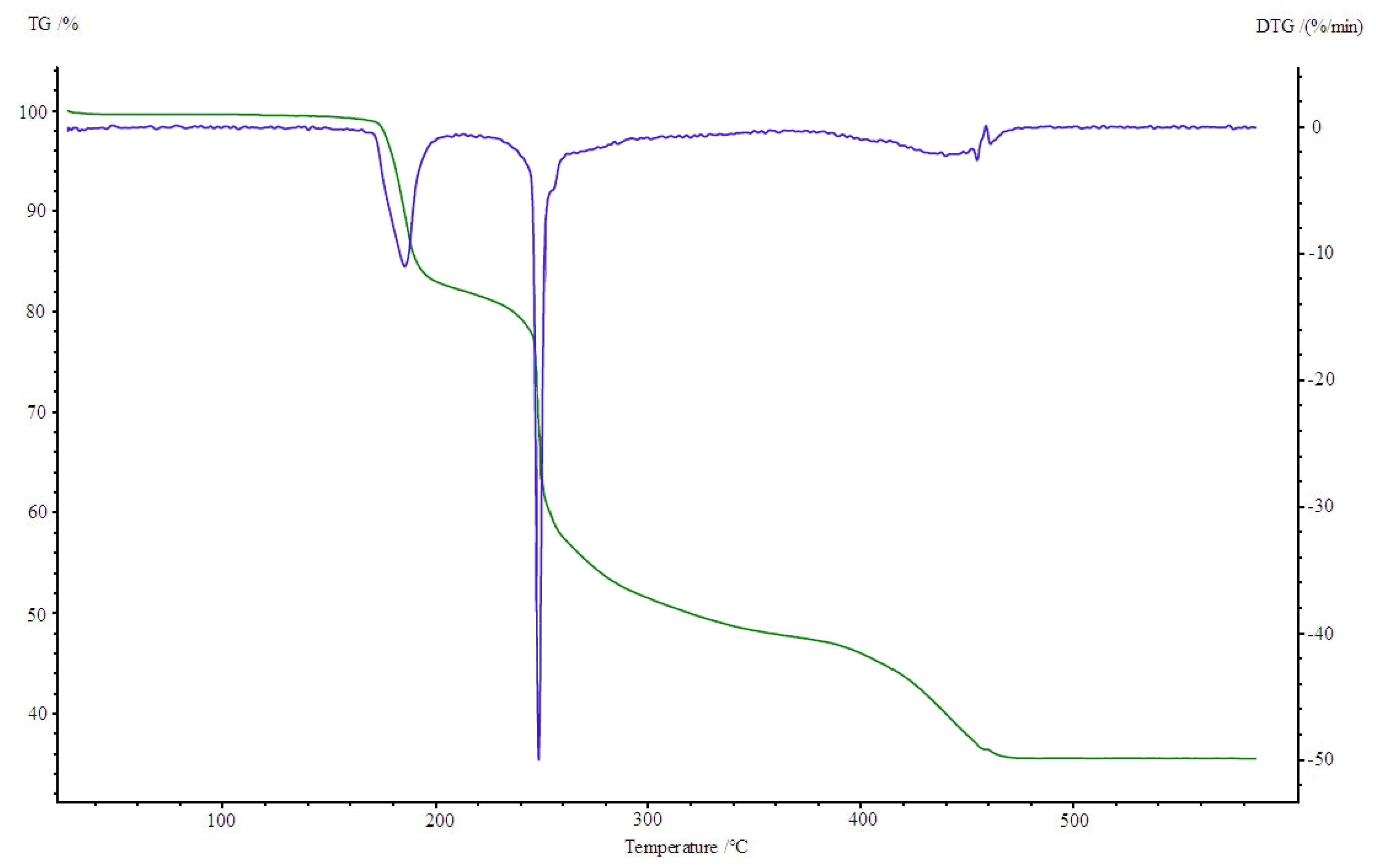
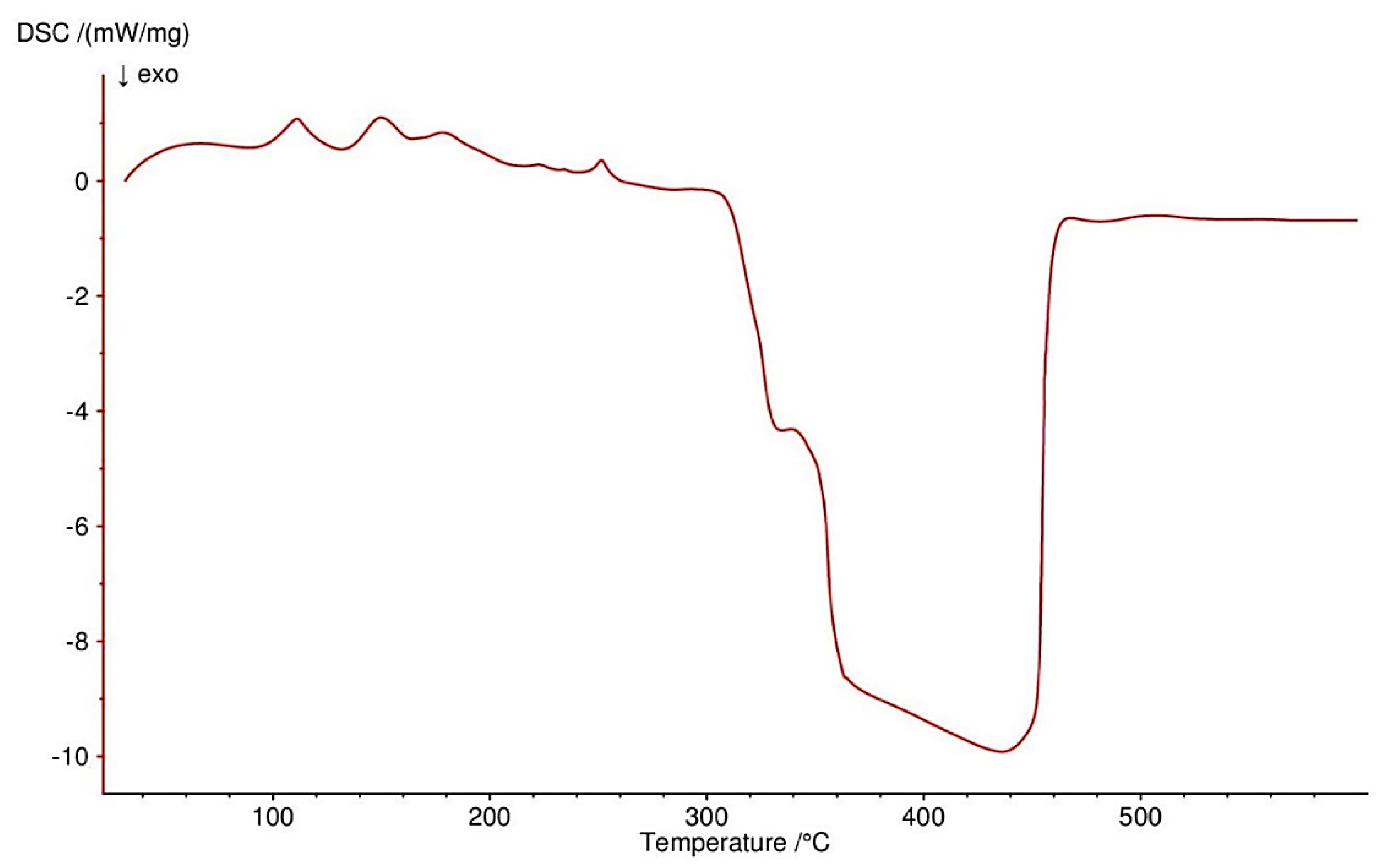
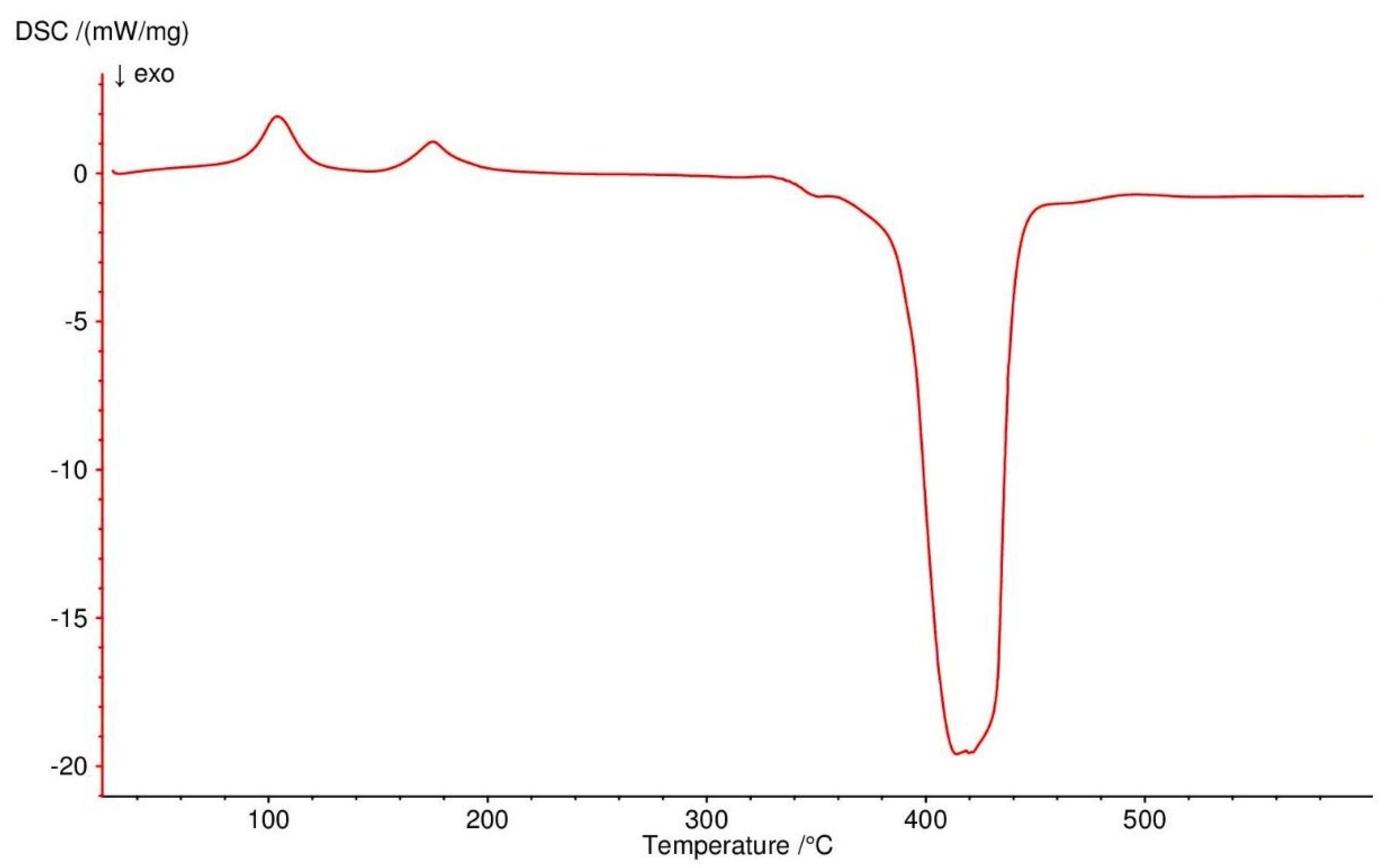
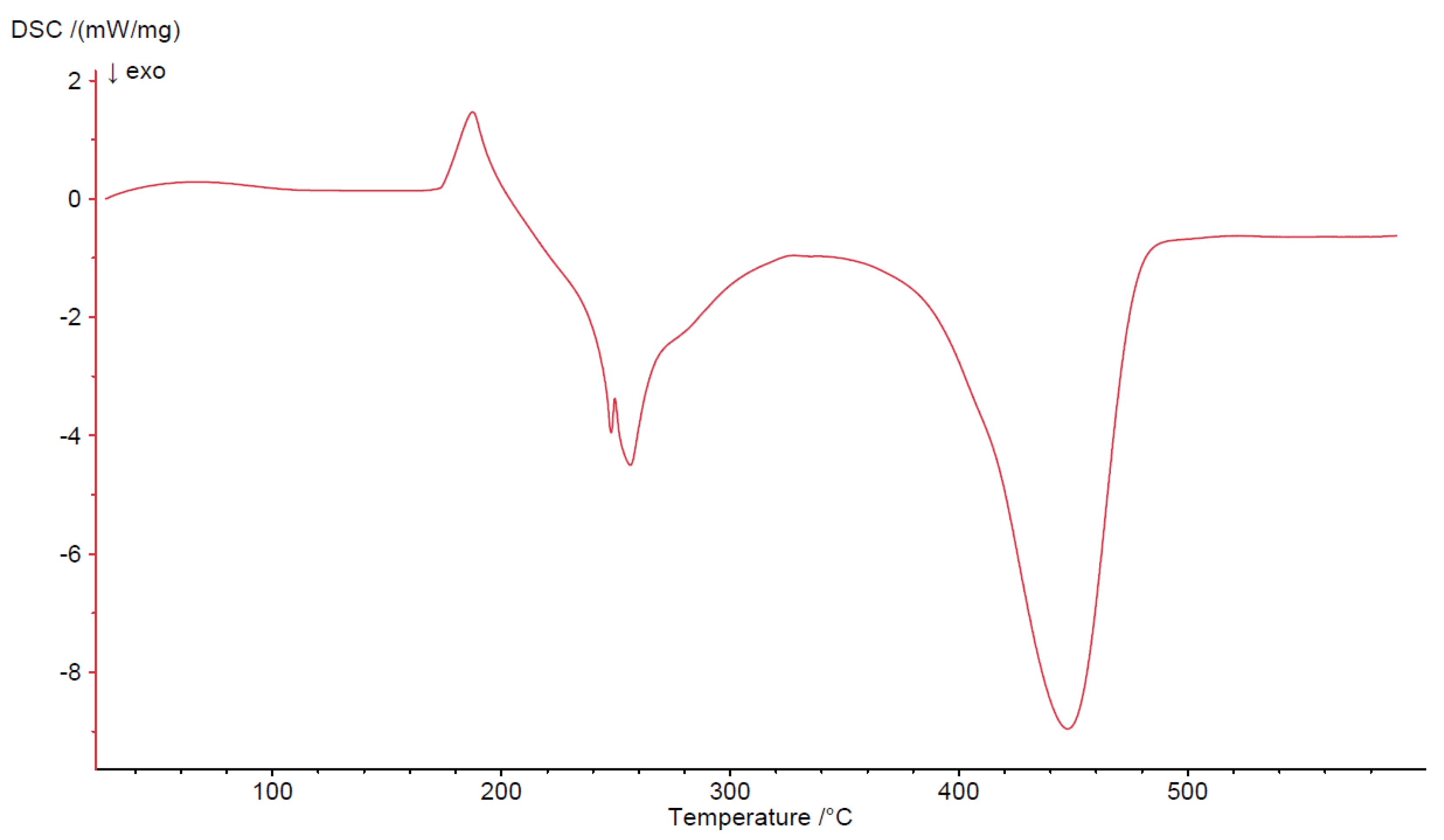
3.4. Thermogravimetric Studies in Air
3.5. TG-FTIR Studies in Air
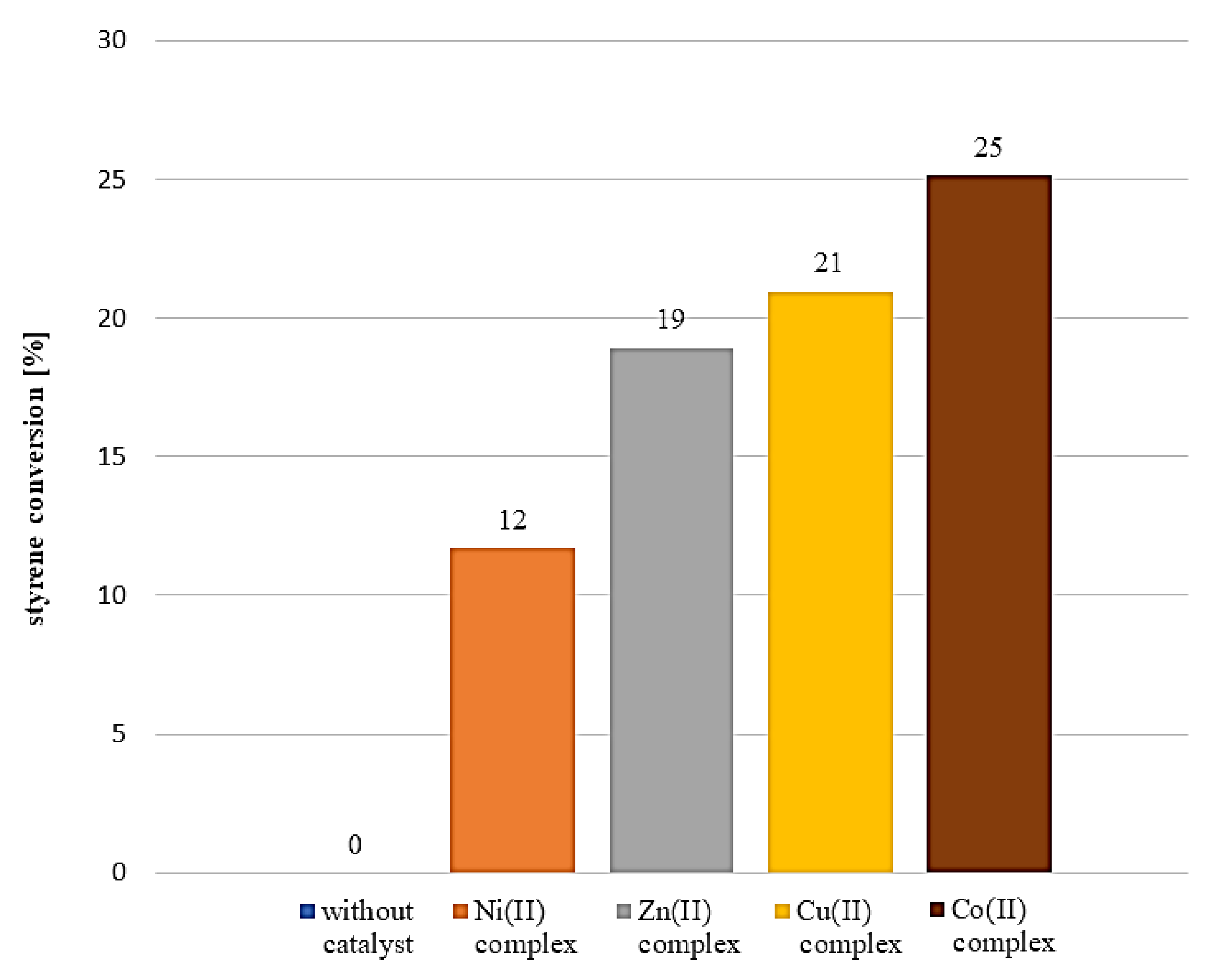
3.6. Activity Tests in Styrene Oxidation Reaction
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Abraham, O.C.; Manavathu, E.K.; Cutright, J.L.; Chandrasekar, P.H. In vitro susceptibilities of Aspergillus species to voriconazole, itraconazole, and amphotericin B. Diagn. Microbiol. Infect. Dis. 1999, 33, 7–11. [Google Scholar] [CrossRef]
- Ally, R.; Schürmann, D.K.W.; Carosi, G.; Aguirrebengoa, K.; Dupont, B.; Hodges, M.; Troke, P.; Romero, A.J. A randomized, double-blind, double-dummy, multicenter trial of voriconazole and fluconazole in the treatment of esophageal candidiasis in immunocompromised patients. Clin. Infect. Dis. 2001, 33, 1447–1454. [Google Scholar] [CrossRef]
- Andes, D.; Marchillo, K.; Stamstad, T.; Conklin, R. In Vivo Pharmacokinetics and Pharmacodynamics of a New Triazole, Voriconazole, in a Murine Candidiasis Model. Antimicrob. Agents Chemother. 2003, 47, 3165–3169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burgess, D.S.; Hastings, R.W.; Summers, K.K.; Hardin, T.C.; Rinaldi, M.G. Pharmacodynamics of fluconazole, itraconazole, and amphotericin B against Candida albicans. Diagn. Microbiol. Infect. Dis. 2000, 36, 13–18. [Google Scholar] [CrossRef]
- Chandrasekar, P.H.; Manavathu, E. Voriconazole: A second-generation triazole. Drugs Today 2001, 37, 135–148. [Google Scholar] [CrossRef] [PubMed]
- Pfaller, M.A.; Sutton, D.A. Review of in vitro activity of sertaconazole nitrate in the treatment of superficial fungal infections. Diagn. Microbiol. Infect. Dis. 2006, 56, 147–152. [Google Scholar] [CrossRef]
- Washton, H. Review of fluconazole: A new triazole antifungal agent. Diagn. Microbiol. Infect. Dis. 1989, 12, 229–233. [Google Scholar] [CrossRef]
- Wong-Beringera, A.; Hindler, J.; Brankovic, L.; Muehlbauer, L.; Steele-Moore, L. Clinical applicability of antifungal susceptibility testing on non-Candida albicans species in hospitalized patients. Diagn. Microbiol. Infect. Dis. 2001, 39, 25–31. [Google Scholar] [CrossRef]
- Mikuriya, M. Copper (II) acetate as a motif for metal-assembled complexes. Bull. Jpn. Soc. Coord. Chem. 2008, 52, 17–28. [Google Scholar] [CrossRef] [Green Version]
- Deacon, G.B. Relationships between the carbon-oxygen stretching frequencies of carboxylato complexes and the type of carboxylate coordination. Coord. Chem. Rev. 1980, 33, 227–250. [Google Scholar] [CrossRef]
- Czylkowska, A.; Raducka, A.; Mierczynski, P. Synthesis, thermal study and some properties of Zn(II), Cd(II) and Pb(II) compounds with mono-, di- and trichloroacetates. J. Therm. Anal. Calorim. 2017, 128, 937–946. [Google Scholar] [CrossRef] [Green Version]
- Sieron, L.; Czylkowska, A.; Rogalewicz, B. Crystal structure of a one-dimensional coordination polimer of gadolinium dibromoacetate with 4,4’-bipyridine. Eur. J. Chem. 2018, 9, 178–181. [Google Scholar] [CrossRef] [Green Version]
- Czylkowska, A. Synthesis, thermal study and some properties of Gd(III), Tb(III), Dy(III) and Er(III) complexes with 4,4′-bipyridine and dibromoacetates. J. Therm. Anal. Calorim. 2015, 122, 339–347. [Google Scholar] [CrossRef] [Green Version]
- Fiume, M.Z. Final report on the safety assessment of triacetin. Int. J. Toxicol. 2003, 22, 1–10. [Google Scholar]
- Kim, D.; Lim, H.-W.; Kim, S.-H.; Seo, K.H. Development of a real-time PCR assay for rapid screening of acetic acid bacteria as a group in food products. Food Control. 2019, 100, 78–82. [Google Scholar] [CrossRef]
- Chen, X.-M.; Ye, B.-H.; Huang, X.-C.; Xu, Z.-T. Model complexes for the carboxylate–histidine–metal triad systems in metalloenzymes. Synthesis, crystal structures and spectroscopic properties of [M(Him)2(O2CMe)2](M = ZnII or CoII, Him = imidazole). J. Chem. Soc. Dalton Trans. 1996, 16, 3465–3468. [Google Scholar] [CrossRef]
- Naumov, P.; Ristova, M.; Drew, M.G.B.; Ng, S.W. Bis (acetate-O) tetrakis (imidazole-N3) nickel (II). Acta Crystallogr. Sect. C Cryst. Struct. Commun. 2000, 56, e372–e373. [Google Scholar] [CrossRef]
- Henriksson, H.Å. The crystal structure of bis (imidazole) copper (II) diacetate. Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem. 1977, 33, 1947–1950. [Google Scholar] [CrossRef]
- Abuhijleh, A.; Woods, C. Synthesis, spectroscopic and structural characterization of bis (acetato) tetrakis (imidazole) copper (II): A model complex for DNA binding. Inorg. Chim. Acta 1992, 194, 9–14. [Google Scholar] [CrossRef]
- Horrocks, W.D., Jr.; Ishley, J.N.; Holmquist, B.; Thompson, J.S. Structural and electronic mimics of the active site of cobalt (II)-substituted zinc metalloenzymes. J. Inorg. Biochem. 1980, 12, 131–141. [Google Scholar] [CrossRef]
- Chen, X.-M.; Xu, Z.-T.; Huang, X.-C. Dalton communications. A model complex for the carboxylate–histidine–zinc system in zinc enzymes. Crystal structure of [Zn(Him)2(MeCO2)2](Him = imidazole). J. Chem. Soc. Dalton Trans. 1994, 15, 2331–2332. [Google Scholar] [CrossRef]
- Horrocks, W.D.; Ishley, J.N.; Whittle, R.R. Models for cobalt (II)-substituted zinc metalloenzymes. 2. Comparisons of the crystal structures of complexes of the type [M(RCOO)2(2-X-Im)2] (Im = imidazole; M = Co, Zn; R = CH3, C2H5, C3H7; X = CH3, C2H5). An unusual type of linkage isomerism. Inorg. Chem. 1982, 21, 3270–3274. [Google Scholar] [CrossRef]
- Gadet, A.L. Structure cristalline du complexe cobalt(lmidazole)2(acelate)2. Acta Cryst. 1974, 30, 349–353. [Google Scholar] [CrossRef]
- Schubert, D.M.; Visi, M.Z.; Knobler, C.B. Acid-catalyzed synthesis of zinc imidazolates and related bimetallic metal-organic framework compounds. Main Group Chem. 2008, 7, 311–322. [Google Scholar] [CrossRef]
- Brown, D.; Glass, W.; Brown, D.; Kemp, T.; Errington, W.; Clarkson, G.; Haase, W.; Karsten, F.; Mahdy, A. Structural variations in dinuclear model hydrolases and hydroxamate inhibitor models: Synthetic, spectroscopic and structural studies. Inorg. Chim. Acta 2004, 357, 1411–1436. [Google Scholar] [CrossRef]
- Ye, B.H.; Williams, I.D.; Li, X.Y. Syntheses and characterization of aqua-bridged dimetallic complexes, M2(μ-H2O)(μ-OAc)2(Im)4(OAc)2 (M=Mg2+, Mn2+ and Ni2+). Structural models for the active sites of dimetallic hydrolases. J. Inorg. Biochem. 2002, 92, 128–136. [Google Scholar] [CrossRef]
- Bao-Hui, Y.; Xiao-Ming, C. Syntheses, Characterization and Equilibrium between Mono-and Aqua-bridged Dicobalt (II) Complexes. A Structural Model for Methionine Aminopeptidase. Chin. J. Chem. 2010, 21, 531–536. [Google Scholar] [CrossRef]
- Masciocchi, N.; Ardizzoia, G.A.; LaMonica, G.; Maspero, A.; Galli, S.; Sironi, A. Metal imidazolato complexes: Synthesis, characterization, and X-ray powder diffraction studies of group 10 coordination polymers. Inorg. Chem. 2001, 40, 6983–6989. [Google Scholar] [CrossRef]
- Stamatatos, T.C.; Perlepes, S.P.; Raptopoulou, C.P.; Terzis, A.; Patrickios, C.S.; Tasiopoulos, A.J.; Boudalis, A.K. Alcoholysis/hydrolysis of 1,1′-carbonyldiimidazole as a means of preparing unprecedented, imidazole-containing one-dimensional coordination polymers of copper (II). Dalton Trans. 2009, 17, 3354–3362. [Google Scholar] [CrossRef]
- Adam, F.; Iqbal, A. The oxidation of styrene by chromium–silica heterogeneous catalyst prepared from rice husk. Chem. Eng. J. 2010, 160, 742–750. [Google Scholar] [CrossRef]
- Ratnasamy, P.; Kumar, R. Selective oxidation with redox metallosilicates in the production of fine chemicals. Adv. Pharmacol. 1995, 97, 367–376. [Google Scholar] [CrossRef]
- Powder Diffraction File, PDF-2; The International Centre for Diffraction Data (ICDD): 12 Campus Boulevard, Newton Square, PA, USA, 2004.
- Morzyk-Ociepa, B.; Różycka-Sokołowska, E.; Michalska, D. Revised crystal and molecular structure, FT-IR spectra and DFT studies of chlorotetrakis (imidazole) copper (II) chloride. J. Mol. Struct. 2012, 1028, 49–56. [Google Scholar] [CrossRef]
- Vlaicu, I.D.; Olar, R.; Scaeteanu, G.V.; Silvestro, L.; Maurer, M.; Stanica, N.; Badea, M. Thermal, spectral and biological investigation of new nickel complexes with imidazole derivatives. J. Therm. Anal. Calorim. 2018, 134, 503–512. [Google Scholar] [CrossRef]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds; Wiley and Sons: New York, NY, USA, 2009. [Google Scholar]
- Alcock, N.W.; Tracy, V.M.; Waddington, T.C. Acetates and acetato-complexes. Part 2. Spectroscopic studies. J. Chem. Soc. Dalton Trans. 1976, 21, 2243–2246. [Google Scholar] [CrossRef]
- Brzyska, W.; Ożga, W. Spectral, magnetic and thermal investigations of some d-electron element 3-methoxy-4-methylbenzoates. J. Therm. Anal. Calorim. 2006, 84, 385–389. [Google Scholar] [CrossRef]
- Zeleňák, V.; Vargová, Z.; Györyová, K. Correlation of infrared spectra of zinc (II) carboxylates with their structures. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2006, 66, 262–272. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.; Gao, Q. Selective oxidation of styrene to benzaldehyde over VSB-5 and isomorphously substituted cobalt VSB-5. Catal. Commun. 2007, 8, 681–685. [Google Scholar] [CrossRef]
- Titinchi, S.J.; Von Willingh, G.; Abbo, H.S.; Prasad, R. Tri- and tetradentate copper complexes: A comparative study on homogeneous and heterogeneous catalysis over oxidation reactions. Catal. Sci. Technol. 2015, 5, 325–338. [Google Scholar] [CrossRef]
- Aberkouks, A.; Mekkaoui, A.A.; Boualy, B.; El Houssame, S.; Ali, M.A.; El Firdoussi, L. Selective oxidation of styrene to benzaldehyde by Co-Ag codoped ZnO catalyst and H2O2 as oxidant. Adv. Mater. Sci. Eng. 2018, 1–7. [Google Scholar] [CrossRef] [Green Version]






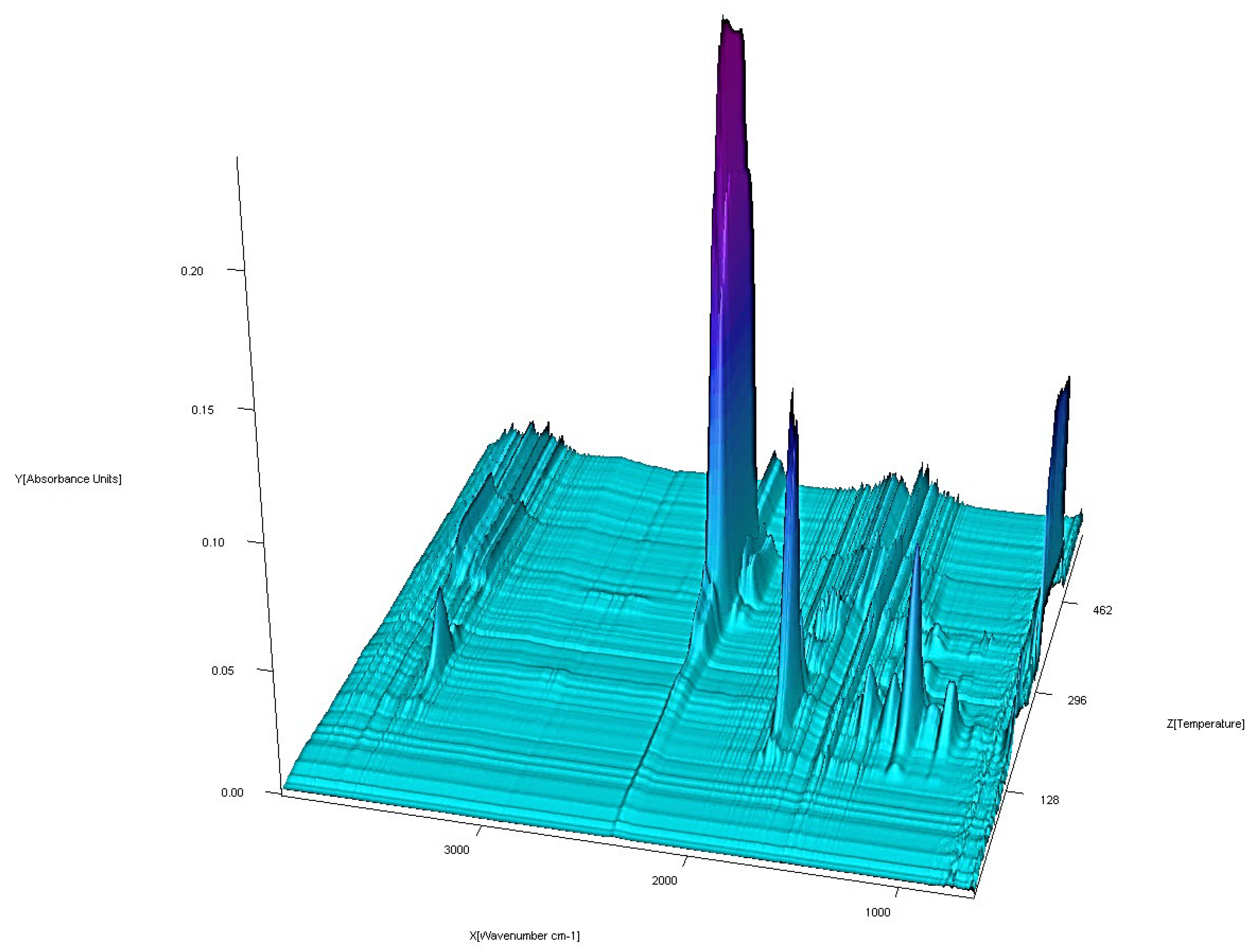
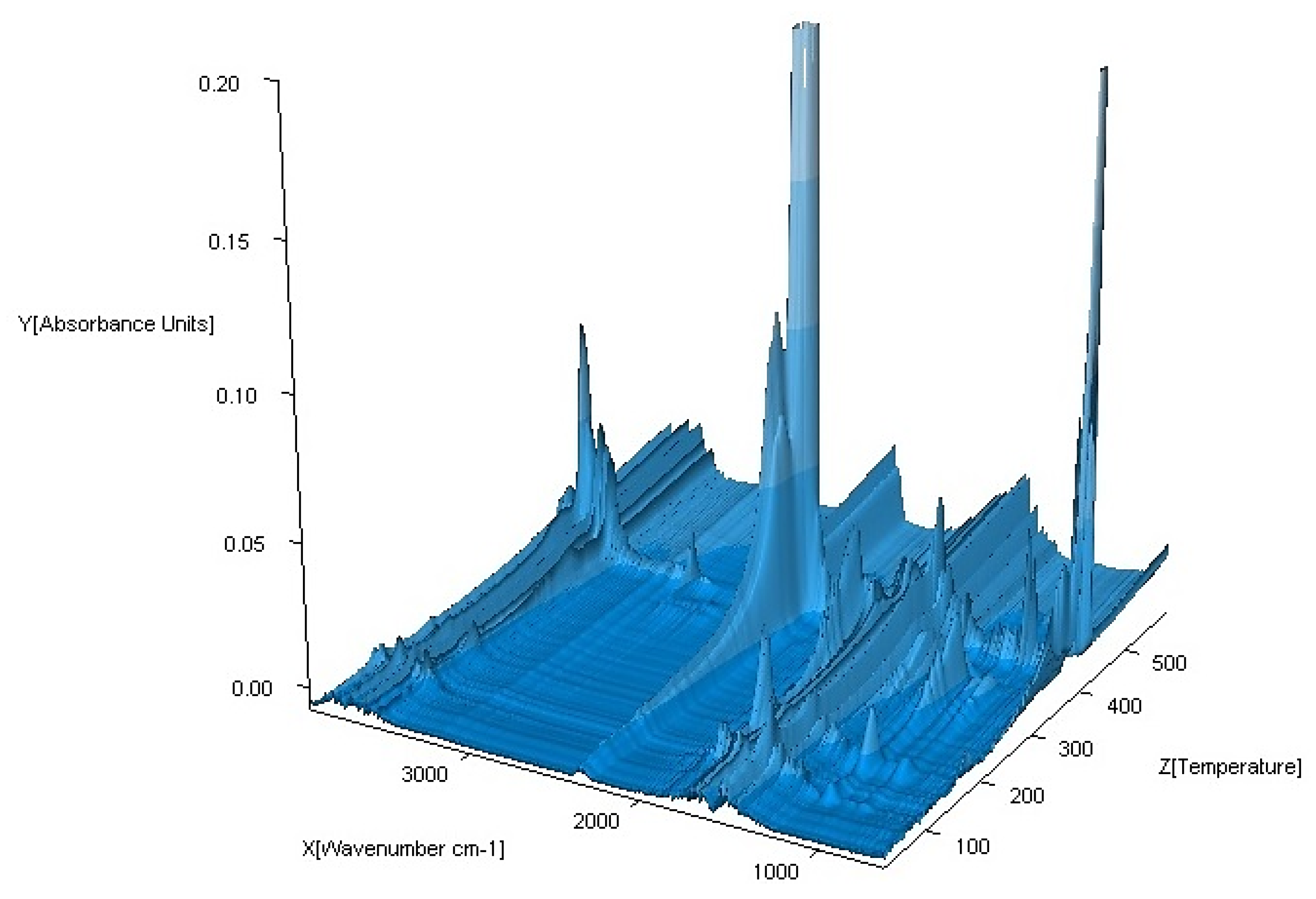
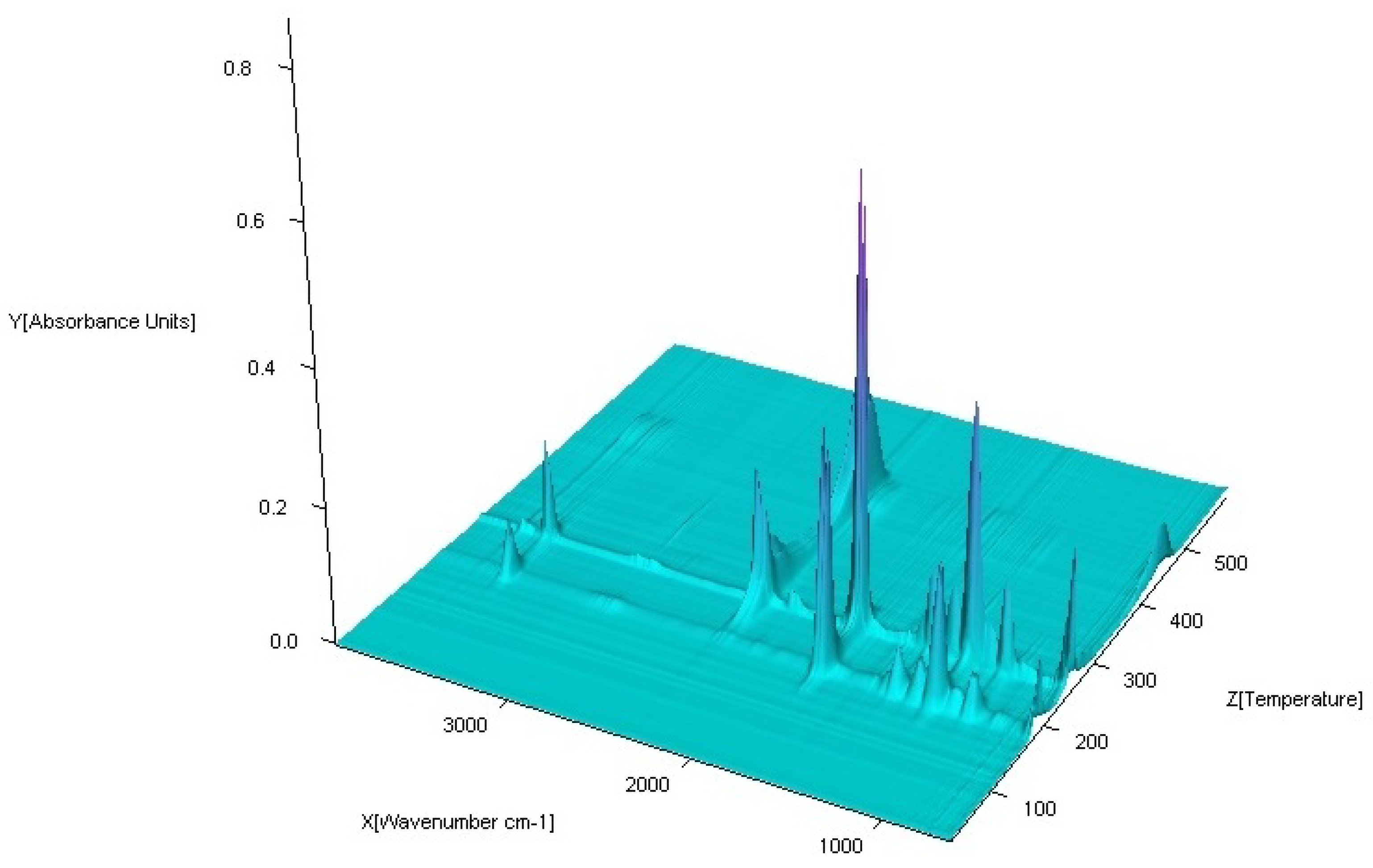
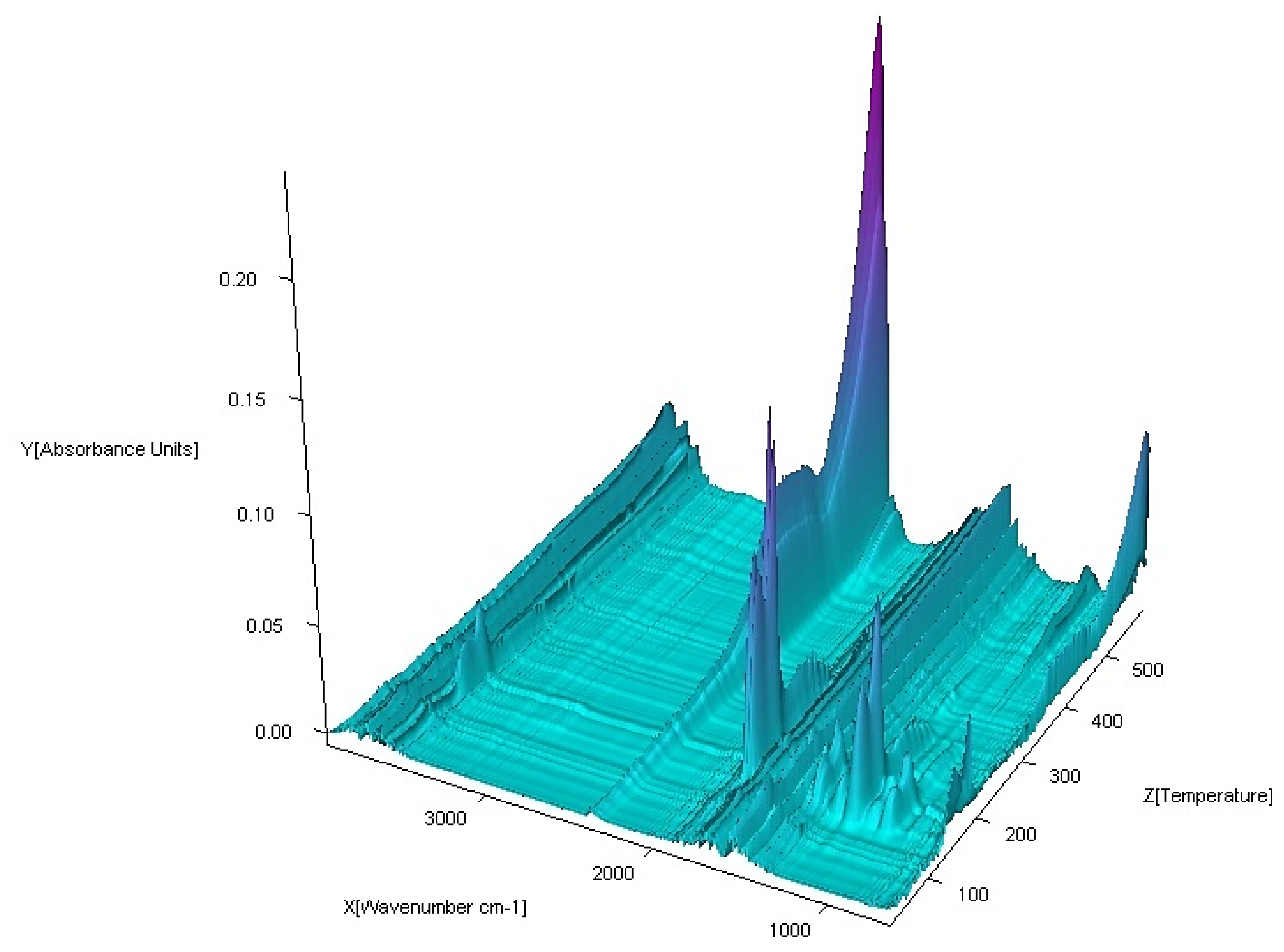
| No. | Compound | Analysis: (Found) Calculated/% | |||
|---|---|---|---|---|---|
| M | C | H | N | ||
| (I) | Co(OAc)2(Im)·H2O | 22.40 (22.43) | 31.95 (31.90) | 4.56 (4.59) | 10.64 (10.59) |
| (II) | Ni(OAc)2(Im)1.5·2H2O | 18.64 (18.59) | 32.39 (32.48) | 5.08 (5.06) | 13.34 (13.42) |
| (III) | Cu2(OAc)4(Im) | 29.46 (28.94) | 30.63 (31.18) | 3.53 (3.50) | 6.49 (6.45) |
| (IV) | Zn(OAc)2(Im)·H2O | 24.25 (24.19) | 31.15 (31.05) | 4.45 (4.43) | 10.39 (10.43) |
| Compound | ν(NH) | ν(CH) | ν(CN) | δ(NH), ν(CC), ν(CN) | δ(CH), δ(imidazole ring) | π(CH), δ(imidazole ring) | νas(COO) | νs(COO) |
|---|---|---|---|---|---|---|---|---|
| NaOAc | − | − | − | − | − | − | 1578 | 1414 |
| imidazole | 3124 | 3014 | 1645 | 1540 | 937 | 894 | − | − |
| Co(OAc)2(Im)·H2O | 3139 3118 | 3058 | 1600 | 1546 | 952 | 883 | 1571 | 1415 1409 |
| Ni(OAc)2(Im)1.5·2H2O | 3120 | 3047 | 1612 | 1537 | 910 | 868 | • | 1415 |
| Cu2(OAc)4(Im) | 3143 3118 | 3056 | 1627 | 1552 | 916 | 869 | 1519 | 1409 1360 |
| Zn(OAc)2(Im)·H2O | 3139 3114 | 3058 | 1621 1610 | 1548 | 954 | 887 | 1577 | 1419 |
| Compound | Range of Decomposition/°C | DSC Peaks/°C | Mass Loss/% | Intermediate and Residue Solid Products | |
|---|---|---|---|---|---|
| Found | Calc. | ||||
| Co(OAc)2(Im)·H2O | 80–125 125–225 225–410 | 110 endo 150, 180 endo 250 endo, 330–460 exo | 4.0 17.0 49.5 | 3.43 16.35 49.72 | Co(OAc)2(Im)·0.5H2O Co(OAc)2(Im)0.5 Co3O4 |
| Ni(OAc)2(Im)1.5·2H2O | 50–125 125–160 160–375 375–440 | 105 endo - 175 endo 350–440 exo | 8.0 3.5 32.0 32.5 | 8.58 2.86 32.43 32.42 | Ni(OAc)2(Im)1.5·0.5H2O Ni(OAc)2(Im)1.5 Ni(OAc)2 NiO |
| Cu2(OAc)4(Im) | 165–200 200–340 340–490 | 190 endo 250, 260 exo 450 exo | 16.0 34.0 13.5 | 15.78 34.23 13.12 | Cu2(OAc)4 Cu2(OAc)1.5 CuO |
| Zn(OAc)2(Im)·H2O | 50–100 125–310 310–650 | 80 endo 140, 170 endo 330 endo, 430–600 exo | 4.0 28.0 37.5 | 3.34 28.62 37.86 | Zn(OAc)2(Im)·0.5H2O Zn(OAc)2 ZnO |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Czylkowska, A.; Rogalewicz, B.; Raducka, A.; Błaszczyk, N.; Maniecki, T.; Wieczorek, K.; Mierczyński, P. Synthesis, Spectroscopic, Thermal and Catalytic Properties of Four New Metal (II) Complexes with Selected N- and O-Donor Ligands. Materials 2020, 13, 3217. https://doi.org/10.3390/ma13143217
Czylkowska A, Rogalewicz B, Raducka A, Błaszczyk N, Maniecki T, Wieczorek K, Mierczyński P. Synthesis, Spectroscopic, Thermal and Catalytic Properties of Four New Metal (II) Complexes with Selected N- and O-Donor Ligands. Materials. 2020; 13(14):3217. https://doi.org/10.3390/ma13143217
Chicago/Turabian StyleCzylkowska, Agnieszka, Bartłomiej Rogalewicz, Anita Raducka, Natalia Błaszczyk, Tomasz Maniecki, Kinga Wieczorek, and Paweł Mierczyński. 2020. "Synthesis, Spectroscopic, Thermal and Catalytic Properties of Four New Metal (II) Complexes with Selected N- and O-Donor Ligands" Materials 13, no. 14: 3217. https://doi.org/10.3390/ma13143217
APA StyleCzylkowska, A., Rogalewicz, B., Raducka, A., Błaszczyk, N., Maniecki, T., Wieczorek, K., & Mierczyński, P. (2020). Synthesis, Spectroscopic, Thermal and Catalytic Properties of Four New Metal (II) Complexes with Selected N- and O-Donor Ligands. Materials, 13(14), 3217. https://doi.org/10.3390/ma13143217






