The Synergistic Effect of Ionic Liquid-Modified Expandable Graphite and Intumescent Flame-Retardant on Flame-Retardant Rigid Polyurethane Foams
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
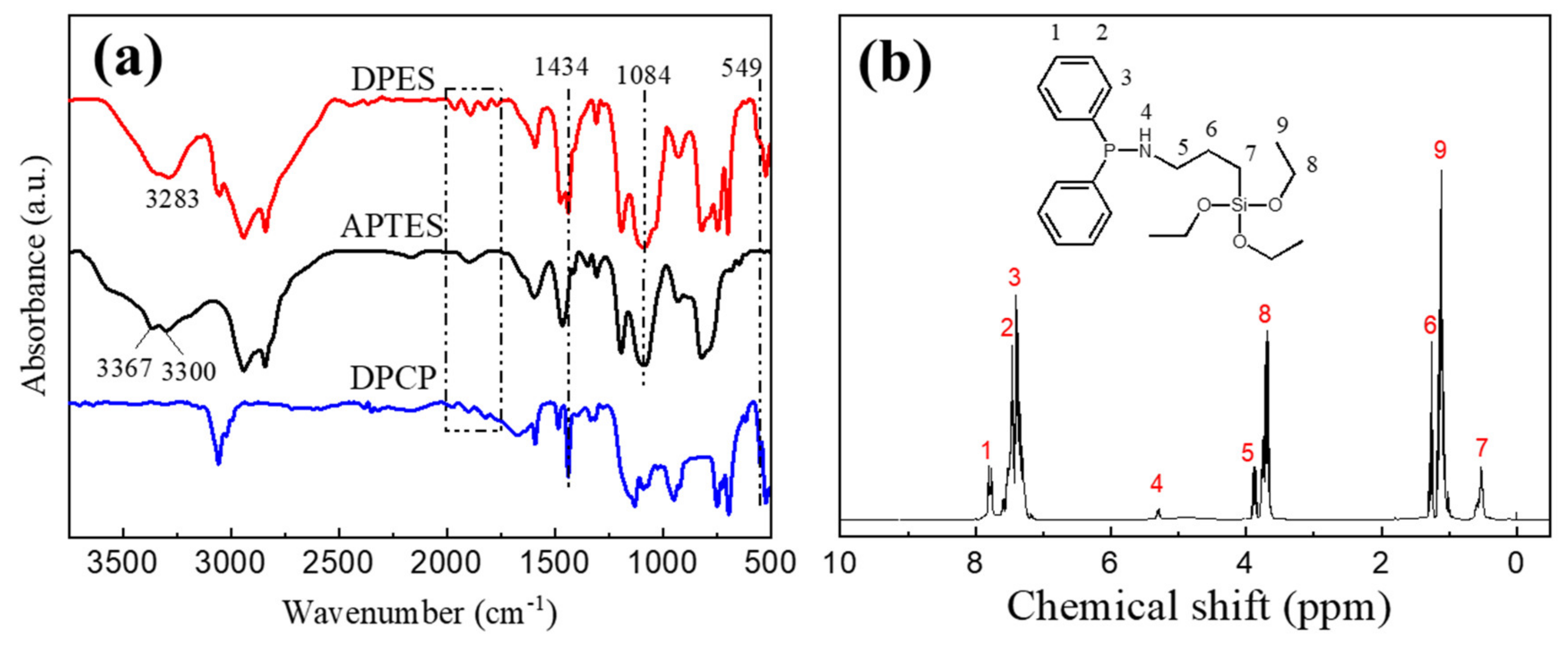
2.2. Preparation of DPES
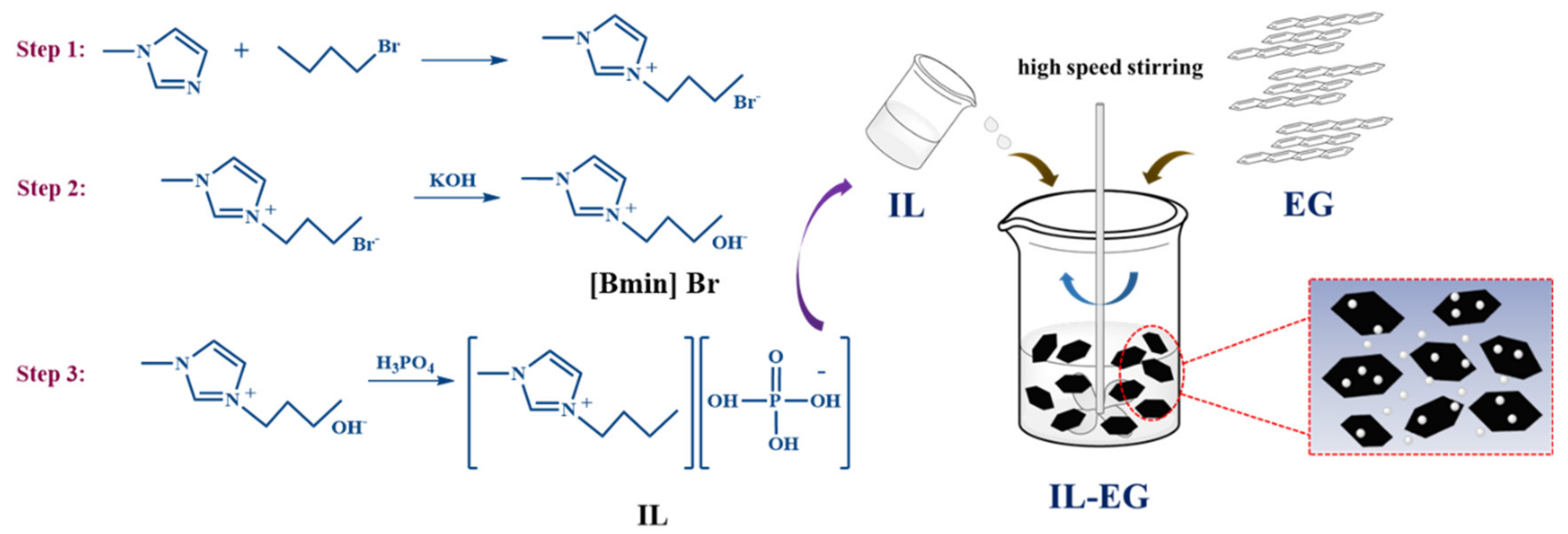
2.3. Preparation of IL-EG
2.4. Preparation of Flame-Retardant RPUF
2.5. Characterization
3. Results and Discussion
3.1. Structural Characterization of DPES
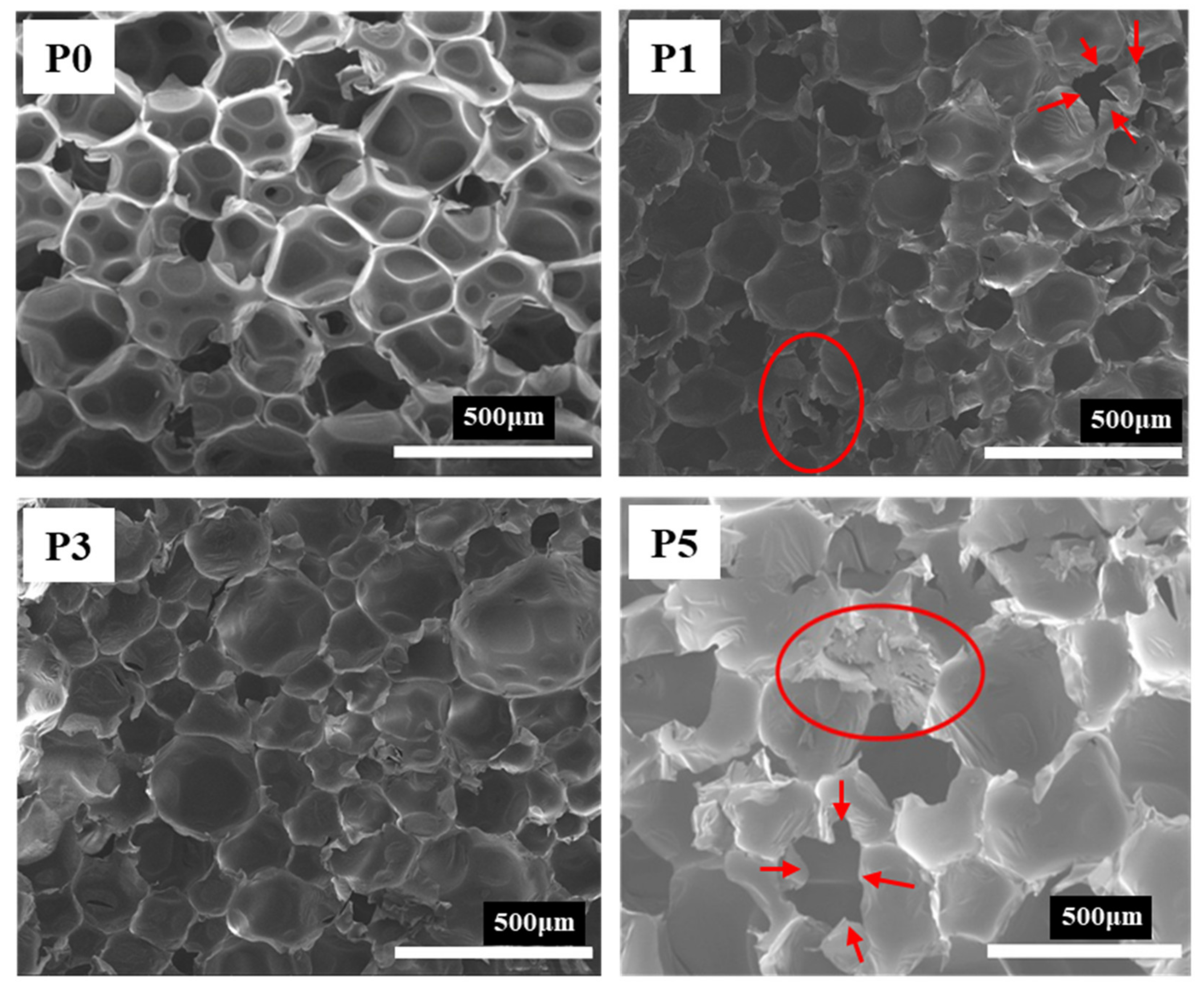
3.2. Effects on Pore Morphology of RPUF with Different Flame-Retardant Systems
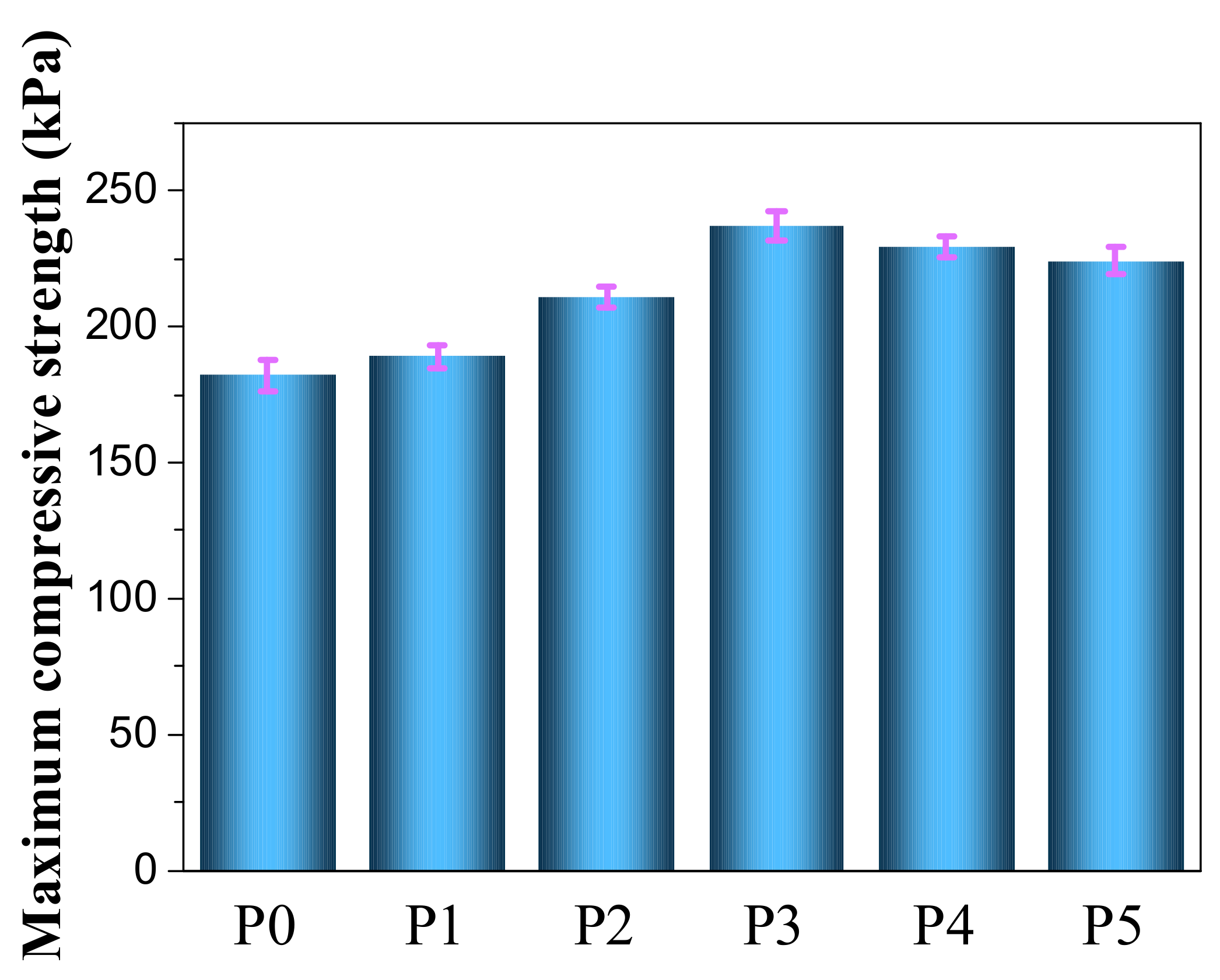
3.3. Mechanical Properties of IL-EG/DPES/RPUF Composites
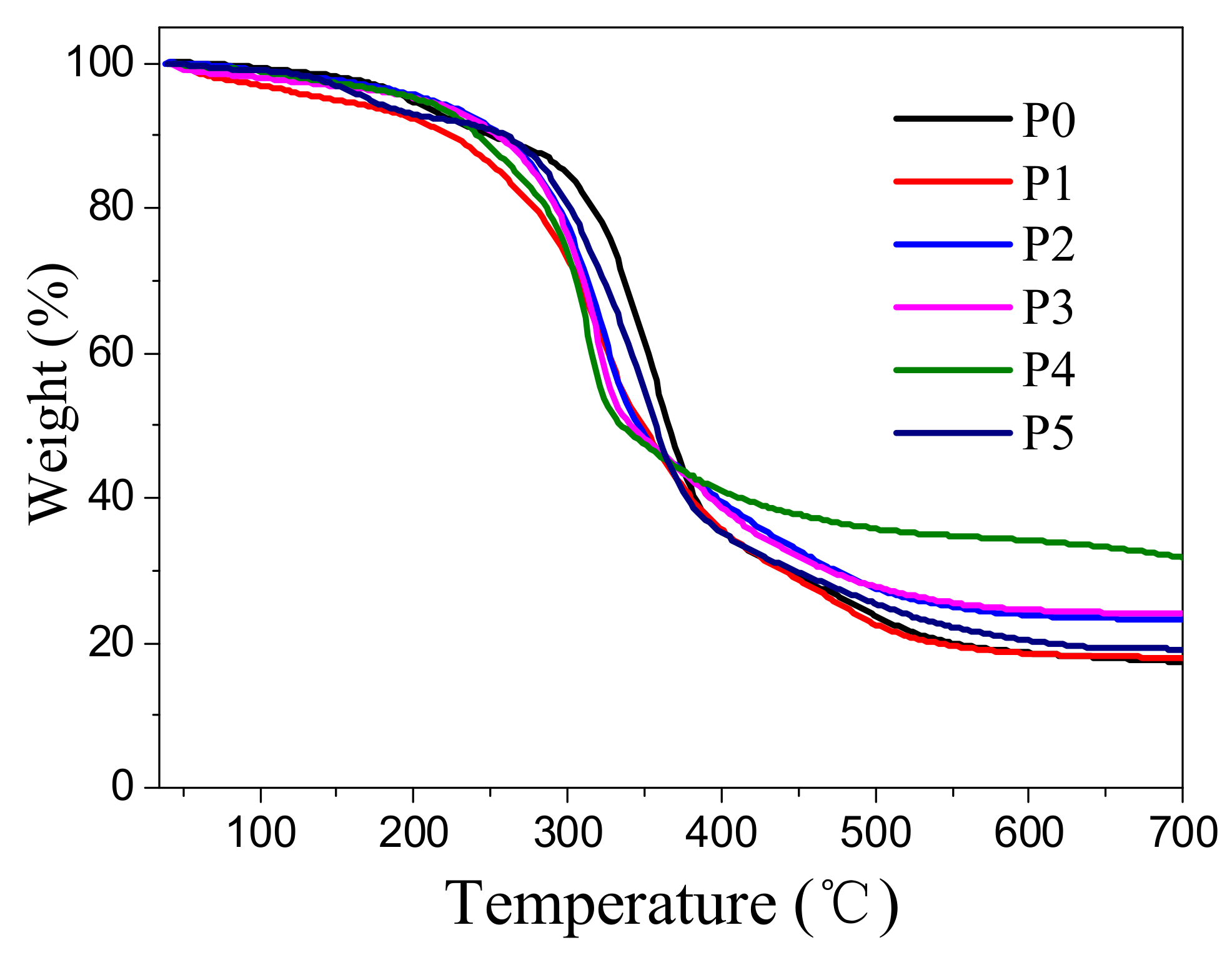
3.4. Thermal Degradation Behavior of Flame-Retardant RPUF
3.5. Effect of IL-EG/DPES on RPUF Flame-Retardancy
3.5.1. CONE Calorimeter Analysis
3.5.2. Horizontal Burning Performance
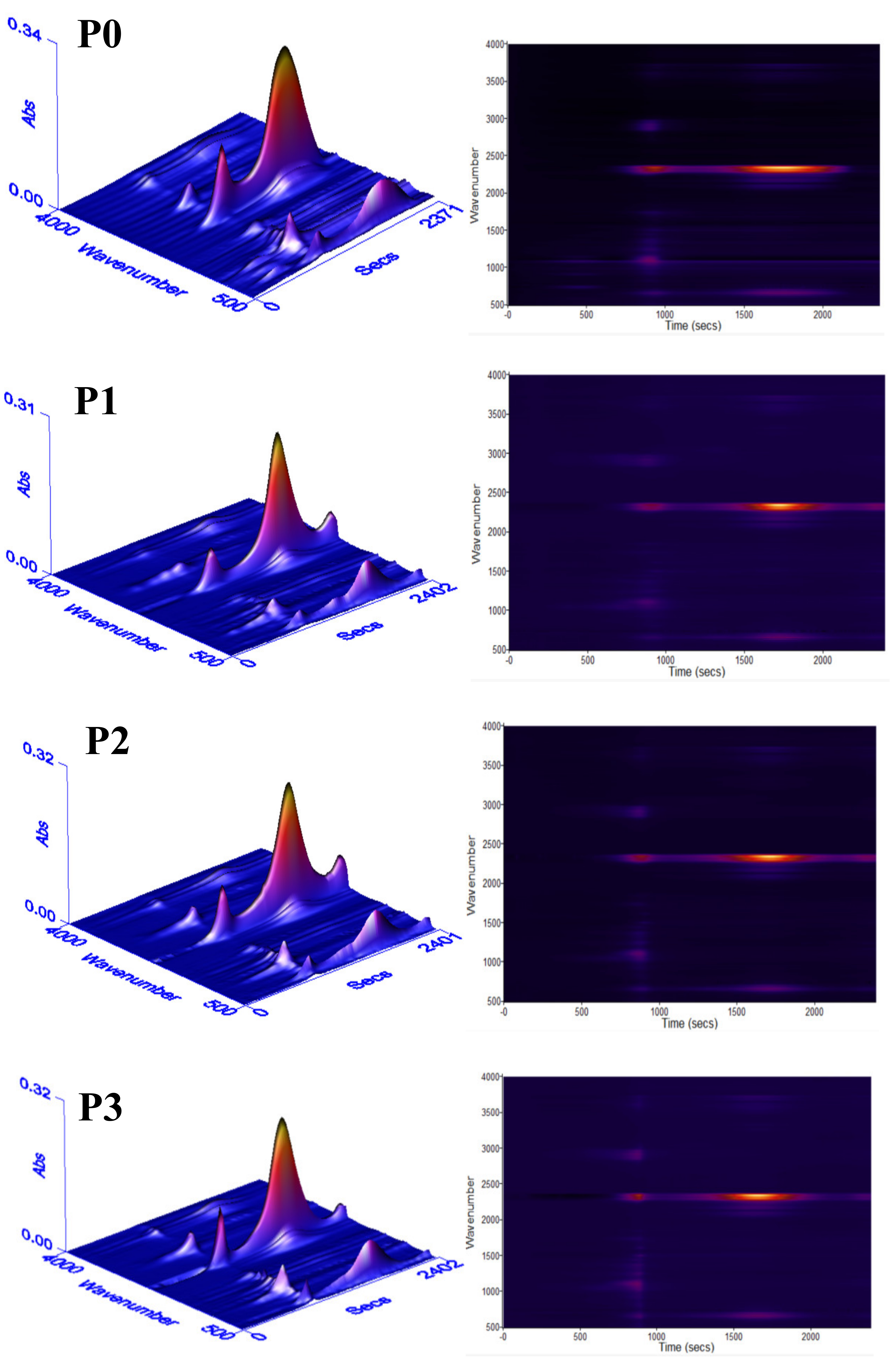
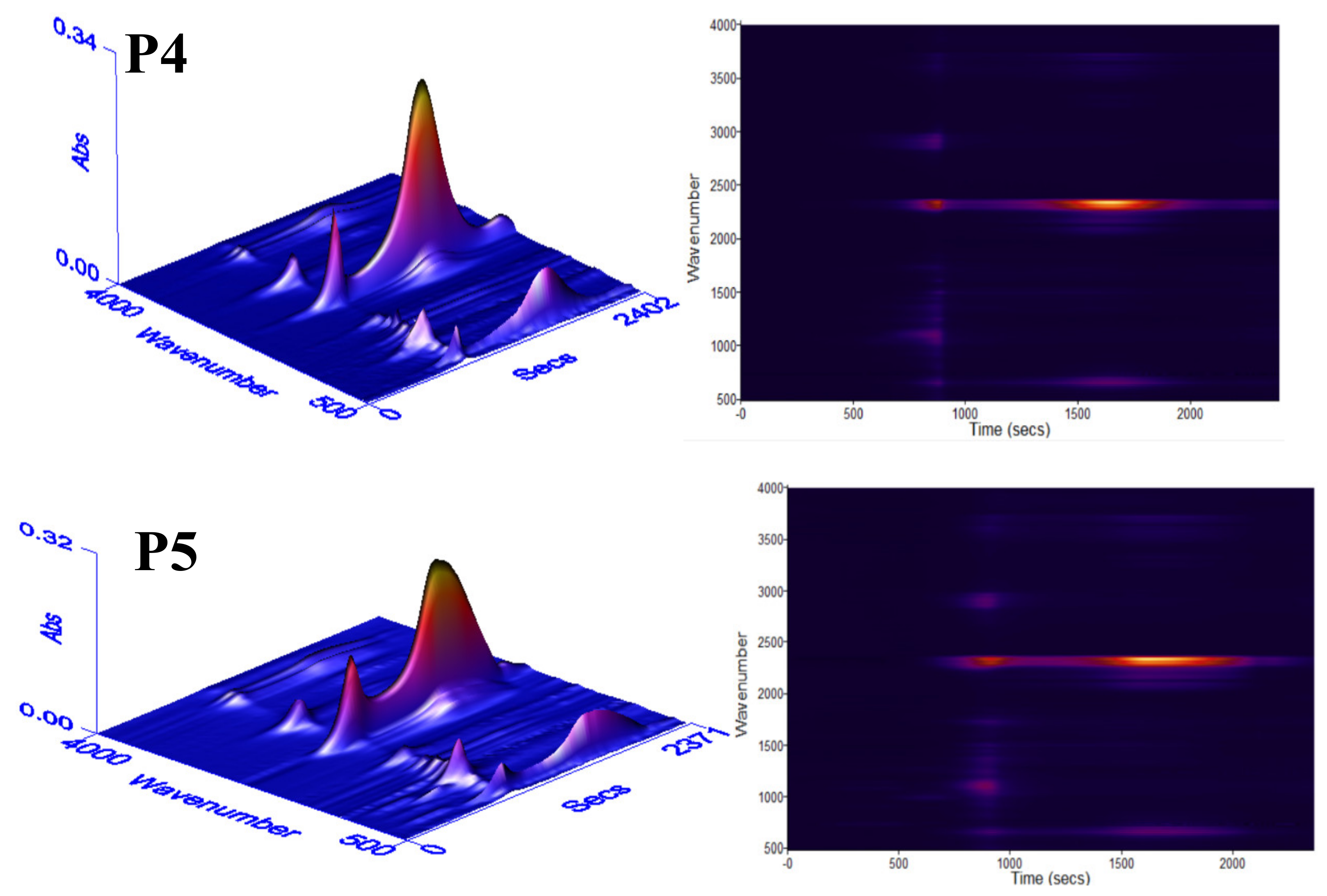
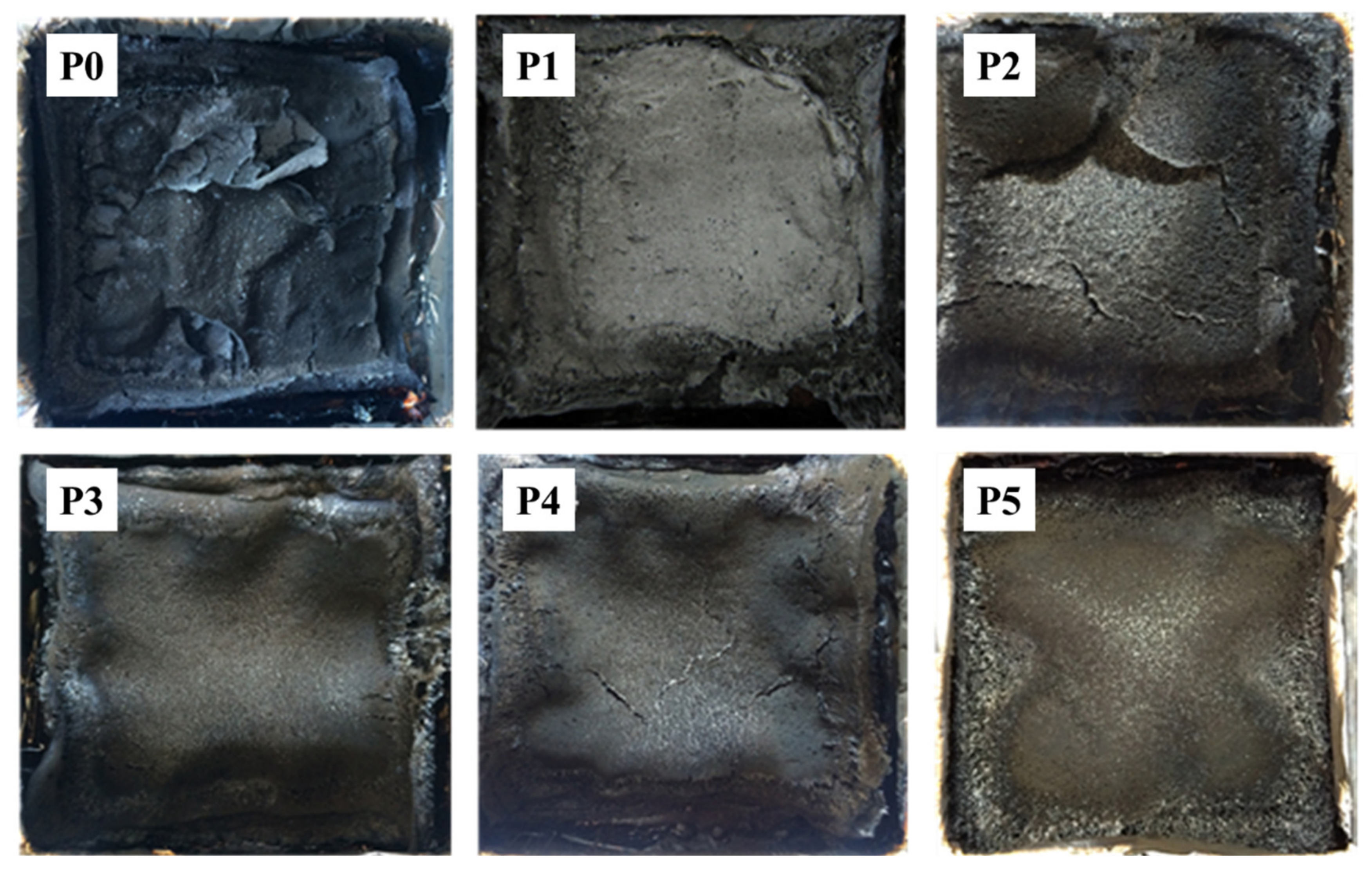
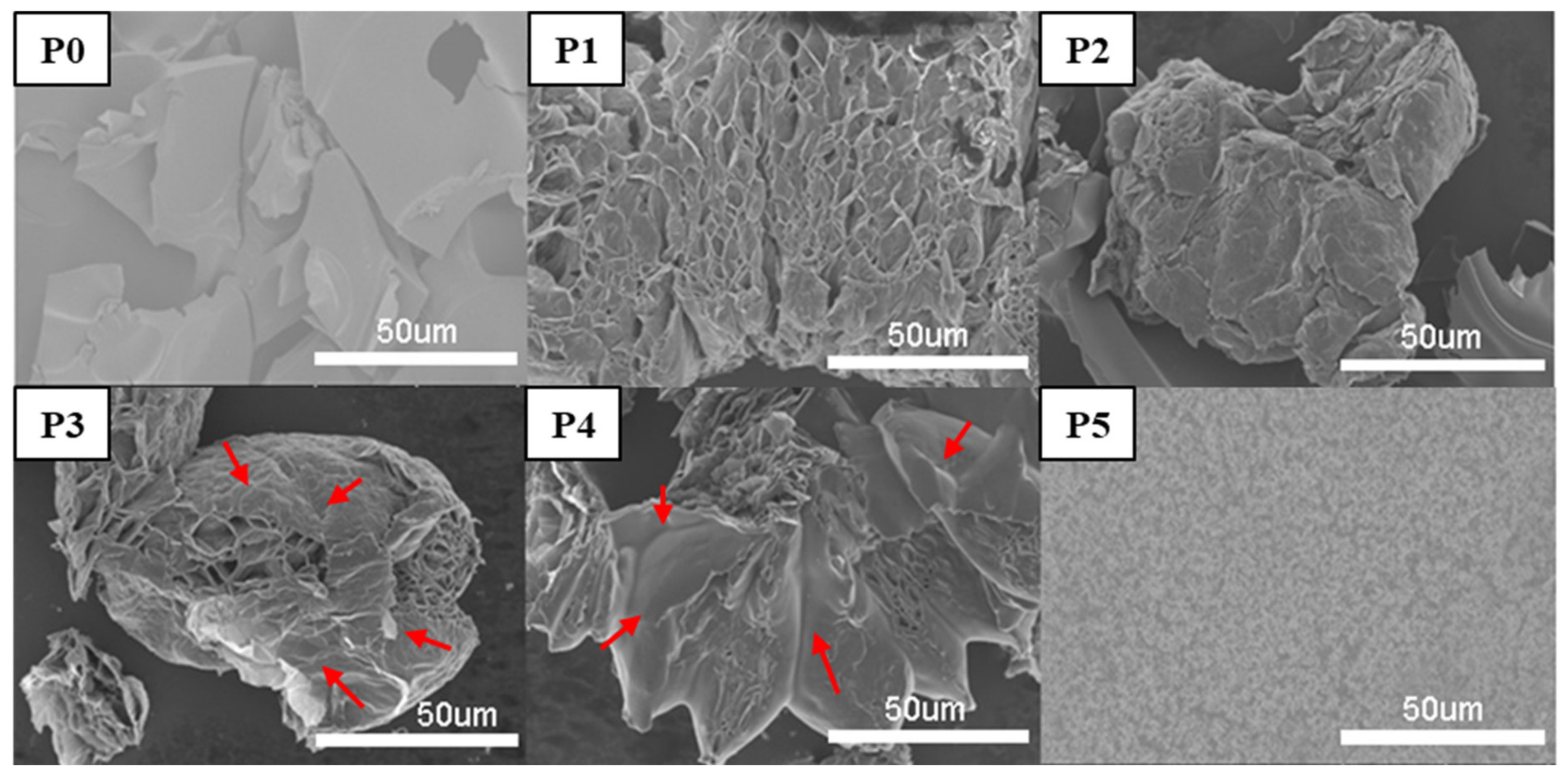
3.6. Char Residue Analysis and Flame-Retardant Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cao, Z.; Dong, X.; Fu, T.; Deng, S.; Liao, W.; Wang, Y. Coated vs. naked red phosphorus: A comparative study on their fire retardancy and smoke suppression for rigid polyurethane foams. Polym. Degrad. Stab. 2017, 136, 103–111. [Google Scholar] [CrossRef]
- Li, J.; Mo, X.; Li, Y.; Zou, H.; Liang, M.; Chen, Y. Influence of expandable graphite particle size on the synergy flame-retardant property between expandable graphite and ammonium polyphosphate in semi-rigid polyurethane foam. Polym. Bull. 2018, 75, 5287–5304. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, D.; Xu, M.; Li, B. Synthesis of a novel phosphorus and nitrogen-containing flame-retardant and its application in rigid polyurethane foam with expandable graphite. Polym. Degrad. Stab. 2020, 173, 109077. [Google Scholar] [CrossRef]
- Xi, C.; Yu, J.; Chuan, J. Smoke suppression properties of ferrite yellow on flame-retardant thermoplastic polyurethane based on ammonium polyphosphate. J. Hazard. Mater. 2014, 266, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Sheng, H.; Zhi, T.; Fei, C.; Jun, L.; Qiang, S.; Zhi, H.; Lian, Z. Flame-retardant properties and synergistic effect of ammonium polyphosphate/aluminum hydroxide/mica/silicone rubber composites. Fire Mater. 2020, 2831, 1–10. [Google Scholar]
- Zhang, M.; Zhang, J.; Chen, S.; Zhou, Y. Synthesis and fire properties of rigid polyurethane foams made from a polyol derived from melamine and cardanol. Polym. Degrad. Stab. 2014, 110, 27–34. [Google Scholar] [CrossRef]
- Chao, W.; Yi, W.; Ying, L.; Qian, S.; Xing, Y.; Cui, H.; Zhe, W.; Zhen, L.; Zhan, G. Flame-retardant rigid polyurethane foam with a phosphorus-nitrogen single intumescent flame-retardant. Polym. Advan. Technol. 2018, 29, 668–676. [Google Scholar]
- Beaugendre, A.; Lemesle, C.; Bellayer, S.; Degoutin, S.; Duquesne, S.; Casetta, M.; Pierlot, C.; Jaime, F.; Kim, T.; Jimenez, M. Flame-retardant and weathering resistant self-layering epoxy-silicone coatings for plastics. Prog. Org. Coat. 2019, 136, 105269. [Google Scholar] [CrossRef]
- Qiang, L.; Ping, J.; Zhan, S.; Ping, W.; Geng, W.; Xiao, T. Synergistic effect of phosphorus, nitrogen, and silicon on flame-retardant properties and char yield in polypropylene. J. Appl. Polym. Sci. 2005, 96, 854–860. [Google Scholar]
- Yin, W.; Feng, W.; Quan, D.; Ming, X.; Guo, Z. Core-shell expandable graphite @aluminum hydroxide as a flame-retardant for rigid polyurethane foams. Polym. Degrad. Stabil. 2017, 146, 267–276. [Google Scholar]
- Qian, W.; Qian, Z.; Li, Z.; Shi-Neng, L.; Long-Cheng, T. A novel and facile strategy for highly flame-retardant polymer foam composite materials: Transforming silicone resin coating into silica self-extinguishing layer. J. Hazard. Mater. 2017, 336, 222–231. [Google Scholar]
- Han, Z.; Ping, W.; Ping, J.; Dan, W.; Gen, W. Synthesis and characteristics of a novel silicon-containing flame-retardant and its application in poly[2,2-propane-(bisphenol)carbonate]/acrylonitrile butadiene styrene. J. Polym. Sci. Pol. Phys. 2007, 45, 1542–1551. [Google Scholar]
- Xiao, Z.; Guo, W.; Wei, X. Roles of organically-modified montmorillonite and phosphorous flame-retardant during the combustion of rigid polyurethane foam. Polym. Degrad. Stabil. 2014, 101, 32–39. [Google Scholar]
- Hai, L.; Wen, R.; Peng, Z.; Liang, W.; Yuan, L.; Chuan, Y. An efficient organic/inorganic phosphorus-nitrogen-silicon flame-retardant towards low-flammability epoxy resin. Polym. Degrad. Stabil. 2020, 178, 109195. [Google Scholar]
- Frederic, B.; Serge, B.; Sophie, D.; Charaf, J.; Michel, B.; Christine, P.; Murielle, R. Heat and fire resistance of polyurethane-polydimethylsiloxane hybrid material. Polym. Advan. Technol. 2006, 17, 304–311. [Google Scholar]
- Casetta, M.; Michaux, G.; Ohl, B.; Duquesne, S.; Bourbigot, S. Key role of magnesium hydroxide surface treatment in the flame retardancy of glass fiber reinforced polyamide 6. Polym. Degrad. Stabil. 2018, 148, 95–103. [Google Scholar] [CrossRef]
- Zhang, Y.; He, J.; Yang, R. The effects of phosphorus-based flame-retardants and octaphenyl polyhedral oligomeric silsesquioxane on the ablative and flame-retardation properties of room temperature vulcanized silicone rubber insulating composites. Polym. Degrad. Stabil. 2015, 125, 140–147. [Google Scholar]
- Xi, C.; Chuan, J.; Jun, Z. Microencapsulation of ammonium polyphosphate with hydroxyl silicone oil and its flame retardance in thermoplastic polyurethane. J. Therm. Anal. Calorim. 2011, 104, 1037–1043. [Google Scholar]
- Ning, W.; Xiang, L. Flame retardancy and synergistic flame-retardant mechanisms of acrylonitrile-butadiene-styrene composites based on aluminum hypophosphite. Polym. Degrad. Stabil. 2014, 105, 265–276. [Google Scholar]
- Mark, G.; Robert, H. Halogenated flame-retardant concentrations in settled dust, respirable and inhalable particulates and polyurethane foam at gymnastic training facilities and residences. Environ. Int. 2015, 79, 106–114. [Google Scholar]
- Jelena, V.; Ivan, J.; Gregor, J.; Jenny, A.; Giulio, M.; Milena, Z.; Brigita, T.; Barbara, S. Functionalization of cellulose fibres with DOPO-polysilsesquioxane flame-retardant nanocoating. Cellulose 2015, 22, 1893–1910. [Google Scholar]
- Li, G.; Zhen, G.; Mu, H.; Yun, L. Halogen-free flame-retardant waterborne polyurethane with a novel cyclic structure of phosphorus-nitrogen synergistic flame-retardant. J. Appl. Polym. Sci. 2015, 132, 41288. [Google Scholar]
- Wang, X.; Li, Q.; Zhi, H.; Yan, C.; Lin, L. Continuous flame-retardant actions of two phosphate esters with expandable graphite in rigid polyurethane foams. Polym. Degrad. Stabil. 2016, 130, 97–102. [Google Scholar]
- Xiao, C.; Zong, H.; Xiu, X.; Jia, L.; Xin, F.; Zhan, W. Synergistic effect of carbon and phosphorus flame-retardants in rigid polyurethane foams. Fire Mater. 2018, 42, 447–453. [Google Scholar]
- Xian, F.; Xu, H.; Zhen, L.; Li, C.; Su, C. Synthesis of new superhydrophobic nanosilica and investigation of their performance in reinforcement of polysiloxane. Polym. Composite. 2010, 31, 1628–1636. [Google Scholar]
- Sarita, K.; Susheel, K.; Annamaria, C.; James, N.; Youssef, H.; Rajesh, K. Surface modification of inorganic nanoparticles for development of organic-inorganic nanocomposites-A review. Prog. Polym. Sci. 2013, 38, 1232–1261. [Google Scholar]
- Xi, C.; Cui, M.; Chuan, J. Enhancement of flame-retardant performance of thermoplastic polyurethane with the incorporation of aluminum hypophosphite and iron-graphene. Polym. Degrad. Stabil. 2016, 129, 275–285. [Google Scholar]
- Yong, C.; Yuan, L.; Xiao, G.; Li, C.; Ti, X.; De, J. Structure and flame-retardant actions of rigid polyurethane foams with expandable graphite. Polymers 2019, 11, 686. [Google Scholar]
- Ji, L.; Hang, L.; Hai, C.; Yun, H.; Ai, X.; Bing, P. Structure and thermal property of intumescent char produced by flame-retardant high-impact polystyrene/expandable graphite/microencapsulated red phosphorus composite. Fire Mater. 2019, 43, 971–980. [Google Scholar]
- Khalili, P.; Liu, X.; Zhao, Z.; Blinzler, B. Fully Biodegradable Composites: Thermal, Flammability, Moisture Absorption and Mechanical Properties of Natural Fibre-Reinforced Composites with Nano-Hydroxyapatite. Materials 2019, 12, 1145. [Google Scholar] [CrossRef]
- Agnė, K.; Arūnas, K.; Giedrius, B.; Sylwia, C.; Anna, S. Closed cell rigid polyurethane foams based on low functionality polyols: Research of dimensional stability and standardised performance properties. Materials 2020, 13, 1438. [Google Scholar]
- Li, X.; Cao, H.; Zhang, Y. Thermal degradation kinetics of rigid polyurethane foams blown with water. J. Appl. Polym. Sci. 2006, 102, 4149–4156. [Google Scholar] [CrossRef]
- Sylwia, C.; Anna, S.; Krzysztof, S.; Agnė, K.; Arūnas, K. Bio-based polyurethane composite foams with improved mechanical, thermal, and antibacterial properties. Materials 2020, 13, 1108. [Google Scholar]
- Gao, M.; Wu, W.; Liu, S.; Wang, Y.; Shen, T. Thermal degradation and flame retardancy of rigid polyurethane foams containing a novel intumescent flame-retardant. J. Therm. Anal. Calorim. 2014, 117, 1419–1425. [Google Scholar] [CrossRef]
- Shi, L.; Li, Z.; Xie, B.; Wang, J.; Tian, C.; Yang, M. Flame retardancy of different-sized expandable graphite particles for high-density rigid polyurethane foams. Polym. Int. 2010, 55, 862–871. [Google Scholar] [CrossRef]


| Sample | P0 (phpp a) | P1 (phpp) | P2 (phpp) | P3 (phpp) | P4 (phpp) | P5 (phpp) |
|---|---|---|---|---|---|---|
| HF-4110H | 70 | 70 | 70 | 70 | 70 | 70 |
| HF-4110 | 30 | 30 | 30 | 30 | 30 | 30 |
| H2O | 3 | 3 | 3 | 3 | 3 | 3 |
| AK-8803 | 2 | 2 | 2 | 2 | 2 | 2 |
| A33 | 2 | 2 | 2 | 2 | 2 | 2 |
| GI | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| PAPI | 138 | 138 | 138 | 138 | 138 | 138 |
| IL-EG | 0 | 0 | 5 | 10 | 15 | 20 |
| DPES | 0 | 20 | 15 | 10 | 5 | 0 |
| Element | C | H | N | P | Si |
|---|---|---|---|---|---|
| Theoretical value (wt %) | 61.37 | 7.97 | 4.03 | 7.67 | 6.93 |
| Measured value (wt %) | 61.03 | 7.72 | 3.64 | 7.50 | 7.05 |
| Sample | T5% (°C) | T10% (°C) | T50% (°C) | Tonset (°C) | Tmax (°C) | Residue at 700 °C (wt%) |
|---|---|---|---|---|---|---|
| P0 | 196.5 | 249.8 | 364.8 | 180.0 | 356.5 | 17.3 |
| P1 | 145.9 | 223.4 | 348.4 | 125.7 | 315.1 | 17.9 |
| P2 | 210.7 | 256.7 | 345.7 | 196.7 | 321.2 | 22.2 |
| P3 | 205.2 | 255.2 | 340.2 | 188.4 | 320.7 | 24.1 |
| P4 | 203.6 | 242.3 | 333.5 | 189.4 | 314.4 | 31.9 |
| P5 | 171.1 | 259.8 | 357.3 | 150.0 | 352.0 | 19.3 |
| Sample | TTI (s) | PHRR (kW/m2) | THR (MJ/m2) | TSR (m2) |
|---|---|---|---|---|
| P0 | 4 ± 1 | 243 ± 15 | 21.8 ± 5 | 11.6 ± 2 |
| P1 | 7 ± 2 | 190 ± 11 | 16.8 ± 3 | 8.7 ± 1 |
| P2 | 8 ± 2 | 143 ± 10 | 14.6 ± 4 | 6.7 ± 1 |
| P3 | 10 ± 1 | 96 ± 6 | 12.0 ± 1 | 3.6 ± 0.5 |
| P4 | 9 ± 2 | 111 ± 9 | 13.2 ± 3 | 4.9 ± 1 |
| P5 | 8 ± 2 | 108 ± 7 | 13.9 ± 3 | 4.1 ± 1 |
| Sample | P0 | P1 | P2 | P3 | P4 | P5 |
|---|---|---|---|---|---|---|
| Horizontal burning rate/mm·min−1 | 51.5 ± 9 | 55.9 ± 8 | 41.7 ± 6 | 25.6 ± 3 | 33.6 ± 4 | 37.2 ± 3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Luo, Y.; Guo, X.; Chen, L.; Jia, D. The Synergistic Effect of Ionic Liquid-Modified Expandable Graphite and Intumescent Flame-Retardant on Flame-Retardant Rigid Polyurethane Foams. Materials 2020, 13, 3095. https://doi.org/10.3390/ma13143095
Chen Y, Luo Y, Guo X, Chen L, Jia D. The Synergistic Effect of Ionic Liquid-Modified Expandable Graphite and Intumescent Flame-Retardant on Flame-Retardant Rigid Polyurethane Foams. Materials. 2020; 13(14):3095. https://doi.org/10.3390/ma13143095
Chicago/Turabian StyleChen, Yongjun, Yuanfang Luo, Xiaohui Guo, Lijuan Chen, and Demin Jia. 2020. "The Synergistic Effect of Ionic Liquid-Modified Expandable Graphite and Intumescent Flame-Retardant on Flame-Retardant Rigid Polyurethane Foams" Materials 13, no. 14: 3095. https://doi.org/10.3390/ma13143095
APA StyleChen, Y., Luo, Y., Guo, X., Chen, L., & Jia, D. (2020). The Synergistic Effect of Ionic Liquid-Modified Expandable Graphite and Intumescent Flame-Retardant on Flame-Retardant Rigid Polyurethane Foams. Materials, 13(14), 3095. https://doi.org/10.3390/ma13143095





