Surface Complexation Models of Pertechnetate on Biochar/Montmorillonite Composite—Batch and Dynamic Sorption Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials Preparation
2.2. Materials Characterization
2.3. Batch Sorption Study
2.4. Dynamic Sorption Study
3. Results and Discussion
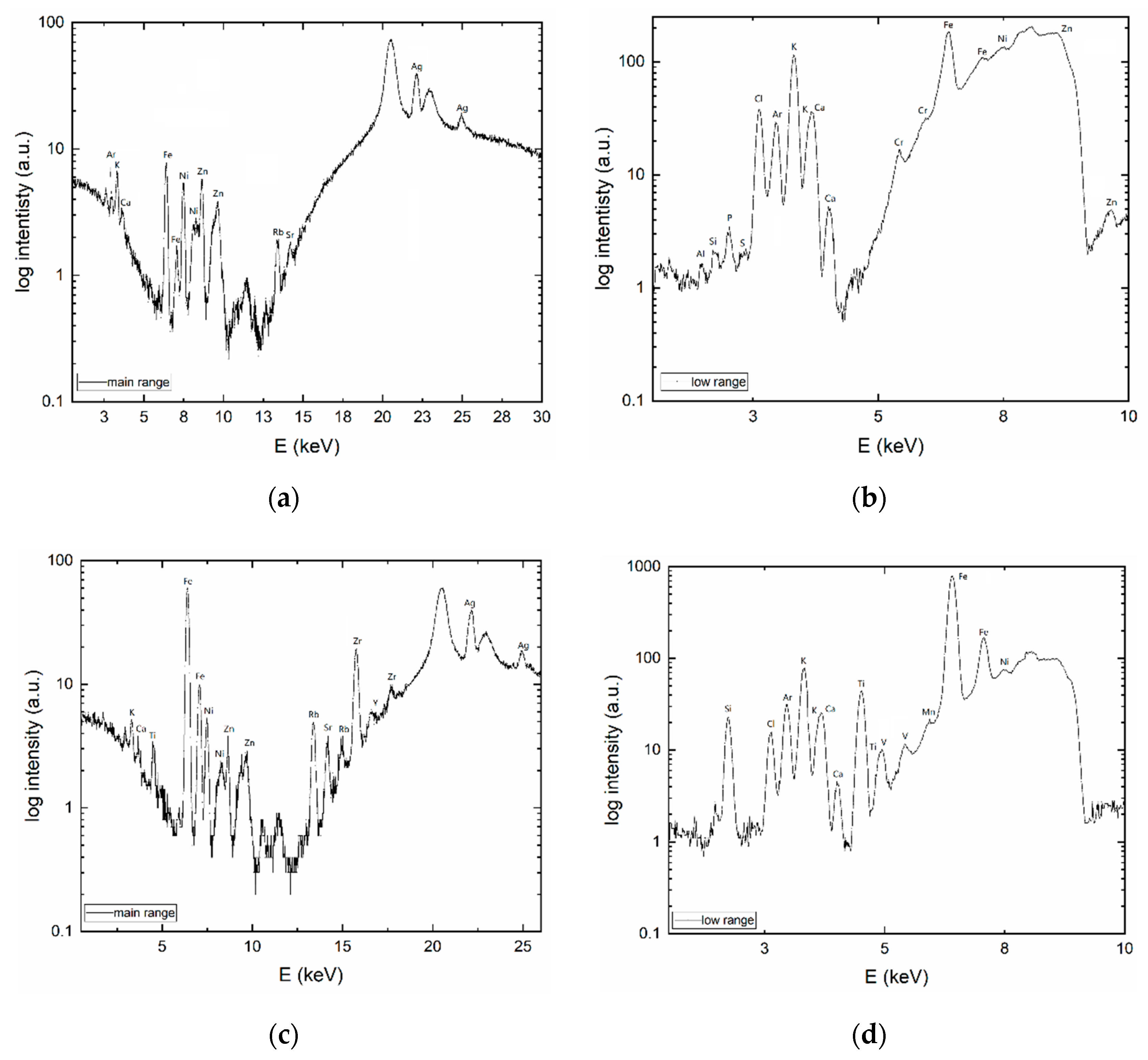
3.1. Material Characterization
3.1.1. XRF
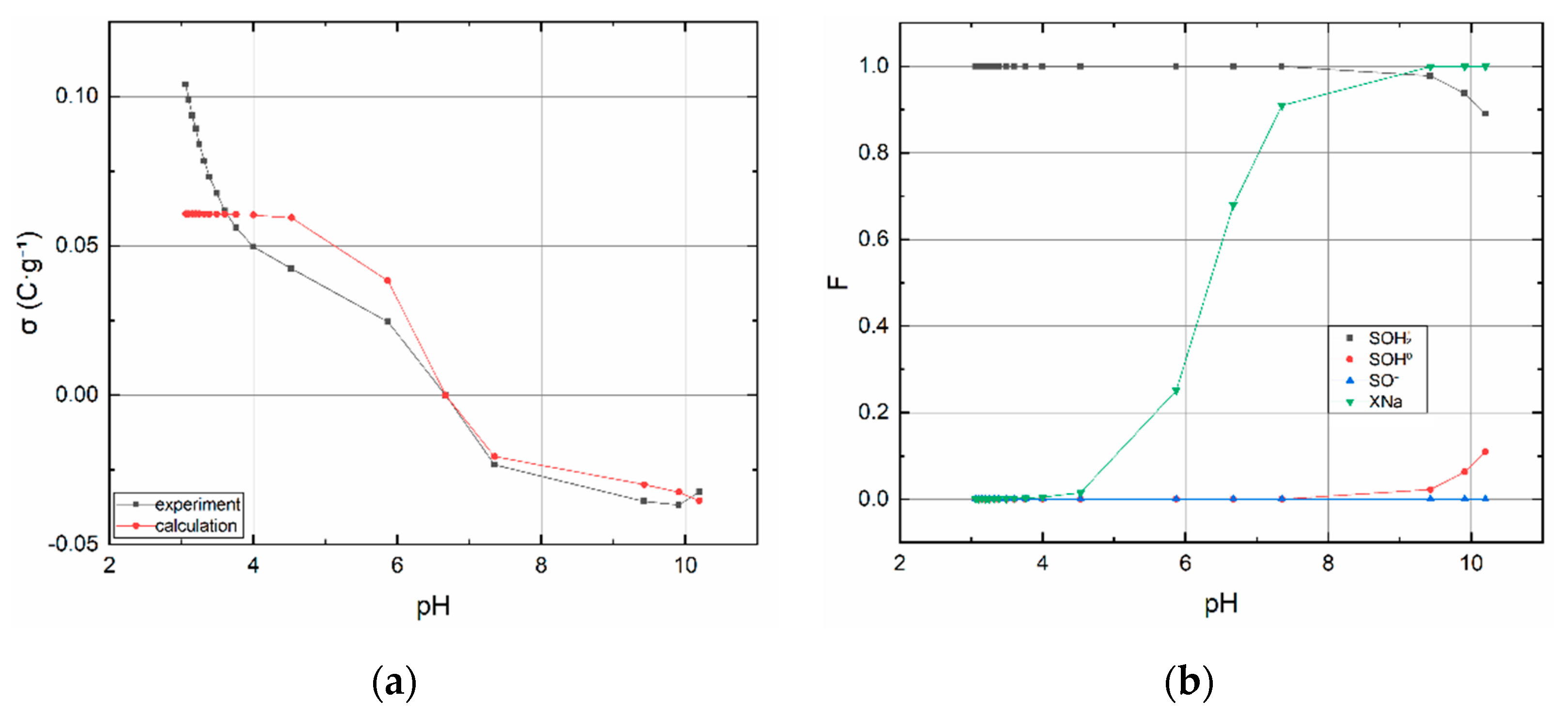
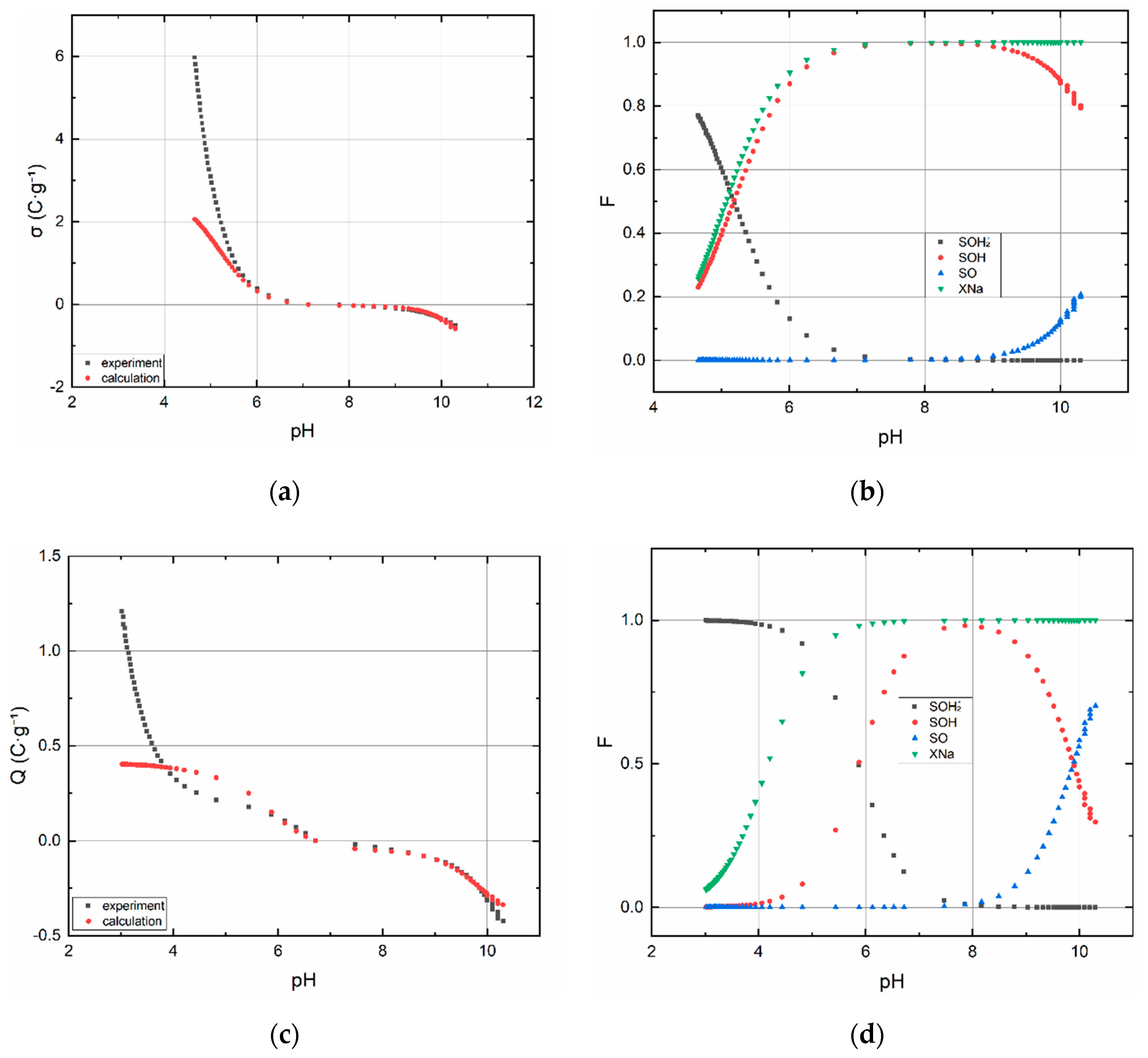
3.1.2. Acid–Base Titration
3.2. Batch Adsorption Experiments
3.2.1. Influence of pH
3.2.2. Influence of Contact Time
3.2.3. Equilibrium Study
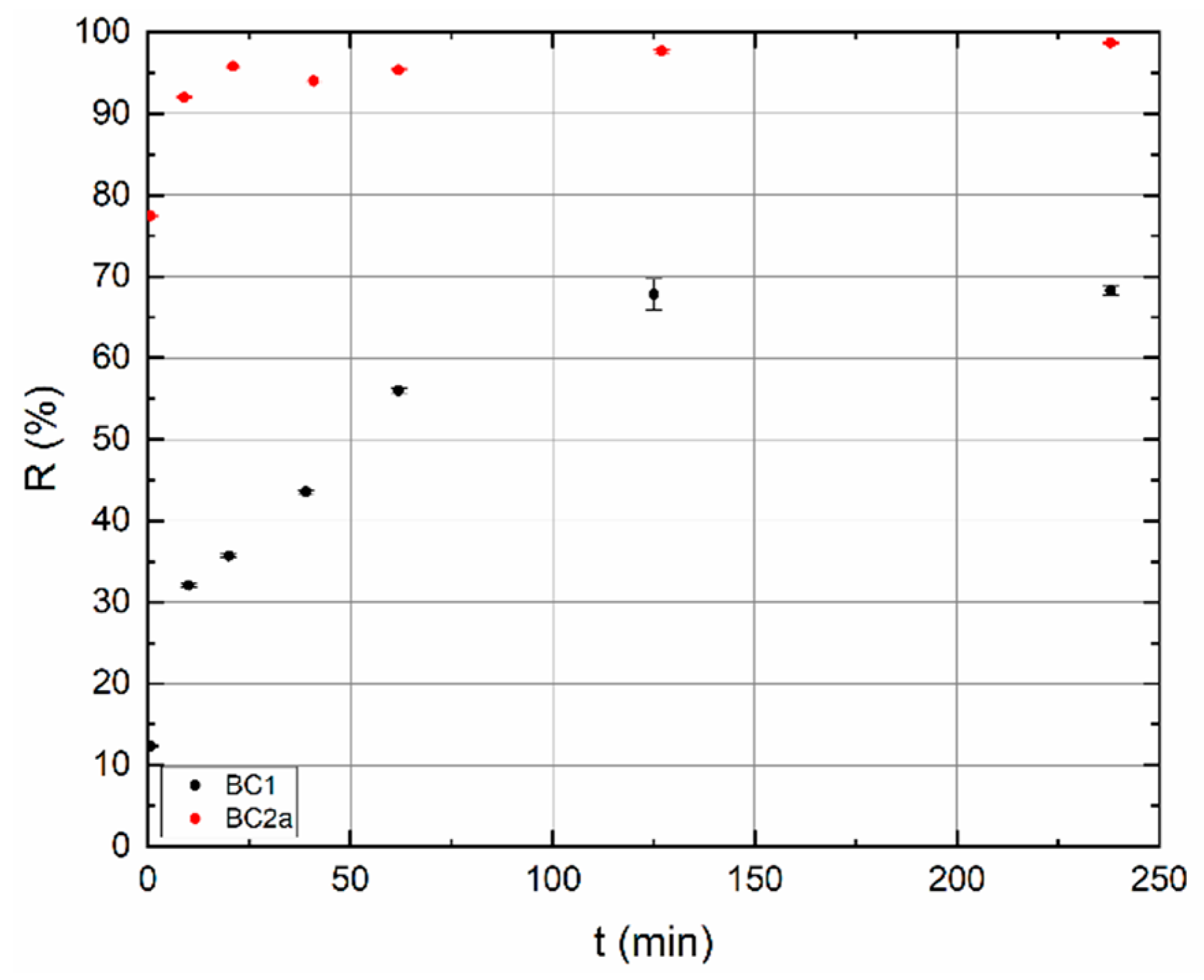
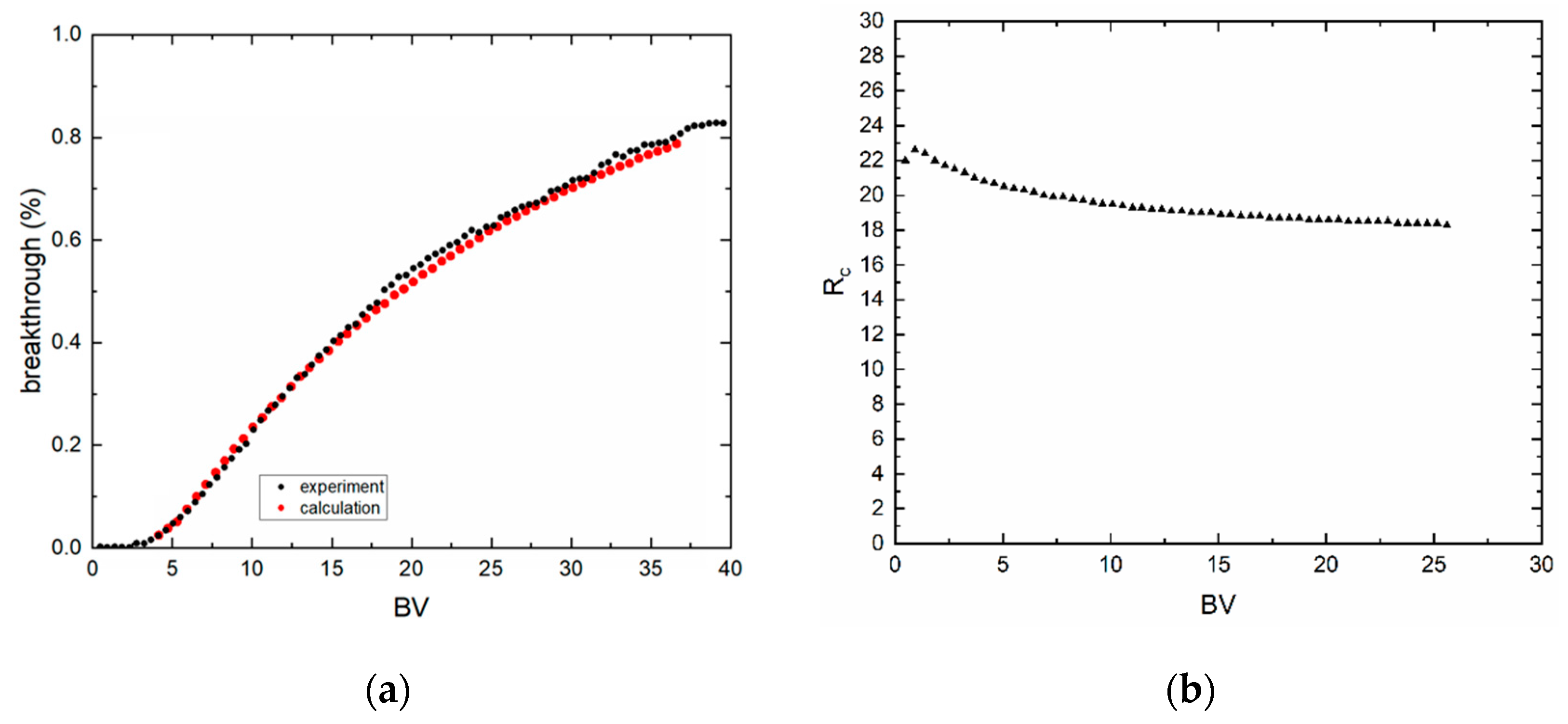
3.3. Dynamic Sorption Experiments
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Li, D.; Seaman, J.C.; Kaplan, D.I.; Heald, S.M.; Sun, C. Pertechnetate (TcO4−) sequestration from groundwater by cost-effective organoclays and granular activated carbon under oxic-environmental conditions. Chem. Eng. J. 2019, 360, 1–9. [Google Scholar] [CrossRef]
- Hu, H.; Jiang, B.; Wu, H.; Zhang, J.; Chen, X.H. Bamboo (acidosasa edulis) shoot shell biochar: Its potential isolation and mechanism to perrhenate as a chemical surrogate for pertechnetate. J. Environ. Radioac. 2016, 165, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Rajec, P.; Rosskopfova, O.; Galamboš, M.; Frišták, V.; Soja, G.; Dafnomili, A.; Noli, F.; Ðukicć, A.; Matović, L.J. Sorption and desorption of pertechnetate on biochar under static batch and dynamic conditions. J. Radioanal. Nucl. Chem. 2016, 310, 253–261. [Google Scholar] [CrossRef]
- Chen, L.; Yin, X.; Yu, Q.; Lu, S.; Meng, F.; Ning, S.; Wang, X.; Wei, Y. Rapid and selective capture of perrhenate anion from simulated groundwater by a mesoporous silica-supported anion exchanger. Microporous Mesoporous Mater. 2019, 274, 155–162. [Google Scholar] [CrossRef]
- Nicholson, S.; Sanders, T.W.; Blaine, L.M. The determination of low levels of 99Tc in environmental samples by inductively coupled plasma-mass spectrometry. Sci. Total. Environ. 1993, 130–131, 275–284. [Google Scholar] [CrossRef]
- Chen, Q.; Dahlgaard, H.; Hansen, H.J.M.; Aarkrog, A. Determination of 99Tc in environmental samples by anion exchange and liquid-liquid extraction at controlled valency. Anal. Chim. Acta 1990, 228, 163–167. [Google Scholar] [CrossRef]
- Attrep, M.; Enochs, J.A.; Broz, L.D. Atmospheric technetium-99. Environ. Sci. Technol. 1971, 5, 344–345. [Google Scholar] [CrossRef]
- Serne, R.J.; Crum, J.V.; Riley, B.J.; Levitskaia, T.G. Options for the Separation and Immobilization of Technetium; Technical Report for the U.S. Department of Energy Under Contract DE-AC05-76RL01830; Pacific Northwest National Lab. (PNNL): Richland, WA, USA, September 2016. [Google Scholar]
- TrisKem International. Extraction Chromatography. Technical Documentation. Available online: https://www.triskem-international.com/scripts/files/5addcf96423962.97324869/technical_doc_all-products_web-0.pdf (accessed on 16 May 2020).
- Viglašová, E.; Daňo, M.; Galamboš, M.; Rosskopfová, O.; Rajec, P.; Novák, I. Column studies for the separation of 99mTc using activated carbon. J. Radioanal. Nucl. Chem. 2016, 307, 591–597. [Google Scholar] [CrossRef]
- Shi, K.; Hou, X.; Roos, P.; Wu, W. Determination of technetium-99 in environmental samples: A review. Anal. Chim. Acta 2012, 709, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Cornett, R.J.; Zhao, X.-L.; Hou, X.-L.; Kieser, W.E. A preliminary study of 99Tc measurement using matrix-assisted low energy AMS. Nucl. Instrum. Methods Phys. Res. Sec. B 2019, 455, 181–189. [Google Scholar] [CrossRef]
- Bergquist, B.A.; Marchetti, A.A.; Martinelli, R.E.; McAninch, J.E.; Nimz, G.J.; Proctor, I.D.; Southon, J.R.; Vogel, J.S. Technetium measurements by accelerator mass spectrometry at LLNL. Nucl. Instrum. Methods Phys. Res. Sec. B 2000, 172, 328–332. [Google Scholar] [CrossRef] [Green Version]
- Povinec, P. Analysis of Environmental Radionuclides; Elsevier: Amsterdam, The Netherlands, 2011; ISBN 9780080553375. [Google Scholar]
- Triskem. TEVA Resin, Product Sheet. 10.12.2015. Available online: https://www.triskem-international.com/scripts/files/5c5855b887c4f4.23796223/PS_TEVA-Resin_EN_160927.pdf (accessed on 16 May 2020).
- Eichrom Analytical Procedure. Technetium-99 in Water. Available online: https://www.eichrom.com/eichrom/methods/eichrom-methods/ (accessed on 16 May 2020).
- Viglašová, E.; Galamboš, M.; Dankovaá, Z.; Krivosudský, L.; Lengauer, C.L.; Hood-Nowotny, R.; Soja, G.; Rompel, A.; Matík, M.; Briančin, J. Production, characterization and adsorption studies of bamboo-based biochar/montmorillonite composite for nitrate removal. Waste Manag. 2018, 79, 385–394. [Google Scholar] [CrossRef]
- Chacón, F.J.; Sánchez-Monedero, M.A.; Lezama, L.; Cayuela, M.L. Enhancing biochar redox properties through feedstock selection, metal preloading and post-pyrolysis treatments. Chem. Eng. J. 2020, 395, 125100. [Google Scholar] [CrossRef]
- Wu, L.; Jing, S.Z.; Ding, X.W. Phosphorus retention using iron (II/III) modified biochar in saline-alkaline soils: Adsorption, column and field tests. Environ. Pollut. 2020, 261, 114223. [Google Scholar] [CrossRef]
- Pandey, D.; Daverey, A.; Arunachalam, K. Biochar: Production, properties and emerging role as a support for enzyme immobilization. J. Clean. Prod. 2020, 255, 120267. [Google Scholar] [CrossRef]
- Khan, M.B.; Cui, X.; Jilan, G.; Lu, L.T.M.; Cao, X.; Sahito, Z.A.; Hamid, Y.; Hussain, B.; Yang, X.; He, Z. New insight into the impact of biochar during vermi-stabilization of divergent biowastes: Literature synthesis and research pursuits. Chemosphere 2020, 238, 124679. [Google Scholar] [CrossRef] [PubMed]
- Filipská, H.; Štamberg, K. Mathematical modeling of a Cs(I)-Sr(II)-bentonite-magnetite sorption system, simulating the processes taking place in a deep geological repository. Acta Polytech. 2005, 45, 11–18. [Google Scholar]
- Wanner, H.; Albinsson, Y.; Karnland, O.; Wieland, E.; Wersin, P.; Charlet, L. The acid-base chemistry of montmorillonite. Radiochim. Acta 1994, 66, 157–162. [Google Scholar] [CrossRef]
- Dvořák, L.; Ledvinka, M.; Sobotka, M. Famulus 3.5. Software; Charles University: Prague, Czech Republic, 1993. [Google Scholar]
- Rahmani, A.; Mousavi, H.Z.; Fazli, M. Effect of nanostructure alumina on adsorption of heavy metals. Desalination 2010, 253, 94–100. [Google Scholar] [CrossRef]
- Palágyi, Š.; Štamberg, K. Modeling of transport of radionuclides in beds of crushed crystalline rocks under equilibrium non-linear sorption isotherm conditions. Radiochim. Acta 2010, 98, 359–365. [Google Scholar] [CrossRef]
- Palágyi, Š.; Štamberg, K.; Vopálka, D. Simplified modeling in dynamic column technique for the determination of radionuclide transport parameters in systems of solid granular materials and groundwater. J. Radioanal. Nucl. Chem. 2017, 311, 1059–1073. [Google Scholar] [CrossRef]
- Štamberg, K.; Palágyi, Š. Effect of grain size on the sorption and desorption of 137Cs in crushed granite columns and groundwater system under dynamic conditions. J. Radioanal. Nucl. Chem. 2011, 293, 127–134. [Google Scholar] [CrossRef]
- Nartey, O.D.; Zhao, B. Biochar preparation, characterization, and adsorptive capacity and its effect on bioavailability of contaminants: An overview. Adv. Matter. Sci. Eng. 2014, 2014, 12. [Google Scholar] [CrossRef] [Green Version]
- Batista, E.M.C.C.; Shultz, J.; Matos, T.T.S.; Fornari, M.R.; Ferreira, T.M.; Szpoganicz, B.; De Freitas, R.A.; Mangrich, A.S. Effect of surface and porosity of biochar on water holding capacity aiming indirectly at preservation of the Amazon biome. Sci. Rep. 2018, 8, 10677. [Google Scholar] [CrossRef]
- Wu, M.; Feng, Q.; Sun, X.; Wang, H.; Gielen, G.; Wu, W. Rice (Oryza sativa L) plantation affects the stability of biochar in paddy soil. Sci. Rep. 2015, 5, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; He, Z.; Uchimiya, M. Comparison of biochar formation from various agricultural by-products using FTIR spectroscopy. Mod. Appl. Sci. 2015, 9, 246–253. [Google Scholar] [CrossRef]
- Zhao, J.; Shen, X.-J.; Domene, X.; Alcañiz, J.-M.; Liao, X.; Palet, C. Comparison of biochars derived from diferent types of feedstock and their potential for heavy metal removal in multiple-metal solutions. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef] [Green Version]
- IBI (International Biochar Iniciative). Standardized Product Definition and Product Testing Guidelines for Biochar That Is Used in Soil. Available online: https://biochar-international.org/wp-content/uploads/2020/06/IBI_Biochar_Standards_V2.1_Final2.pdf (accessed on 7 July 2020).
- Wijitkosum, S.; Jiwnok, P. Elemental composition of biochar obtained from agricultural waste for soil amendment and carbon sequestration. Appl. Sci. 2019, 9, 3980. [Google Scholar] [CrossRef] [Green Version]
- Waqas, M.; Aburiazaiza, A.; Miandad, R.; Rehan, M.; Barakat, M.; Nizami, A.-S. Development of biochar as fuel and catalyst in energy recovery technologies. J. Clean. Prod. 2018, 188, 477–488. [Google Scholar] [CrossRef]
- Manna, S.; Singh, N.; Purakayastha, T.; Berns, A.E.E. Effect of deashing on physico-chemical properties of wheat and rice straw biochars and potential sorption of pyrazosulfuron-ethyl. Arab. J. Chem. 2020, 13, 1247–1258. [Google Scholar] [CrossRef]
- Sen, T.K. Point of Zero Charge and Effect of Solution pH. In Air, Gas, and Water Pollution Control Using Industrial and Agricultural Solid Waste Adsorbents; Chapter 12.3.2; CRC Press: Boca Raton, FL, USA, 2017; ISBN 9781351230698. [Google Scholar]
- Guo, Y.; Yu, X. Characterizing the surface charge of clay minerals with Atomic Force Microscope (AFM). AIMS Mater. Sci. 2017, 4, 582–593. [Google Scholar] [CrossRef]
- Liu, J.; Gaikwad, R.; Hande, A.; Das, S.; Thundat, T. Mapping and quantifying surface charges on clay nanoparticles. Langmuir 2015, 31, 10469–10476. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Lu, X.; Sprik, M.; Cheng, J.; Meijer, E.J.; Wang, R. Acidity of edge surface sites of montmorillonite and kaolinite. Geochim. Cosmochim. Acta 2013, 117, 180–190. [Google Scholar] [CrossRef] [Green Version]
- Demiral, H.; Gündüzoğlu, G. Removal of nitrate from aqueous solutions by activated carbon prepared from sugar beet bagasse. Bioresour. Technol. 2010, 101, 1675–1680. [Google Scholar] [CrossRef] [PubMed]


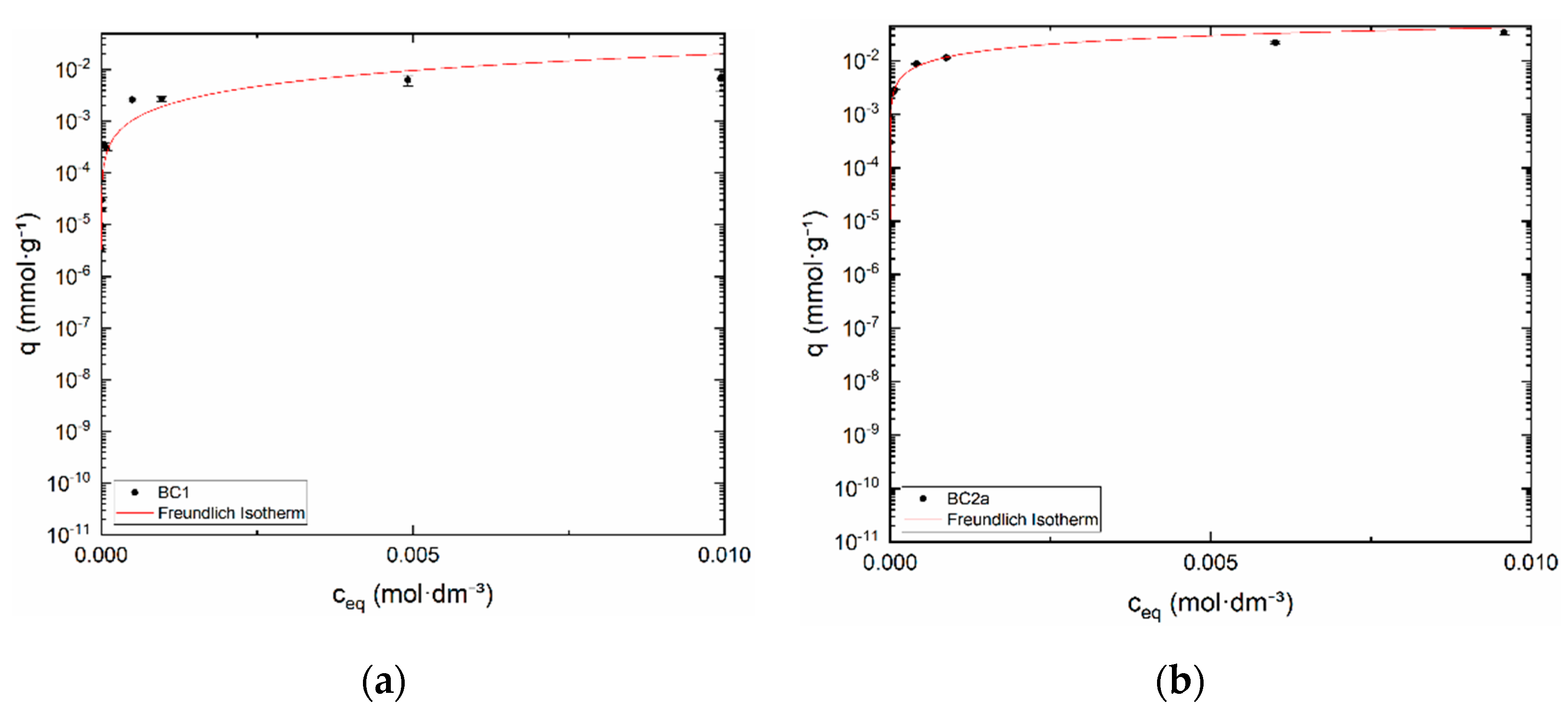

| Sample | log (K1) | log (K2) | log (Kex) | [SOH]tot (mol·kg−1) | [S]tot (mol·kg−1) | I (mol·dm−3) |
|---|---|---|---|---|---|---|
| BC1 | 15 | 10 | 5 | 0.1 | 0.1 | 0.1 |
| BC2a | 10 | 6 | 5 | 1 | 0.5 | 0.1 |
| BC2b | 10 | 6 | 3 | 0.4 | 0.06 | 0.1 |
| K1 | K2 | Kex | [SOH]tot (mol·kg−1) | |
| BC1 | (1.54 ± 24.9) × 1015 | (1.53 ± 0.16) × 1011 | (8.71 ± 13.8) × 10−1 | (6.07 ± 0.03) × 10−2 |
| BC2a | (9.54 ± 0.15) × 1010 | (1.97 ± 0.22) × 105 | (1.06 ± 11.0) × 104 | (2.69 ± 0.35) × 100 |
| BC2b | (9.94 ± 0.79) × 10⁹ | (9.60 ± 0.25) × 105 | (1.49 ± 0.44) × 103 | (4.06 ± 0.54) × 10−1 |
| WSOS/DF | χ2 | σi | [S]tot (mol·kg−1) | |
| BC1 | 10.3 | 1.34 × 102 | 1 × 10−1 | (8.94 ± 0.05) × 10−2 |
| BC2a | 17.5 | 1.15 × 103 | 1 × 10−1 | (3.07 ± 8.91) × 10−2 |
| BC2b | 15.4 | 9.06 × 102 | 1 × 10−1 | (5.04 ± 0.77) × 10−2 |
| Sample | kf,S | Pe | nf,S | Rc | L (cm) | u (cm·h−1) |
| BC1 | 100 | 5 | 1 | 100 | 2.1 | 0.185 |
| BC2a | 1 | 1 | 20 | 10 | 2.1 | 0.223 |
| Sample | c (ReO4−) (mol·dm−3) | ε (cm3·cm−3) | ξ (g·cm−3) | - | ||
| BC1 | 0.001 | 0.877 | 0.351 | |||
| BC2a | 0.0001 | 0.752 | 0.323 | |||
| kf,S | Pe | nf,S | |
| BC1 | (1.95 ± 0.17) × 101 | (1.93 ± 0.06) × 100 | (8.71 ± 0.14) × 10−1 |
| BC2a | (5.69 ± 0.53) × 10−1 | (3.65 ± 0.03) × 10−1 | (6.41 ± 0.11) × 10−1 |
| WSOS/DF | χ2 | σi | |
| BC1 | 0.301 | 16 | 0.1 |
| BC2a | 0.004 | 1.53 | 0.1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Daňo, M.; Viglašová, E.; Galamboš, M.; Štamberg, K.; Kujan, J. Surface Complexation Models of Pertechnetate on Biochar/Montmorillonite Composite—Batch and Dynamic Sorption Study. Materials 2020, 13, 3108. https://doi.org/10.3390/ma13143108
Daňo M, Viglašová E, Galamboš M, Štamberg K, Kujan J. Surface Complexation Models of Pertechnetate on Biochar/Montmorillonite Composite—Batch and Dynamic Sorption Study. Materials. 2020; 13(14):3108. https://doi.org/10.3390/ma13143108
Chicago/Turabian StyleDaňo, Martin, Eva Viglašová, Michal Galamboš, Karel Štamberg, and Jan Kujan. 2020. "Surface Complexation Models of Pertechnetate on Biochar/Montmorillonite Composite—Batch and Dynamic Sorption Study" Materials 13, no. 14: 3108. https://doi.org/10.3390/ma13143108
APA StyleDaňo, M., Viglašová, E., Galamboš, M., Štamberg, K., & Kujan, J. (2020). Surface Complexation Models of Pertechnetate on Biochar/Montmorillonite Composite—Batch and Dynamic Sorption Study. Materials, 13(14), 3108. https://doi.org/10.3390/ma13143108









