The Tensile Properties, Scratch Behaviors and Sliding Wear of Oxide Scale Formed on Titanium Grade 2
Abstract
1. Introduction
2. Experimental Procedures
- Fn—indenter load;
- Ft—resistance to indenter penetration;
- Pd—depth of indenter penetration under load;
- Rd—scratch depth after unloading the indenter.
- Vv—wear factor (mm3/N·m);
- V—volume of the material removed during tests (mm3);
- F—load (N);
- s—length of the path of friction (m).
- V—volume of the material removed (mm3);
- b—diameter of the wear track of the ball, measured in two perpendicular directions (mm);
- R—diameter of the ball (mm).
3. Results and Discussion
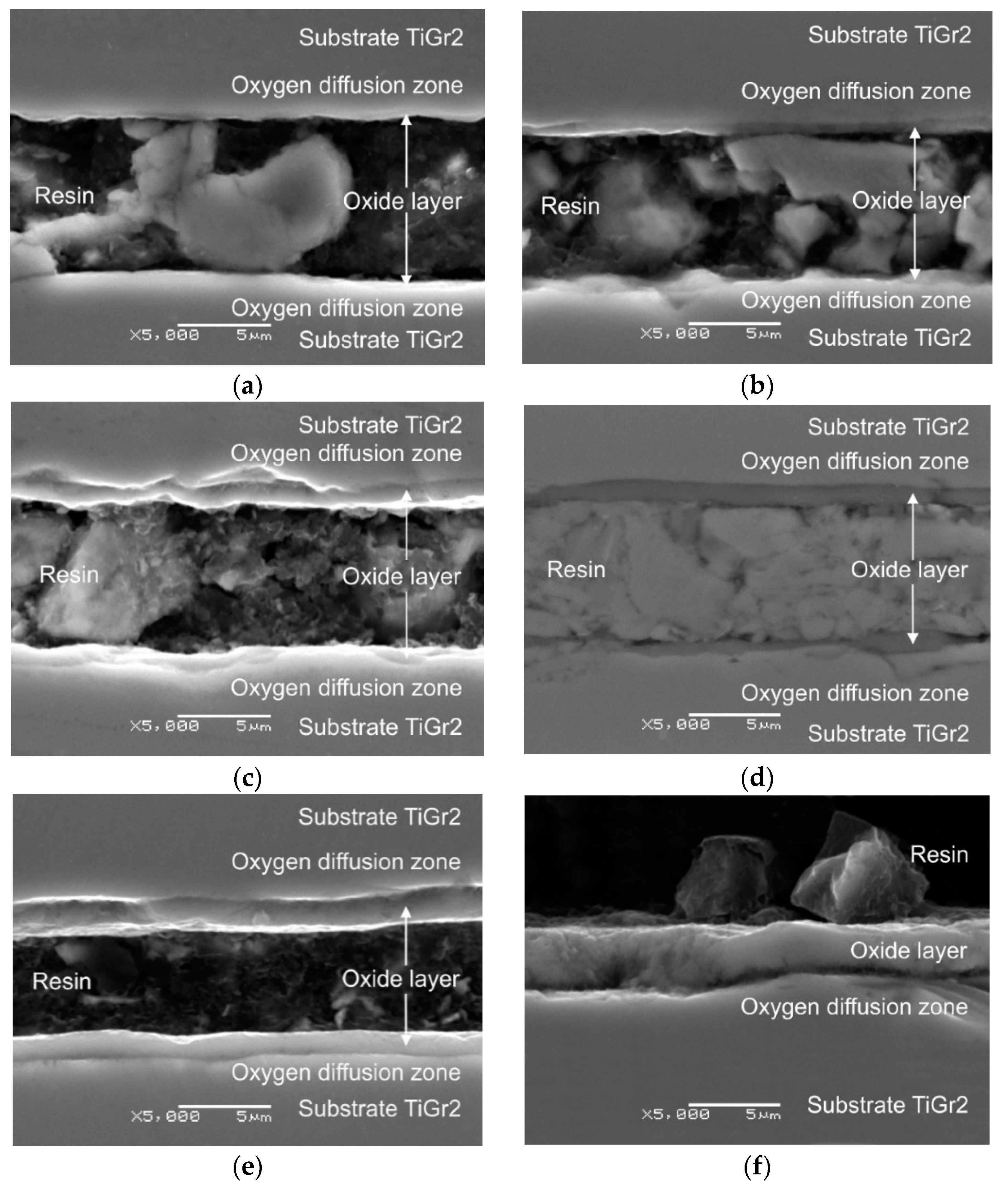
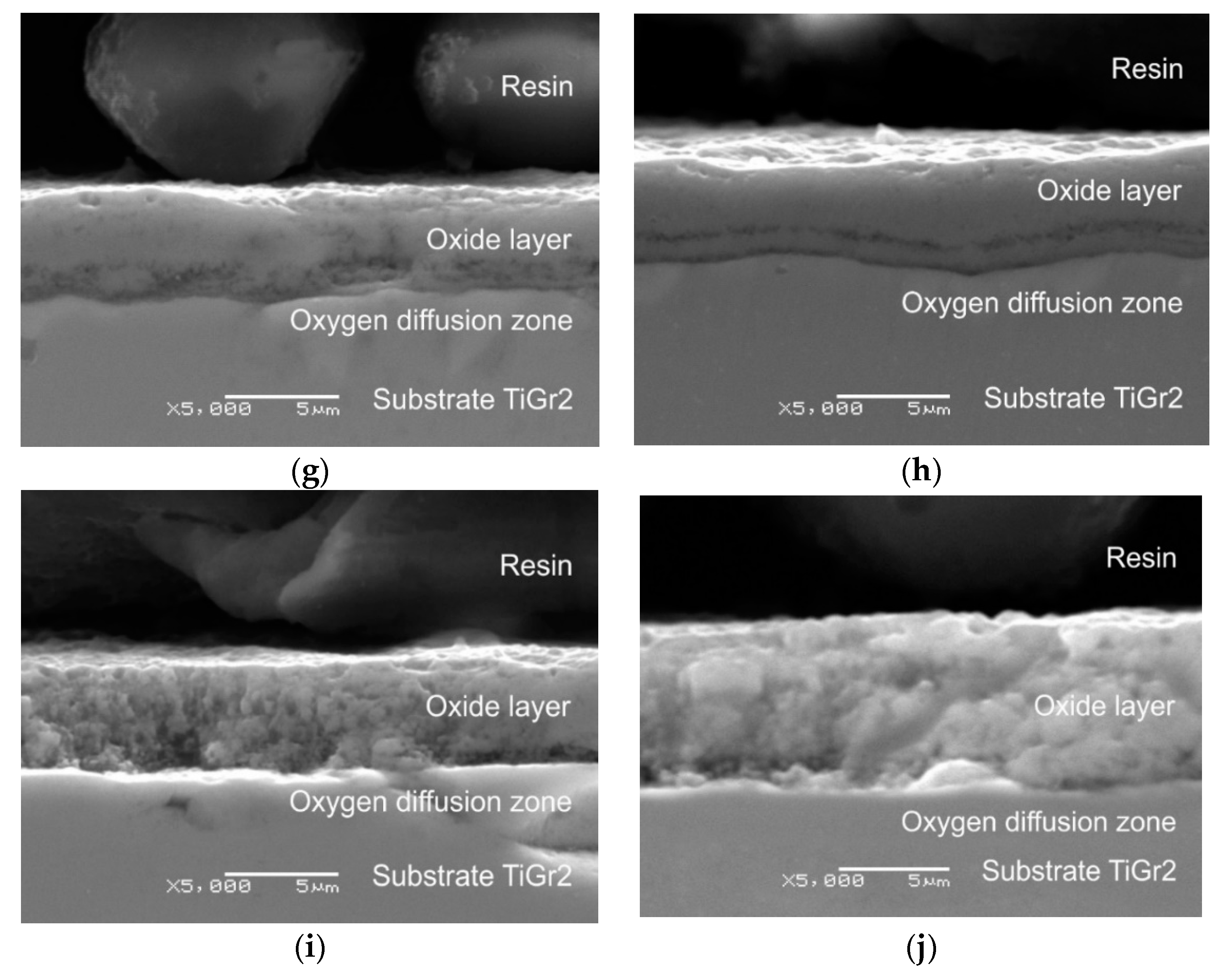
3.1. Oxide Scale Thickness
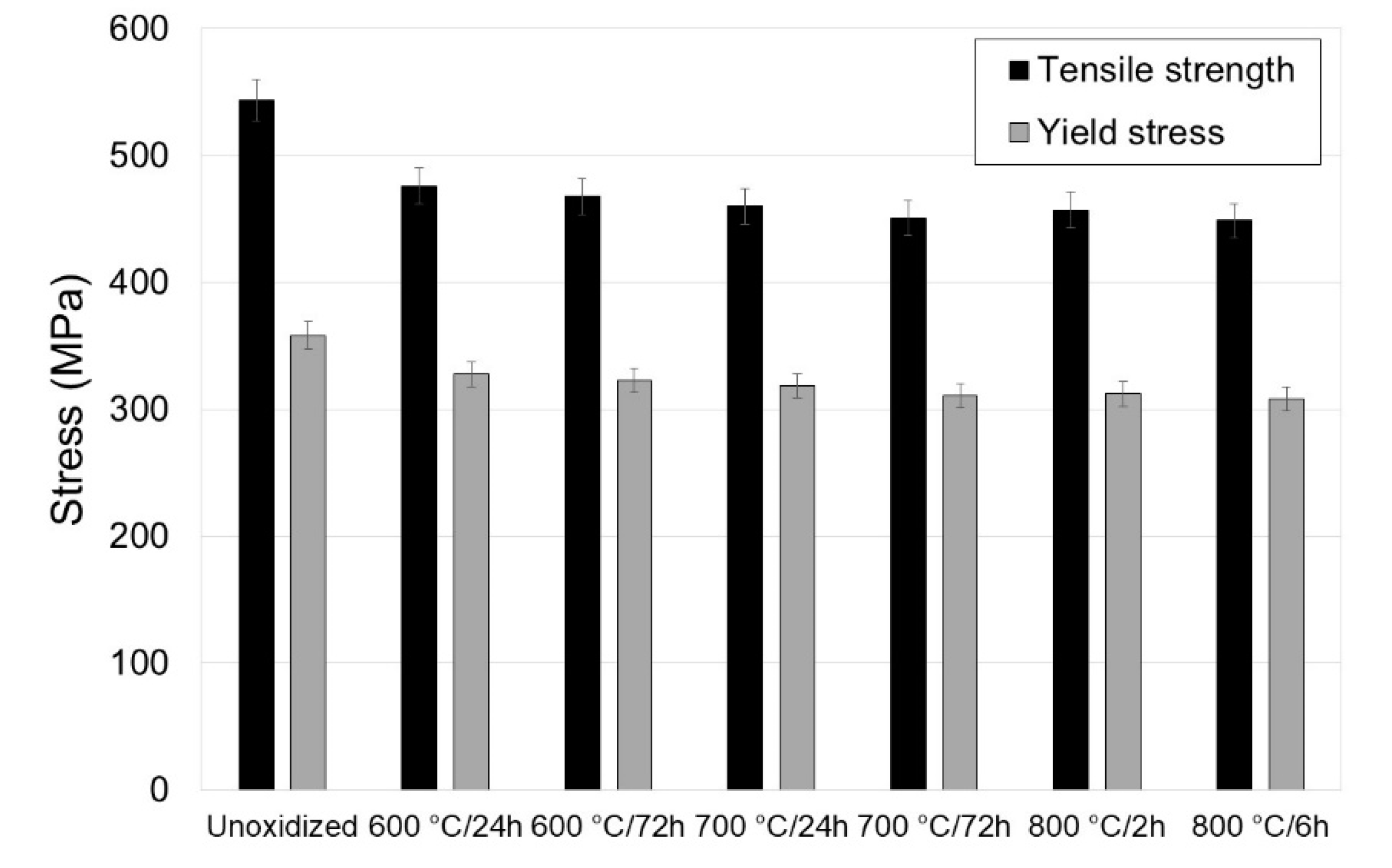
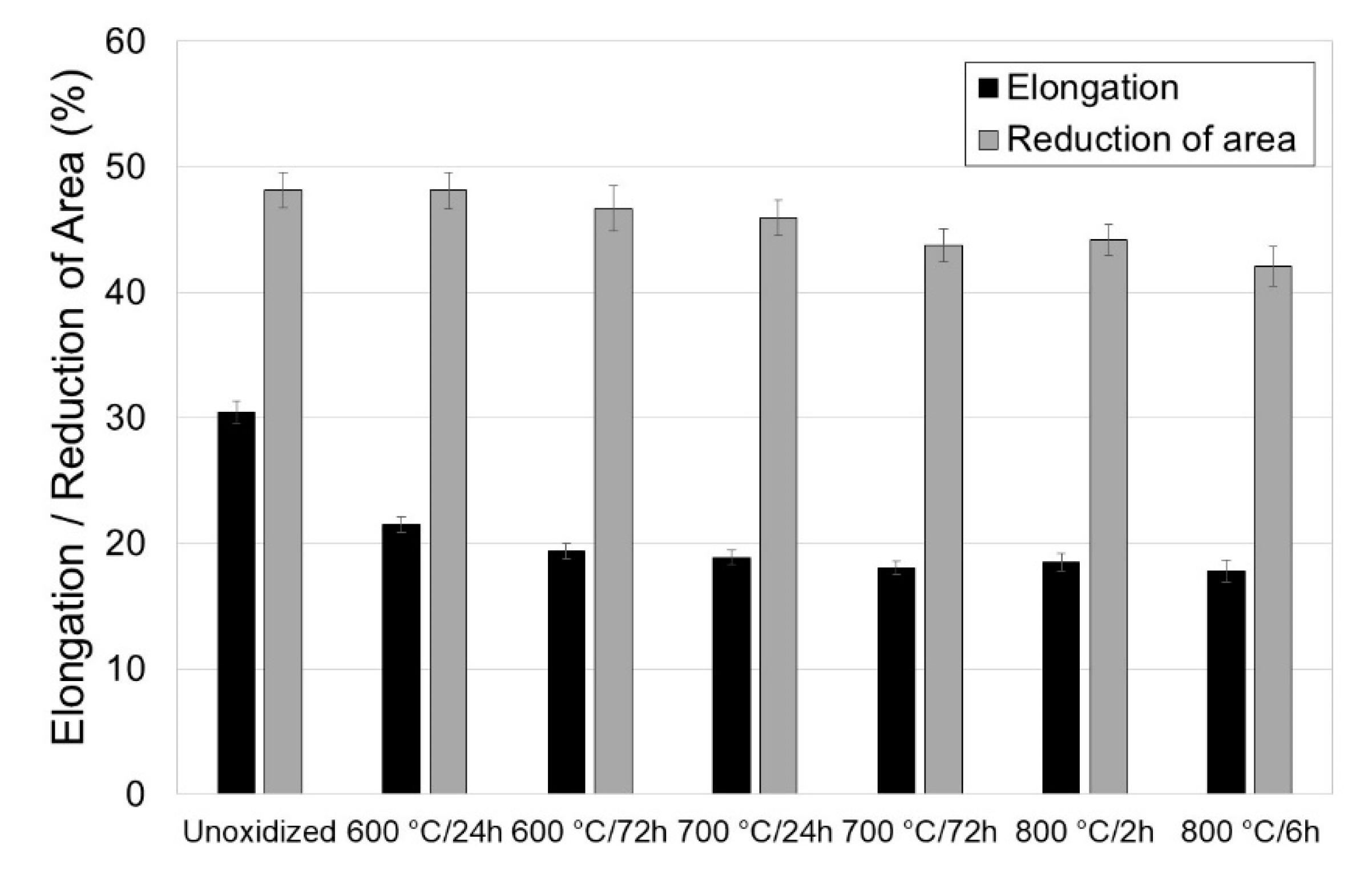
3.2. Tensile Properties of Titanium after Annealing
3.3. Examination of Adhesive Properties by the Scratch Test Method
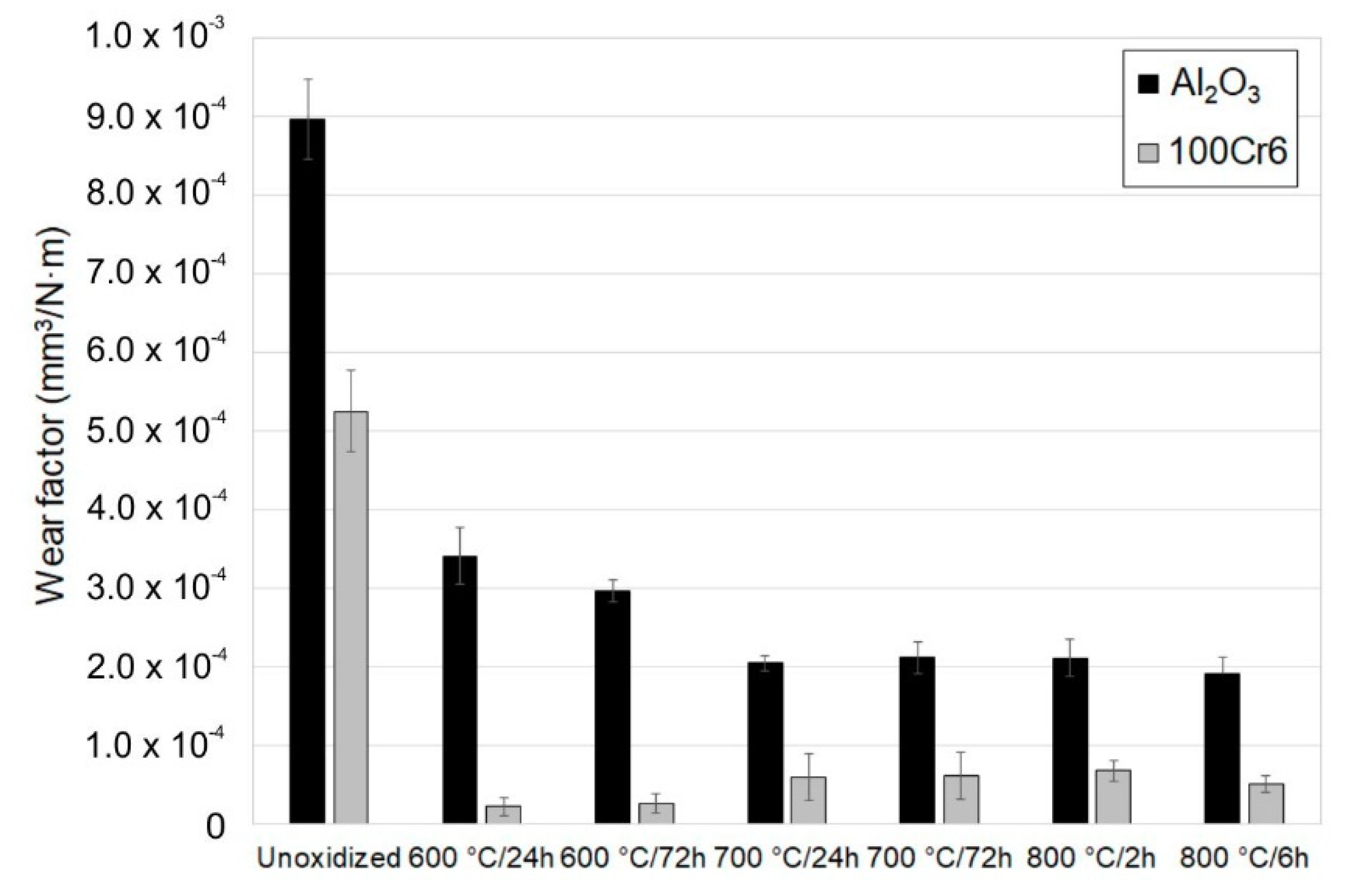
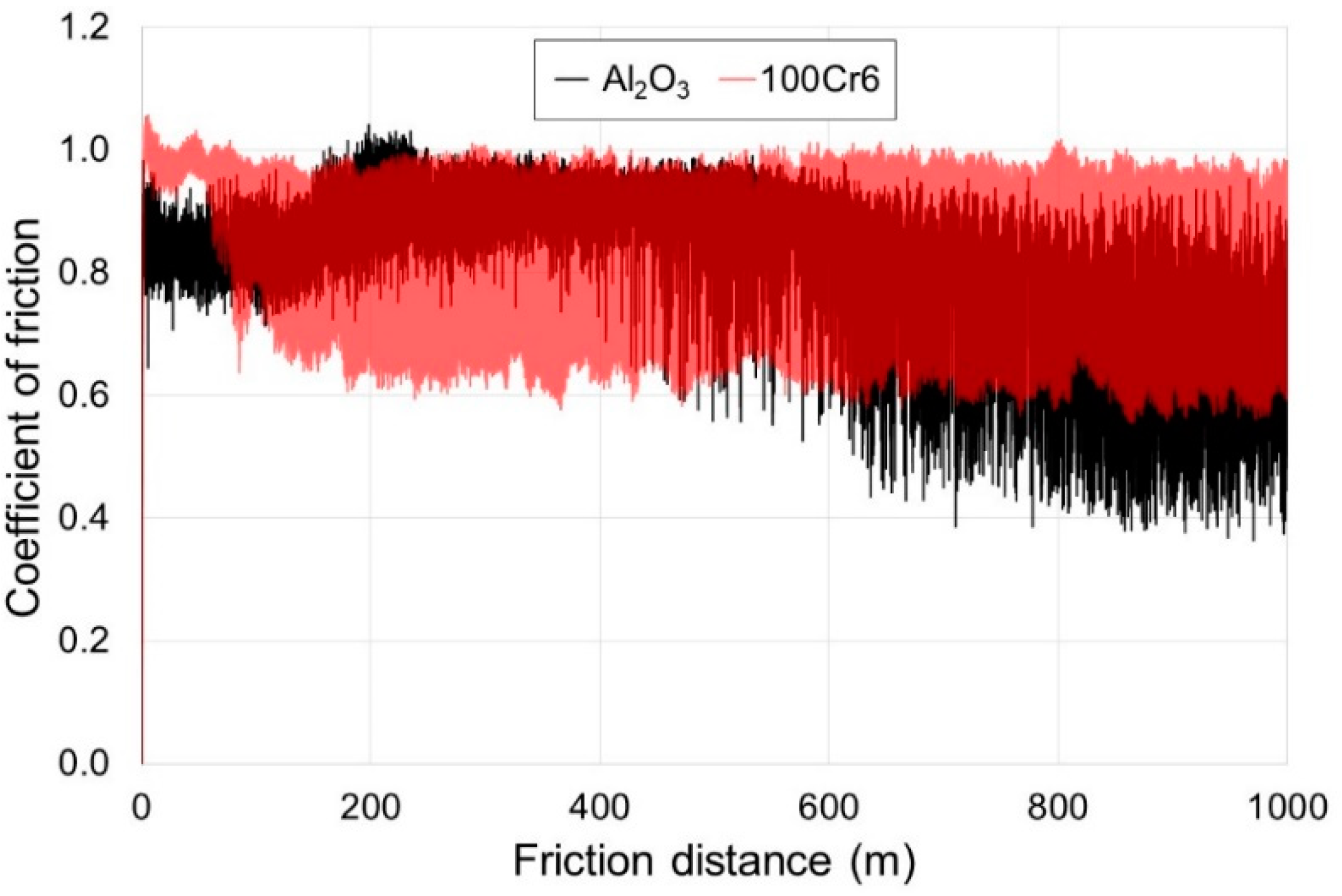
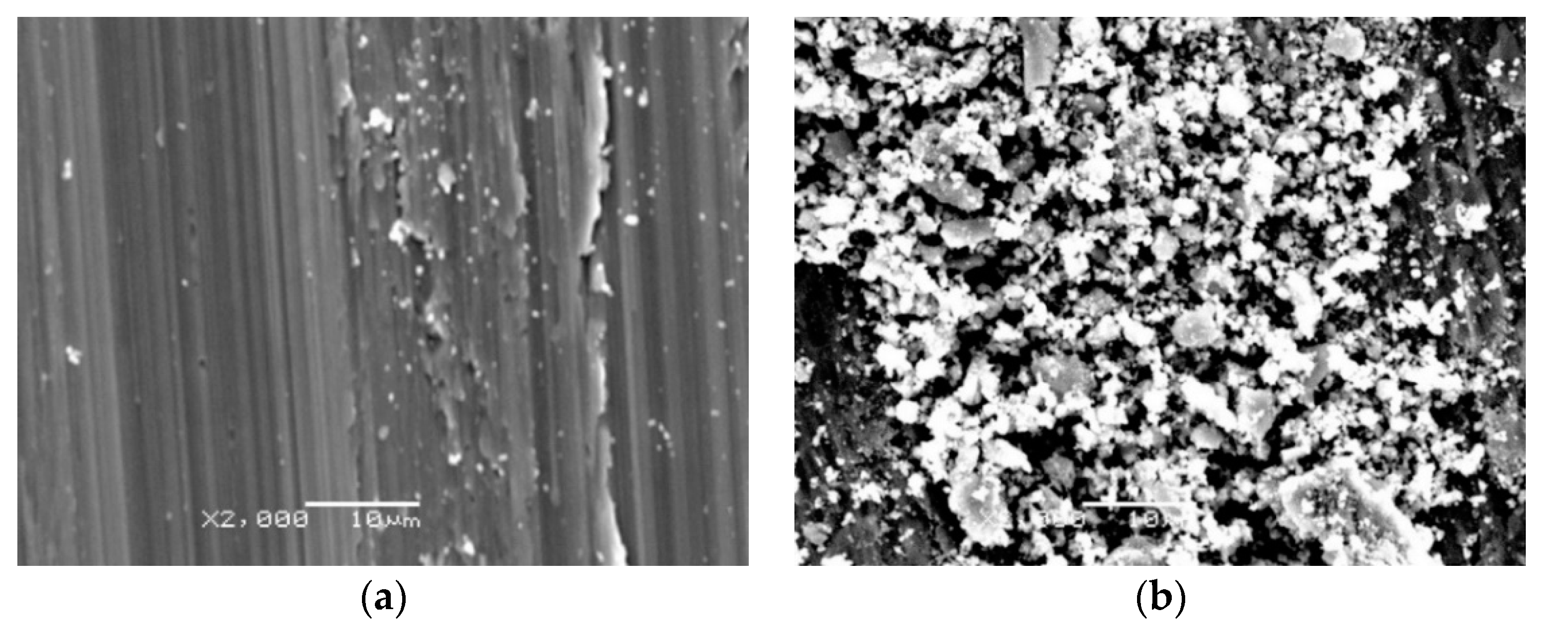
3.4. Tribological Properties of the Oxide Scale
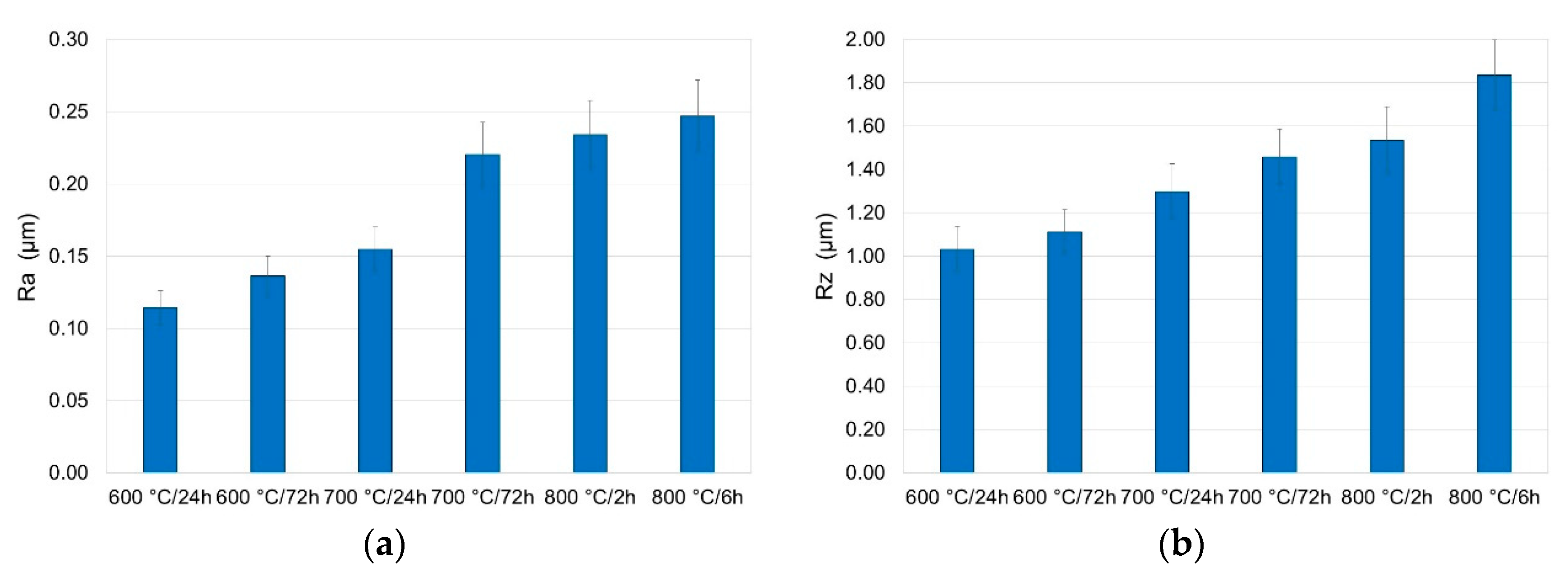
3.5. Surface Roughness Measurement after Oxidation
4. Conclusions
- The isothermal oxidation process allowed producing on titanium an oxide scale of good quality, which was characterized by a homogeneous structure and varied thickness depending on the oxidation parameters;
- The parameters of isothermal oxidation had a significant influence on the course of the oxidation process. It was demonstrated that the oxidation intensity was higher at higher annealing temperatures. This translated directly into the thickness of the oxides produced. The oxide layer obtained after annealing at 800 °C over a period of 6 h had the greatest thickness (8.1 µm);
- Isothermal oxidation has an adverse effect on the tensile properties of titanium Grade 2. It was found that the maximum reduction in tensile strength was approximately 17.5%, and of the yield point, approximately 13.9%. Isothermal oxidation also had an adverse effect on plastic parameters (reduction in elongation by approximately 12.7%, and reduction of area by approximately 6.1%);
- Examination of scratch behaviors of the oxide layers by means of a scratch test showed that the layers formed had different adhesion properties. The best scratch resistance was exhibited by the oxide scales obtained at 800 °C, in particular, the layer produced after 6-h oxidation (Lc1 = 63 N, Lc2 = 85 N);
- Titanium after isothermal oxidation was characterized by more advantageous tribological properties. The reason for the significant improvement in resistance to sliding wear was the presence of oxides distinguished by their high hardness. At the same time, it was shown that the oxide scale caused a significant increase in the friction coefficient, which was strictly connected with the increased surface roughness after isothermal oxidation. It was found that the principal wear mechanism was abrasive wear;
- Roughness measurements showed that an increase in oxidation temperature is a factor which intensely increases surface roughness. It was found that extending the oxidation time also increases surface roughness, but to a lesser extent than the oxidation temperature.
Author Contributions
Funding
Conflicts of Interest
References
- Lederer, S.; Lutz, P.; Fürbeth, W. Surface modification of Ti 13Nb 13Zr by plasma electrolytic oxidation. Surf. Coat. Technol. 2018, 335, 62–71. [Google Scholar] [CrossRef]
- Hu, H.Y.; Zhang, L.; He, Z.Y.; Jiang, Y.H.; Tan, J. Microstructure evolution, mechanical properties, and enhanced bioactivity of Ti-13Nb-13Zr based calcium pyrophosphate composites for biomedical applications. Mater. Sci. Eng. C 2019, 98, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Garbacz, H.; Wieciński, P.; Kuczyńska, D.; Kubacka, D.; Kurzydłowski, K.J. The effect of grain size on the surface properties of titanium grade 2 after different treatments. Surf. Coat. Technol. 2018, 335, 13–24. [Google Scholar] [CrossRef]
- Koizumi, H.; Takeuchi, Y.; Imai, H.; Kawai, T.; Yoneyama, T. Application of titanium and titanium alloys to fixed dental prostheses. J. Prosthodont. Res. 2019, 63, 266–270. [Google Scholar] [CrossRef]
- Gravinaa, A.N.; Rubert, A.A.; Bertuola, M.; Fernández Lorenzo de Mele, M. Bioactivity enhancement of cerium-containing titanium oxide nanotubes. Relationship between surface reactivity and nanostructuring process. Surf. Coat. Technol. 2019, 378, 124968. [Google Scholar] [CrossRef]
- Jebieshia, T.R.; Kim, J.M.; Kang, J.W.; Son, S.W.; Kim, H.D. Microstructural and very high cycle fatigue (vhcf) behavior of Ti6Al4V—A comparative study. Materials 2020, 13, 1948. [Google Scholar] [CrossRef]
- Salguero, J.; Del Sol, I.; Vazquez-Martinez, J.M.; Schertzer, M.J.; Iglesias, P. Effect of laser parameters on the tribological behavior of Ti6Al4V titanium microtextures under lubricated conditions. Wear 2019, 426–427, 1272–1279. [Google Scholar] [CrossRef]
- Redmore, E.; Li, X.; Dong, H. Tribological performance of surface engineered low-cost beta titanium alloy. Wear 2019, 426–427, 952–960. [Google Scholar] [CrossRef]
- Hong, X.; Tan, Y.-F.; Wang, X.-L.; Tan, H.; Xu, T. Effects of nitrogen flux on microstructure and tribological properties of in-situ TiN coatings deposited on TC11 titanium alloy by electrospark deposition. Trans. Nonferrous Met. Soc. China 2015, 25, 3329–3338. [Google Scholar] [CrossRef]
- Budinski, K.G. Tribological properties of titanium alloys. Wear 1991, 151, 203–217. [Google Scholar] [CrossRef]
- Miller, P.D.; Holladay, J.W. Friction and wear properties of titanium. Wear 1958, 2, 133–140. [Google Scholar] [CrossRef]
- An, Q.; Chen, J.; Tao, Z.; Ming, W.; Chen, M. Experimental investigation on tool wear characteristics of PVD and CVD coatings during face milling of Ti-6242S and Ti-555 titanium alloys. Inter. J. Refrac. Met. Hard Mater. 2020, 86, 105091. [Google Scholar] [CrossRef]
- Acciari, H.A.; Palma, D.P.S.; Codaro, E.N.; Zhou, Q.; Wang, J.; Ling, Y.; Zhang, J.; Zhang, Z. Surface modifications by both anodic oxidation and ion beam implantation on electropolished titanium substrates. App. Surf. Sci. 2019, 487, 1111–1120. [Google Scholar] [CrossRef]
- Alcázar, J.C.B.; Lemos, R.M.J.; Conde, M.C.M.; Chisini, L.A.; Salas, M.M.S.; Noremberg, B.S.; Da Motta, F.V.; Demarco, F.F.; Tarquinio, S.B.C.; Carreño, N.L.V. Preparation, characterization, and biocompatibility of different metal oxide/PEG-based hybrid coating synthesized by sol–gel dip coating method for surface modification of titanium. Prog. Org. Coat. 2019, 130, 206–213. [Google Scholar] [CrossRef]
- Borgioli, F.; Galvanetto, E.; Iozzelli, F.; Pradelli, G. Improvement of wear resistance of Ti–6Al–4V alloy by means of thermal oxidation. Mater. Lett. 2005, 59, 2159–2162. [Google Scholar] [CrossRef]
- Tillmann, W.; Grisales, D.; Stangier, D.; Jebara, I.B.; Kang, H. Influence of the etching processes on the adhesion of TiAlN coatings deposited by DCMS, HiPIMS and hybrid techniques on heat treated AISI H11. Surf. Coat. Technol. 2019, 378, 125075. [Google Scholar] [CrossRef]
- Zhang, X.; Tian, X.-B.; Zhao, Z.-W.; Gao, J.-B.; Zhou, Y.-W.; Gao, P.; Guo, Y.-Y.; Lv, Z. Evaluation of the adhesion and failure mechanism of the hard CrN coatings on different substrates. Surf. Coat. Technol. 2019, 364, 135–143. [Google Scholar] [CrossRef]
- Aniołek, K.; Kupka, M. Mechanical, tribological and adhesive properties of oxide layers obtained on the surface of the Ti–6Al–7Nb alloy in the thermal oxidation process. Wear 2019, 432, 202929. [Google Scholar] [CrossRef]
- Aniołek, K.; Kupka, M.; Barylski, A.; Dercz, G. Mechanical and tribological properties of oxide layers obtained on titanium in the thermal oxidation process. App. Surf. Sci. 2015, 357, 1419–1426. [Google Scholar] [CrossRef]
- Camarano, A.; Giuranno, D.; Narciso, J. SiC-IrSi3 for high oxidation resistance. Materials 2020, 13, 98. [Google Scholar] [CrossRef]
- Bailey, R.; Sun, Y. Unlubricated sliding friction and wear characteristics of thermally oxidized commercially pure titanium. Wear 2013, 308, 61–70. [Google Scholar] [CrossRef]
- Biswas, A.; Majumdar, J.D. Surface characterization and mechanical property evaluation of thermally oxidized Ti–6Al–4V. Mater. Character. 2009, 60, 513–518. [Google Scholar] [CrossRef]
- Dalili, N.; Edrisy, A.; Farokhzadeh, K.; Li, J.; Lo, J.; Riahi, A.R. Improving the wear resistance of Ti–6Al–4V/TiC composites through thermal oxidation (TO). Wear 2010, 269, 590–601. [Google Scholar] [CrossRef]
- Aniołek, K.; Barylski, A.; Kupka, M. Modelling the structure and mechanical properties of oxide layers obtained on biomedical Ti-6Al-7Nb alloy in the thermal oxidation process. Vacuum 2018, 154, 309–314. [Google Scholar] [CrossRef]
- Jiang, H.; Hirohasi, M.; Lu, Y.; Imanari, H. Effect of Nb on the high temperature oxidation of Ti–(0–50 at.%) Al. Scr. Mater. 2020, 46, 639–643. [Google Scholar] [CrossRef]
- Abduluyahed, A.A.; Kurzydłowski, K.J. Tensile properties of a type 316 stainless steel strained in air and vacuum. Mater. Sci. Eng. A 1998, 256, 34–38. [Google Scholar] [CrossRef]
- Wang, S.; Liao, Z.; Liu, Y.; Liu, W. Influence of thermal oxidation temperature on the microstructural and tribological behavior of Ti6Al4V alloy. Surf. Coat. Technol. 2014, 240, 470–477. [Google Scholar] [CrossRef]
- Arslan, E.; Totik, Y.; Demirci, E.; Alsaran, A. Influence of surface roughness on corrosion and tribological behavior of CP-Ti after thermal oxidation treatment. J. Mater. Eng. Perform. 2010, 19, 428–433. [Google Scholar] [CrossRef]
- Fellah, M.; Assala, O.; Labaïz, M.; Dekhil, L.; Iost, A. Friction and wear behavior of Ti-6Al-7Nb biomaterial alloy. J. Biomater. Nanobiotech 2013, 4, 374–384. [Google Scholar] [CrossRef]
- Aniołek, K.; Kupka, M.; Barylski, A.; Mieszczak, Ł. Characteristic of oxide layers obtained on titanium in the process of thermal oxidation. Arch. Metall. Mater. 2016, 61, 853–856. [Google Scholar] [CrossRef]
- Guleryuz, H.; Cimenoglu, H. Surface modification of a Ti–6Al–4V alloy by thermal oxidation. Surf. Coat. Technol. 2005, 192, 164–170. [Google Scholar] [CrossRef]
- Siva Rama Krishna, D.; Brama, Y.L.; Sun, Y. Thick rutile layer on titanium for tribological applications. Tribol. Int. 2007, 40, 329–334. [Google Scholar] [CrossRef]
- Wang, S.; Liao, Z.; Liu, Y.; Liu, W. Influence of thermal oxidation duration on the microstructure and fretting wear behavior of Ti6Al4V alloy. Mater. Chem. Phys. 2015, 159, 139–151. [Google Scholar] [CrossRef]
- Dearnley, P.A.; Dahm, K.L.; Çimenoglu, H. The corrosion–wear behaviour of thermally oxidised CP-Ti and Ti–6Al–4V. Wear 2004, 256, 469–479. [Google Scholar] [CrossRef]
- Duarte, M.; Vragovic, I.; Molina, J.M.; Prieto, R.; Narciso, J.; Louis, E. 1/f Noise in Sliding Friction under Wear Conditions: The Role of Debris. Phys. Rev. Lett. 2009, 102, 045501. [Google Scholar] [CrossRef] [PubMed]
- Duarte, M.; Molina, J.M.; Prieto, R.; Louis, E.; Narciso, J. Self-similar fluctuations and 1/f noise in dry friction dynamics. Metall. Mater. Trans. A 2007, 38, 298–305. [Google Scholar] [CrossRef]










| Specimen Condition | Critical Load [N] | |
|---|---|---|
| Lc1 | Lc2 | |
| 600 °C/24 h | 39 | 75 |
| 600 °C/72 h | 35 | 75 |
| 700 °C/24 h | 34 | 67 |
| 700 °C/72 h | 47 | 81 |
| 800 °C/2 h | 50 | 82 |
| 800 °C/6 h | 63 | 85 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aniołek, K.; Barylski, A.; Kupka, M.; Leszek, I. The Tensile Properties, Scratch Behaviors and Sliding Wear of Oxide Scale Formed on Titanium Grade 2. Materials 2020, 13, 3048. https://doi.org/10.3390/ma13143048
Aniołek K, Barylski A, Kupka M, Leszek I. The Tensile Properties, Scratch Behaviors and Sliding Wear of Oxide Scale Formed on Titanium Grade 2. Materials. 2020; 13(14):3048. https://doi.org/10.3390/ma13143048
Chicago/Turabian StyleAniołek, Krzysztof, Adrian Barylski, Marian Kupka, and Iwona Leszek. 2020. "The Tensile Properties, Scratch Behaviors and Sliding Wear of Oxide Scale Formed on Titanium Grade 2" Materials 13, no. 14: 3048. https://doi.org/10.3390/ma13143048
APA StyleAniołek, K., Barylski, A., Kupka, M., & Leszek, I. (2020). The Tensile Properties, Scratch Behaviors and Sliding Wear of Oxide Scale Formed on Titanium Grade 2. Materials, 13(14), 3048. https://doi.org/10.3390/ma13143048







