Carbonyl-Terminated Quinoidal Oligothiophenes as p-Type Organic Semiconductors
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Experimental Methods
2.2. OFET Device Fabrication and Evaluation
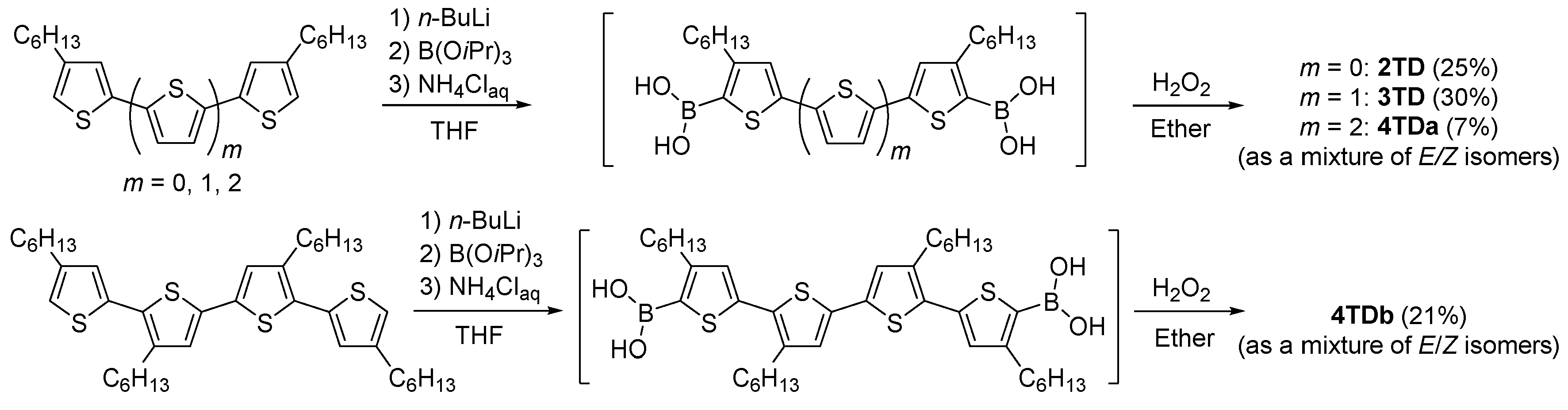
2.3. Synthesis
2.3.1. 4,4′-Dihexyl-5H,5′H-[2,2′-bithiophenylidene]-5,5′-dione (2TD)
2.3.2. 4,4″-Dihexyl-5H,5″H-[2,2′:5′,2″-terthiophene]-5,5″-dione (3TD)
2.3.3. 4,4′′′-Dihexyl-5H,5′′′H-[2,2′:5′,2″:5″,2′′′-quaterthiophene]-5,5′′′-dione (4TDa)
2.3.4. 3′,4,4″,4′′′-Tetrahexyl-5H,5′′′H-[2,2′:5′,2″:5″,2′′′-quaterthiophene]-5,5′′′-dione (4TDb)
3. Results
3.1. Theoretical Calculations
3.2. Synthesis and Chatacterization
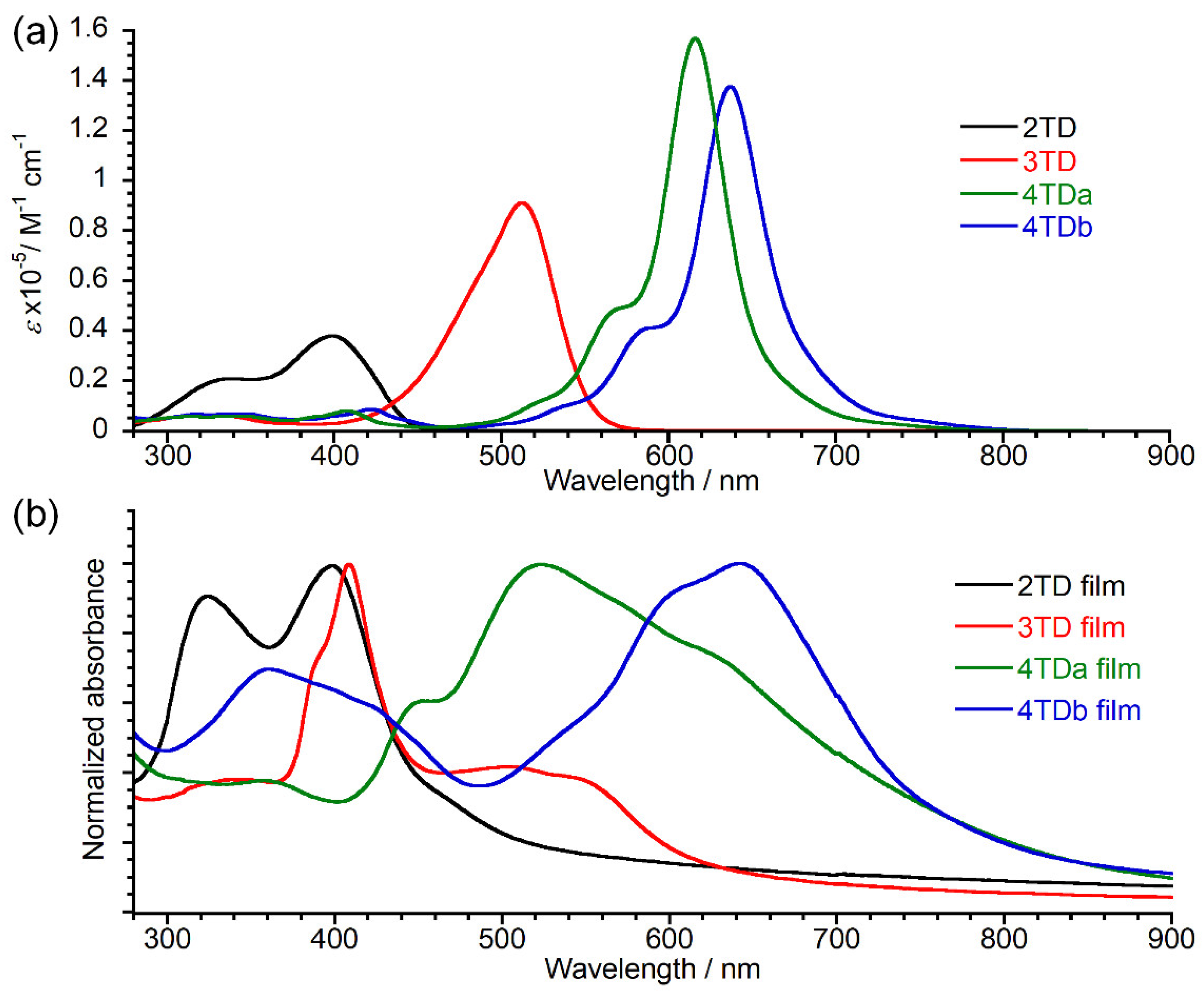
3.3. Physicochemical Properties
3.4. Electronic Structure in the Solid State
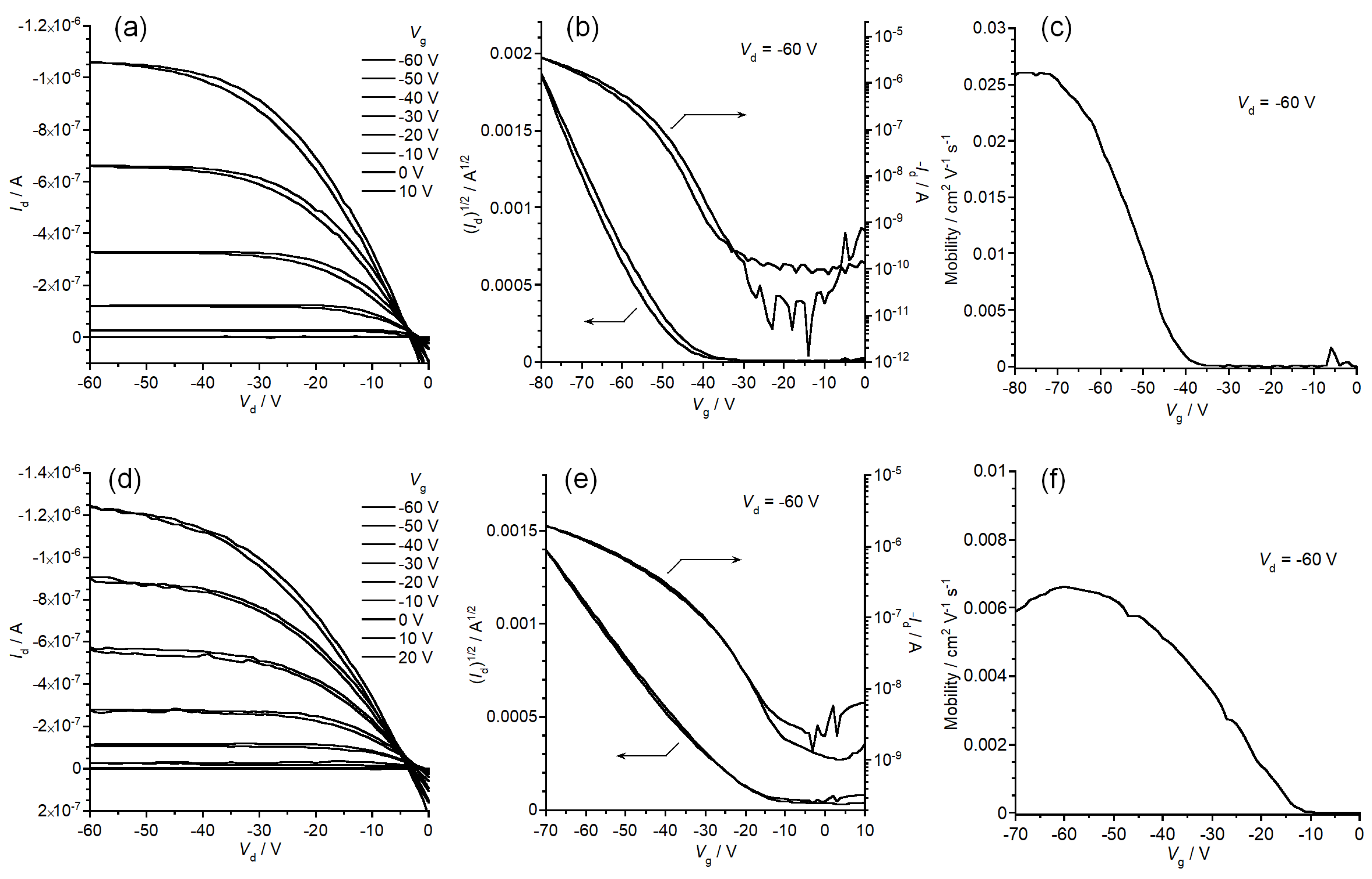
3.5. Fabrication and Evaluation of Thin Film Transistors
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sirringhaus, H. 25th Anniversary Article: Organic Field-Effect Transistors: The Path Beyond Amorphous Silicon. Adv. Mater. 2014, 26, 1319–1335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dou, L.; You, J.; Hong, Z.; Xu, Z.; Li, G.; Street, R.A.; Yang, Y. 25th Anniversary Article: A Decade of Organic/Polymeric Photovoltaic Research. Adv. Mater. 2013, 25, 6642–6671. [Google Scholar] [CrossRef] [PubMed]
- Sasabe, H.; Kido, J. Development of high performance OLEDs for general lighting. J. Mater. Chem. C 2013, 1, 1699–1707. [Google Scholar] [CrossRef]
- Fichou, D. Structural order in conjugated oligothiophenes and its implications on opto-electronic devices. J. Mater. Chem. 2000, 10, 571–588. [Google Scholar] [CrossRef]
- Schoonveld, W.A.; Wildeman, J.; Fichou, D.; Bobbert, P.A.; van Wees, B.J.; Klapwijk, T.M. Coulomb-blockade transport in single-crystal organic thin-film transistors. Nature 2000, 404, 977–980. [Google Scholar] [CrossRef]
- Mishra, A.; Ma, C.-Q.; Bäuerle, P. Functional Oligothiophenes: Molecular Design for Multidimensional Nanoarchitectures and Their Applications. Chem. Rev. 2009, 109, 1141–1276. [Google Scholar] [CrossRef]
- Ferraris, J.; Cowan, D.O.; Walatka, V.; Perlstein, J.H. Electron transfer in a new highly conducting donor-acceptor complex. J. Am. Chem. Soc. 1973, 95, 948–949. [Google Scholar] [CrossRef]
- Casado, J.; Ponce Ortiz, R.; Lopez Navarrete, J.T. Quinoidal oligothiophenes: New properties behind an unconventional electronic structure. Chem. Soc. Rev. 2012, 41, 5672–5686. [Google Scholar] [CrossRef]
- Takimiya, K.; Kawabata, K. Thienoquinoidal System: Promising Molecular Architecture for Optoelectronic Applications. J. Synth. Org. Chem. Jpn. 2018, 76, 1176–1184. [Google Scholar] [CrossRef]
- Yui, K.; Aso, Y.; Otsubo, T.; Ogura, F. New electron acceptors for organic metals: Extensively conjugated homologues of thiophene–7,7,8,8-tetracyanoquinodimethane (TCNQ). J. Chem. Soc. Chem. Commun. 1987, 1816–1817. [Google Scholar] [CrossRef]
- Pappenfus, T.M.; Chesterfield, R.J.; Frisbie, C.D.; Mann, K.R.; Casado, J.; Raff, J.D.; Miller, L.L. A π-Stacking Terthiophene-Based Quinodimethane Is an n-Channel Conductor in a Thin Film Transistor. J. Am. Chem. Soc. 2002, 124, 4184–4185. [Google Scholar] [CrossRef] [PubMed]
- Janzen, D.E.; Burand, M.W.; Ewbank, P.C.; Pappenfus, T.M.; Higuchi, H.; da Silva Filho, D.A.; Young, V.G.; Brédas, J.-L.; Mann, K.R. Preparation and Characterization of π-Stacking Quinodimethane Oligothiophenes. Predicting Semiconductor Behavior and Bandwidths from Crystal Structures and Molecular Orbital Calculations. J. Am. Chem. Soc. 2004, 126, 15295–15308. [Google Scholar] [CrossRef]
- Handa, S.; Miyazaki, E.; Takimiya, K.; Kunugi, Y. Solution-Processible n-Channel Organic Field-Effect Transistors Based on Dicyanomethylene-Substituted Terthienoquinoid Derivative. J. Am. Chem. Soc. 2007, 129, 11684–11685. [Google Scholar] [CrossRef]
- Ribierre, J.C.; Watanabe, S.; Matsumoto, M.; Muto, T.; Hashizume, D.; Aoyama, T. Thickness Dependence of the Ambipolar Charge Transport Properties in Organic Field-Effect Transistors based on a Quinoidal Oligothiophene Derivative. J. Phys. Chem. C 2011, 115, 20703–20709. [Google Scholar] [CrossRef]
- Zhang, C.; Zang, Y.; Gann, E.; McNeill, C.R.; Zhu, X.; Di, C.A.; Zhu, D. Two-Dimensional π-Expanded Quinoidal Terthiophenes Terminated with Dicyanomethylenes as n-Type Semiconductors for High-Performance Organic Thin-Film Transistors. J. Am. Chem. Soc. 2014, 136, 16176–16184. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Fan, H.; Huang, D.; Yuan, D.; Di, C.A.; Zhu, X. Dithienoindophenines: P-Type Semiconductors Designed by Quinoid Stabilization for Solar-Cell Applications. Chem. Eur. J. 2016, 22, 17136–17140. [Google Scholar] [CrossRef] [PubMed]
- Nishinaga, T. Introduction. In Organic Redox Systems; John Wiley & Sons: Hoboken, NJ, USA, 2015; pp. 1–12. [Google Scholar]
- Sheldrick, G. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. Sec. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Sheldrick, G. Crystal structure refinement with SHELXL. Acta Crystallogr. Sec. C 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16 Rev. C.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- ADF: Powerful DFT Code for Modeling Molecules. Scien-tific Computing and Modeling: Amsterdam, The Netherlands. Available online: https://www.scm.com/product/adf/ (accessed on 3 July 2020).
- Saito, H.; Chen, J.; Kuwabara, J.; Yasuda, T.; Kanbara, T. Facile one-pot access to π-conjugated polymers via sequential bromination/direct arylation polycondensation. Polym. Chem. 2017, 8, 3006–3012. [Google Scholar] [CrossRef] [Green Version]
- Li, J.-C.; Lee, S.-H.; Hahn, Y.-B.; Kim, K.-J.; Zong, K.; Lee, Y.-S. Synthesis and characterization of triphenylamine-3-hexylthiophene oligomer hybrids: A triphenylamine core carrying three terthiophene branches and triphenylamine end-capped quaterthiophene. Synth. Met. 2008, 158, 150–156. [Google Scholar] [CrossRef]
- Garnier, F.; Hajlaoui, R.; El Kassmi, A.; Horowitz, G.; Laigre, L.; Porzio, W.; Armanini, M.; Provasoli, F. Dihexylquaterthiophene, a Two-Dimensional Liquid Crystal-like Organic Semiconductor with High Transport Properties. Chem. Mater. 1998, 10, 3334–3339. [Google Scholar] [CrossRef]
- Katz, H.E.; Laquindanum, J.G.; Lovinger, A.J. Synthesis, Solubility, and Field-Effect Mobility of Elongated and Oxa-Substituted α, ω-Dialkyl Thiophene Oligomers. Extension of “Polar Intermediate” Synthetic Strategy and Solution Deposition on Transistor Substrates. Chem. Mater. 1998, 10, 633–638. [Google Scholar] [CrossRef]
- Ponce Ortiz, R.; Casado, J.; Hernández, V.; López Navarrete, J.T.; Viruela, P.M.; Ortí, E.; Takimiya, K.; Otsubo, T. On the Biradicaloid Nature of Long Quinoidal Oligothiophenes: Experimental Evidence Guided by Theoretical Studies. Angew. Chem. Int. Ed. 2007, 46, 9057–9061. [Google Scholar] [CrossRef]
- Ponce-Ortiz, R.; Casado, J.; Rodríguez-González, S.; Hernández, V.; López-Navarrete, J.T.; Viruela, P.M.; Ortí , E.; Takimiya, K.; Otsubo, T. Quinoidal Oligothiophenes: Towards Biradical Ground-State Species. Chem. Eur. J. 2010, 16, 470–484. [Google Scholar] [CrossRef] [PubMed]
- Takimiya, K.; Nakano, M.; Sugino, H.; Osaka, I. Design and elaboration of organic molecules for high field-effect-mobility semiconductors. Synth. Met. 2016, 217, 68–78. [Google Scholar] [CrossRef]
- Kawabata, K.; Saito, M.; Osaka, I.; Takimiya, K. Very Small Bandgap pi-Conjugated Polymers with Extended Thienoquinoids. J. Am. Chem. Soc. 2016, 138, 7725–7732. [Google Scholar] [CrossRef] [PubMed]
- Kawabata, K.; Osaka, I.; Sawamoto, M.; Zafra, J.L.; Mayorga-Burrezo, P.; Casado, J.; Takimiya, K. Dithienyl Acenedithiophenediones as New π-Extended Quinoidal Cores: Synthesis and Properties. Chem. Eur. J. 2017, 23, 4579–4589. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Miyazaki, E.; Takimiya, K. ((Alkyloxy)carbonyl)cyanomethylene-Substituted Thienoquinoidal Compounds: A New Class of Soluble n-Channel Organic Semiconductors for Air-Stable Organic Field-Effect Transistors. J. Am. Chem. Soc. 2010, 132, 10453–10466. [Google Scholar] [CrossRef] [PubMed]






| Cmpd | EHOMO (eV) | ELUMO (eV) | Eg (eV) | λhole (eV) | λelectron (eV) |
|---|---|---|---|---|---|
| 2TD | −6.41 | −3.30 | 3.10 | 0.310 | 0.538 |
| 3TD | −5.71 | −3.44 | 2.26 | 0.224 | 0.446 |
| 4TDa | −5.30 | −3.53 | 1.77 | 0.190 | 0.388 |
| 4TDb | −5.19 | −3.45 | 1.73 | 0.184 | 0.381 |
| 2T | −5.73 | −1.51 | 4.22 | 0.376 | 0.334 |
| 3T | −5.38 | −1.94 | 3.44 | 0.332 | 0.286 |
| 4T | −5.20 | −2.17 | 3.03 | 0.303 | 0.259 |
| Cmpd | Eox1/2 (V) | Ered1/2 (V) | EHOMOCV a (eV) | ELUMOCV b (eV) | Egc (eV) |
|---|---|---|---|---|---|
| 2TD | >1.1 | −1.36 | <−5.9 | −3.44 | >2.4 |
| 3TD | 0.73 | −1.30 | −5.53 | −3.50 | 2.03 |
| 4TDa | 0.33 | −1.10 | −5.13 | −3.70 | 1.43 |
| 4TDb | 0.39 | 1.02 | −5.19 | −3.75 | 1.44 |
| Cmpd | In Solution | Film | ||||||
|---|---|---|---|---|---|---|---|---|
| λmax (nm) | λonset (nm) | ε (Lcm−1mol−1) | Egopt (eV) | λmax (nm) | λonset (nm) | Egopt (eV) | IP (eV) | |
| 2TD | 398 | 445 | 3.9×104 | 2.79 | 397 | 451 | 2.75 | >6.2 |
| 3TD | 513 | 556 | 9.2×104 | 2.23 | 408 | 620 | 2.00 | 5.8 |
| 4TDa | 616 | 704 | 1.6×105 | 1.76 | 523 | 756 | 1.64 | 5.3 |
| 4TDb | 637 | 724 | 1.4×105 | 1.71 | 642 | 760 | 1.63 | 5.1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asoh, T.; Kawabata, K.; Takimiya, K. Carbonyl-Terminated Quinoidal Oligothiophenes as p-Type Organic Semiconductors. Materials 2020, 13, 3020. https://doi.org/10.3390/ma13133020
Asoh T, Kawabata K, Takimiya K. Carbonyl-Terminated Quinoidal Oligothiophenes as p-Type Organic Semiconductors. Materials. 2020; 13(13):3020. https://doi.org/10.3390/ma13133020
Chicago/Turabian StyleAsoh, Takato, Kohsuke Kawabata, and Kazuo Takimiya. 2020. "Carbonyl-Terminated Quinoidal Oligothiophenes as p-Type Organic Semiconductors" Materials 13, no. 13: 3020. https://doi.org/10.3390/ma13133020
APA StyleAsoh, T., Kawabata, K., & Takimiya, K. (2020). Carbonyl-Terminated Quinoidal Oligothiophenes as p-Type Organic Semiconductors. Materials, 13(13), 3020. https://doi.org/10.3390/ma13133020