3D Printing of Cell Culture Devices: Assessment and Prevention of the Cytotoxicity of Photopolymers for Stereolithography
Abstract
:1. Introduction
2. Materials and Methods
2.1. Resins
2.2. Additive Manufacturing
2.3. Post-Processing
2.4. Sterilization
2.5. Cell Culture
2.6. Viability Assays
2.7. Live/Dead Cell Staining
2.8. Statistical Analysis
3. Results
3.1. Additive Manufacturing
3.2. Cytotoxicity Testing
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lantada, A.D.; Morgado, P.L. Rapid Prototyping for Biomedical Engineering: Current Capabilities and Challenges. Annu. Rev. Biomed. Eng. 2012, 14, 73–96. [Google Scholar] [CrossRef] [Green Version]
- Bessler, N.; Ogiermann, D.; Buchholz, M.-B.; Santel, A.; Heidenreich, J.; Ahmmed, R.; Zaehres, H.; Brand-Saberi, B.; Zähres, H. Nydus One Syringe Extruder (NOSE): A Prusa i3 3D printer conversion for bioprinting applications utilizing the FRESH-method. HardwareX 2019, 6, e00069. [Google Scholar] [CrossRef]
- Shallan, A.; Smejkal, P.; Corban, M.; Guijt, R.; Breadmore, M.C. Cost-Effective Three-Dimensional Printing of Visibly Transparent Microchips within Minutes. Anal. Chem. 2014, 86, 3124–3130. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.M.B.; Ng, S.; Li, K.H.H.; Yoon, Y.-J. 3D printed microfluidics for biological applications. Lab Chip 2015, 15, 3627–3637. [Google Scholar] [CrossRef] [PubMed]
- Do, A.-V.; Khorsand, B.; Geary, S.M.; Salem, A.K. 3D Printing of Scaffolds for Tissue Regeneration Applications. Adv. Healthc. Mater. 2015, 4, 1742–1762. [Google Scholar] [CrossRef] [Green Version]
- An, J.; Teoh, J.E.M.; Suntornnond, R.; Chua, C.K. Design and 3D Printing of Scaffolds and Tissues. Engineering 2015, 1, 261–268. [Google Scholar] [CrossRef] [Green Version]
- Lücking, T.H.; Sambale, F.; Schnaars, B.; Bulnes-Abundis, D.; Beutel, S.; Scheper, T. 3D-printed individual labware in biosciences by rapid prototyping: In vitro biocompatibility and applications for eukaryotic cell cultures. Eng. Life Sci. 2014, 15, 57–64. [Google Scholar] [CrossRef] [Green Version]
- Gulyas, M.; Csiszer, M.; Mehes, E.; Czirok, A. Software tools for cell culture-related 3D printed structures. PLoS ONE 2018, 13, e0203203. [Google Scholar] [CrossRef]
- Egger, D.; Fischer, M.; Clementi, A.; Ribitsch, V.; Hansmann, J.; Kasper, C. Development and Characterization of a Parallelizable Perfusion Bioreactor for 3D Cell Culture. Bioengineering 2017, 4, 51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Putame, G.; Terzini, M.; Carbonaro, D.; Pisani, G.; Serino, G.; Di Di Meglio, F.; Castaldo, C.; Massai, D. Application of 3D Printing Technology for Design and Manufacturing of Customized Components for a Mechanical Stretching Bioreactor. J. Healthc. Eng. 2019, 2019, 1–9. [Google Scholar] [CrossRef]
- Baume, A.; Boughton, P.; Coleman, N.; Ruys, A. Sterilization of tissue scaffolds. In Characterisation and Design of Tissue Scaffolds; Tomlins, P., Ed.; Woodhead Publishing: Sawston, UK, 2016; pp. 225–244. [Google Scholar]
- Williams, D.F. Specifications for Innovative, Enabling Biomaterials Based on the Principles of Biocompatibility Mechanisms. Front. Bioeng. Biotechnol. 2019, 7, 255. [Google Scholar] [CrossRef] [PubMed]
- Alifui-Segbaya, F.; Varma, S.; Lieschke, G.J.; George, R. Biocompatibility of Photopolymers in 3D Printing. 3D Print. Addit. Manuf. 2017, 4, 185–191. [Google Scholar] [CrossRef] [Green Version]
- Berger, C.; Bjørlykke, Y.; Hahn, L.; Mühlemann, M.; Kress, S.; Walles, H.; Luxenhofer, R.; Ræder, H.; Metzger, M.; Zdzieblo, D. Matrix decoded—A pancreatic extracellular matrix with organ specific cues guiding human iPSC differentiation. Biomaterials 2020, 244, 119766. [Google Scholar] [CrossRef] [PubMed]
- Massia, S.P.; Stark, J.; Letbetter, D.S. Surface-immobilized dextran limits cell adhesion and spreading. Biomaterials 2000, 21, 2253–2261. [Google Scholar] [CrossRef]
- Carve, M.; Wlodkowic, D. 3D-Printed Chips: Compatibility of Additive Manufacturing Photopolymeric Substrata with Biological Applications. Micromachines 2018, 9, 91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, F.; Friedrich, T.; Nugegoda, D.; Kaslin, J.; Wlodkowic, D. Assessment of the biocompatibility of three-dimensional-printed polymers using multispecies toxicity tests. Biomicrofluidics 2015, 9, 061103. [Google Scholar] [CrossRef] [Green Version]
- Williams, D.F. Regulatory biocompatibility requirements for biomaterials used in regenerative medicine. J. Mater. Sci. Mater. Med. 2015, 26, 89. [Google Scholar] [CrossRef]
- Van den Driesche, S.; Lucklum, F.; Bunge, F.; Vellekoop, M.J. 3D Printing Solutions for Microfluidic Chip-to-World Connections. Micromachines 2018, 9, 71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cieślik, M.; Kot, M.; Reczyński, W.; Engvall, K.; Rakowski, W.; Kotarba, A. Parylene coatings on stainless steel 316L surface for medical applications—Mechanical and protective properties. Mater. Sci. Eng. C 2012, 32, 31–35. [Google Scholar] [CrossRef]
- Yu, H.; Deng, R.; Tong, W.H.; Huan, L.; Way, N.C.; IslamBadhan, A.; Iliescu, C.; Yu, H. A perfusion incubator liver chip for 3D cell culture with application on chronic hepatotoxicity testing. Sci. Rep. 2017, 7, 14528. [Google Scholar] [CrossRef] [Green Version]
- Romero-Morales, A.I.; O’Grady, B.J.; Balotin, K.M.; Bellan, L.M.; Lippmann, E.S.; Gama, V. Spin∞: An updated miniaturized spinning bioreactor design for the generation of human cerebral organoids from pluripotent stem cells. HardwareX 2019, 6, e00084. [Google Scholar] [CrossRef]
- Moreno-Rivas, O.; Hernández-Velázquez, D.; Piazza, V.; Marquez, S. Rapid prototyping of microfluidic devices by SL 3D printing and their biocompatibility study for cell culturing. Mater. Today Proc. 2019, 13, 436–445. [Google Scholar] [CrossRef]
- Rimington, R.P.; Capel, A.J.; Player, D.J.; Bibb, R.J.; Christie, S.D.R.; Lewis, M.P. Feasibility and Biocompatibility of 3D-Printed Photopolymerized and Laser Sintered Polymers for Neuronal, Myogenic, and Hepatic Cell Types. Macromol. Biosci. 2018, 18, e1800113. [Google Scholar] [CrossRef] [Green Version]
- Kurzmann, C.; Janjić, K.; Shokoohi-Tabrizi, H.; Edelmayer, M.; Pensch, M.; Moritz, A.; Agis, H. Evaluation of Resins for Stereolithographic 3D-Printed Surgical Guides: The Response of L929 Cells and Human Gingival Fibroblasts. BioMed. Res. Int. 2017, 2017, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Soldatow, V.Y.; Lecluyse, E.; Griffith, L.G.; Rusyn, I. In vitro models for liver toxicity testing. Toxicol. Res. 2013, 2, 23–39. [Google Scholar] [CrossRef] [Green Version]
- Pan, C.; Kumar, C.; Bohl, S.; Klingmüller, U.; Mann, M. Comparative proteomic phenotyping of cell lines and primary cells to assess preservation of cell type-specific functions. Mol. Cell. Proteom. 2008, 8, 443–450. [Google Scholar] [CrossRef] [Green Version]
- Swain, R.J.; Kemp, S.J.; Goldstraw, P.; Tetley, T.D.; Stevens, M.M. Assessment of Cell Line Models of Primary Human Cells by Raman Spectral Phenotyping. Biophys. J. 2010, 98, 1703–1711. [Google Scholar] [CrossRef] [Green Version]
- Alge, C.S.; Hauck, S.M.; Priglinger, S.G.; Kampik, A.; Ueffing, M. Differential Protein Profiling of Primary versus Immortalized Human RPE Cells Identifies Expression Patterns Associated with Cytoskeletal Remodeling and Cell Survival. J. Proteome Res. 2006, 5, 862–878. [Google Scholar] [CrossRef]
- Squillaro, T.; Peluso, G.; Galderisi, U. Clinical Trials with Mesenchymal Stem Cells: An Update. Cell Transplant. 2016, 25, 829–848. [Google Scholar] [CrossRef] [Green Version]
- Uder, C.; Brückner, S.; Winkler, S.; Tautenhahn, H.-M.; Christ, B. Mammalian MSC from selected species: Features and applications. Cytom. Part A 2017, 93, 32–49. [Google Scholar] [CrossRef] [Green Version]
- Han, Y.; Li, X.; Zhang, Y.; Han, Y.; Chang, F.; Ding, J. Mesenchymal Stem Cells for Regenerative Medicine. Cells 2019, 8, 886. [Google Scholar] [CrossRef] [Green Version]
- ISO10993-5:2009. International Organization for Standardization ISO 10993-5: 2009. Biological Evaluation of Medical Devices–Part 5: Tests for in Vitro Cytotoxicity; International Organization for Standardization ISO: Geneva, Switzerland, 2009. [Google Scholar]
- ISO10993-10:2010. International Organization for Standardization ISO 10993-10: 2010. Biological Evaluation of Medical Devices–Part 10: Tests for Irritation and Skin Sensitization; International Organization for Standardization ISO: Geneva, Switzerland, 2010. [Google Scholar]
- ISO10993-1:2009. International Organization for Standardization ISO 10993-1: 2009. Biological Evaluation of Medical Devices–Part 1: Evaluation and Testing within a Risk Management Process; International Organization for Standardization ISO: Geneva, Switzerland, 2009. [Google Scholar]
- Materials Data Sheet. Available online: https://formlabs-media.formlabs.com/datasheets/XL-DataSheet-June2019update.pdf (accessed on 21 June 2020).
- Formlabs Safety Data Sheet Black Resin. Available online: https://formlabs-media.formlabs.com/datasheets/1801033-SDS-ENEU-0.pdf (accessed on 21 June 2020).
- Formlabs Dental SG Safety Data Sheet. Available online: https://formlabs-media.formlabs.com/datasheets/Safety_Data_Sheet_EN-EU_-_Dental_SG.pdf (accessed on 21 June 2020).
- Formlabs Dental LT Clear Safety Data Sheet. Available online: https://formlabs-media.formlabs.com/datasheets/Safety_Data_Sheet_EN-EU_-_Dental_LT_Clear.pdf (accessed on 21 June 2020).
- Formlabs Safety Data Sheet High Temp V2 Resin. Available online: https://formlabs-media.formlabs.com/datasheets/2001047-SDS-ENEU-0.pdf (accessed on 21 June 2020).
- Formlabs Safety Data Sheet Flexible Resin. Available online: https://formlabs-media.formlabs.com/datasheets/1801044-SDS-ENEU-0.pdf (accessed on 21 June 2020).
- Form Cure Time and Temperature Settings. Available online: https://support.formlabs.com/s/article/Form-Cure-Time-and-Temperature-Settings?language=en (accessed on 21 June 2020).
- Egger, D.; Schwedhelm, I.; Hansmann, J.; Kasper, C. Hypoxic Three-Dimensional Scaffold-Free Aggregate Cultivation of Mesenchymal Stem Cells in a Stirred Tank Reactor. Bioengineering 2017, 4, 47. [Google Scholar] [CrossRef] [Green Version]
- Yang, T.; Martin, P.; Fogarty, B.; Brown, A.; Schurman, K.; Phipps, R.; Yin, V.P.; Lockman, P.; Bai, S. Exosome delivered anticancer drugs across the blood-brain barrier for brain cancer therapy in Danio rerio. Pharm. Res. 2015, 32, 2003–2014. [Google Scholar] [CrossRef]
- Franciozi, C.E.D.S.; Vangsness, C.T.; Tibone, J.E.; Martinez, J.C.; Rodger, D.; Chou, T.-C.; Tai, Y.-C.; Brant, R.; Wu, L.; Abdalla, R.J.; et al. Parylene scaffold for cartilage lesion. Biomed. Microdevices 2017, 19, 26. [Google Scholar] [CrossRef]
- Brancato, L.; Decrop, D.; Lammertyn, J.; Puers, R. Surface Nanostructuring of Parylene-C Coatings for Blood Contacting Implants. Materials 2018, 11, 1109. [Google Scholar] [CrossRef] [Green Version]
- Nowak, D.; Ortyl, J.; Kamińska-Borek, I.; Kukuła, K.; Topa, M.; Popielarz, R. Photopolymerization of hybrid monomers. Polym. Test. 2017, 64, 313–320. [Google Scholar] [CrossRef]
- Popov, V.; Evseev, A.V.; Ivanov, A.L.; Roginski, V.V.; Volozhin, A.I.; Howdle, S.M. Laser stereolithography and supercritical fluid processing for custom-designed implant fabrication. J. Mater. Sci. Mater. Med. 2004, 15, 123–128. [Google Scholar] [CrossRef]
- Ngan, C.; O’Connell, C.D.; Blanchard, R.; Boyd-Moss, M.; Williams, R.J.; Bourke, J.L.; Quigley, A.; McKelvie, P.; Kapsa, R.M.I.; Choong, P.F. Optimising the biocompatibility of 3D printed photopolymer constructs in vitro and in vivo. Biomed. Mater. 2019, 14, 035007. [Google Scholar] [CrossRef]
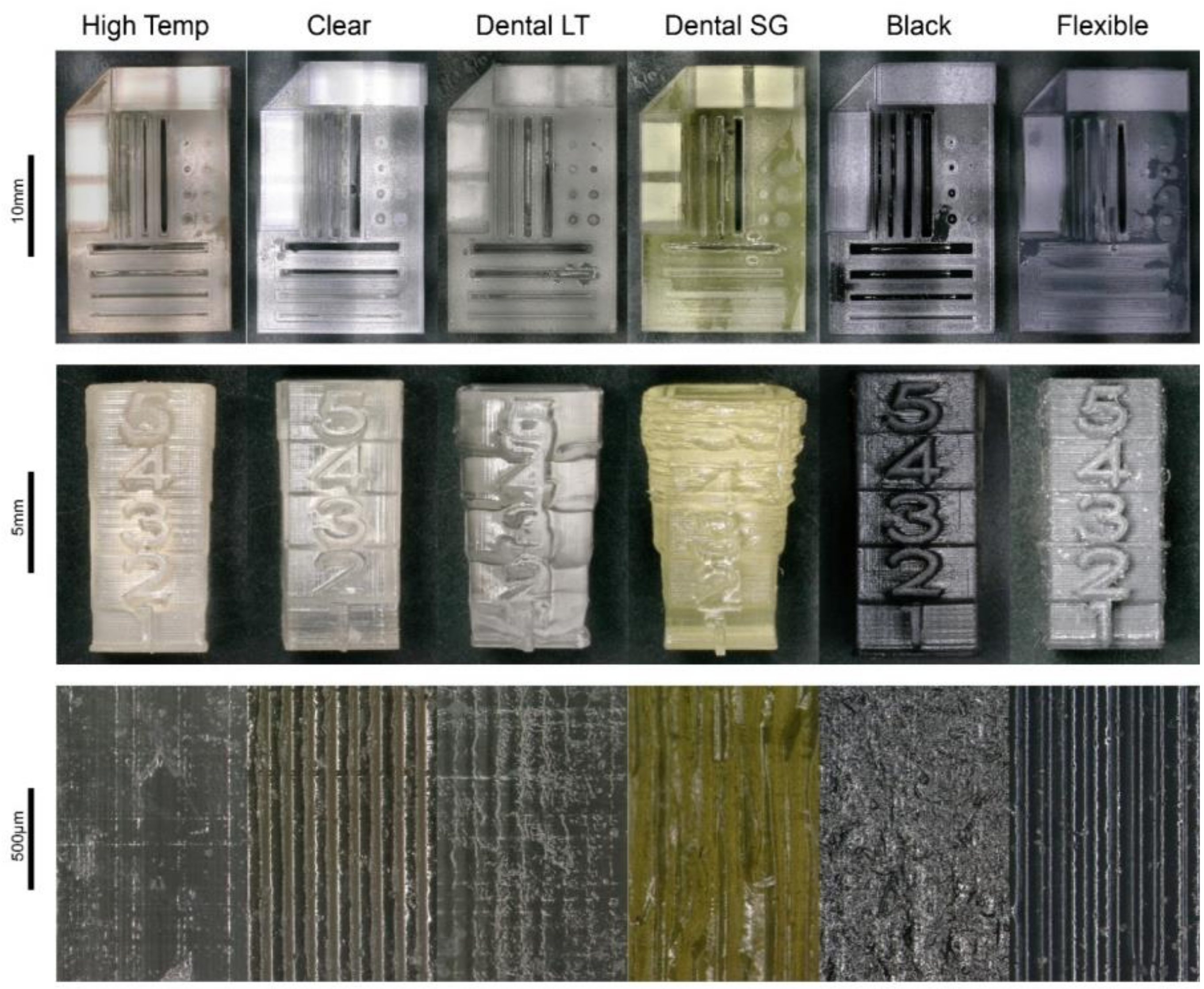

| Photopolymer | Order Number | Properties |
|---|---|---|
| High Temp | FLHTAM02 | Heat resistant up to 238 °C. |
| Clear | FLGPCL04 | Optical transparency, high resolution |
| Dental SG | FLSGAM01 | Class I Medical Device, biocompatible (not cytotoxic, no irritation, no sensitization) according to EN ISO 10993-5:2009 [33], ISO 10993-10:2010/(R)2014 [34]) |
| Dental LT | FLDLCL01 | Biocompatible according to EN-ISO 10993-1:2009/AC:2010 [35] |
| Black | FLGPBK04 | High resolution |
| Flexible | FLFLGR02 | High heat resistance, Vicat softening point of 230 °C |
| Photopolymer | Translucent | Autoclavable | Reproducibility | Level of Detail | Cytotoxicity |
|---|---|---|---|---|---|
| High Temp | Medium | Yes | High | High | High |
| Clear | Yes | No | High | High | Medium |
| Dental SG | No | Yes | Low | Low | Very low |
| Dental LT | Medium | Yes | Medium | Medium | Low |
| Black | No | No | High | High | High |
| Flexible | No | No | Low | Low | High |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kreß, S.; Schaller-Ammann, R.; Feiel, J.; Priedl, J.; Kasper, C.; Egger, D. 3D Printing of Cell Culture Devices: Assessment and Prevention of the Cytotoxicity of Photopolymers for Stereolithography. Materials 2020, 13, 3011. https://doi.org/10.3390/ma13133011
Kreß S, Schaller-Ammann R, Feiel J, Priedl J, Kasper C, Egger D. 3D Printing of Cell Culture Devices: Assessment and Prevention of the Cytotoxicity of Photopolymers for Stereolithography. Materials. 2020; 13(13):3011. https://doi.org/10.3390/ma13133011
Chicago/Turabian StyleKreß, Sebastian, Roland Schaller-Ammann, Jürgen Feiel, Joachim Priedl, Cornelia Kasper, and Dominik Egger. 2020. "3D Printing of Cell Culture Devices: Assessment and Prevention of the Cytotoxicity of Photopolymers for Stereolithography" Materials 13, no. 13: 3011. https://doi.org/10.3390/ma13133011
APA StyleKreß, S., Schaller-Ammann, R., Feiel, J., Priedl, J., Kasper, C., & Egger, D. (2020). 3D Printing of Cell Culture Devices: Assessment and Prevention of the Cytotoxicity of Photopolymers for Stereolithography. Materials, 13(13), 3011. https://doi.org/10.3390/ma13133011







