An Electrically Rechargeable Zinc/Air Cell with an Aqueous Choline Acetate Electrolyte
Abstract
:1. Introduction
| Zinc electrode: Zn + 4OH− → [Zn(OH)4]2− + 2e− | (E0 = −1.26 V) |
| [Zn(OH)4]2− → ZnO + H2O + 2OH− | |
| Air electrode: O2 + 2H2O + 4e− → 4OH− | (E0 = +0.4 V) |
| Overall reaction: 2Zn + O2 → 2ZnO | (E0 = +1.66 V) |
2. Materials and Methods
2.1. Hydrothermal Synthesis of MnCo2O4 (MCO) and NiCo2O4 (NCO)
2.2. Fabrication of Gas Diffusion Electrode (GDE)
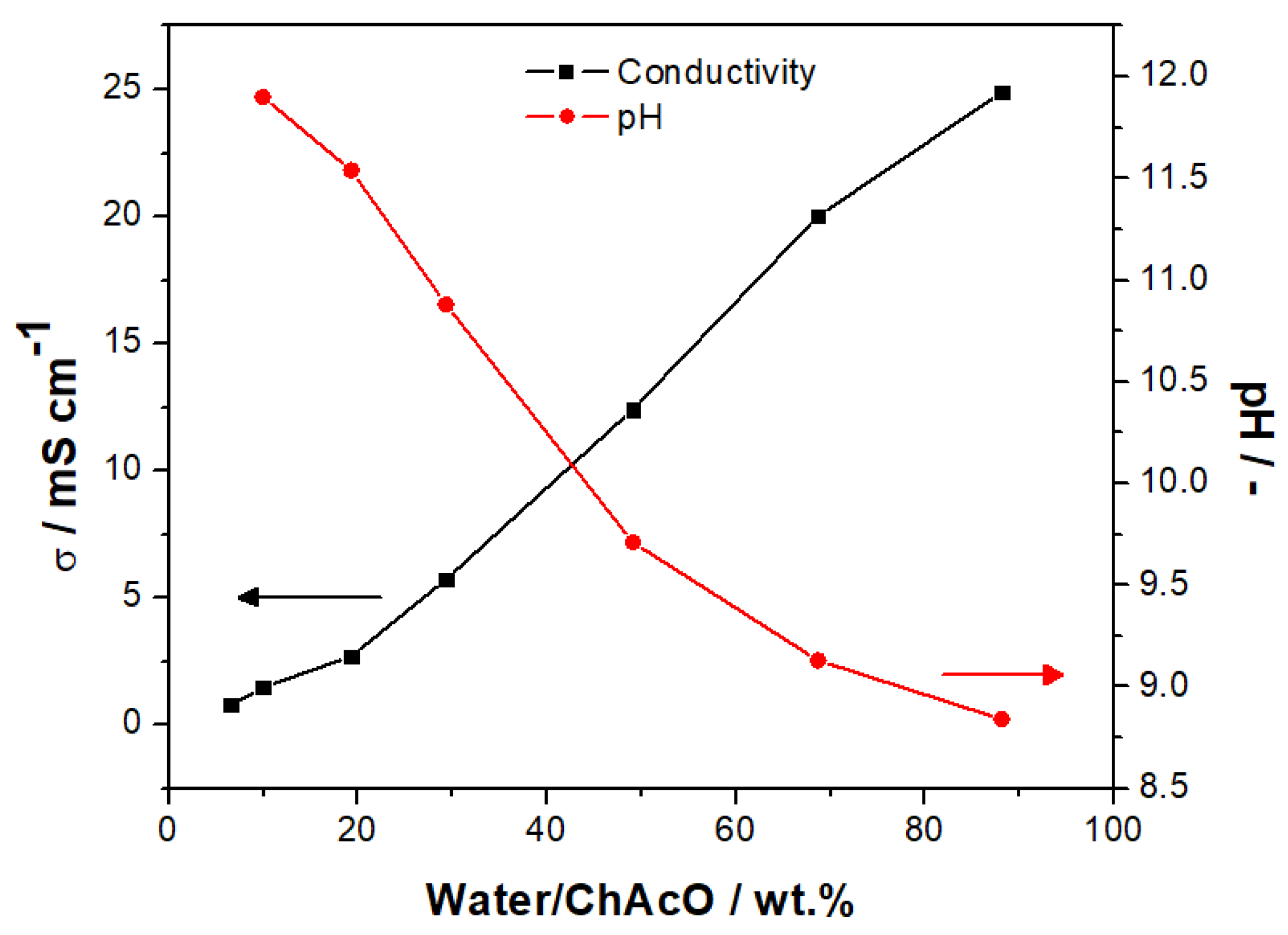
2.3. Preparation and Characterization of Aqueous Ionic Liquid Electrolyte
2.4. Structural Characterization Techniques
2.5. Half-Cell Measurements
2.5.1. Catalyst Activity for ORR/OER Reactions
2.5.2. Zn Redox Reaction
2.6. Full-Cell Measurements
2.6.1. Current-Voltage and Charge/Discharge Characteristics in the El-Cell
2.6.2. Charge/Discharge Characteristic in the Coin Cell
2.7. In-Situ Raman Investigation During Zn/Air Discharge Step in IL
3. Results and Discussion
3.1. Physicochemical Characterization of Catalyst Powder
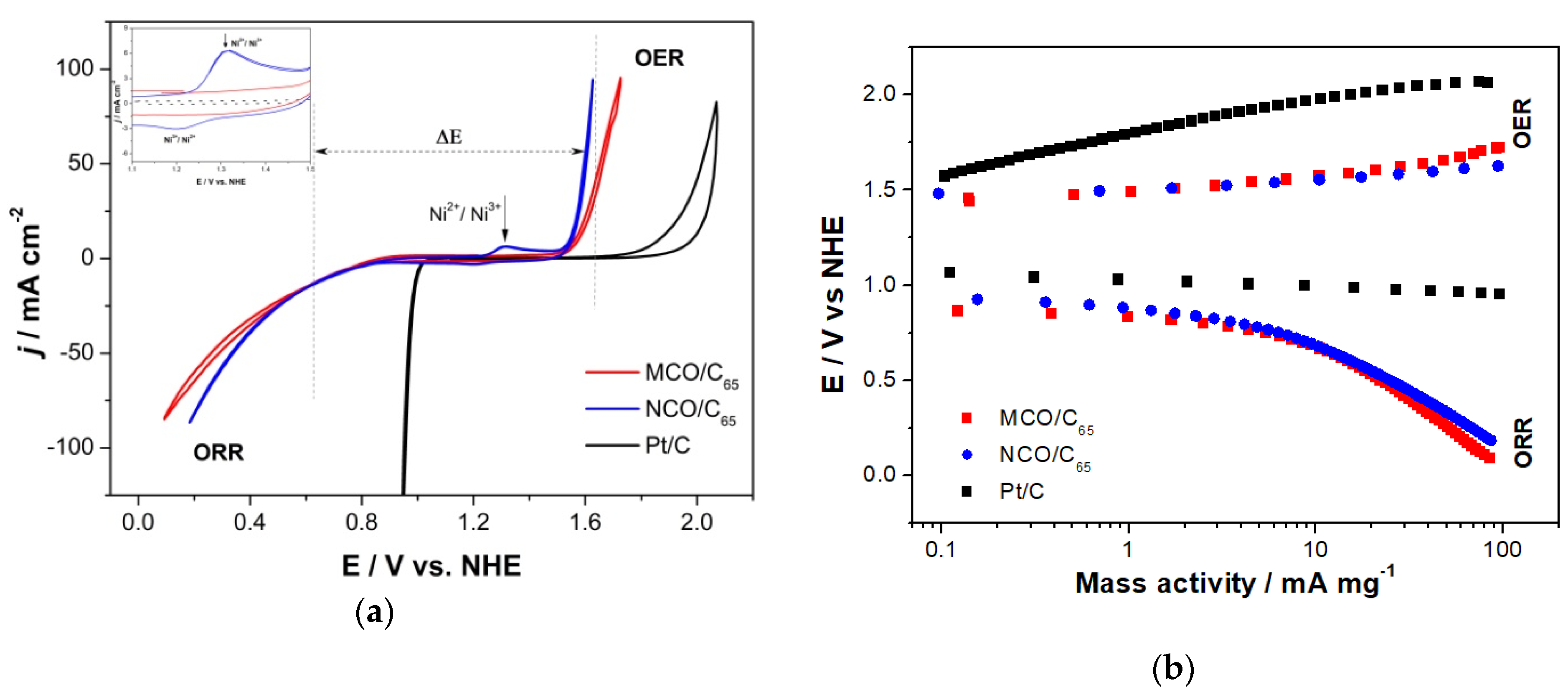
3.2. Half-Cell Investigations
3.2.1. Activity of MCO and NCO Spinel GDEs for ORR/OER in 7 M KOH with Synthetic Dry Air
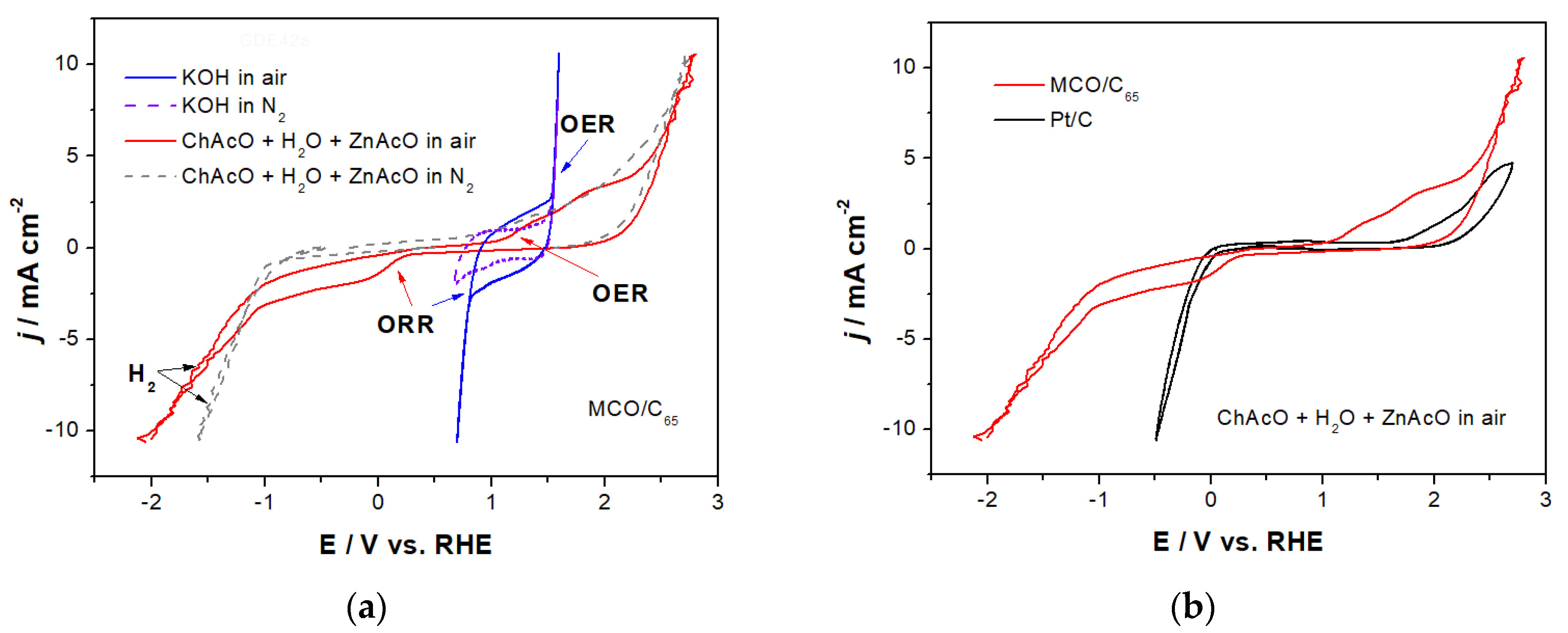
3.2.2. Activity of MCO for OER/ORR in ChAcO and KOH in Dry Air
3.2.3. Long-Term Activity of MCO for OER/ORR in ChAcO in Dry Air
3.2.4. Activity of Redox Zinc Reactions in ChAcO and KOH Electrolytes
3.3. Zn/air Full-Cell Measurements
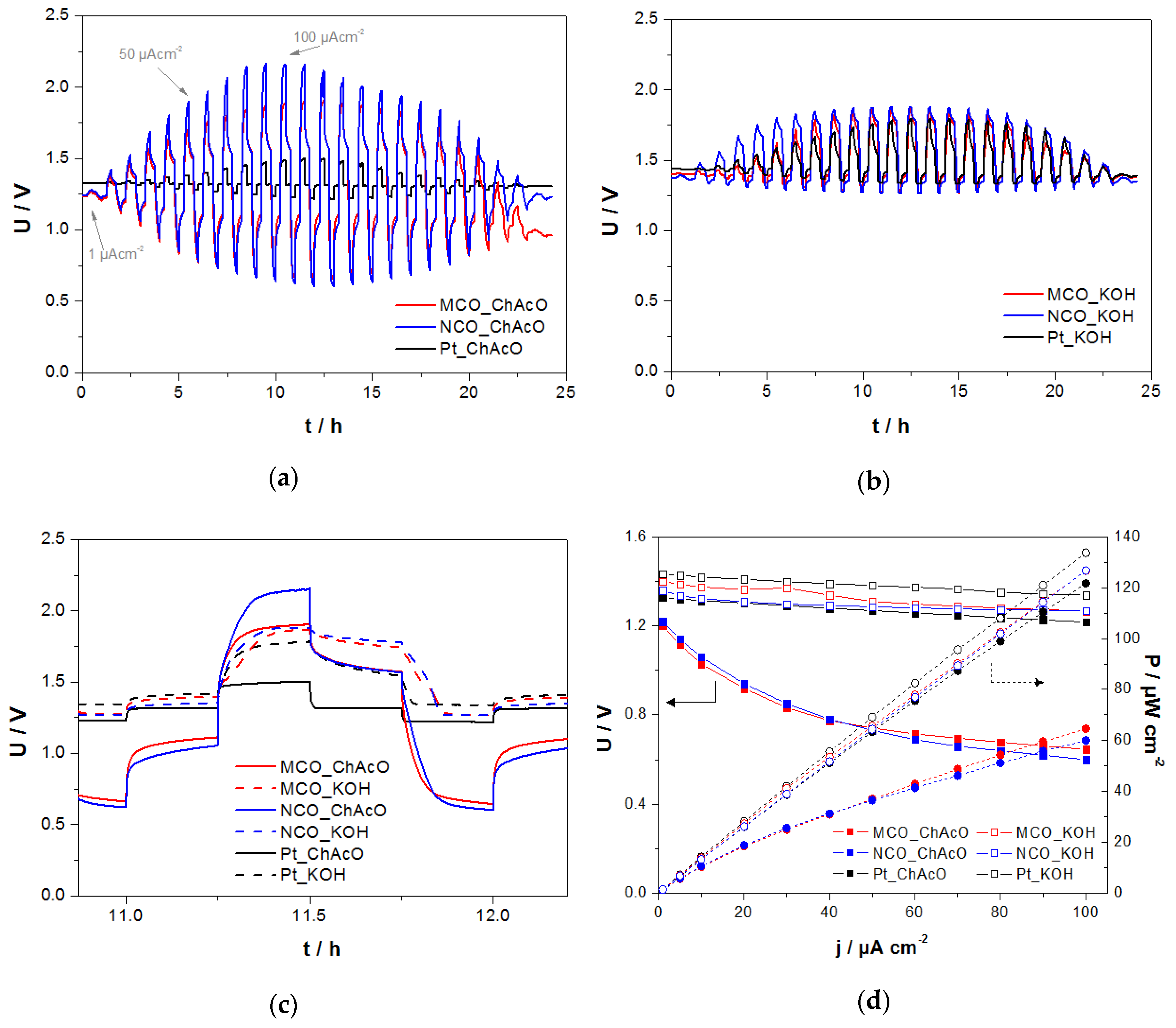
3.3.1. Zn/Air U/I Tests in ChAcO and KOH with Synthetic Dry Air in El-Cell
3.3.2. Zn/Air I-U Tests in ChAcO with Ambient Air in El-Cell
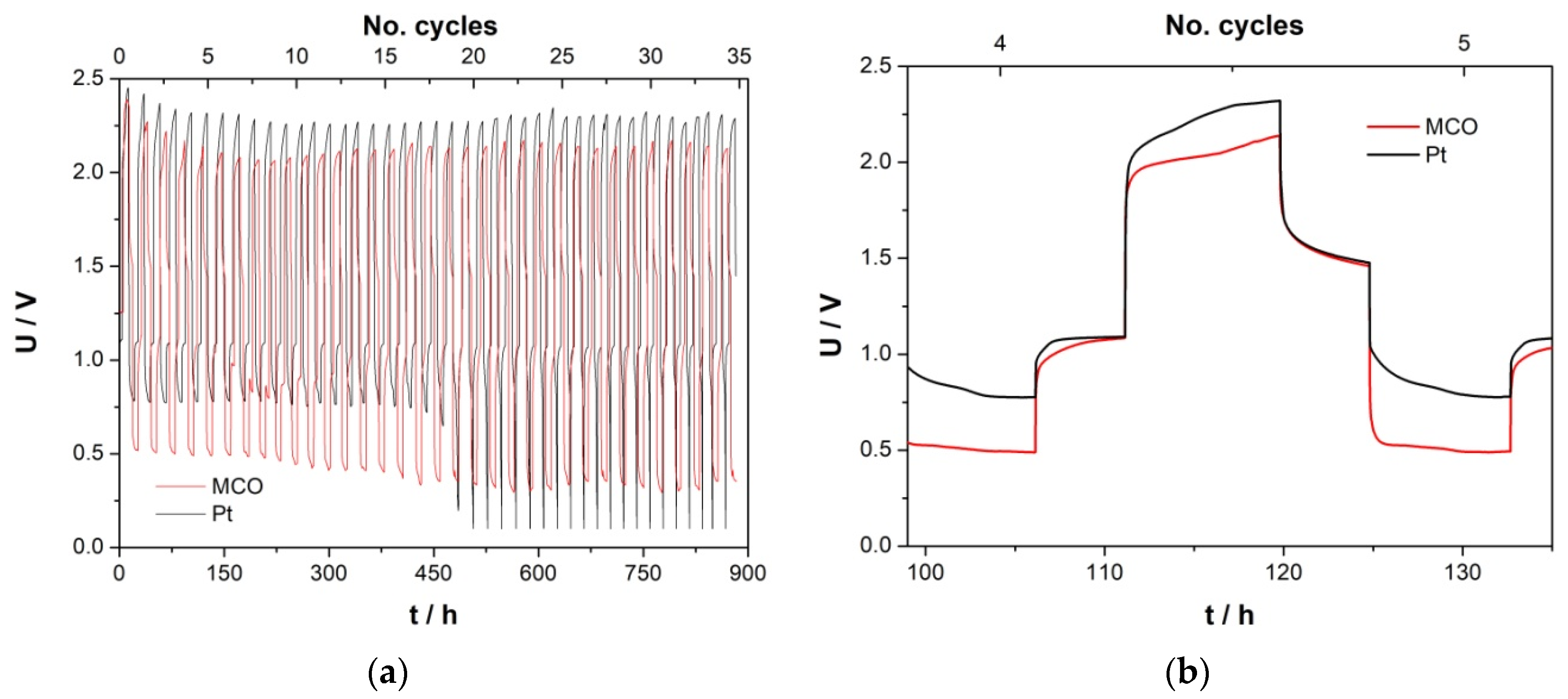
3.3.3. Zn/Air Charge/Discharge Test in ChAcO for 26 h/Cycle in the El-Cell
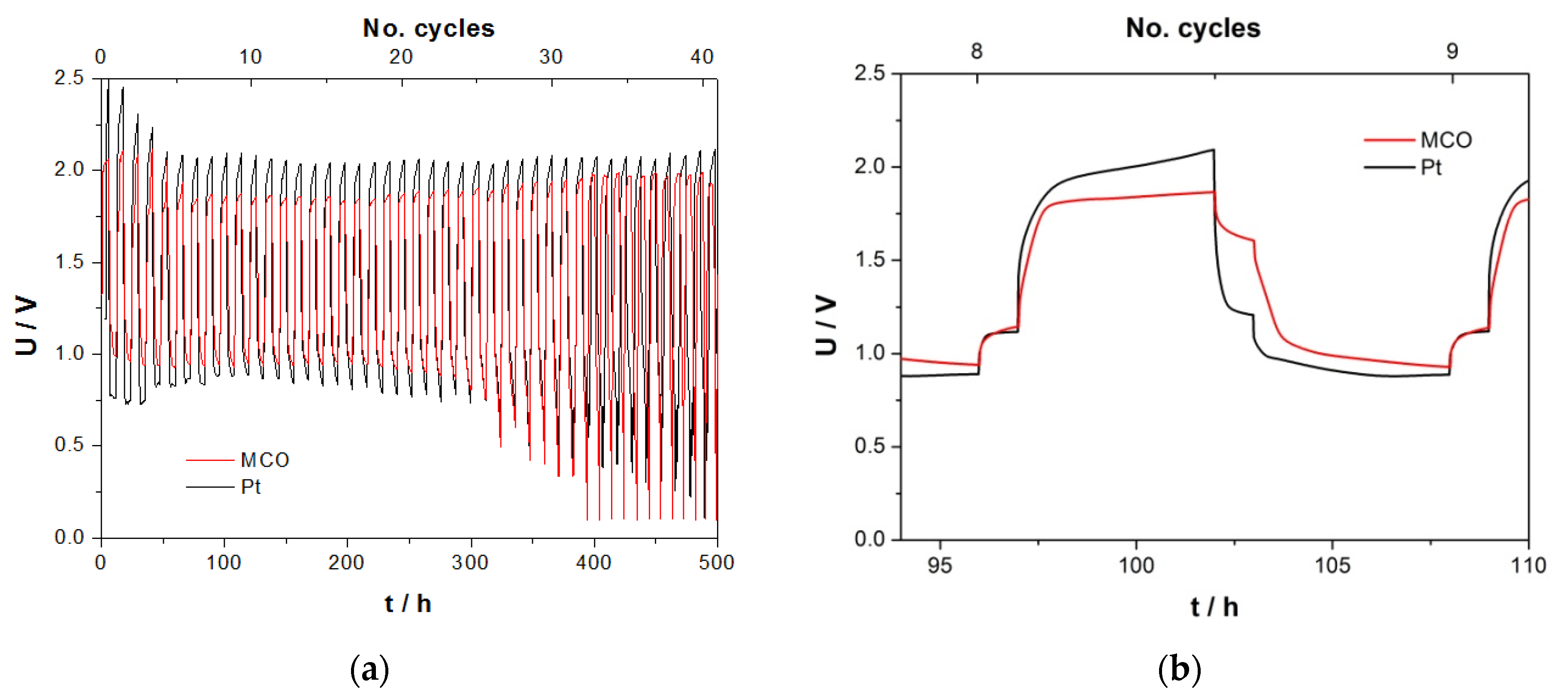
3.3.4. Zinc/Air Charge/Discharge Tests in ChAcO for 10 Days/Cycle in the EL-Cell
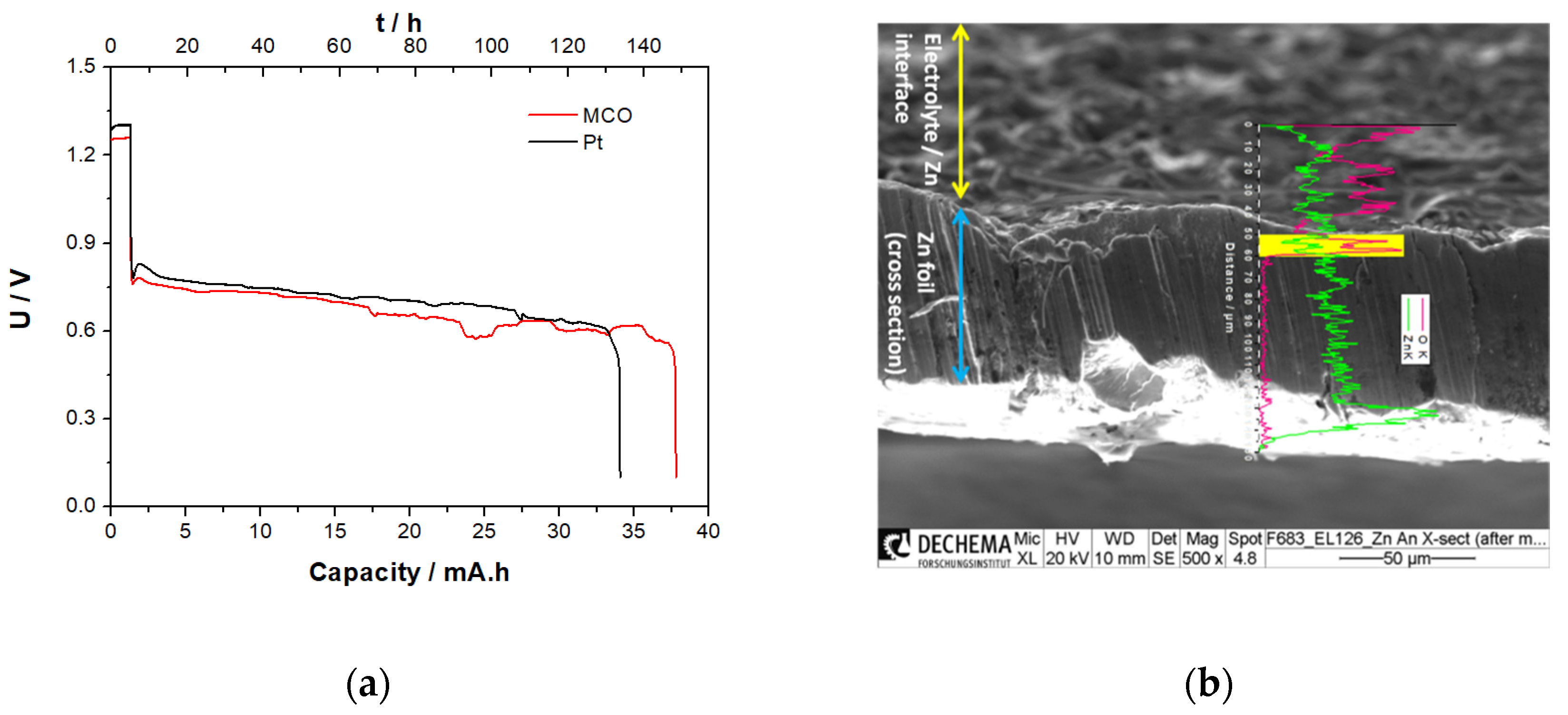
3.3.5. Zn/Air Single Discharge Step in ChAcO for 12 h/Cycle in El-Cell
3.3.6. Zn/Air Charge/Discharge Steps for 12 h/Cycle in CR2032 Coin Cell
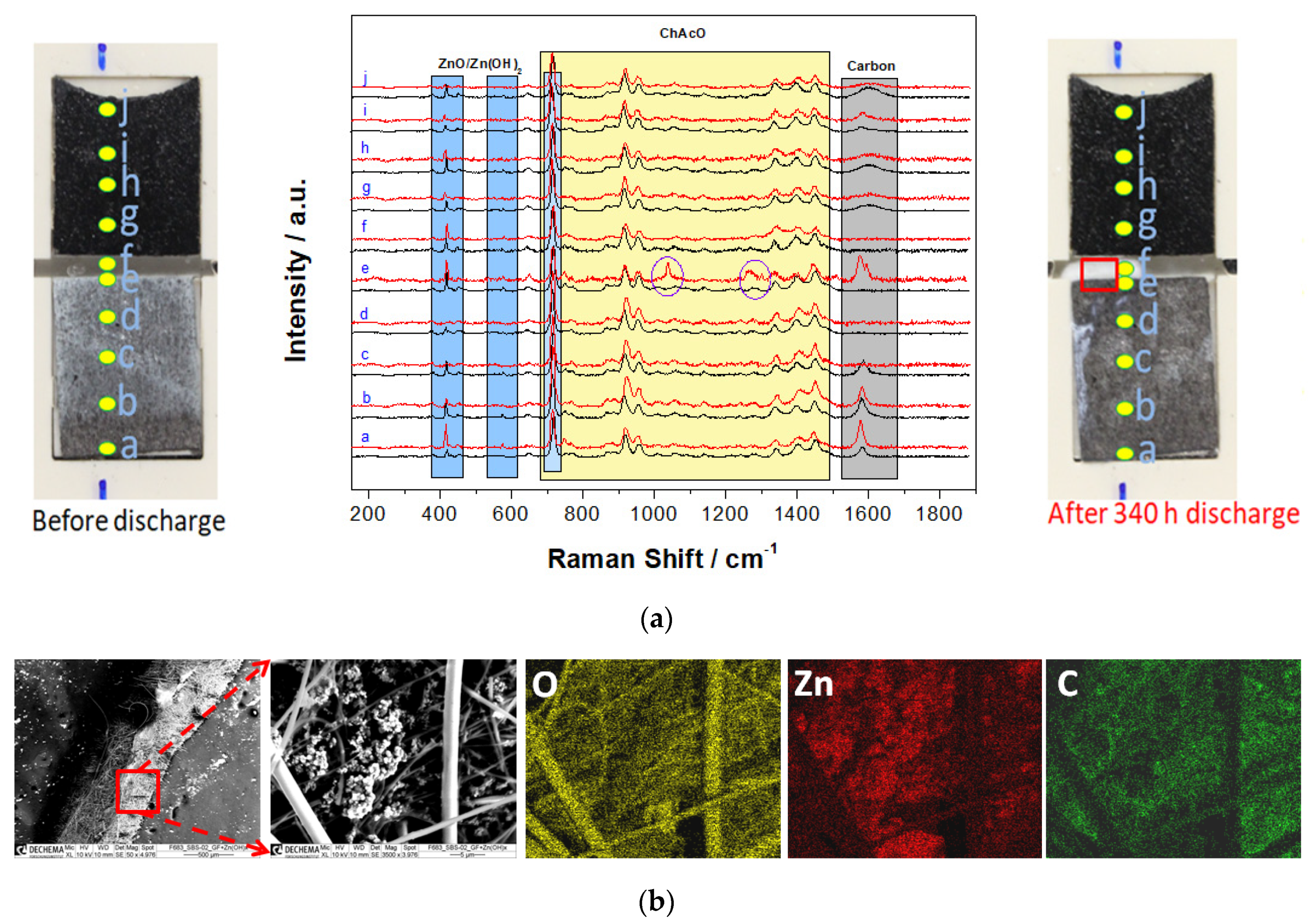
3.4. In-Situ Raman Investigation in the Side-by-Side Zn/Air Cell
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lee, J.S.; Kim, S.T.; Cao, R.; Choi, N.S.; Liu, M.; Lee, K.T.; Cho, J. Metal–air batteries with high energy density: Li–air versus Zn–air. Adv. Energy Mater. 2011, 1, 34–50. [Google Scholar] [CrossRef]
- Rahman, A.; Wang, X.; Wen, C.; Rani, J.V.; Kanakaiah, V.; Dadmal, T.; Bhavanarushi, S.; Rao, M.S. High energy density metal-air batteries: A review. J. Electrochem. Soc. 2013, 160, A1759–A1771. [Google Scholar] [CrossRef]
- Drillet, J.F.; Holzer, F.; Kallis, T.; Müller, S.; Schmidt, V.M. Influence of CO2 on the stability of bifunctional oxygen electrodes for rechargeable zinc/air batteries and study of different CO2 filter materials. Phys. Chem. Chem. Phys. 2001, 3, 368–371. [Google Scholar] [CrossRef]
- Neburchilov, V.; Wang, H.; Martin, J.J.; Qu, W. A review on air cathodes for zinc-air fuel cells. J. Power Sources 2010, 195, 1271–1291. [Google Scholar] [CrossRef]
- Toussaint, G.; Stevens, P.; Akrour, L.; Rouget, R.; Fourgeot, F. Development of a Rechargeable Zinc-Air Battery. ECS Trans. 2010, 28, 25–34. [Google Scholar] [CrossRef]
- Suntivich, J.; May, K.J.; Gasteiger, H.A.; Goodenough, J.B.; Shao-Horn, Y. A perovskite oxide optimized for oxygen evolution catalysis from molecular orbital principles. Science 2011, 334, 1383–1385. [Google Scholar] [CrossRef]
- Bogolowski, N.; Ngaleu, O.; Sakthivel, M.; Drillet, J.-F. Long-life bifunctional BaSrCoFeO3/C gas diffusion electrode. Carbon 2017, 119, 511–518. [Google Scholar] [CrossRef]
- Jörissen, L. Bifunctional oxygen/air electrodes. J. Power Sources 2006, 155, 23–32. [Google Scholar] [CrossRef]
- Pletcher, D.; Li, X.; Price, S.W.T.; Russell, A.E.; Sönmez, T.; Thompson, S.J. Comparison of the SpinelsCo3O4 and NiCo2O4 as Bifunctional Oxygen Catalysts in Alkaline Media. Electrochim. Acta 2016, 188, 286–293. [Google Scholar] [CrossRef] [Green Version]
- Gebremariam, T.T.; Chen, F.; Wang, Q.; Wang, J.; Liu, Y.; Wang, X.; Qaseem, A. Bimetallic Mn–Co Oxide Nanoparticles Anchored on Carbon Nanofibers Wrapped in Nitrogen-Doped Carbon for Application in Zn–Air Batteries and Supercapacitors. ACS Appl. Energy Mater. 2018, 1, 1612–1625. [Google Scholar] [CrossRef]
- Prabu, M.; Ramakrishnan, P.; Shanmugam, S. CoMn2O4 nanoparticles anchored on nitrogen-doped graphene nanosheets as bifunctional electrocatalyst for rechargeable zinc–air battery. Electrochem. Commun. 2014, 41, 59–63. [Google Scholar] [CrossRef]
- Davari, E.; Johnson, A.D.; Mittal, A.; Xiong, M.; Ivey, D.G. Manganese-cobalt mixed oxide film as a bifunctional catalyst for rechargeable zinc-air batteries. Electrochim. Acta 2016, 211, 735–743. [Google Scholar] [CrossRef]
- Wang, Z.; Xiao, S.; An, Y.; Long, X.; Zheng, X.; Lu, X.; Tong, Y.; Yang, S. Co(II)1-xCo(0)x/3Mn(III)2x/3S Nanoparticles Supported on B/N-Codoped Mesoporous Nanocarbon as a Bifunctional Electrocatalyst of Oxygen Reduction/Evolution for High-Performance Zinc-Air Batteries. ACS Appl. Mater. Interfaces 2016, 8, 13348–13359. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhao, Z.; Xia, Z.; Dai, L. A metal-free bifunctional electrocatalyst for oxygen reduction and oxygen evolution reactions. Nat. Nanotechnol. 2015, 10, 444–452. [Google Scholar] [CrossRef]
- Zhan, J.; Cai, M.; Zhang, C.; Wang, C. Synthesis of mesoporous NiCo2O4 fibers and their electrocatalytic activity on direct oxidation of ethanol in alkaline media. Electrochim. Acta 2015, 154, 70–76. [Google Scholar] [CrossRef]
- Ma, C.Y.; Xu, N.N.; Qiao, J.L.; Jian, S.S.; Zhang, J.J. Facile synthesis of NiCo2O4 nanosphere-carbon nanotubes hybrid as an efficient bifunctional electrocatalyst for rechargeable Zn–air batteries. Int. J. Hydrogen Energy 2016, 41, 9211–9218. [Google Scholar] [CrossRef]
- Geng, D.S.; Ding, N.N.; Hor, T.S.A.; Chien, S.W.; Liu, Z.L.; Zong, Y. Cobalt sulfide nanoparticles impregnated nitrogen and sulfur co-doped graphene as bifunctional catalyst for rechargeable Zn-air batteries. RSC Adv. 2015, 5, 7280–7284. [Google Scholar] [CrossRef]
- Ishihara, T.; Ishihara, T.; Yokoe, K.; Miyano, T.; Kusaba, H. Mesoporous MnCo2O4 spinel oxide for a highly active and stable air electrode for Zn-air rechargeable battery. Electrochim. Acta 2019, 300, 455–460. [Google Scholar] [CrossRef]
- Wang, W.; Kuai, L.; Cao, W.; Huttula, M.; Ollikkala, S.; Ahopelto, T.; Honkanen, A.P.; Huotari, S.; Yu, M.; Geng, B. Mass-Production of Mesoporous MnCo2O4 Spinels with Manganese (IV)- and Cobalt (II)-Rich Surfaces for Superior Bifunctional Oxygen Electrocatalysis. Angew. Chem. Int. Ed. 2017, 56, 14977–14981. [Google Scholar] [CrossRef] [Green Version]
- Walsh, D.A.; Ejigu, A.; Muhammad, S.; Licence, P. The formation and role of oxide layers on Pt during hydrazine oxidation in protic ionic liquids. ChemElectroChem 2014, 1, 281–288. [Google Scholar] [CrossRef]
- Endres, F.; Zein El Abedin, S. Air and water stable ionic liquids in physical chemistry. Phys. Chem. Chem. Phys. 2006, 8, 2101–2116. [Google Scholar] [CrossRef] [PubMed]
- Haerens, K.; Matthijs, E.; Chmielarz, A.; Bruggen, B.V.D. The use of ionic liquids based on choline chloride for metaldeposition: A green alternative? J. Environ. Manag. 2009, 90, 3245–3252. [Google Scholar] [CrossRef] [PubMed]
- Mainar, A.R.; Iruin, E.; Colmenares, L.C.; Kvasha, A.; Meatza, I.; Bengoechea, M.; Leonet, O.; Boyano, I.; Zhang, Z.; Blazquez, J.A. An overview of progress in electrolytes for secondary zinc-air batteries and other storage systems based on zinc. J. Energy Storage 2018, 15, 304–328. [Google Scholar] [CrossRef]
- Ingale, P.; Sakthivel, M.; Drillet, J.-F. Test of Diethylmethylammonium Trifluoromethanesulfonate Ionic Liquid as Electrolyte in Electrically Rechargeable Zn/Air Battery. J. Electrochem. Soc. 2017, 164, H5224–H5229. [Google Scholar] [CrossRef]
- Ge, X.; Liu, Y.; Goh, F.T.; Hor, T.A.; Zong, Y.; Xiao, P.; Zhang, Z.; Lim, S.H.; Li, B.; Wang, X.; et al. Dual-Phase Spinel MnCo2O4 and Spinel MnCo2O4/Nanocarbon Hybrids for Electrocatalytic Oxygen Reduction and Evolution. ACS Appl. Mater. Interfaces 2014, 6, 12684–12691. [Google Scholar] [CrossRef]
- Liu, W.; Bao, J.; Xu, L.; Guan, M.; Wang, Z.; Qiu, J.; Huang, Y.; Xia, J.; Lei, Y.; Li, H. NiCo2O4 ultrathin nanosheets with oxygen vacancies as bifunctional electrocatalysts for Zn-air battery. Appl. Surf. Sci. 2019, 478, 552–559. [Google Scholar] [CrossRef]
- Vreese, P.D.; Skoczylas, A.; Matthijs, E.; Fransaer, J.; Binnemans, K. Electrodeposition of copper–zinc alloys from an ionic liquid-like choline acetate electrolyte. Electrochim. Acta 2013, 108, 788–794. [Google Scholar] [CrossRef]
- Liu, Z.; Pulletikurthi, G.; Endres, F. A Prussian Blue/Zinc Secondary Battery with a Bio-Ionic Liquid–Water Mixture as Electrolyte. ACS Appl. Mater. Interfaces 2016, 8, 12158–12164. [Google Scholar] [CrossRef]
- Kaur, A.; Bansal, S.; Chauhan, D.; Bhasin, K.K.; Chaudhary, G.R. The study of molecular interactions of aqueous solutions of Choline Acetate at different temperatures. J. Mol. Liq. 2019, 286, 110878–110884. [Google Scholar] [CrossRef]
- Yang, X.; Yu, X.; Yang, Q.; Zhao, D.; Zhang, K.; Yao, J.; Li, G.; Zhou, H.; Zuo, X. Controllable synthesis and magnetic properties of hydrothermally synthesized NiCo2O4 nano-sphere. Ceram. Int. 2017, 43, 8585–8589. [Google Scholar] [CrossRef]
- Baji, D.S.; Jadhav, H.S.; Nair, S.V.; Rai, A.K. Porous MnCo2O4 as superior anode material over MnCo2O4 nanoparticles for rechargeable lithium ion batteries. J. Solid State Chem. 2018, 262, 191–198. [Google Scholar] [CrossRef]
- Vadivel, S.; Balaji, G.; Rathinavel, S. High performance ethanol and acetone gas sensor based nanocrystalline MnCo2O4 using clad-modified fiber optic gas sensor. Opt. Mater. 2018, 85, 267–274. [Google Scholar] [CrossRef]
- Ding, R.; Qi, L.; Jia, M.; Wang, H. Simple hydrothermal synthesis of mesoporous spinel NiCo2O4 nanoparticles and their catalytic behavior in CH3OH electro-oxidation and H2O2 electro-reduction. Catal. Sci. Technol. 2013, 3, 3207–3215. [Google Scholar] [CrossRef]
- Mehrez, J.A.A.; Owusu, K.A.; Chen, Q.; Li, L.; Hamwi, K.; Luo, W.; Mai, L. Hierarchical MnCo2O4@NiMoO4 as free-standing core–shell nanowire arrays with synergistic effect for enhanced supercapacitor performance. Inorg. Chem. Front. 2019, 6, 857–865. [Google Scholar] [CrossRef]
- Karkera, G.; Chandrappa, S.G.; Prakash, A.S. Viable Synthesis of Porous MnCo2O4/Graphene Composite by Sonochemical Grafting: A High-Rate-Capable Oxygen Cathode for Li–O2 Batteries. Chem. Eur. J. 2018, 24, 17303–17310. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Jiang, L. Wettability by ionic liquids. Small 2016, 12, 9–15. [Google Scholar] [CrossRef]
- Michael, M.S.; Kawamura, J.; Kuwata, N.; Prabaharan, S.R.S. Nanoengineered Lithium–Air Secondary Batteries: Fundamental Understanding and the Current Status of Development; Prabaharan, S.R.S., Michael, M.S., Eds.; Taylor & Francis: New York, NY, USA, 2013; pp. 89–103. [Google Scholar]
- Sakthivel, M.; Bhandari, S.; Drillet, J.F. On Activity and Stability of Rhombohedral LaNiO3 Catalyst towards ORR and OER in Alkaline Electrolyte. ECS Electrochem. Lett. 2015, 4, A56–A58. [Google Scholar] [CrossRef]
- Wang, L.; Gu, C.; Ge, X.; Zhang, J.; Zhu, H.; Tu, J. Highly Efficient Bifunctional Catalyst of NiCo2O4@NiO@Ni Core/Shell Nanocone Array for Stable Overall Water Splitting. Part. Part. Syst. Charact. 2017, 34, 1700228–1700244. [Google Scholar] [CrossRef]
- Xiao, C.; Li, Y.; Lu, X.; Zhao, C. Bifunctional Porous NiFe/NiCo2O4/Ni Foam Electrodes with Triple Hierarchy and Double Synergies for Efficient Whole Cell Water Splitting. Adv. Funct. Mater. 2016, 26, 3515–3523. [Google Scholar] [CrossRef]
- Song, M.K.; Park, S.; Alamgir, F.M.; Choand, J.; Liu, M. Nanostructured electrodes for lithium-ion and lithium-air batteries: The latest developments, challenges, and perspectives. Mater. Sci. Eng. R 2011, 72, 203–252. [Google Scholar] [CrossRef]
- Liu, Z.; Zein El Abedin, S.; Endres, F. Electrodeposition of zinc films from ionic liquids and ionic liquid/water mixtures. Electrochim. Acta 2013, 89, 635–643. [Google Scholar] [CrossRef]
- Cai, W.-B.; Shi, Q.; Mansuetto, M.F.; Scherson, D.A. In situ Raman spectroscopy on an operating AA Zn-MnO2 battery under high discharge currents. Electrochem. Solid-State Lett. 2000, 3, 319–320. [Google Scholar] [CrossRef]
- Ferraro, J.R. Low Frequency Vibration of Inorganic and Coordination Compounds; Springer: New York, NY, USA, 1971. [Google Scholar]
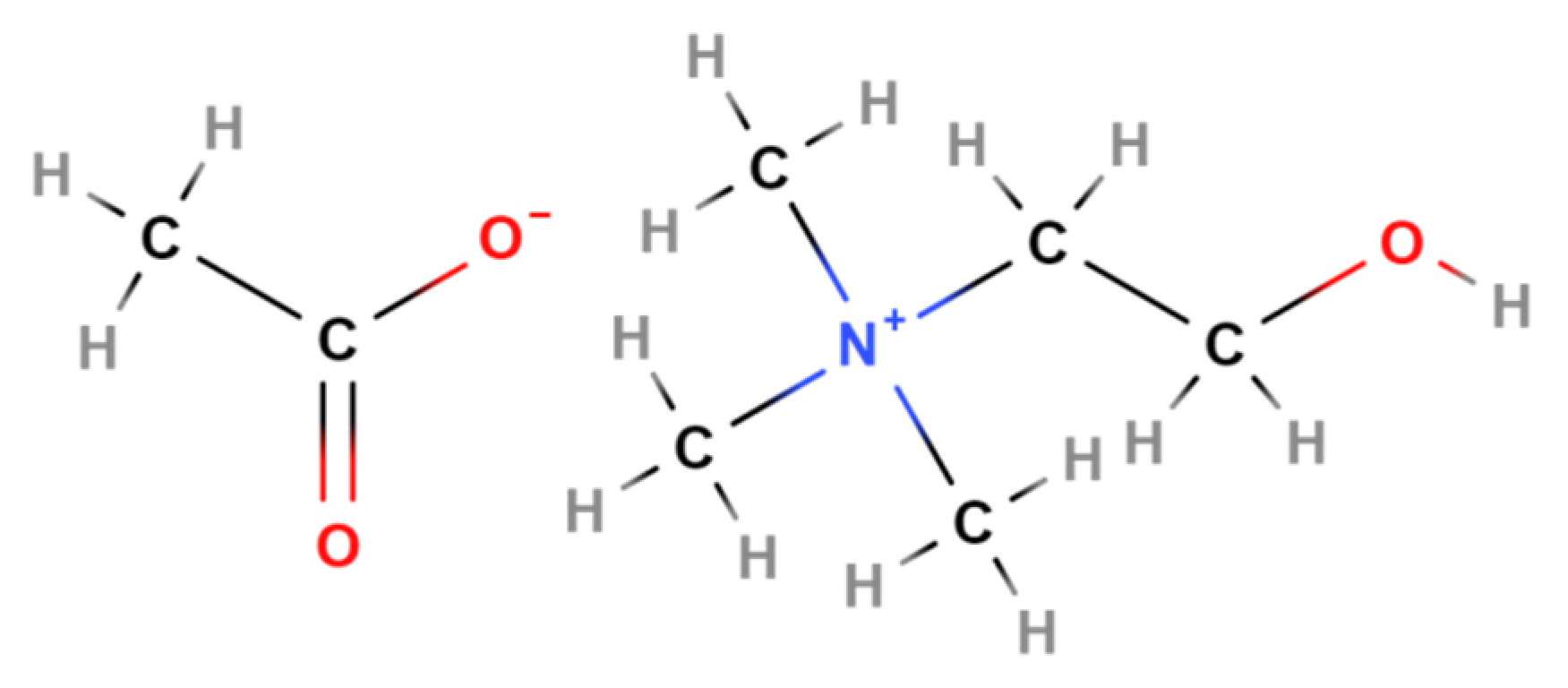
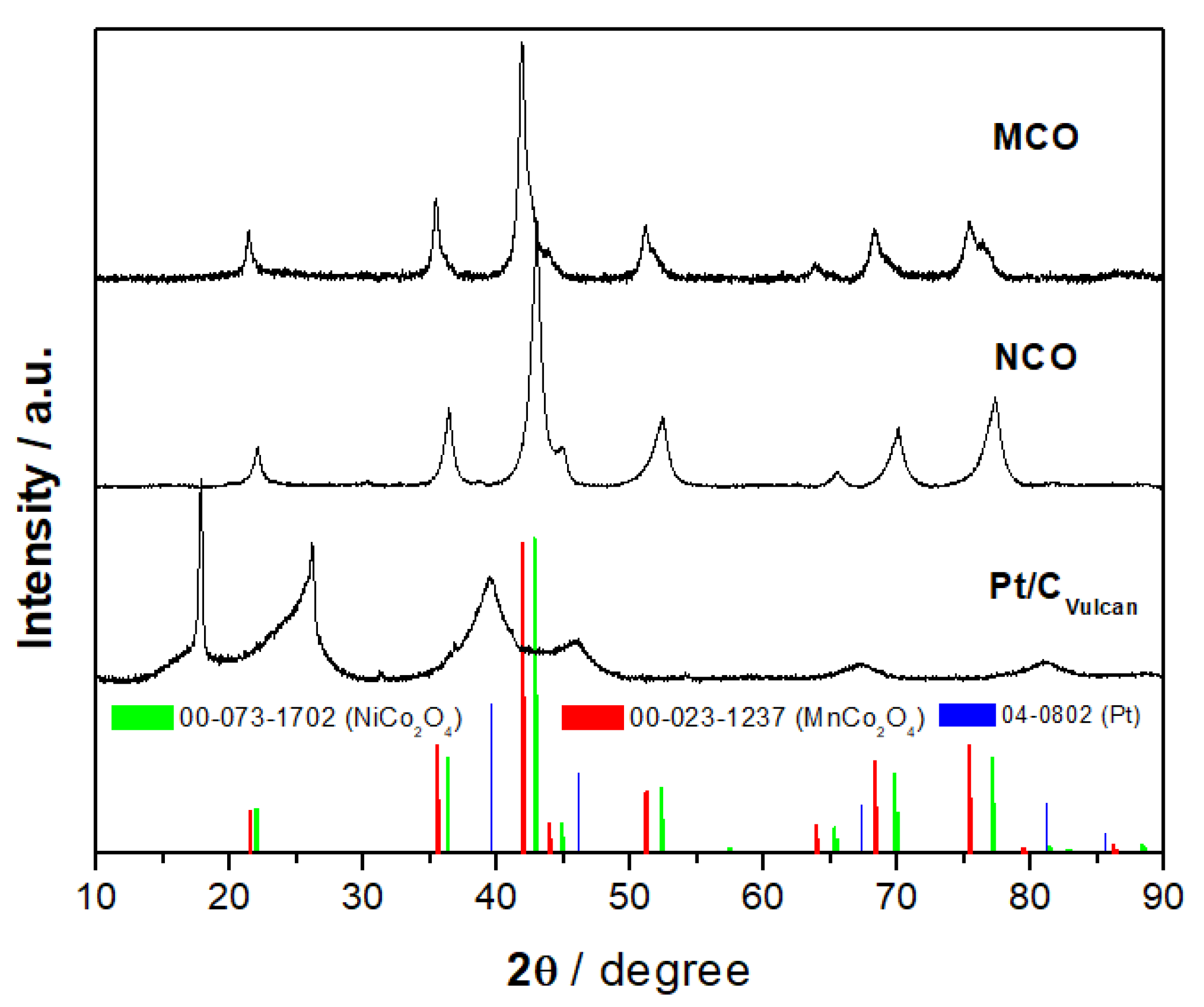
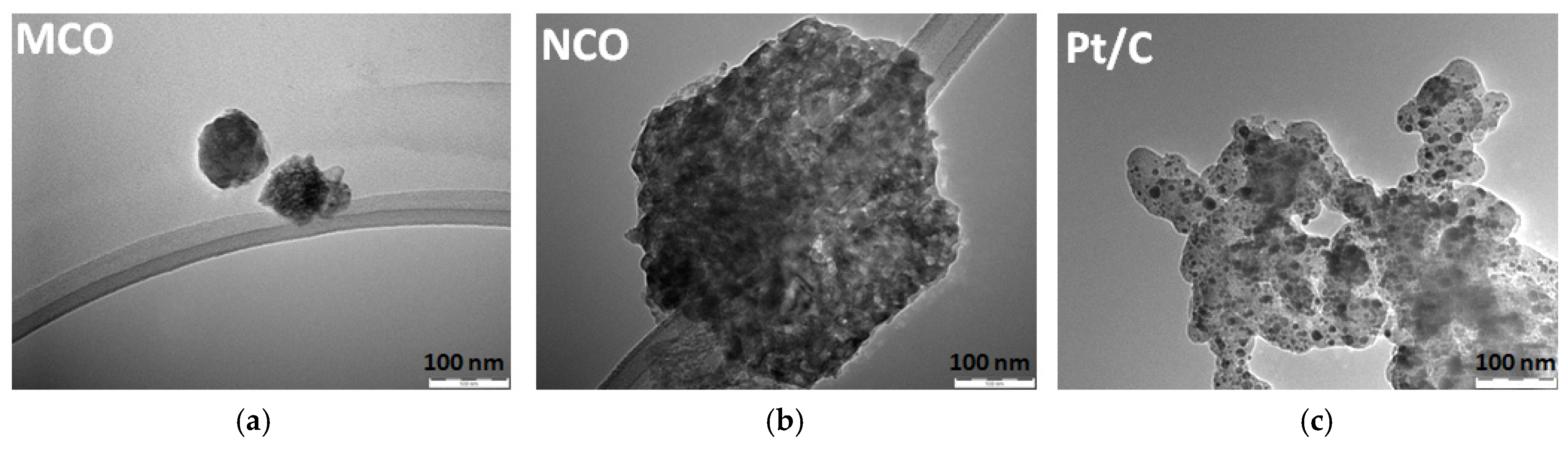

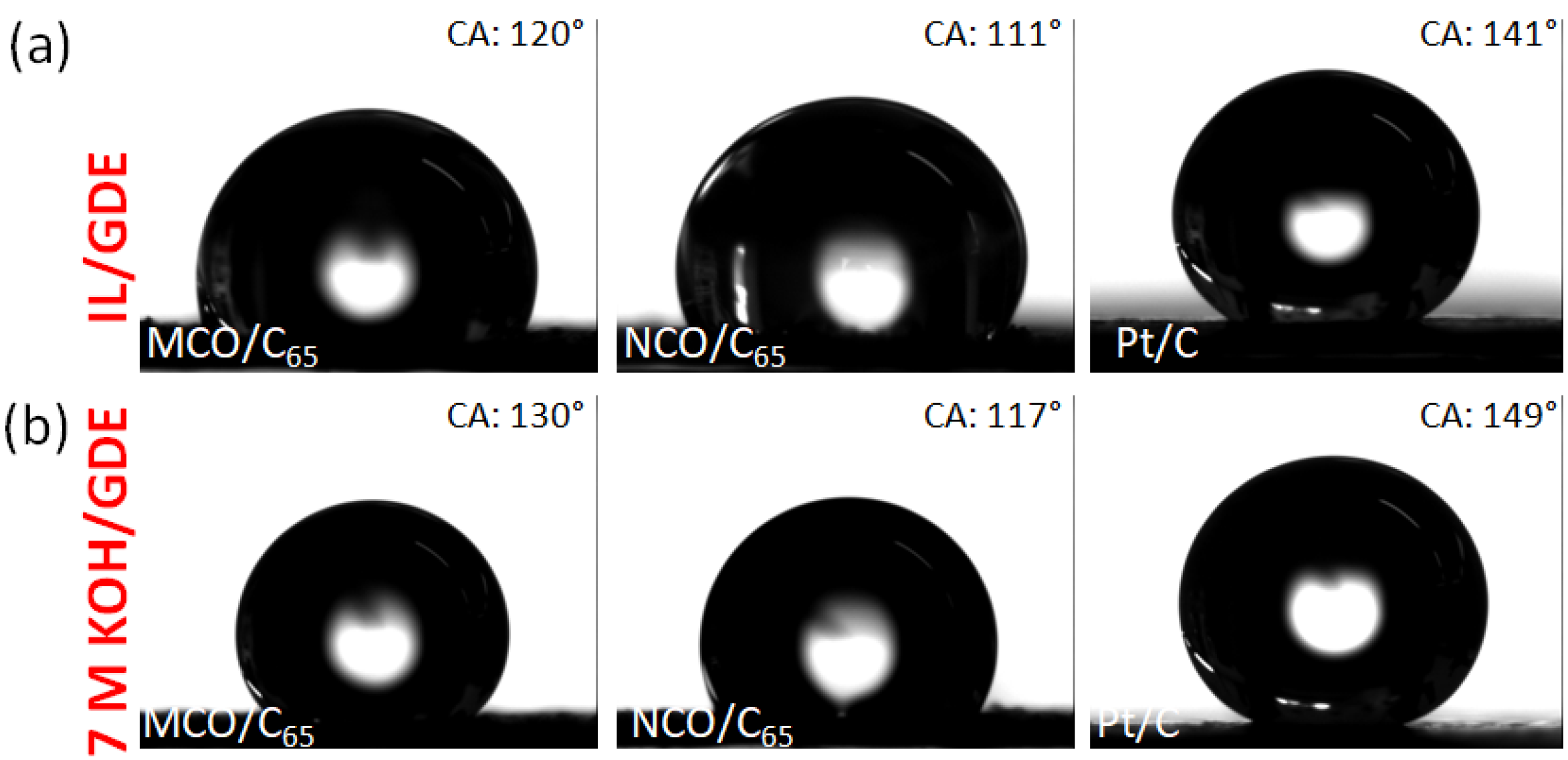





| Catalyst | BET m2 g−1 | Pore Size + nm | D # nm | CA * ° | CA ** ° |
|---|---|---|---|---|---|
| MCO/C65 | 126 | 17 | 26 | 120 | 130 |
| NCO/C65 | 108 | 3.8 | 100 | 111 | 117 |
| Pt/C | 55 | 36 | 10 | 141 | 149 |
| Catalyst | MAORR at 0.6 V mA mg−2 | MAOER at 1.65 V mA mg−2 | TafelORR mV dec−1 | TafelOER mV dec−1 | ΔE at 10 mA cm−2 V |
|---|---|---|---|---|---|
| MCO/C65 | 15 | 47 | 70–185 # | 77–113 # | 0.9 |
| NCO/C65 | 16 | 74 | 84–186 # | 30–70 # | 0.86 |
| Pt/C | 98 * | 1.5 | 60–150 + | 178–330 + | 0.86 |
| GDE Catalyst Electrolyte | MCO IL | MCO KOH | Pt IL | Pt KOH |
|---|---|---|---|---|
| OPOER/mV # | 1200 | 1500 | 1725 | 1800 |
| OPORR/mV # | 200 | 750 | 35 | 1000 |
| ΔE/mV | 1000 | 750 | 1690 | 800 |
| Design | El-Cell | Coin Cell | ||||
|---|---|---|---|---|---|---|
| Current/µA | 254 | 200 | ||||
| Time per cycle/h | 26 | 240 | 12 | |||
| Rev. capacity/mAh | 2.03 | 25.40 | 1.01 | |||
| DOD/% | 2.2 | 28.0 | 1.4 | |||
| Air catalyst | Pt | MCO | Pt | MCO | Pt | MCO |
| Cycles number/ *- | 1–17 | 1–33 | 1–6 | 1–7 | 1–30 | 1–33 |
| Duration/h * | 440 | 850 | 1300 | 1500 | 360 | 396 |
| Coulombic efficiency/% * | 100 | 100 | 100 | 100 | 100 | 100 |
| Energy efficiency/%* | 28–35 | 15–24 | 37–51 | 29–54 | 39–48 | 33–56 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sakthivel, M.; Batchu, S.P.; Shah, A.A.; Kim, K.; Peters, W.; Drillet, J.-F. An Electrically Rechargeable Zinc/Air Cell with an Aqueous Choline Acetate Electrolyte. Materials 2020, 13, 2975. https://doi.org/10.3390/ma13132975
Sakthivel M, Batchu SP, Shah AA, Kim K, Peters W, Drillet J-F. An Electrically Rechargeable Zinc/Air Cell with an Aqueous Choline Acetate Electrolyte. Materials. 2020; 13(13):2975. https://doi.org/10.3390/ma13132975
Chicago/Turabian StyleSakthivel, Mariappan, Sai Praneet Batchu, Abbas Ali Shah, Kwangmin Kim, Willi Peters, and Jean-Francois Drillet. 2020. "An Electrically Rechargeable Zinc/Air Cell with an Aqueous Choline Acetate Electrolyte" Materials 13, no. 13: 2975. https://doi.org/10.3390/ma13132975
APA StyleSakthivel, M., Batchu, S. P., Shah, A. A., Kim, K., Peters, W., & Drillet, J.-F. (2020). An Electrically Rechargeable Zinc/Air Cell with an Aqueous Choline Acetate Electrolyte. Materials, 13(13), 2975. https://doi.org/10.3390/ma13132975






