Investigation on Fabrication of Reduced Graphene Oxide-Sulfur Composite Cathodes for Li-S Battery via Hydrothermal and Thermal Reduction Methods
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of Graphene Oxide (GO)
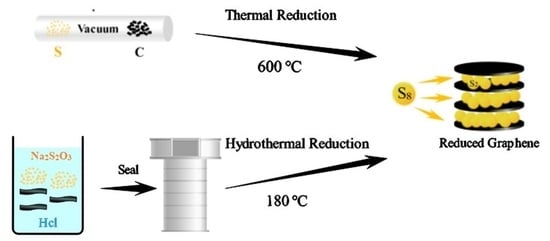
2.2. Synthesis of rGO-S Composites by One Step Hydrothermal Method (rGO-S-HT)
2.3. Synthesis of rGO-S Composites by In-Situ Thermal Reduction Method (rGO-S-T)
2.4. Characterizations
2.5. Electrode Preparation and Electrochemical Measurements
3. Results and Discussion
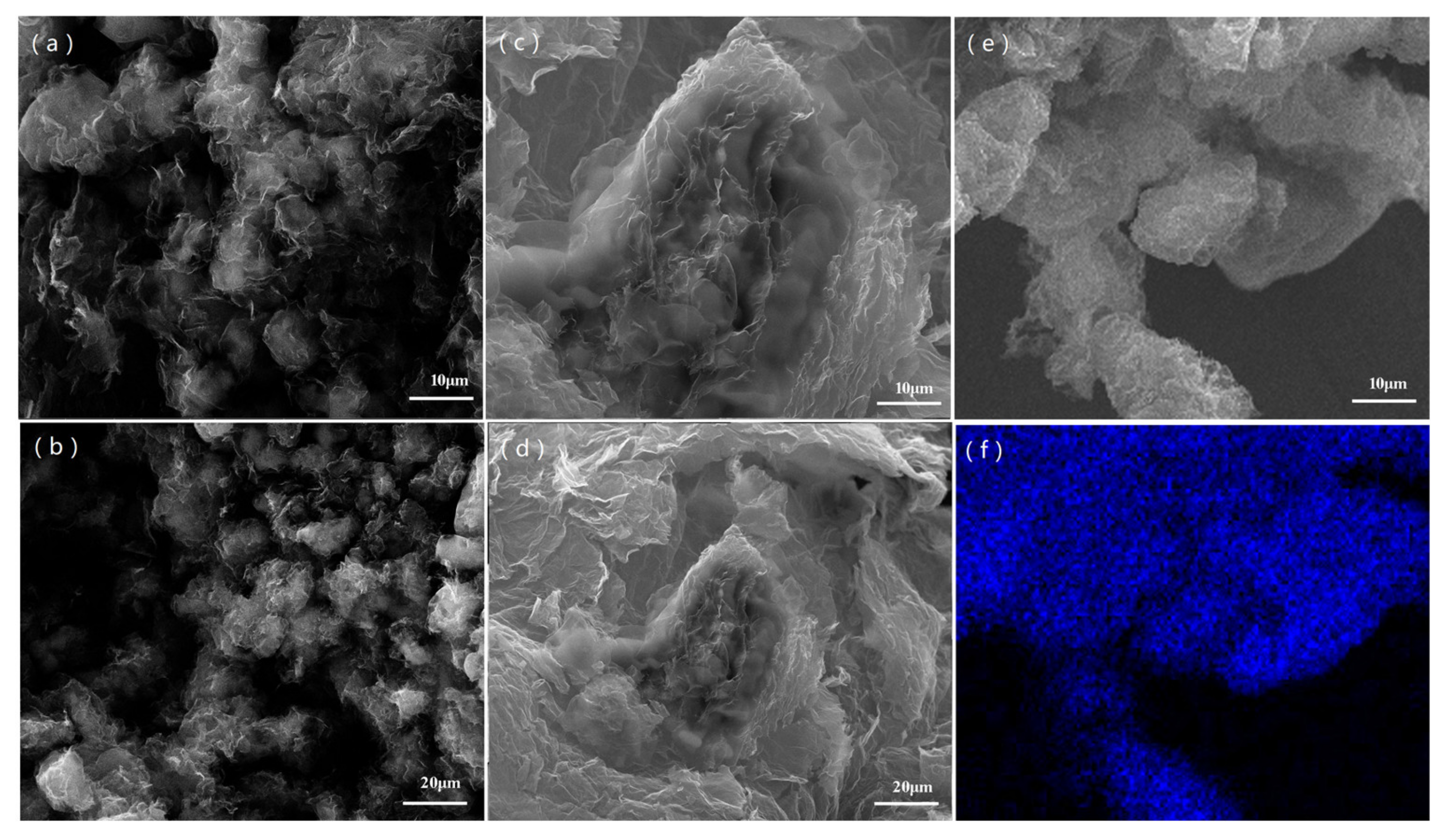
3.1. Morphology
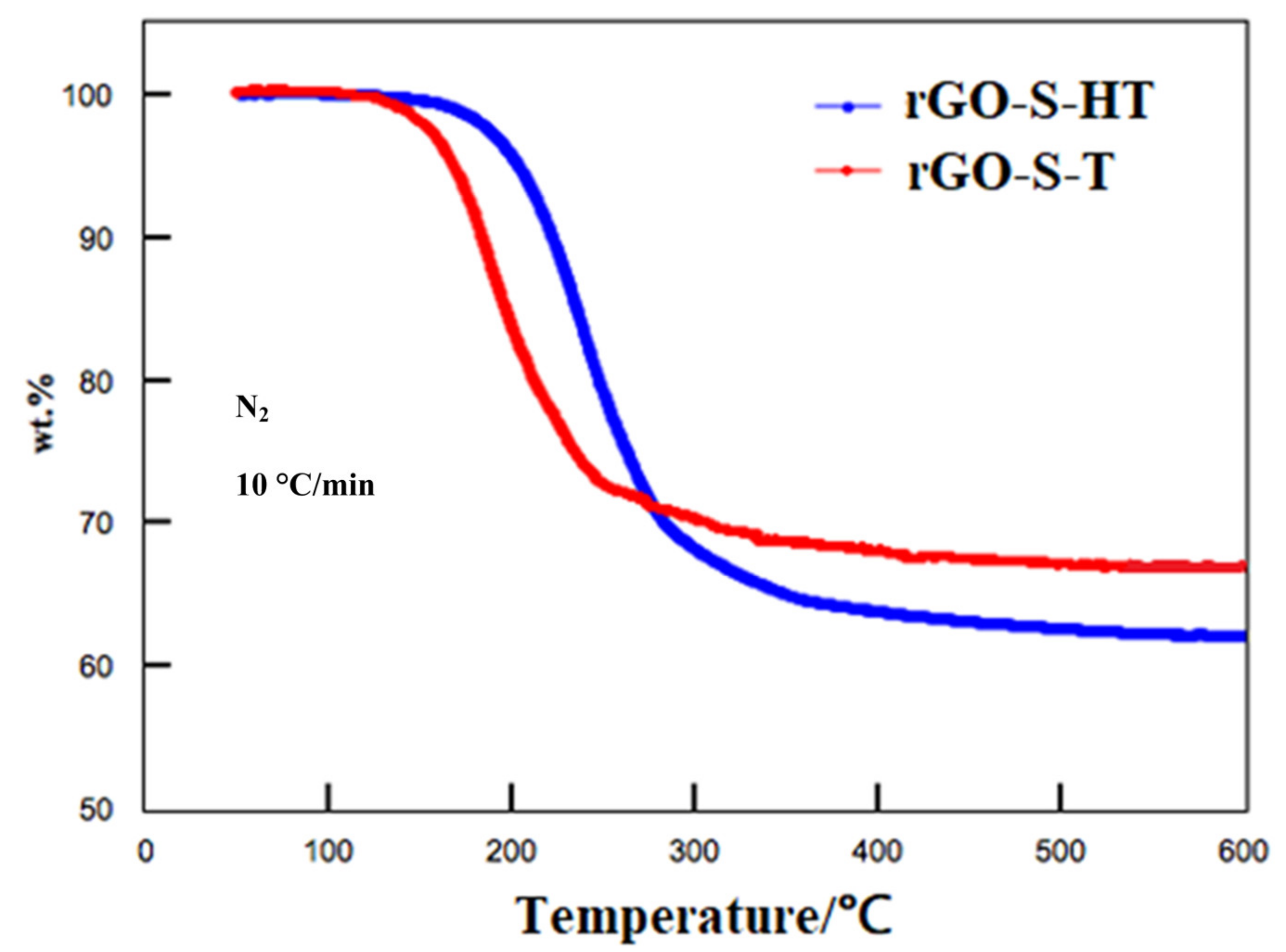
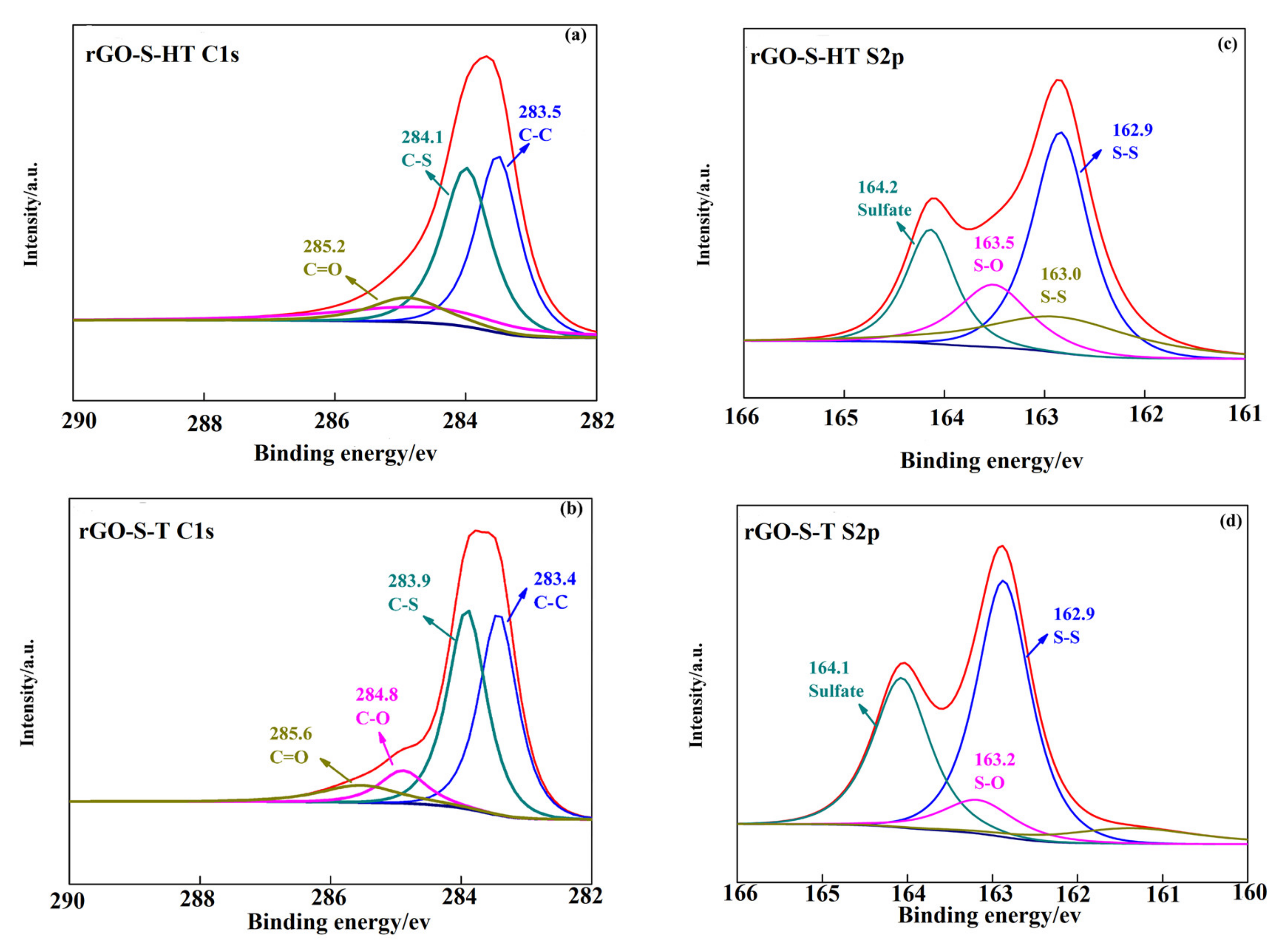
3.2. Structural Characteristics
3.3. Electrochemical Performance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Winter, M.; Brodd, J.R. What Are Batteries, Fuel Cells, and Supercapacitors. Chem. Rev. 2004, 104, 4245–4269. [Google Scholar] [CrossRef] [PubMed]
- Dunn, B.; Kamath, H.; Tarascon, J.-M. Electrical Energy Storage for the Grid: A Battery of Choices. Science 2011, 334, 928–935. [Google Scholar] [CrossRef]
- Ji, X.; Lee, K.T.; Nazar, L.F. A highly ordered nanostructured carbon-sulphur cathode forlithium-sulphur batteries. Nat. Mater. 2009, 8, 500–506. [Google Scholar] [CrossRef]
- Yang, Y.; Zheng, G.; Cui, Y. Nanostructured sulfur cathodes. Chem. Soc. Rev. 2013, 42, 3018–3032. [Google Scholar] [CrossRef]
- Peng, H.J.; Huang, J.Q.; Cheng, X.B.; Zhang, Q. Review on high-loading and high energy lithium-sulfur batteries. Adv. Energy Mater. 2017, 7, 1700260. [Google Scholar] [CrossRef]
- Ji, X.; Nazar, L.F. Advances in Li-S batteries. J. Mater. Chem. 2010, 20, 9821–9826. [Google Scholar] [CrossRef]
- Manthiram, A.; Fu, Y.; Su, Y.-S. Challenges and prospects of lithium-sulfur batteries. Acc. Chem. Res. 2013, 46, 1125–1134. [Google Scholar] [CrossRef]
- Diao, Y.; Xie, K.; Xiong, S.; Hong, X. Analysis of polysulfide dissolved in electrolyte in discharge-charge process of Li-S battery. J. Electrochem. Soc. 2012, 159, A421–A425. [Google Scholar] [CrossRef]
- Mikhaylik, Y.V.; Akridge, J.R. Polysulfide shuttle study in the Li/S battery system. J. Electrochem. Soc. 2004, 151, A1969–A1976. [Google Scholar] [CrossRef]
- Song, M.K.; Cairns, E.J.; Zhang, Y. Lithium/sulfur batteries with high specific energy: Old challenges and new opportunities. Nanoscale 2013, 5, 2186–2204. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Lee, J.T.; Nitta, N.; Kim, H.; Borodin, O.; Yushin, G. Lithium iodide as a promising electrolyte additive for lithium–sulfur batteries: Mechanisms of performance enhancement. Adv. Mater. 2015, 27, 101–108. [Google Scholar] [CrossRef]
- Zhang, S.; Ueno, K.; Dokko, K.; Watanabe, M. Recent advances in electrolytes for lithium–sulfur batteries. Adv. Energy Mater. 2015, 5, 201400981. [Google Scholar] [CrossRef]
- Liu, S.; Li, G.R.; Gao, X.P. Lanthanum nitrate as electrolyte additive to stabilize the surface morphology of lithium anode for lithium-sulfur battery. ACS Appl. Mater. Interfaces 2016, 8, 7783–7789. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.-S.; Manthiram, A. Lithium–sulphur batteries with a microporous carbon paper as a bifunctional interlayer. Nat. Commun. 2012, 3, 1166. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.-S.; Manthiram, A. A new approach to improve cycle performance of rechargeable lithium–sulfur batteries by inserting a free-standing MWCNT interlayer. Chem. Commun. 2012, 48, 8817–8819. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Ding, X.; Wang, C.; Feng, Z.; Xu, L.; Gao, X.; Zhai, Y.; Wang, D. A multi-shelled CoP nanosphere modified separator for highly efficient Li-S batteries. Nanoscale 2018, 10, 13694–13701. [Google Scholar] [CrossRef] [PubMed]
- Lai, Z.; Chen, Y.C.; Tan, C.; Zhang, X.; Zhang, H. Self-assembly of two-dimensional nanosheets into one-dimensional nanostructures. Chem 2016, 1, 59–77. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, J.T.; Chen, Y.M.; Li, J.; Lou, X.W. Pie-like electrode design for high-energy density lithium-sulfur batteries. Nat. Commun. 2015, 6, 8850. [Google Scholar] [CrossRef]
- Zhou, G.; Paek, E.; Hwang, G.S.; Manthiram, A. Long-life Li/polysulphide batteries with high sulfur loading enabled by lightweight three-dimensional nitrogen/sulphur-codoped graphene sponge. Nat. Commun. 2015, 6, 7760–7770. [Google Scholar] [CrossRef]
- Zhang, J.; Hu, H.; Li, Z.; Lou, X.W. Double-shelled nanocages with cobalt hydroxide inner shell and layered double hydroxides outer shell as high-efficiency polysulfide mediator for lithium-sulfur batteries. Angew. Chem. 2016, 55, 3982–3986. [Google Scholar] [CrossRef]
- Xiao, L.; Cao, Y.; Xiao, J.; Schwenzer, B.; Engelhard, M.H.; Saraf, L.V.; Nie, Z.; Exarhos, G.J.; Liu, J. A soft approach to encapsulate sulfur: Polyaniline nanotubes for lithium-sulfur batteries with long cycle life. Adv. Mater. 2012, 24, 1176–1181. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.J.; Wu, F.; Chen, J.Z.; Wu, S.X.; Li, L.; Chen, S.; Zhao, T. Sulfur/polythiophene with a core/shell structure: Synthesis and electrochemical properties of the cathode for rechargeable lithium batteries. J. Phys. Chem. 2011, 115, 6057–6063. [Google Scholar]
- Yang, Y.; Yu, G.H.; Cha, J.J.; Wu, H.; Vosgueritchian, M.; Yao, Y.; Bao, Z.A.; Cui, Y. Improving the performance of lithium-sulfur batteries by conductive polymer coating. ACS Nano 2011, 5, 9187–9193. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wan, W.; Chen, J.; Zhou, H.; Zhang, X.; Yuan, L.; Huang, Y. Dual core–shell structured sulfur cathode composite synthesized by a one-pot route for lithium sulfur batteries. J. Mater. Chem. 2013, 1, 1716–1721. [Google Scholar] [CrossRef]
- Li, G.; Li, G.; Ye, S.; Gao, X. A polyaniline-coated sulfur/carbon composite with an enhanced high-rate capability as a cathode material for lithium/sulfur batteries. Adv. Energy Mater. 2012, 2, 1238–1245. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, J.; Lou, X.W. Hollow carbon nanofibers filled with MnO2 nanosheets as efficient sulfur hosts for lithium-sulfur batteries. Angew. Chem. 2015, 54, 12886–12890. [Google Scholar] [CrossRef]
- Zheng, G.; Yang, Y.; Cha, J.J.; Hong, S.S.; Cui, Y. Hollow Carbon nanofiber-encapsulated sulfur cathodes for high specific capacity rechargeable lithium batteries. Nano Lett. 2011, 11, 4462–4467. [Google Scholar] [CrossRef]
- Zheng, S.; Chen, Y.; Xu, Y.; Yi, F.; Zhu, Y.; Liu, Y.; Yang, J.; Wang, C. In situ formed lithium sulfide/microporous carbon cathodes for lithium-ion batteries. ACS Nano 2013, 7, 10995–11003. [Google Scholar] [CrossRef]
- Liang, C.D.; Dudney, N.J.; Howe, J.Y. Hierarchically structured sulfur/carbon nanocomposite material for high-energy lithium battery. Chem. Mater. 2009, 21, 4724–4730. [Google Scholar] [CrossRef]
- Yoo, E.; Kim, J.; Hosono, E.; Zhou, H.S.; Kudo, T.; Honma, I. Large reversible Li storage of graphene nanosheet families for use in rechargeable lithium ion batteries. Nano Lett. 2008, 8, 2277–2282. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Yu, C.; Liu, S.; Yang, J.; Fan, X.; Huang, H.; Qiu, J. 3D porous n-doped graphene frameworks made of interconnected nanocages for ultrahigh-rate and long-life Li-O2 batteries. Adv. Funct. Mater. 2015, 25, 6913–6920. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, G.; Lai, Y.; Li, J. A freestanding hollow carbon nanofiber/reduced graphene oxide interlayer for high-performance lithium-sulfur batteries. J. Alloys Compd. 2016, 663, 501–506. [Google Scholar] [CrossRef]
- Jiang, Y.; Lu, M.; Ling, X.; Jiao, Z.; Chen, L.; Hu, P.; Zhao, B. One-step hydrothermal synthesis of three-dimensional porous graphene aerogels/sulfur nanocrystals for lithium-sulfur batteries. J. Alloys Compd. 2015, 645, 509–516. [Google Scholar] [CrossRef]
- Li, S.; Cen, Y.; Xiang, Q.; Aslam, M.K.; Hu, B.; Li, W.; Tang, Y.; Yu, Q.; Liu, Y.; Chen, C. Vanadium dioxide-reduced graphene oxide binary host as an efficient polysulfide plague for high-performance lithium-sulfur batteries. J. Mater. Chem. 2019, 7, 1658–1668. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, R.; Tang, W.; Zhan, L.; Zhao, S.; Kang, Q.; Wang, Y.; Yang, S. Efficient polysulfide barrier of a graphene aerogel-carbon nanofibers-Ni network for high-energy-density lithium-sulfur batteries with ultrahigh sulfur content. J. Mater. Chem. 2018, 10, 20926–20938. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, N.; Yang, X.; Li, S.; Yao, J.; Cai, Y. Hollow sulfur@graphene oxide core–shell composite for high-performance Li-S batteries. J. Alloys Compd. 2015, 650, 604–609. [Google Scholar] [CrossRef]
- Zu, C.; Manthiram, A. Hydroxylated graphene-sulfur nanocomposites for high-rate lithium-sulfur batteries. Adv. Energy Mater. 2013, 3, 1008–1012. [Google Scholar] [CrossRef]
- Sun, Y.; Zheng, G.; She, Z.W.; Liu, N.; Wang, S.; Sun, J.; Lee, H.R.; Cui, Y. Graphite-encapsulated Li-metal hybrid anodes for high-capacity Li batteries. Chem 2016, 2, 287–297. [Google Scholar] [CrossRef]
- Zheng, S.Y.; Wen, Y.; Zhu, Y.; Han, Z.; Wang, J.; Yang, J.; Wang, C. In situ sulfur reduction and intercalation of graphite oxides for Li-S battery cathodes. Adv. Energy Mater. 2015, 4, 1400482. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, R.E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Zhang, J.; Li, J.; Wang, W.; Zhang, X.; Tan, X.; Chu, W.; Guo, Y. Microemulsion assisted assembly of 3D porous S/graphene@g-C3N4 hybrid sponge as free-standing cathodes for high energy density Li-S batteries. Adv. Energy Mater. 2018, 8, 1702839. [Google Scholar] [CrossRef]
- Kaiser, M.R.; Ma, Z.; Wang, X.; Han, F.; Gao, T.; Fan, X.; Wang, J.Z.; Liu, H.K.; Dou, S.; Wang, C. Reverse microemulsion synthesis of sulfur/gaphene composite for lithium/sulfur batteries. ACS Nano 2017, 11, 9048–9056. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, Y.; Konarov, A.; Gosselink, D.; Soboleski, H.G.; Chen, P. A novel nano-sulfur/polypyrrole/graphene nanocomposite cathode with a dual-layered structure for lithium rechargeable batteries. J. Power Sour. 2013, 241, 517–521. [Google Scholar] [CrossRef]
- Zhang, C.; Lv, W.; Zhang, W.; Zheng, X.; Wu, M.; Wei, W.; Tao, Y.; Li, Z.; Yang, Q. Reduction of graphene oxide by hydrogen sulfide: A promising strategy for pollutant control and as an electrode for Li-S batteries. Adv. Energy Mater. 2014, 4, 175–182. [Google Scholar] [CrossRef]
- Ji, L.; Rao, M.; Zheng, H.; Zhang, L.; Li, Y.; Duan, W.; Guo, J.; Cairns, E.J.; Zhang, Y. Graphene oxide as a sulfur immobilizer in high performance lithium/sulfur cells. J. Am. Chem. Soc. 2011, 133, 18522–18527. [Google Scholar] [CrossRef]
- Wang, Z.; Dong, Y.; Li, H.; Zhao, Z.; Bin, W.H.; Hao, C.; Liu, S.; Qiu, J.; Lou, X.W. Enhancing lithium-sulphur battery performance by strongly binding the discharge products on amino-functionalized reduced graphene oxide. Nat. Commun. 2014, 5, 5002–5008. [Google Scholar] [CrossRef] [PubMed]
- Benitez, A.; Di Lecce, D.; Elia, G.A.; Caballero, A.; Morales, J.; Hassoun, J. A lithiumion battery using a 3D-array nanostructured graphene-sulfur cathode and a silicon oxide-based anode. ChemSusChem 2018, 11, 1512–1520. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Sun, H.; Pang, Y.; Yu, M.; Zheng, S. Investigation on Fabrication of Reduced Graphene Oxide-Sulfur Composite Cathodes for Li-S Battery via Hydrothermal and Thermal Reduction Methods. Materials 2021, 14, 861. https://doi.org/10.3390/ma14040861
Li Z, Sun H, Pang Y, Yu M, Zheng S. Investigation on Fabrication of Reduced Graphene Oxide-Sulfur Composite Cathodes for Li-S Battery via Hydrothermal and Thermal Reduction Methods. Materials. 2021; 14(4):861. https://doi.org/10.3390/ma14040861
Chicago/Turabian StyleLi, Zhiqi, Hao Sun, Yuepeng Pang, Mingming Yu, and Shiyou Zheng. 2021. "Investigation on Fabrication of Reduced Graphene Oxide-Sulfur Composite Cathodes for Li-S Battery via Hydrothermal and Thermal Reduction Methods" Materials 14, no. 4: 861. https://doi.org/10.3390/ma14040861
APA StyleLi, Z., Sun, H., Pang, Y., Yu, M., & Zheng, S. (2021). Investigation on Fabrication of Reduced Graphene Oxide-Sulfur Composite Cathodes for Li-S Battery via Hydrothermal and Thermal Reduction Methods. Materials, 14(4), 861. https://doi.org/10.3390/ma14040861