Zr(OH)4/GO Nanocomposite for the Degradation of Nerve Agent Soman (GD) in High-Humidity Environments
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
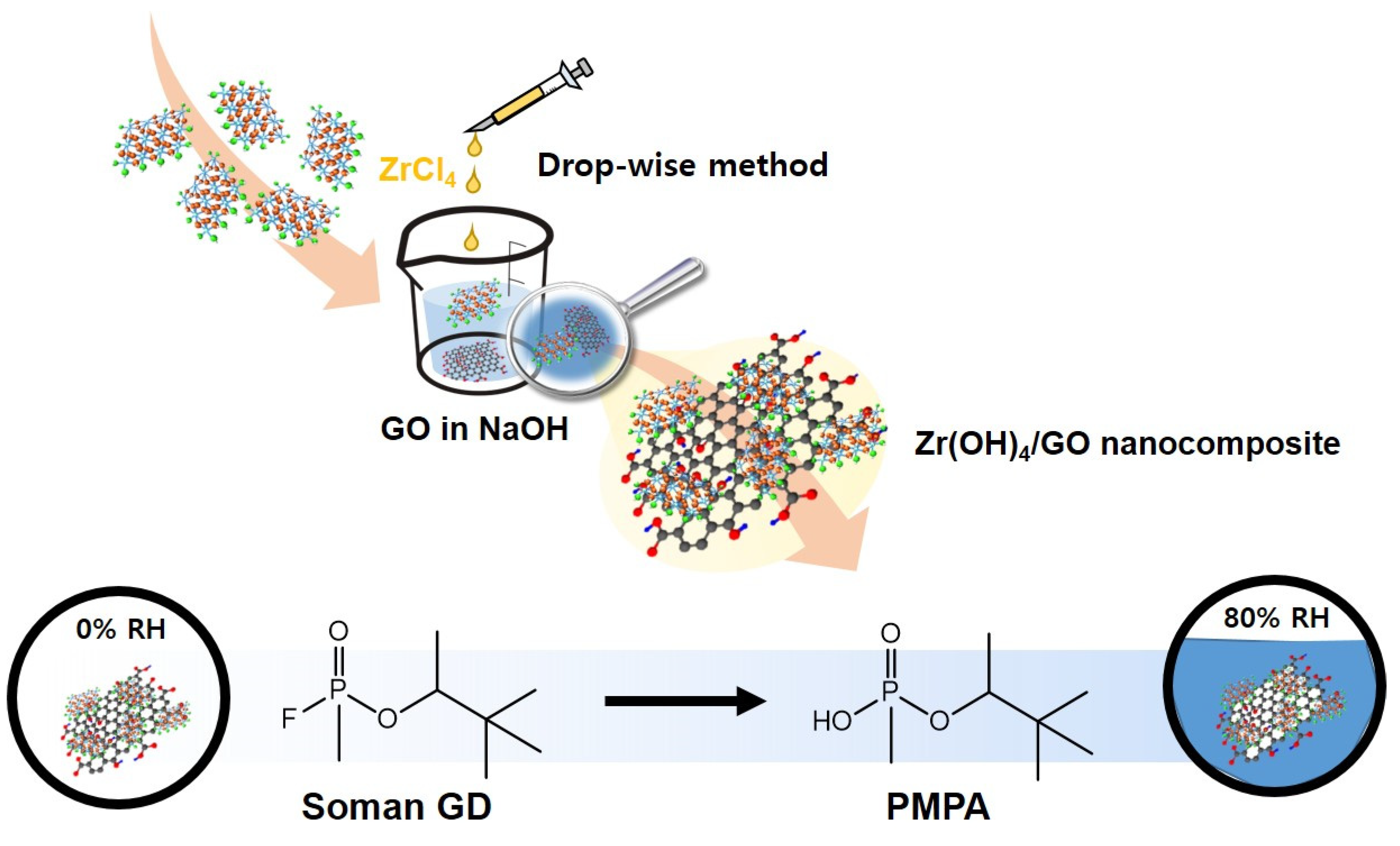
2.2. Preparation of Zr(OH)4/GO Nanocomposite and Zr(OH)4
2.3. Characterization
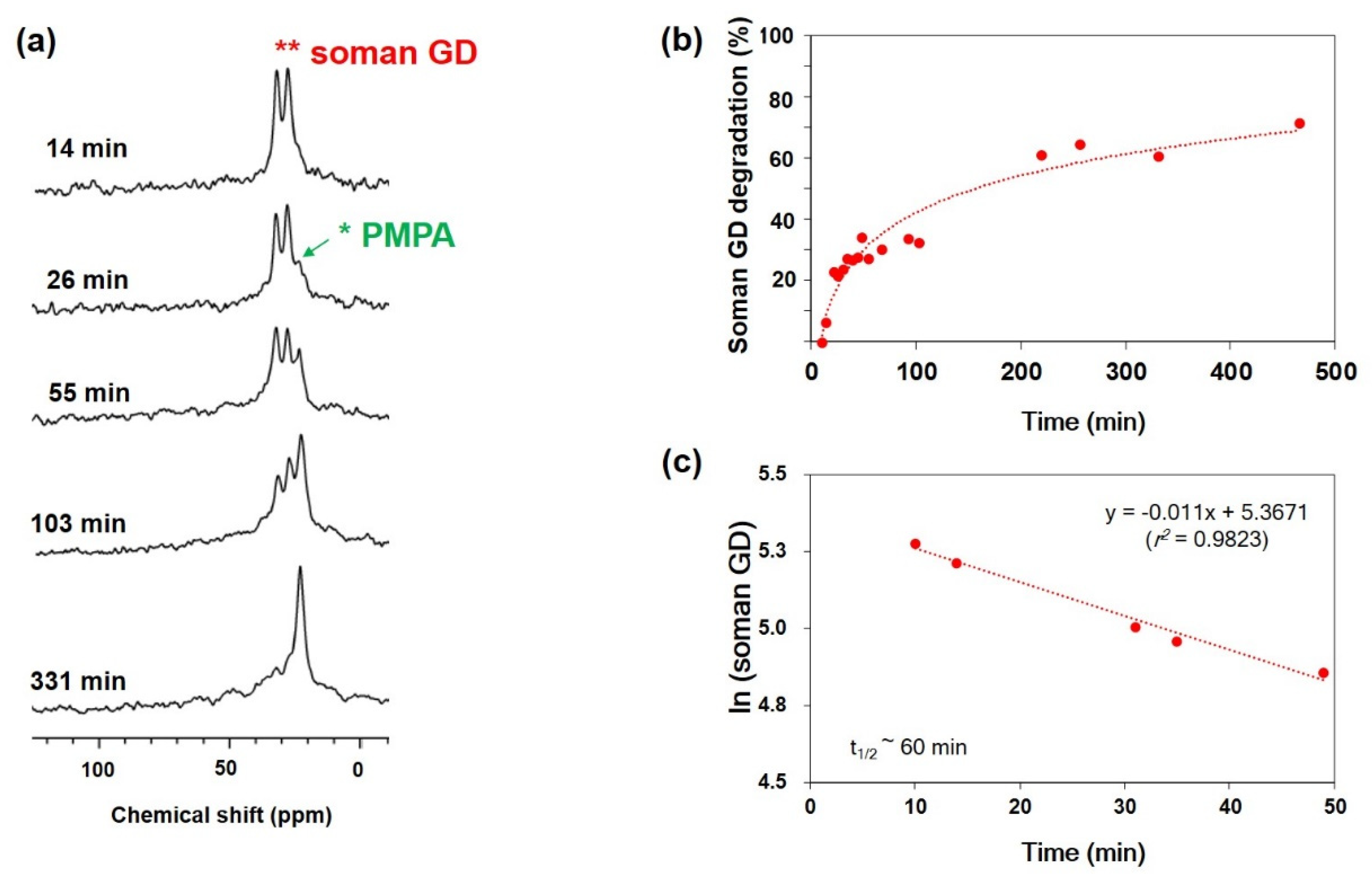
2.4. Degradation of Nerve Agent Soman (GD)
2.5. Reaction Products Analysis
2.6. 31P SS-MAS NMR Analysis
3. Results and Discussion
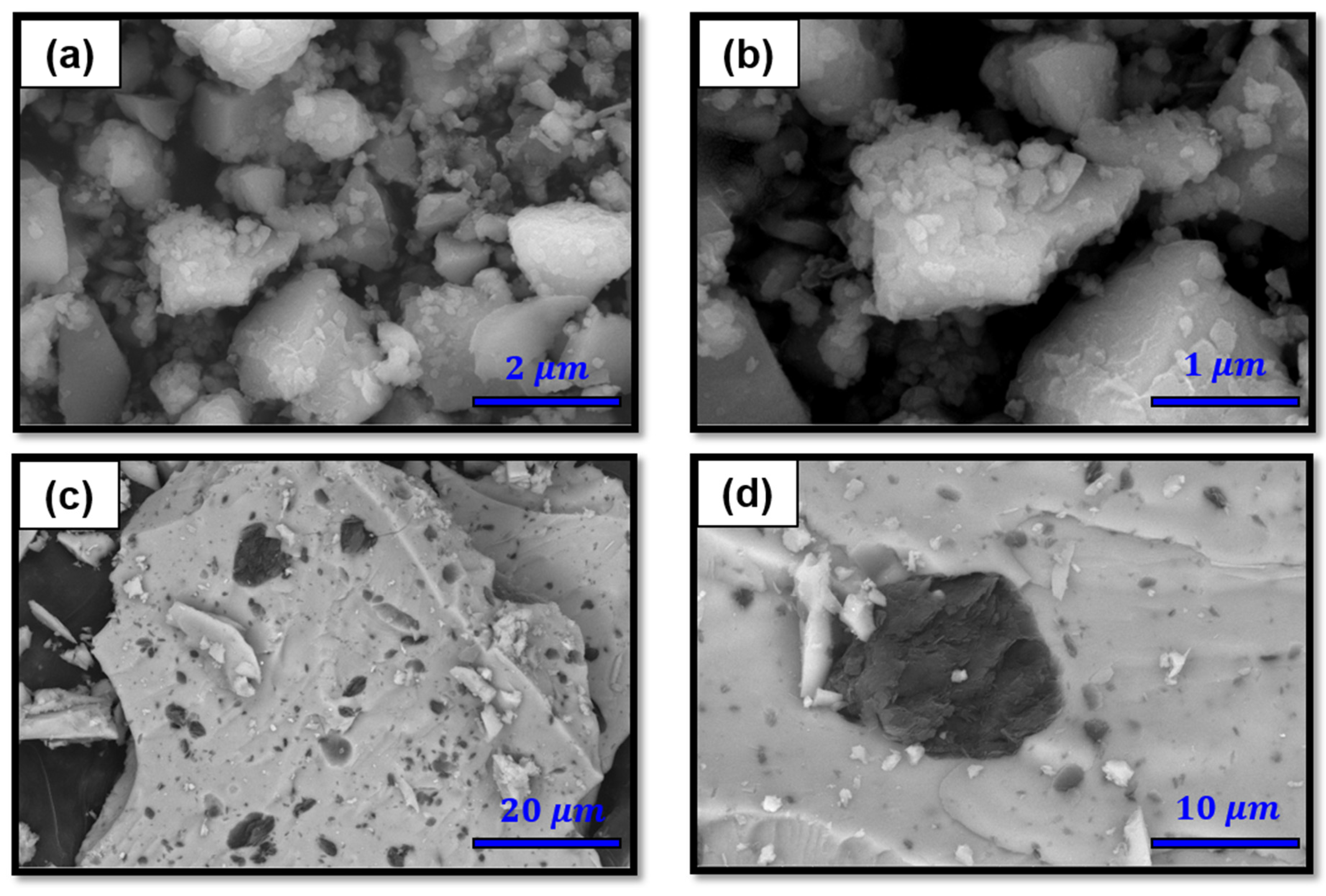
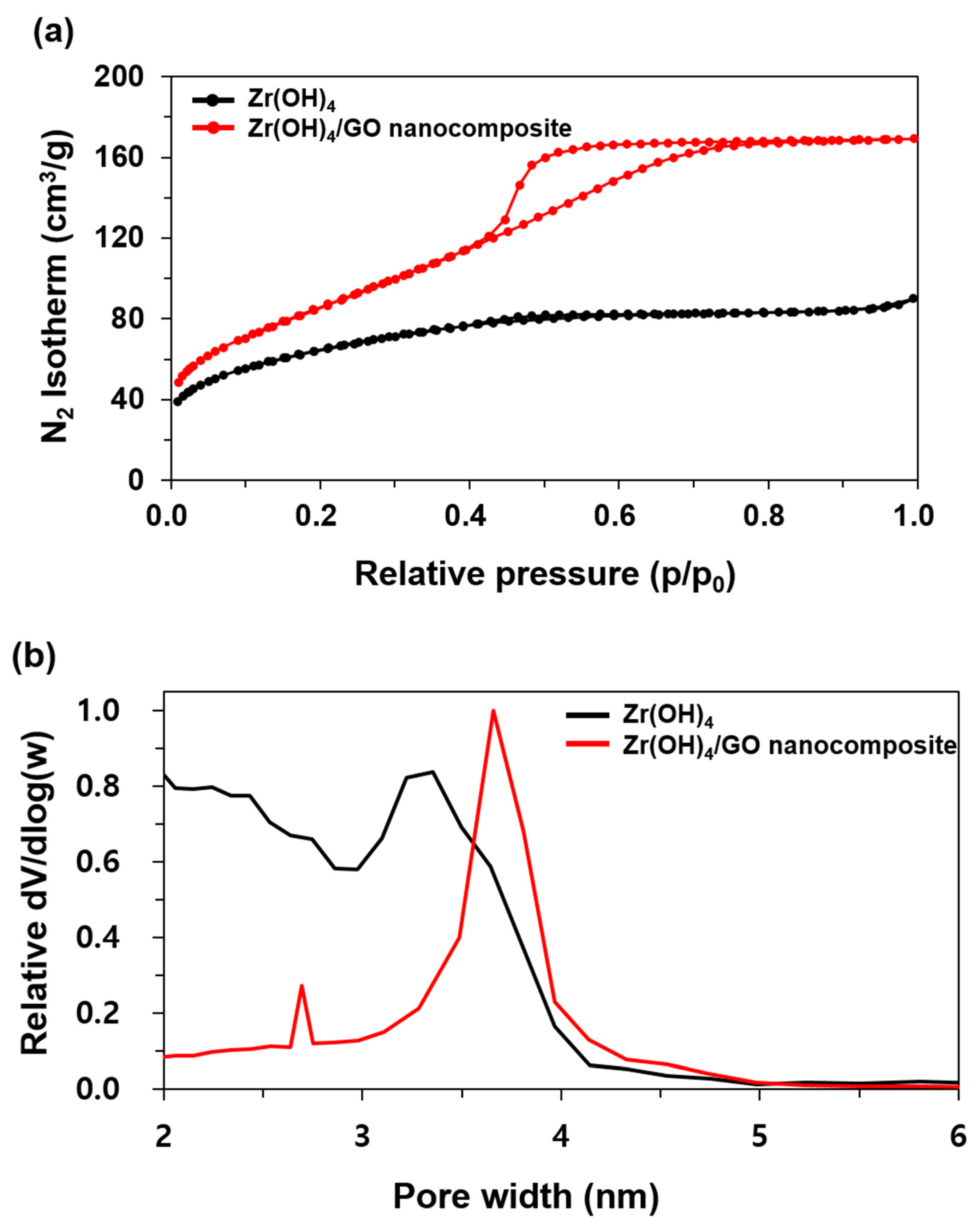
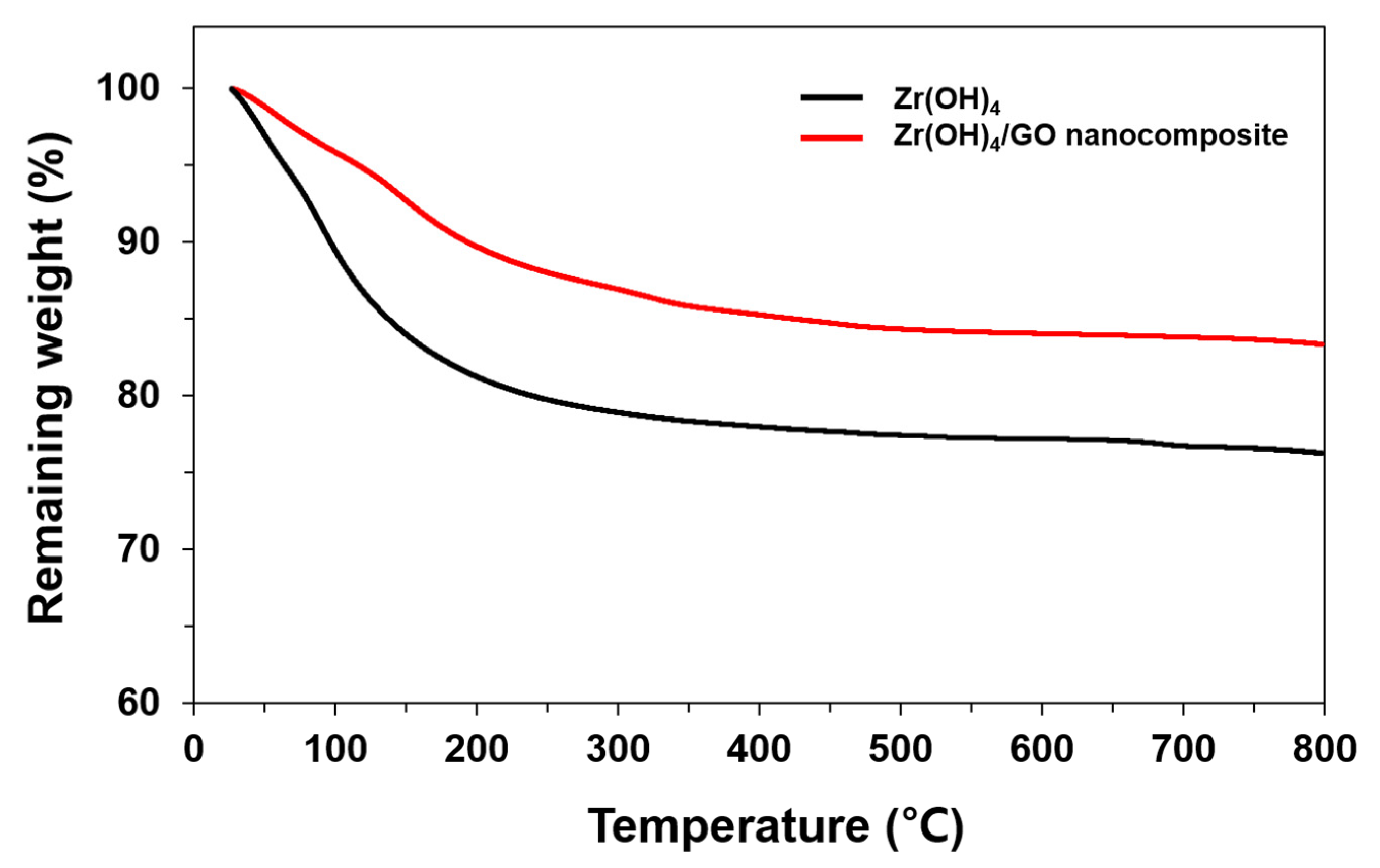
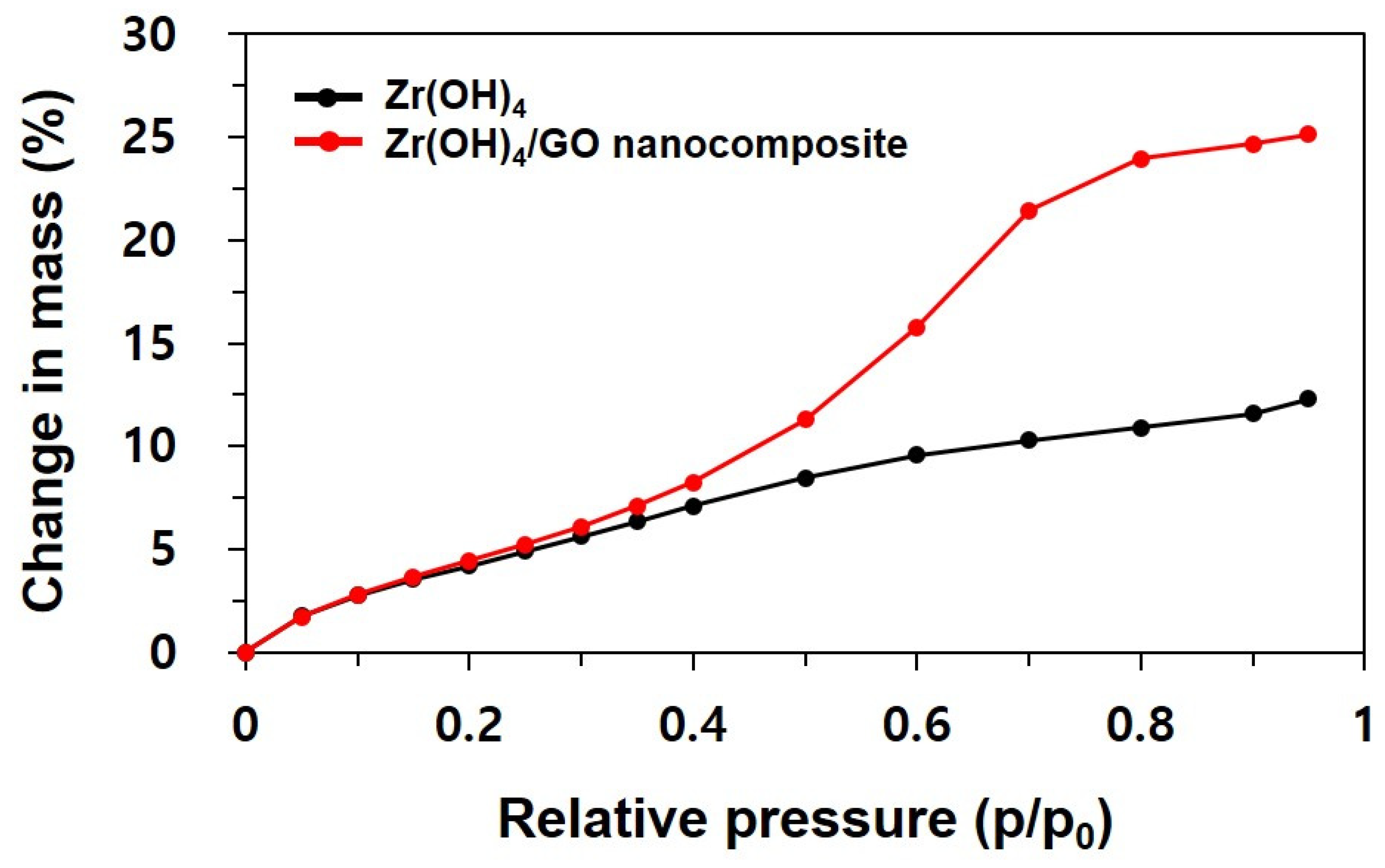
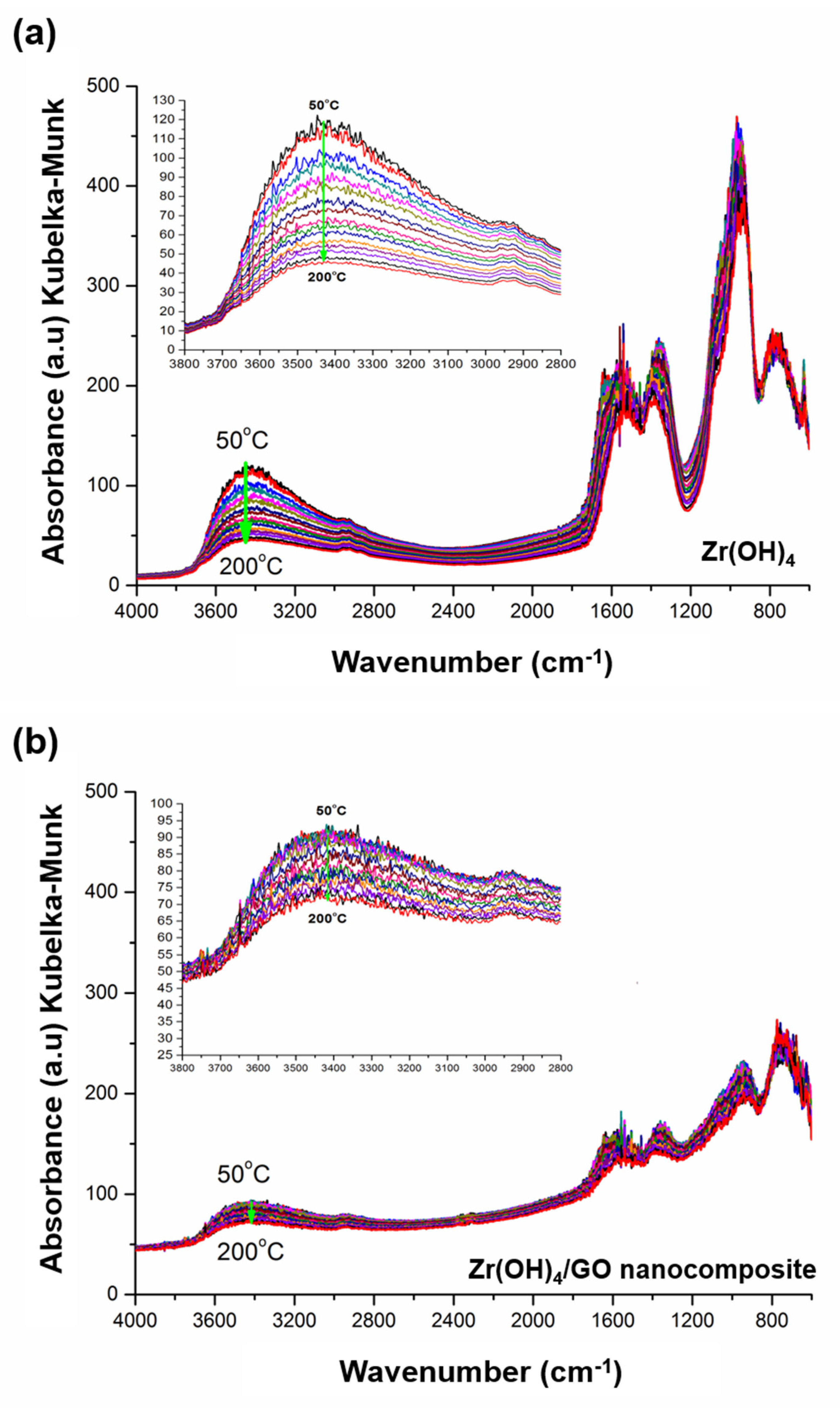
3.1. Characterization of Zr(OH)4/GO Nanocomposite and Pristine
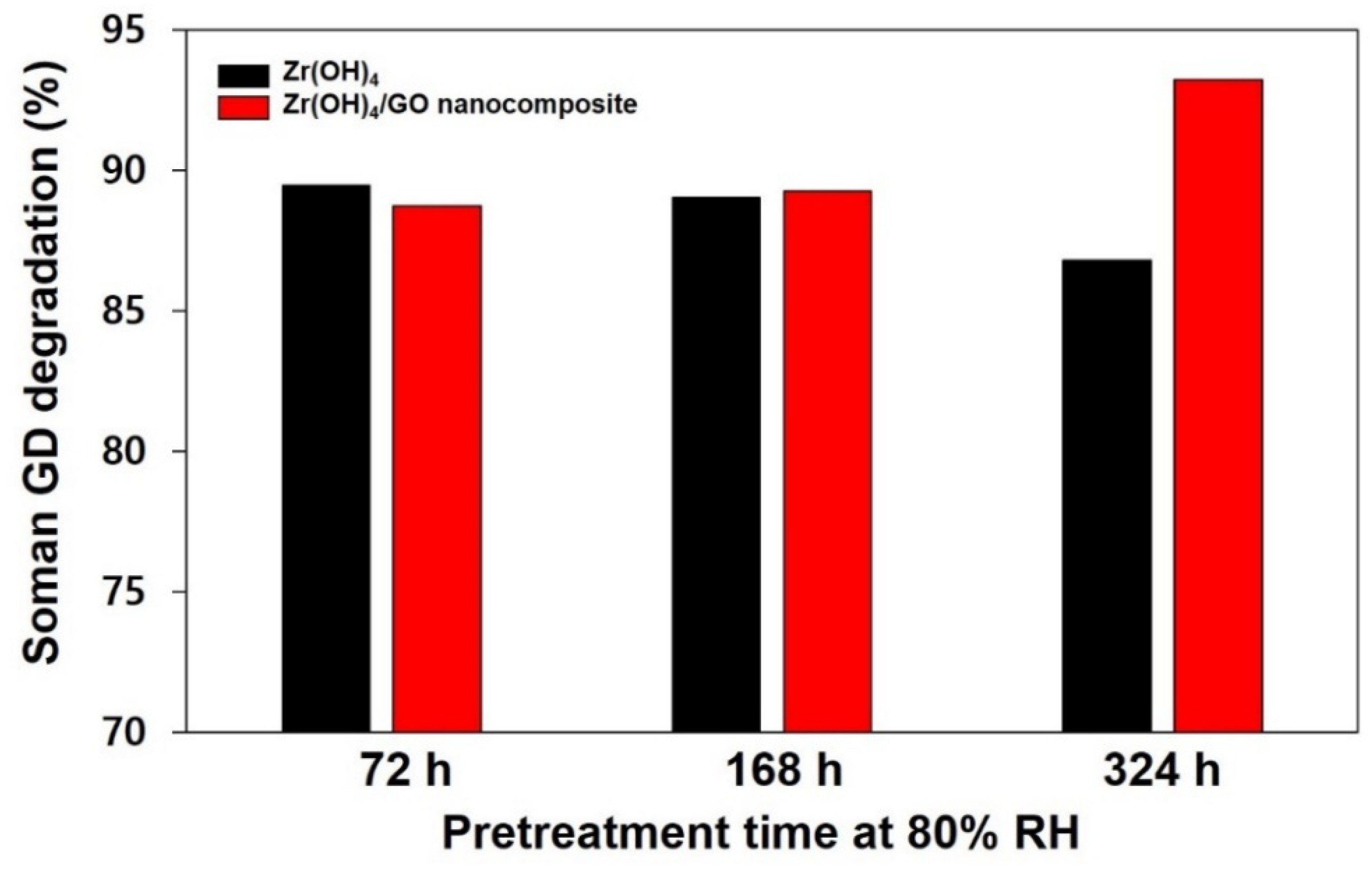
3.2. Hydrolytic Degradation of Soman (GD) by Zr(OH)4/GO Nanocomposite and Pristine in High Humidity
3.3. Discussion of Water Adsorption Isotherms and DRIFT Spectra of Zr(OH)4/GO Nanocomposite and Pristine Zr(OH)4
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Barba-Bon, A.; Martinez-Manez, R.; Sancenon, F.; Costero, A.M.; Gil, S.; Perez-Pla, F.; Llopis, E. Towards the design of organocatalysts for nerve agents remediation: The case of the active hydrolysis of DCNP (a Tabun mimic) catalyzed by simple amine-containing derivatives. J. Hazard Mater. 2015, 298, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, M.; Emadodin, D.; Darchini, M.; Mahdi, B.M. Advances in toxicology and medical treatment of chemical warfare nerve agents. DARU J. Pham. Sci. 2012, 20, 81. [Google Scholar]
- Organization for the Prohibition of Chemical Weapons Convention on the Prohibition of the Development, Production, Stockpiling and Use of Chemical Weapons and on Their Destruction. Available online: https://www.opcw.org/chemical-weapons-convention (accessed on 27 September 2005).
- Picard, B.; Chataigner, I.; Maddaluno, J.; Legros, J. Introduction to chemical warfare agents, relevant simulants and modern neutralisation methods. Org. Biomol. Chem. 2019, 17, 6528–6537. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.J.; Kim, K.; Tsay, O.G.; Atwood, D.A.; Churchill, D.G. Update 1 of: Destruction and Detection of Chemical Warfare Agents. Chem. Rev. 2015, 115, PR1–PR76. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.C.; Baker, J.A.; Ward, J.R. Decontamination of chemical warfare agents. Chem. Rev. 1992, 92, 1729–1743. [Google Scholar] [CrossRef]
- Wagner, G.W.; Sorrick, D.C.; Procell Brickhouse, M.D.; Mcvey, I.F.; Schwartz, L.I. Decontamination of VX, GD, and HD on a surface using modified vaporized hydrogen peroxide. Langmuir 2007, 23, 1178–1186. [Google Scholar] [CrossRef] [PubMed]
- Ryu, S.G.; Lee, H.W. Effectiveness and reaction networks of H2O2 vapor with NH3 gas for decontamination of the toxic warfare nerve agent, VX on solid surface. J. Enviro. Sci. Health Part A 2015, 50, 1417–1427. [Google Scholar]
- Scinicz, L. History of chemical and biological warfare agents. Toxicology 2005, 214, 167–181. [Google Scholar] [CrossRef]
- Liu, Y.; Moon, S.Y.; Hupp, J.T.; Farha, O.K. Dual-function metal-organic framework as a versatile catalyst for detoxifying chemical warfare agent simulants. ACS Nano 2015, 9, 12358–12364. [Google Scholar] [CrossRef]
- Seredych, M.; Bandosz, T.J. Effects of surface features on adsorption of SO2 on graphite oxide/Zr(OH)4 composites. J. Phys. Chem. C 2010, 114, 14552–14560. [Google Scholar] [CrossRef]
- Seredych, M.; Bandosz, T.J. Reactive adsorption of hydrogen sulfide on graphite oxide/Zr(OH)4 composites. Composites Chem. Eng. J. 2011, 166, 1032–1038. [Google Scholar] [CrossRef]
- Chanvan, S.; Vitillo, J.G.; Gianolio, D.; Zavorotynska, O.; Civalleri, B.; Jakobsen, S.; Nilsen, M.H.; Valenzano, L.; Lamberti, C.; Lillerud, K.P.; et al. H2 storage in isostructural UiO-67 and UiO-66 MOFs. Phys. Chem. Chem. Phys. 2012, 14, 1614–1626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryu, S.G.; Kim, M.K.; Park, M.K.; Jang, S.O.; Kim, S.H.; Jung, H.S. Availability of Zr-based MOFs for the degradation of nerve agents in all humidity conditions. Micropor. Mesopor. Mat. 2019, 274, 9–16. [Google Scholar] [CrossRef]
- Kandiah, M.; Nilsen, M.H.; Usseglio, S.; Jakobsen, S.; Olsbye, U.; Tilset, M.; Larabi, C.; Quadrelli, E.A.; Bonino, F.; Lillerud, K.P. Synthesis and stability of tagged UiO-66 Zr-MOFs. Chem. Mater. 2010, 22, 6632–6640. [Google Scholar] [CrossRef]
- Zheng, Y.; Zheng, S.; Xue, H.; Pang, H. Metal-organic framework/graphene-based materials: Preparations and applications. Adv. Funct. Mater. 2018, 28, 1804950. [Google Scholar] [CrossRef]
- Balow, R.B.; Lundin, J.G.; Daniels, G.C.; Gordon, W.O.; McEntee, M.; Peterson, G.W.; Wynne, J.H.; Pehrsson, P.E. Environmental effects on zirconium hydroxide nanoparticles and chemical warfare agent decomposition: Implications of atmospheric water and carbon dioxide. ACS Appl Mater. Interfaces 2017, 9, 39747–39757. [Google Scholar] [CrossRef]
- Bandosz, T.J.; Laskoski, M.; Mahle, J.; Mogilevsky, G.; Peterson, G.W.; Rossin, J.A.; Wagner, G.W. Reactions of VX, GD, and HD with Zr(OH)4: Near instantaneous decontamination of VX. J. Phys. Chem. C 2012, 116, 11606–11614. [Google Scholar] [CrossRef]
- Wagner, G.W.; Peterson, G.W.; Mahle, J.J. Effects of adsorbed water and surface hydroxyls on the hydrolysis of VX, GD, and HD on titania materials: The development of self-decontaminating paints. Ind. Eng. Chem. Res. 2012, 51, 3598–3603. [Google Scholar] [CrossRef]
- Giannakoudakis, D.A.; Mitchell, J.K.; Bandosz, T.J. Reactive adsorption of mustard gas surrogate on zirconium (hydr)oxide/graphite oxide composites: The role of surface and chemical features. J. Mater. Chem. A 2016, 4, 1008–1019. [Google Scholar] [CrossRef]
- Peterson, G.W.; Wagner, G.W.; Keller, J.H.; Rossin, J.A. Enhanced cyanogen chloride removal by the reactive zirconium hydroxide substrate. Ind. Eng. Chem. Res. 2010, 49, 11182–11187. [Google Scholar] [CrossRef]
- Peterson, G.W.; Rossin, J.A. Removal of chlorine gases from streams of air using reactive zirconium hydroxide based filtration media. Ind. Eng. Chem. Res. 2012, 51, 2675–2681. [Google Scholar] [CrossRef]
- Colon-Ortiz, J.; Landers, J.M.; Gordon, W.O.; Abalboa, A.; Karwacki, C.J.; Neimark, A.V. Disordered mesoporous zirconium (hydr)oxides for decomposition of dimethyl chlorophosphate. ACS Appl. Mater. Interfaces 2012, 11, 17931–17939. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.M.; Balow, R.B.; Daniels, G.C.; Ko, J.S.; Pehrsson, P.E. Conformal nanoscale zirconium hydroxide films for decomposing chemical warfare agents. ACS App. Nano Mater. 2019, 2, 2295–2307. [Google Scholar] [CrossRef]
- Das, R.S.; Warkhade, S.K.; Kumar, A.; Wankhade, A.V. Graphene oxide-based zirconium oxide nanocomposite for enhanced visible light-driven photocatalytic activity. Res. Chem. Intermediat. 2019, 45, 1689–1705. [Google Scholar] [CrossRef]
- Wang, H.L.; Casalongue, H.S.; Liang, Y.Y.; Liang, H.J.; Dai, H.J. Ni(OH)2 Nanoplates Grown on Graphene as Advanced Electrochemical Pseudocapacitor Materials. J. Am. Chem. Soc. 2010, 132, 7472–7477. [Google Scholar] [CrossRef] [Green Version]
- Tang, J.; Wang, J. Fe-based metal organic framework/graphene oxide composite as an efficient catalyst for Fento-like degradation of methyl orange. RSC Adv. 2017, 7, 50829–50837. [Google Scholar] [CrossRef] [Green Version]
- Zhang, G.; Li, G.; Lu, H.; Lv, Q.; Sun, Z.G. Synthesis, characterization and photocatalytic properties of MIL-53(Fe)-graphene hybrid materials. RSC Adv. 2014, 15, 7594–7600. [Google Scholar] [CrossRef]
- Petit, C.; Bandosz, T.J. MOF-Graphite Oxide Composites: Combining the Uniqueness of Graphene Layers and Metal-Organic Frameworks. Adv. Mater. 2009, 21, 4753–4757. [Google Scholar] [CrossRef]
- Zubir, N.A.; Yacou, C.; Motuzas, J.; Zhang, X.W.; Zhao, X.S.; da Costa, J.C.D. The sacrificial role of graphene oxide in stabilizing a Fenton-like catalyst GO-Fe3O4. Chem. Commun. 2015, 45, 9291–9293. [Google Scholar] [CrossRef]
- Jung, H.S.; Lim, K.C. Fate and degradation of the chemical warfare agent soman on sands. Environ. Chem. Lett. 2016, 14, 367–372. [Google Scholar] [CrossRef]
- Yokota, T.; Hiraga, K.; Yamane, H.; Takai-Iashi, N. Mass spectrometry of trimethylsilyl derivatives of gibberllin glucosides and glucosyl ester. Phytochemistry 1975, 14, 1569–1574. [Google Scholar] [CrossRef]
- ALOthman, Z.A. A Review: Fundamental Aspects of Silicate Mesoporous Materials. Materials 2012, 5, 2874–2902. [Google Scholar] [CrossRef] [Green Version]
- Boer, J.H.D.; Lippens, B.C.; Lisen, B.G.; Broekhoff, J.C.P.; Heuvel, A.V.D.; Osinga, T.J. Thet-curve of multimolecular N2-adsorption. J. Colloid Interf. Sci. 1966, 21, 405–414. [Google Scholar] [CrossRef]
- Wu, Z.; Zhu, H.; Yuan, X.; Wang, H.; Wang, L.; Chen, X.; Zeng, G.; Wu, Y. Adsorptive removal of methylene blue by rhamnolipid-functionalized graphene oxide from wastewater. Water Res. 2014, 67, 330–344. [Google Scholar] [CrossRef]
- Vellingiri, K.; Philip, L.; Kim, K.H. Metal-organic frameworks as media for the catalytic degradation of chemical warfare agents. Coord. Chem. Rev. 2017, 353, 159–179. [Google Scholar] [CrossRef]
- Matito-Martos, I.; Moghadam, P.Z.; Li, A.; Colombo, V.; Navarro, J.A.R.; Calero, S.; Fairen-Jimenez, D. Discovery of an Optimal Porous Crystalline Materials for the Capture of Chemical Warfare Agents. Chem. Mater. 2018, 30, 4571–4579. [Google Scholar] [CrossRef]
- Koning, M.C.D.; Grol, M.V.; Breijaert, T. Degradation of Paraoxon and the Chemical Warfare Agents VX, Tabun, and Soman by the Metal-Organic Frameworks UiO-66-NH2, MOF-808, NU-1000, and PCN-777. Inorg. Chem. 2017, 56, 11804–11809. [Google Scholar] [CrossRef]
- Peterson, G.W.; Rossin, J.A.; Karwacki, C.J.; Glover, T.G. Surface chemistry and morphology of zirconia polymorphs and the influence on sulfur dioxide removal. J. Phys. Chem. C 2011, 115, 9644–9650. [Google Scholar] [CrossRef]
- Wagner, G.W.; Bartram, P.W.; Koper, O.; Klabunde, K.J. Reactions of VX, GD, and HD with nanosize MgO. J. Phys. Chem. B 1999, 103, 3225–3228. [Google Scholar] [CrossRef]
- Katz, M.J.; Klet, R.C.; Moon, S.-Y.; Mondloch, J.E.; Hupp, J.T.; Farha, O.K. One step backward is two steps forward: Enhancing the hydrolysis rate of UiO-66 by decreasing [OH-]. ACS Catal. 2015, 5, 4637–4642. [Google Scholar] [CrossRef]
- Wagner, G.W.; Procell, L.R.; O’Connor, R.J.; Munavalli, S.; Carnes, C.L.; Kapoor, P.N.; Klabunde, K.J. Reactions of VX, GB, GD, and HD with nanosize Al2O3. Formation of aluminophosphonates. J. Am. Chem. Soc. 2001, 123, 1636–1644. [Google Scholar] [CrossRef] [PubMed]
- Wagner, G.W.; Procell, L.R.; Munavalli, S. 27Al, 47,49Ti, 31P, and 13C MAS study of VX, GD, and HD reactions with nanosize Al2O3, conventional Al2O3 and TiO2, and aluminium and titanium metal. J. Phys. Chem. C 2007, 111, 17564–17569. [Google Scholar] [CrossRef]
- Bale, S.; Samanidou, V.; Deliyanni, E. Effect of the reduction degree of graphene oxide on the adsorption of Bisphenol A. Chem. Eng. Res. And Des. 2016, 109, 573–585. [Google Scholar] [CrossRef]
- Roy, A.; Srivastavana, A.K.; Singh, B.; Mahato, T.H.; Shah, D.; Halve, A.K. Degradation of sulfur mustard and 2-chloroethyl sulfide on Cu-BTC metal-organic framework. Micropor. Mesopor. Mater. 2012, 162, 207–212. [Google Scholar] [CrossRef]
- Buttersack, C. Modeling of type IV and V sigmoidal adsorption isotherms. Phys. Chem. Chem. Phys. 2019, 21, 5614–5626. [Google Scholar] [CrossRef] [Green Version]
| Sample | SBET (m2/g) | VT (cm3/g) | Vmeso (cm3/g) | Vmeso/VT |
|---|---|---|---|---|
| Zr(OH)4 | 216 | 0.139 | 0.050 | 0.640 |
| Zr(OH)4/GO Nanocomposite | 274 | 0.262 | 0.251 | 0.958 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jang, S.; Ka, D.; Jung, H.; Kim, M.-K.; Jung, H.; Jin, Y. Zr(OH)4/GO Nanocomposite for the Degradation of Nerve Agent Soman (GD) in High-Humidity Environments. Materials 2020, 13, 2954. https://doi.org/10.3390/ma13132954
Jang S, Ka D, Jung H, Kim M-K, Jung H, Jin Y. Zr(OH)4/GO Nanocomposite for the Degradation of Nerve Agent Soman (GD) in High-Humidity Environments. Materials. 2020; 13(13):2954. https://doi.org/10.3390/ma13132954
Chicago/Turabian StyleJang, Seongon, Dongwon Ka, Hyunsook Jung, Min-Kun Kim, Heesoo Jung, and Youngho Jin. 2020. "Zr(OH)4/GO Nanocomposite for the Degradation of Nerve Agent Soman (GD) in High-Humidity Environments" Materials 13, no. 13: 2954. https://doi.org/10.3390/ma13132954
APA StyleJang, S., Ka, D., Jung, H., Kim, M.-K., Jung, H., & Jin, Y. (2020). Zr(OH)4/GO Nanocomposite for the Degradation of Nerve Agent Soman (GD) in High-Humidity Environments. Materials, 13(13), 2954. https://doi.org/10.3390/ma13132954





